Introduction
Bladder cancer is increasingly common globally and
its morbidity and mortality rates are the fourth and seventh
highest in men as estimated by the American Cancer Society in 2015,
respectively (1). Therefore, bladder
cancer is a major burden to public health. In total, ~75% of newly
diagnosed bladder cancer cases are non-muscle-invasive bladder
cancer (NMIBC) (2,3). The recommended treatment for patients
with NMIBC is transurethral resection of bladder tumor. However,
bladder cancers have a high recurrence rate, and ~25% of patients
with NMIBC develop into muscle-invasive bladder cancer following
treatment (4,5). Although numerous chemotherapeutic drugs
have been demonstrated to inhibit tumor recurrence and progression,
their toxic side effects and chemosensitivity reduce the overall
therapeutic effect for patients with NMIBC (6–9).
Therefore, the identification of novel adjuvants or alternative
agents for patients with NMIBC is urgently required. Previous
studies have revealed that certain plants and microorganisms have
anticancer effects, often characterized by low toxicity and few
side-effects (10,11).
Puerarin is the main isoflavone glycoside isolated
from the traditional Chinese herb Radix pueraria lobate
(12). Puerarin has been widely used
as an antidiuretic, antipyretic and diaphoretic due to its various
medicinal properties (12). Previous
studies have demonstrated that puerarin may be used to treat
neurodegenerative disorders (13,14) and
cardio-cerebrovascular disease (15,16). In
addition, puerarin may inhibit the apoptosis of human osteoblasts
through the extracellular signal-regulated kinase signaling pathway
(17). Puerarin may also exert
anticancer effects and inhibit the growth of esophageal cancer
cells, and this effect is associated with the mitochondrial pathway
(18). It also inhibits proliferation
and induces apoptosis in glioblastoma (19), gastric cancer (20) and colon cancer (21) cell lines. However, the effect of
puerarin on human bladder cancer are unclear, and the underlying
mechanisms remain elusive. Therefore, the present study
investigated the anticancer effects and potential mechanisms
underlying the effect of puerarin on human bladder cancer.
Materials and methods
Cell culture and reagents
Human bladder cancer T24 cell line and its
derivative, the EJ cell line, were purchased from the China Center
for Type Culture Collection (Wuhan University, Wuhan, China)
(22). The cells were maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Puerarin was purchased from Shandong Fangming
Pharmaceutical Group Co., Ltd. (Heze, China; injection grade;
Chinese FDA approval no. H20033292). Dimethyl sulfoxide was
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Fetal bovine serum (FBS) was obtained from Gibco; Thermo Fisher
Scientific, Inc. The bladder cancer T24 and EJ cell lines were
cultured in RPMI-1640 medium with 10% FBS and maintained at 37°C in
a humidified atmosphere of 5% CO2. The medium was
changed every 2–3 days, and cells were subcultured until they
reached 90% confluency prior to being harvested using trypsin.
Cell viability assay with Cell
Counting Kit-8 (CCK-8)
CCK-8 (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was utilized to quantify T24 and EJ cell
viability. Cells were seeded onto 96-well plates at a density of
1×105 cells/well for 24 h, and then incubated with
RPMI-1640 medium containing various dilutions of puerarin (0.01,
0.1, 1, 10 and 100 µmol/l) and negative control (completed
untreated) at 37°C in a 5% CO2 humidified atmosphere for
24, 48 and 72 h. Following incubation for the indicated times, 10
µl CCK-8 solution was added to each well and incubated for 2 h at
37°C to examine the effect of puerarin on bladder cancer cell
proliferation. Colorimetric analysis was performed at a wavelength
of 490 nm. Three independent experiments were performed in
triplicate.
Transwell cell invasion assays
T24 and EJ cells were seeded in 12-well culture
plate at a density of 4×105 cells/well and incubated
with puerarin (100 µmol/l) at 37°C in a 5% CO2
humidified atmosphere for 24, 48 and 72 h, with completely
untreated cells used as the negative control group. The cells were
then suspended in serum free RPMI-1640 medium and plated at a
density of 2×105 cells/well in the upper chamber of
Transwell plates with polycarbonate membranes (pore size, 8 µm) and
diluted Matrigel coating (BD Biosciences, Franklin Lakes, NJ, USA).
Complete medium (10% FBS RMPI-1640; 600 µl) was added to the lower
chamber. Following incubation for 18 h at 37°C in a 5%
CO2 humidified atmosphere, the cells that passed through
the filters into the bottom wells were fixed in 100% methanol for
30 min at 4°C and stained with 0.5% crystal violet for 15 min at
37°C. The number of cells in 10 randomly selected fields
(magnification, ×100) from each well was counted under an optical
microscope (CX21; Olympus Corporation, Tokyo, Japan). The invasion
assays were repeated at least three times.
Transmission electron microscopy
To observe the morphological changes of bladder
cancer cell lines induced by puerarin with different time and
concentration, T24 and EJ cells were pretreated with puerarin (100
µmol/l) for 0, 24, 48 and 72 h at 37°C, or were completely
untreated in the negative control group. Additionally, T24 cells
were treated with different concentrations of puerarin (0, 1, 10
and 100 µmol/l) for 72 h at 37°C. The cells were then collected
with 447.2 × g centrifugation for 5 min at room temperature and
fixed with 2.5% glutaraldehyde for 2 h at 4°C. Then the sample was
treated with 1% osmium tetroxide for 30 min at 4°C and dehydrated
in increasing concentrations of acetone (50, 70, 90 and 100%; cat
no. PYG0013; Boster Biological Technology, Pleasanton, CA, USA) at
room temperature. The sample was embedded in embedding resin (cat
no. 18109; Epon 812 embedding kit; Ted Pella, Inc., CA, USA) for 24
h at 60°C, and a 50 mm ultrathin section was prepared with a
microtome. The ultrastructure of cells was detected by transmission
electron microscopy (Tecnai G2, FEI; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at ×100 magnification.
Cell cycle and apoptosis assay by flow
cytometry (FCM)
T24 cells were seeded in 4- wells plates at a
density of 4×105 cells/well and incubated with puerarin
(100 µmol/l) at 37°C in a 5% CO2 humidified atmosphere
for 0, 24, 48 and 72 h, and control cells being completely
untreated. The cells were then collected by trypsin and
centrifugation. A total of 500 µl binding buffer (including
precooled 70% ethanol and 0.5 mmol/l EDTA) was added to each tube
and incubated overnight in a 4°C refrigerator. Cells were then
resuspended, centrifuged at a speed of 447.2 × g for 5 min at room
temperature and washed twice with PBS. PBS containing 0.1% Triton
X-100 and 50 µg/ml RNAse was applied to the resuspended cells.
Subsequently, cells were incubated in 90 µl propidium iodide (PI)
buffer (Sigma-Aldrich; Merck KGaA) for 30 min at room temperature
in the dark, and cell cycle analyses were performed by FCM within 1
h. Cells were collected and subjected to Annexin V and PI staining
using an Annexin V-fluorescein isothiocyanate apoptosis detection
kit (Vazyme, Piscataway, NJ, USA), following the manufacturer's
protocol. Apoptotic cells were then detected by FCM (BD
FACSCalibur; BD Biosciences).
Cell apoptosis detection by terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
staining
T24 and EJ cells with 4×105/well cell
density were pretreated with puerarin (0 and 100 µmol/l) and
incubated at 37°C in a 5% CO2 humidified atmosphere for
24, 48 and 72 h, with negative control cells being completely
untreated. Cell apoptosis was analyzed by TUNEL assay, according to
the manufacturer's protocol (TUNEL apoptosis assay kit; Roche
Diagnostics GmbH, Mannheim, Germany). Apoptosis of cells was
analyzed by counting the positive cells, as well as the total
number of cells, at 10 randomly selected fields at ×400
magnification in a blinded manner using a fluorescence microscope
(IX71; Olympus Corporation).
Western blot analysis
To observe the effect of puerarin on the expression
level of protein in bladder cancer lines by different time and
concentration, T24 cells, following treatment with puerarin (0 and
100 µmol/l), were incubated for 0, 24, 48 and 72 h, respectively.
Additionally, T24 and EJ cells were treated with different
concentrations of puerarin (0, 1 and 100 µmol/l) for 72 h,
respectively. Cells were then collected using trypsin and were
centrifuged at a speed of 12,745.2 × g for 15 min at 4°C, and lysed
for 30 min at 4°C by lysis buffer containing 50 mM Tris (pH 7.4),
10% glycerol, 50 mM NaCl, 1 mM EDTA and 1% Triton X-100. All
protein extraction processes were performed according to the
product specification. A bicinchoninic protein assay kit (Beyotime
Institute of Biotechnology, Haimen, China) was used to measure the
protein concentration. Equal amounts of protein (50 µg) were then
separated by electrophoresis on 12% SDS-PAGE and proteins were
transferred to polyvinylidene fluoride membranes. The membranes
were blocked in 5% non-fat milk solution for 2–4 h at 4°C in a
freezer and washed twice with TBST solution (0.1% Tween-20).
Membranes were incubated overnight at 4°C with the following
antibodies: Polyclonal rabbit mammalian target of rapamycin (mTOR;
cat no. A00003; 1:1,000, Boster Biological Technology, Pleasanton,
CA, USA); polyclonal rabbit phosphorylated (p-) mTOR (cat no.
20301782-1; 1:2,000; Bioworld Technology, Inc., St. Louis Park, MN,
USA); polyclonal rabbit P70-S6 kinase 1 (p70S6K; cat no.
20313303-1; 1:2,000; Bioworld Technology, Inc.); and polyclonal
rabbit p-p70S6K (cat no. 20314827-1; 1:2,000; Bioworld Technology,
Inc.); β-actin (cat no. BM0626; 1:1,000; Boster Biological
Technology). Membranes were then washed three times with TBST
solution, incubated with horseradish peroxidase-conjugated
secondary antibodies (goat anti-mouse immunoglobulin G; cat no.
BA1051 and cat no. BA1055; 1:800; Boster Biological Technology) for
2 h at 4°C freezer, and the expression of proteins was then
detected with an enhanced chemiluminescence kit (super-sensitive
ECL ready-to-use substance kit; cat no. AR1173; Boster Biological
Technology) with GeneSys imaging software (Gene Gnome, version:
1.5.9.0; Syngene, Frederick, MD, USA).
Statistical analysis
SPSS software (version 16; SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. All data were calculated as
the mean ± standard deviation. One-way ANOVA and the
Student-Neuman-Keuls method were utilized to analyze the results
between treated and control groups, and a Student's t test was used
to compare two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
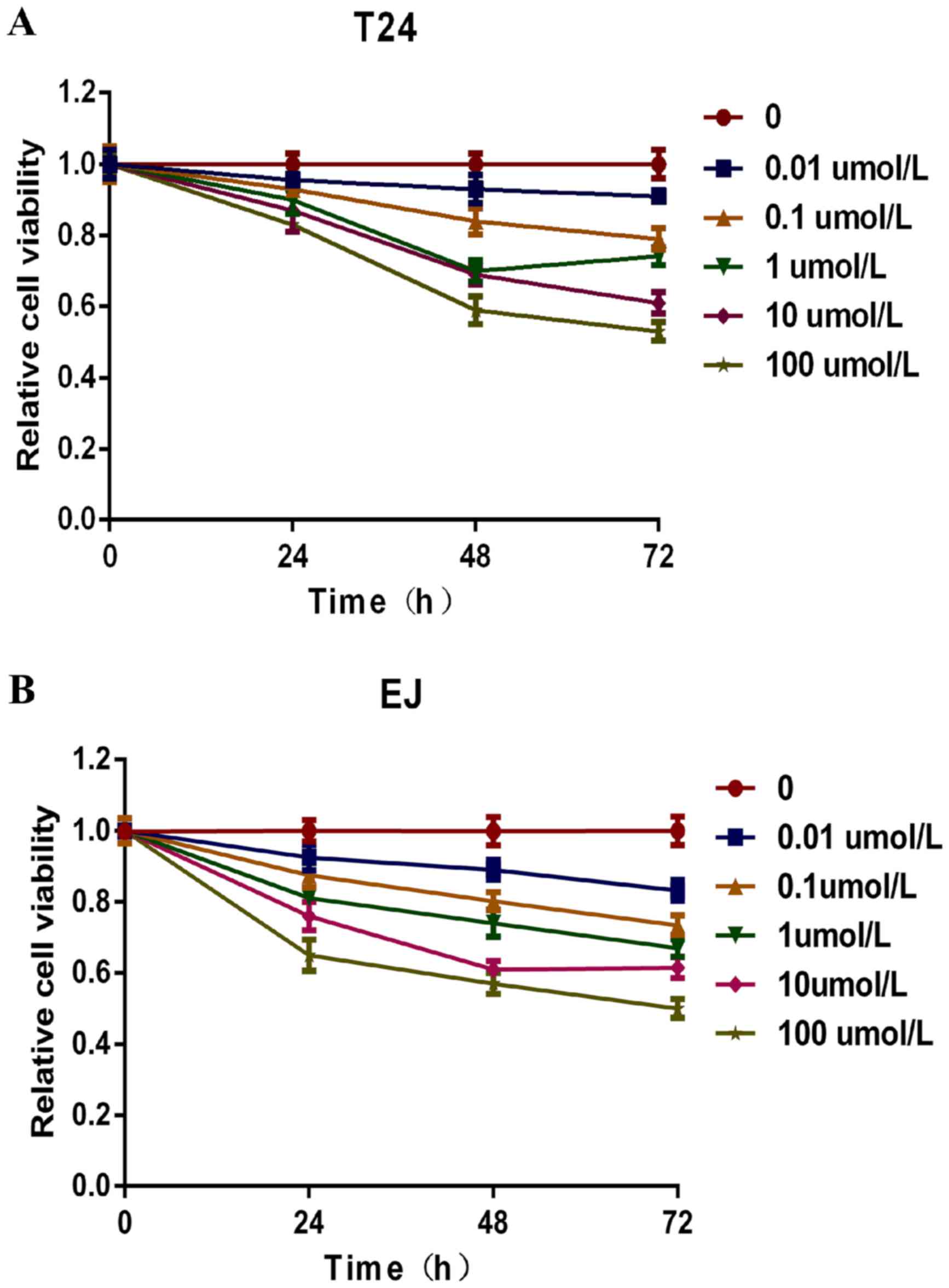
Puerarin inhibits the viability of
bladder cancer cells
Human bladder T24 and EJ cells were pretreated with
puerarin (0, 0.01, 0.1, 1, 10 and 100 µmol/l) for 24, 48 and 72 h.
Subsequently, cell viability was measured using the CCK-8 assay,
and cell viability was revealed to be inhibited in a concentration-
and time-dependent manner by puerarin treatment. As presented in
Fig. 1, the relative viability of T24
and EJ cells following 100 µmol/l puerarin treatment for 72 h was
47 and 50%, respectively, compared with the untreated control
cells. With increased incubation time, the relative cell viability
of T24 and EJ cells treated with puerarin decreased compared with
the untreated control cells. Therefore, a dosage of 100 µmol/l
puerarin and incubation periods of 24, 48 and 72 h were used for
subsequent experiments.
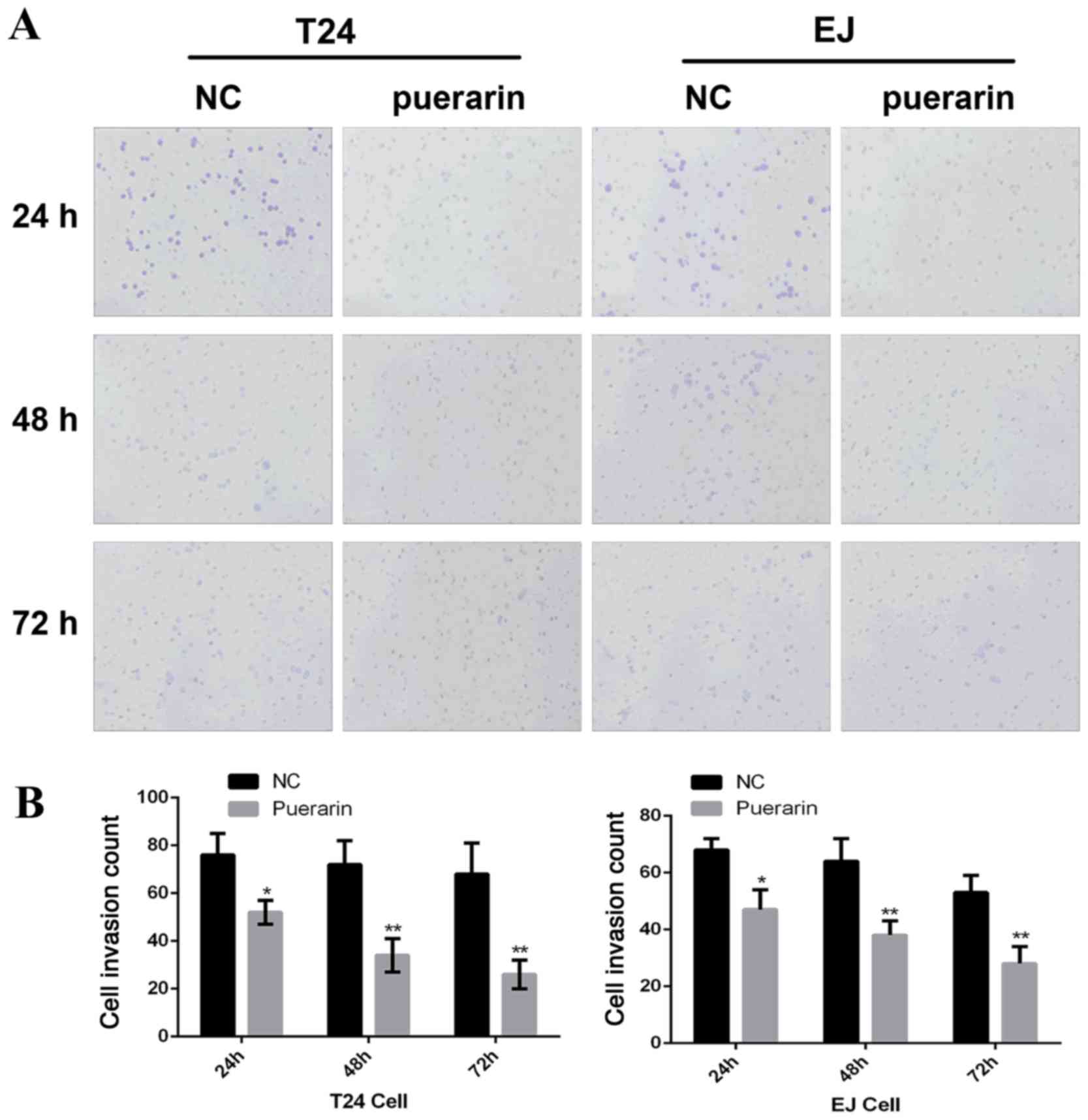
Puerarin inhibits the invasion of
bladder cancer cells
Following pretreatment of human bladder cancer T24
and EJ cells with 0 or 100 µmol/l puerarin for 24, 48 and 72 h,
cell invasion was measured by Transwell assay. As presented in
Fig. 2, puerarin treatment
significantly inhibited the invasion of bladder cancer T24 and EJ
cells, compared with the control group (P<0.05).
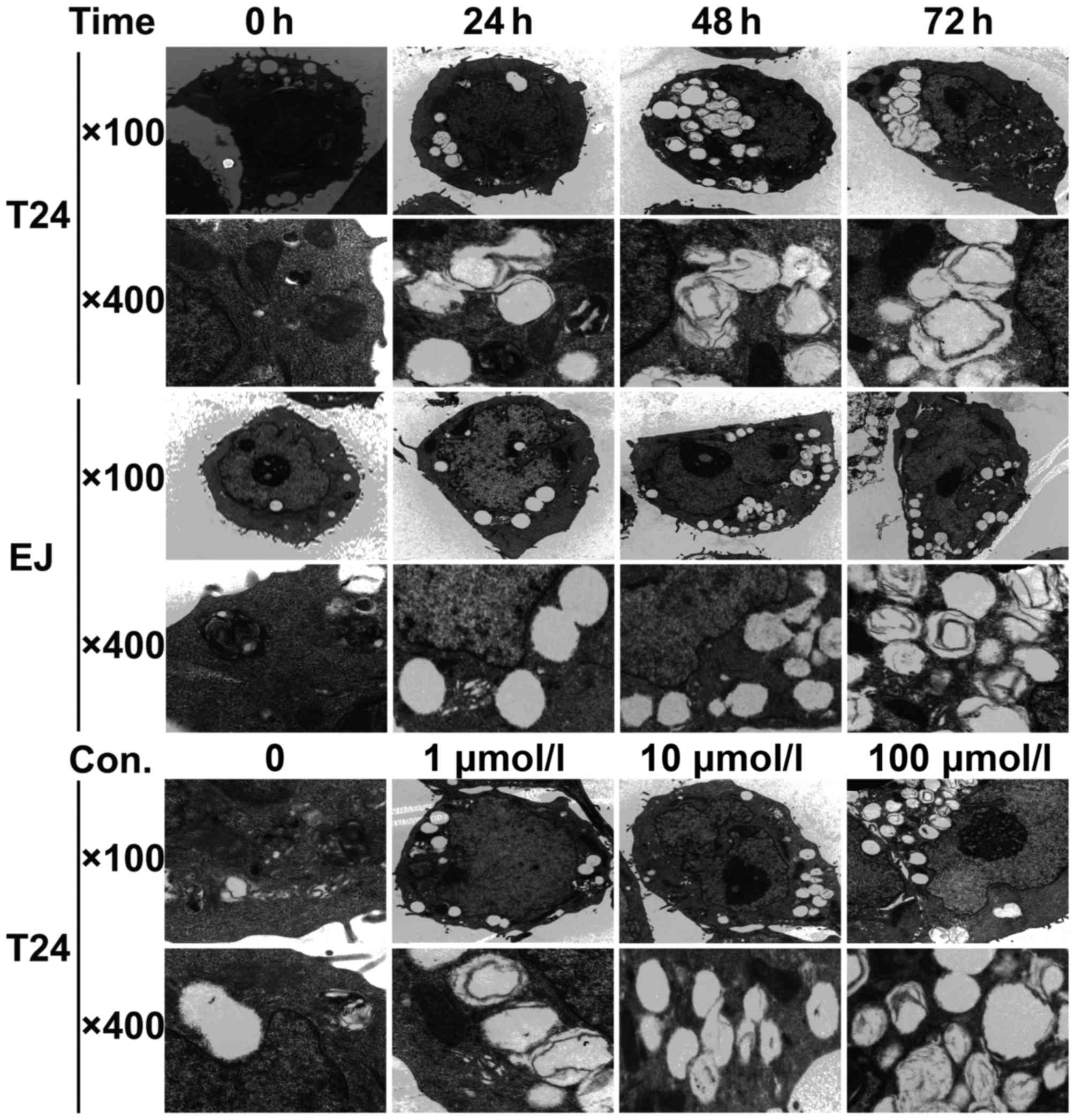
Morphological changes in bladder
cancer cells are induced by puerarin treatment
Transmission electron microscopy was used to examine
the morphological changes induced by puerarin. As presented in
Fig. 3, the untreated control groups
contained cells with intact nuclear membranes, large and circular
nuclei, increased chromatin, abundant mitochondria and endoplasmic
reticulum with normal morphology. However, for T24 and EJ cells
treated with puerarin for 24 h, lumped chromatin accumulation was
observed inside the nuclear membrane, the mitochondria were
impaired due to pyknosis, resulting in membrane ruptures and fewer
mitochondria overall in the puerarin-treated group, and a large
number of autophagocytic vacuoles were formed. Compared with
puerarin treatment for 24 h, when the T24 and EJ cells were
incubated with puerarin for 48 and 72 h, the cells became smaller,
an increased number of organelles were lost, the nuclear membranes
were partially disrupted and the nuclei were broken up (Fig. 3). The T24 cells treated with different
concentrations of puerarin (1, 10 and 100 µmol/l) for 72 h
exhibited the same inhibitory effect (Fig. 3).
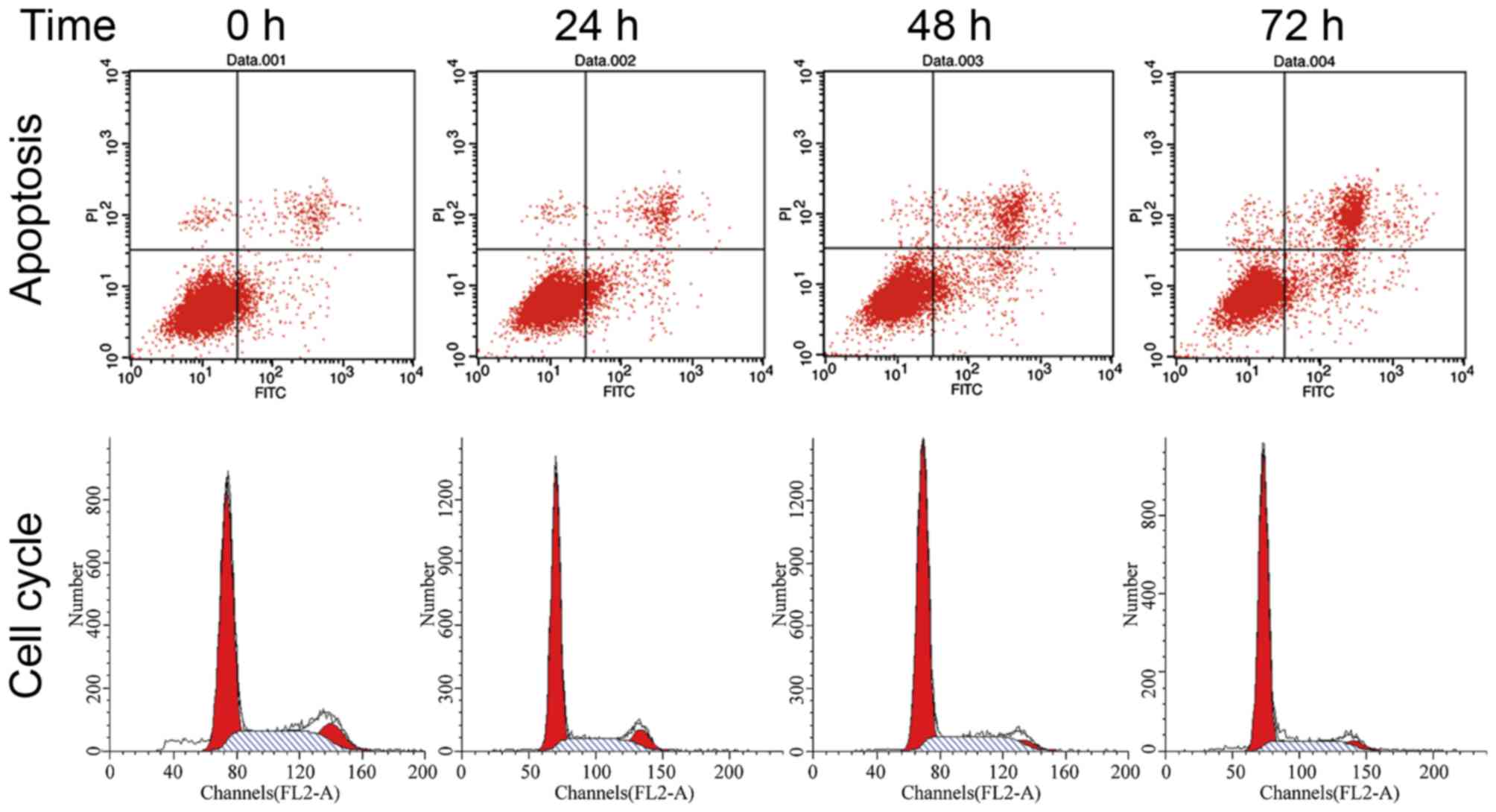
Puerarin affects the cell cycle and
apoptosis of bladder cancer cells
FCM was applied to detect alterations in the cell
cycle distribution induced by puerarin treatment in the T24 cell
line. As presented in Fig. 4,
compared with the 0 h (completed untreated) control group,
cultivating T24 cells with 100 µmol/l puerarin for 24, 48 and 72 h
resulted in 10.5, 12.8 and 17.5% increases in the percentage of
cells in the G0/G1 phase, respectively. This was accompanied by a
decrease in the percentage of cells in the G2/M phase, but no
significant difference was observed in the percentage of cells in
the S phrase, which indicated that puerarin induces cell cycle
arrest at the G0/G1 phase in bladder cancer T24 cells. The results
also revealed that the apoptotic rate of T24 cells increased
following treatment with puerarin for 24, 48 and 72 h (Fig. 4).
Apoptotic effect of puerarin in
bladder cancer cells
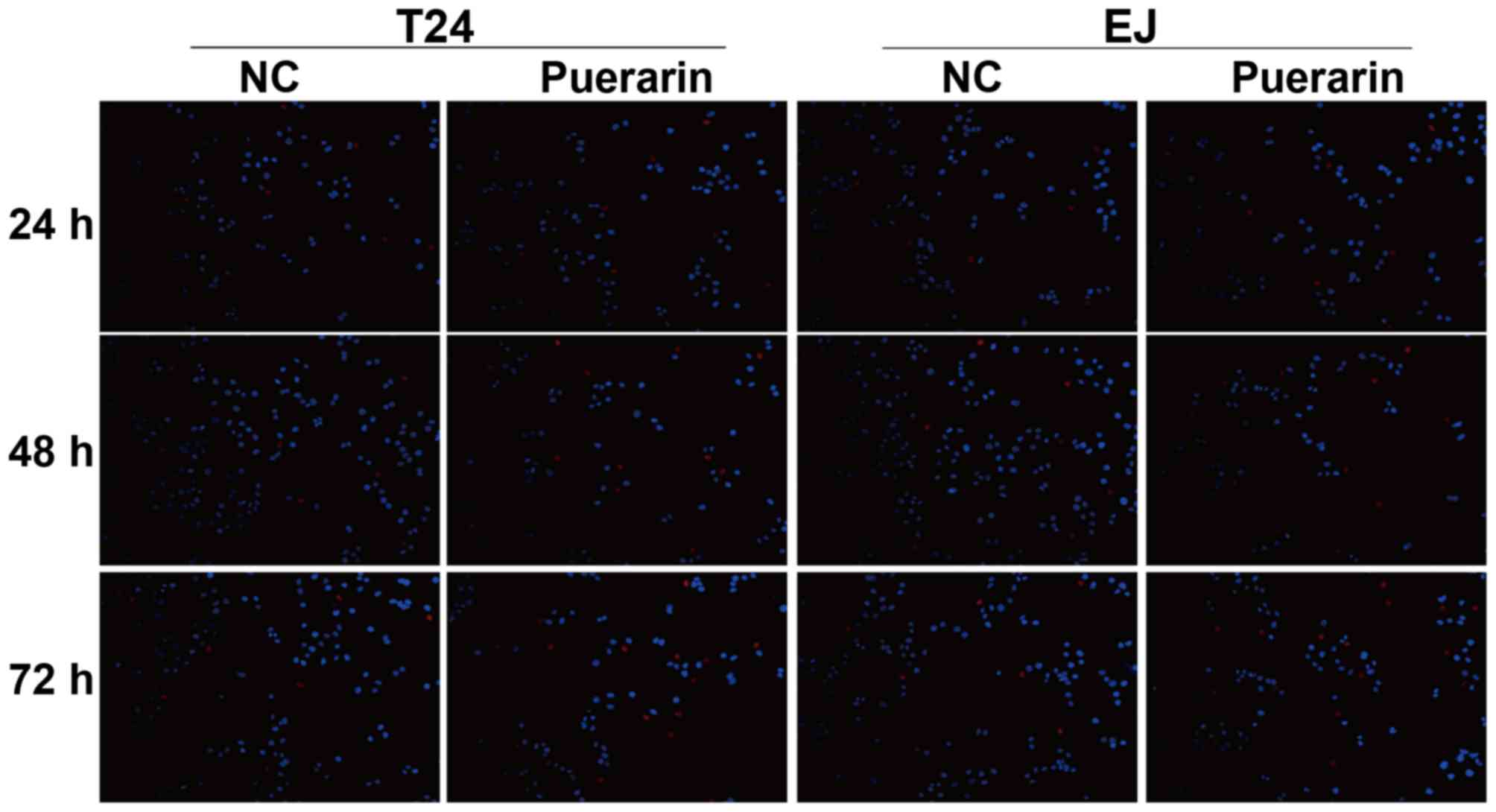
A TUNEL assay was performed to detect the apoptosis
rate of bladder cancer T24 and EJ cells treated with puerarin.
Compared with the control group, the results demonstrated the
apoptosis of T24 and EJ cells was increased following treatment
with puerarin for 24, 48 and 72 h (Fig.
5).
Antitumor effects of puerarin are
mediated by the mTOR signaling pathway
The mTOR signaling pathway is involved in the
tumorigenesis, development and prognosis of bladder urothelium
carcinoma, and is essential for the regulation of autophagy
(23,24). Therefore, to investigate the
mechanisms underlying puerarin-induced cell apoptosis, western
blotting was performed to confirm the expression levels of
associated proteins. As is presented in Fig. 6, compared with the control group, the
protein expression level of p-mTOR and p-p70S6K decreased in a
dose-dependent manner following puerarin-treatment in T24 and EJ
cells, while no changes were observed in the expression level of
mTOR and p70S6K in puerarin-treated T24 and EJ cells. In addition,
the level of p-mTOR and p-p70S6K decreased in a time-dependent
manner following treatment of T24 cells with puerarin, while no
changes were observed in the expression level of mTOR and p70S6K in
puerarin-treated T24 cells (Fig.
6).
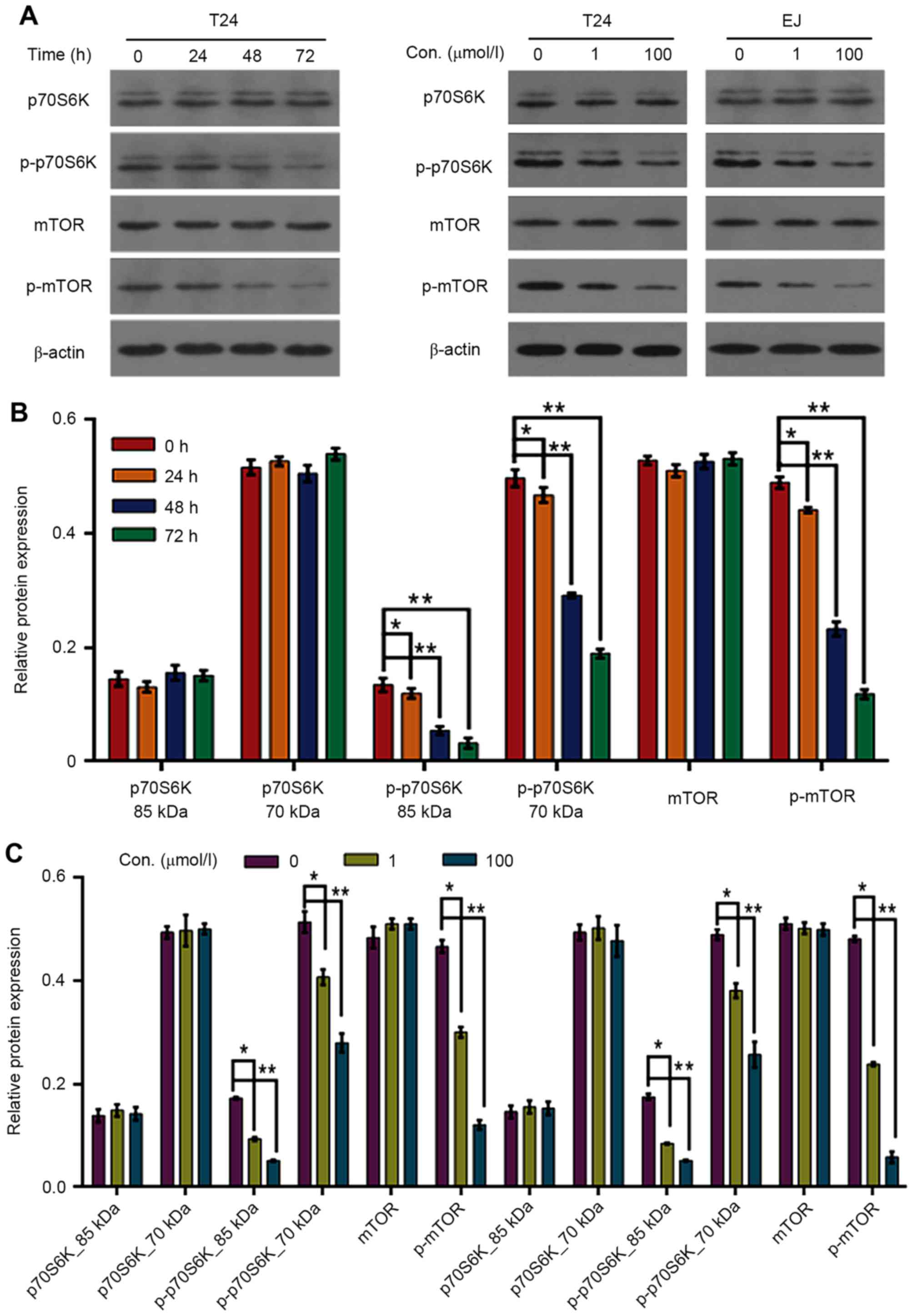 | Figure 6.Effect of puerarin on the expression
levels of mTOR/p70S6K signaling pathway proteins in T24 and EJ cell
lines. (A) Protein levels of mTOR, p70S6K, p-mTOR and p-p70S6K in
T24 and EJ cells following puerarin treatment were detected by
western blot analysis; (B) Relative protein expression following
puerarin treatment in T24 cells for 0, 24, 48 and 72 h. *P<0.05,
**P<0.01 vs. 0 h. (C) Relative protein expression following
puerarin treatment in T24 and EJ cells at 0, 1 and 100 µmol/l.
*P<0.05, **P<0.01 vs. 0 µmol/l. mTOR, mechanistic target of
rapamycin signaling; p-mTOR, phospho-mTOR; p70S6K, p70-S6 kinase;
p-p70S6K, phosphor-P70S6K. |
Discussion
The search for alternative anticancer agents has led
to renewed interest in traditional medicine (25,26).
Puerarin, as a traditional Chinese medicine, has protective effects
on the nervous and cardiovascular system and may additionally
prevent osteoporosis, inflammation and liver injury (14,15,17).
Multiple studies have revealed that puerarin induces cell
apoptosis, suppresses cell proliferation and increases the
chemosensitivity of cancer cells (19–21). Thus,
in the present study, a detailed investigation was conducted on the
effects of puerarin on bladder cancer cells. As revealed by CCK-8
assay, the viability of T24 and EJ cells was inhibited by puerarin
in a dose- and time-dependent manner. The morphological changes
induced by puerarin indicated that cells were undergoing apoptosis.
With the TUNEL assay, puerarin treatment was revealed to
significantly promote the proportion of apoptotic cells. Puerarin
may induce bladder cancer cell autophagy and induce cell cycle
arrest at the G0/G1 phase. In addition, Transwell assays revealed
that puerarin may inhibit cell invasion. These results confirmed
that puerarin treatment resulted in an inhibitory effect on bladder
cancer cells.
The mTOR signaling pathway is involved in cancer
pathogenesis and progression. p70S6K, which is downstream of the
mTOR signaling pathway, is associated with tumor formation
(23,27). Loss of p70S6K promotes cell cycle
progression and cell proliferation (27). p70S6K also mediates the effects of
oncogenic protein kinase B (Akt) signaling on mRNA translation,
cell growth and tumor progression (28). The activation of the mTOR/S6K axis
stimulates protein synthesis and cell growth (29). Notably, the mTOR signaling pathway is
associated with regulation of energy balance and autophagy as a
survival pathway (24). Autophagy is
a major degradation pathway in eukaryotic cells, which is essential
for removing damaged organelles and macromolecules from the
cytoplasm and recycling amino acids during periods of starvation
(30,31). Therefore, the present study
investigated whether inhibition of the mTOR signaling pathway by
puerarin leads to the induction of autophagy. The results indicated
that the protein expression levels of p-mTOR and p-p70S6K decreased
in a time-dependent manner following treatment of bladder cancer
cells with puerarin, and that no changes were observed in the
protein expression level of mTOR and p70S6K following puerarin
treatment in bladder cancer cells.
Yu and Li (21)
reported that puerarin induces apoptosis in colon cancer HT-29
cells and suppressing cell proliferation, and puerarin treatment
increases the expression level of BCL2-associated X protein (Bax)
and decreases the expression level of c-myc and B cell lymphoma-2
(Bcl-2) (21). Yang et al
(19) also reported that puerarin
exerts antitumor effects through suppressing the expression of
p-Akt and Bcl-2, and promotes the expression of Bax in glioblastoma
cells. Puerarin inhibited proliferation and induced apoptosis in
SMMC-7721 hepatocellular carcinoma cells via the
mitochondria-dependent pathway (32).
In addition, combined with or without 5-fluorouracil, puerarin
significantly suppressed proliferation and markedly increased
apoptosis in esophageal cancer cells in vitro and in
vivo (18). These previous
studies demonstrated similar results to the present study, and thus
strengthen them.
A number of previous studies have demonstrated that
cell cycle arrest is associated with the inhibition of cancer cell
proliferation. For example, Lin et al (33) demonstrated that puerarin inhibits the
growth of breast cancer cells through inducing apoptosis and cell
cycle arrest in the G2/M phase. Although the results of the present
study may differ from those of Lin et al (33), Gan and Yin (34) reported that puerarin treatment leads
to cell proliferation inhibition via inducing mantle cell lymphoma
cell cycle arrest in the G1 phase, and this mechanism may involve
the phosphoinositide-3 kinase/Akt and nuclear factor-κB signaling
pathway. In accordance with this previous study, the results of the
present study revealed that puerarin induces bladder cancer cell
cycle arrest at the G0/G1 phase. Due to the aforementioned results,
it may be concluded that puerarin affects bladder cancer cell
viability and induces apoptosis, which is mediated by the
mTOR/p70S6K signaling pathway.
Although puerarin has already been widely applied in
experimental research and clinical trials in China with high
efficiency (14,15,17,35,36),
limitations exist. The low aqueous solubility and intestinal
permeability values may lead to a lower blood concentration
following oral administration of puerarin (37). In order to acquire improved
therapeutic effects of puerarin, investigators are attempting to
design nanoparticles or other puerarin encapsulations and delivery
systems to improve the effect of treatment (38).
In conclusion, the present study demonstrated that
puerarin-induced apoptosis in human bladder cancer cells was
mediated by activation of the mTOR/p70S6K signaling pathway.
Puerarin may serve as a novel therapeutic strategy in the
inhibition of carcinogenesis and progression of bladder cancer.
However, additional studies are required to affirm the effect of
puerarin on bladder cancer in vivo and to verify whether
puerarin may be used as part of an intravesical treatment.
Acknowledgements
The present study was funded by the Hubei Province
Health and Family Planning Scientific Research Project (grant no.
WJ2017M257), Natural Science Foundation of Hubei Province of China
(grant nos. 2014CFC1068 and 2017CFB516) and the Science and
Technology Project of Enshi of China (grant no. 2013, 2014).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A,
Redorta J Palou, et al: EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder: Update 2013. Eur Urol.
64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S and Lotan Y: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schenk-Braat EA and Bangma CH:
Immunotherapy for superficial bladder cancer. Cancer Immunol
Immunother. 54:414–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen Z, Shen T, Wientjes MG, O'Donnell MA
and Au JL: Intravesical treatments of bladder cancer: Review. Pharm
Res. 25:1500–1510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bosschieter J, Nieuwenhuijzen JA, van
Ginkel T, Vis AN, Witte B, Newling D, Beckers GMA and Moorselaar
RJAV: Value of an immediate intravesical instillation of mitomycin
C in patients with non-muscle-invasive bladder cancer: A
prospective multicentre randomised study in 2243 patients. Eur
Urol. July 10–2017.(Epub ahead of print). View Article : Google Scholar
|
|
7
|
Deng T, Liu B, Duan X, Zhang T, Cai C and
Zeng G: Systematic review and cumulative analysis of the
combination of mitomycin C plus Bacillus Calmette-Guérin (BCG) for
non-muscle-invasive bladder cancer. Sci Rep. 7:31722017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hadaschik BA, ter Borg MG, Jackson J,
Sowery RD, So AI, Burt HM and Gleave ME: Paclitaxel and cisplatin
as intravesical agents against non-muscle-invasive bladder cancer.
BJU Int. 101:1347–1355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang SJ, Ye LY and Meng FH: Comparison of
intravesical bacillus Calmette-Guerin and mitomycin C
administration for non-muscle invasive bladder cancer: A
meta-analysis and systematic review. Oncol Lett. 11:2751–2756.
2016.PubMed/NCBI
|
|
10
|
Basmadjian C, Zhao Q, Bentouhami E, Djehal
A, Nebigil CG, Johnson RA, Serova M, de Gramont A, Faivre S,
Raymond E and Désaubry LG: Cancer wars: Natural products strike
back. Front Chem. 2:202014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orang-Ojong BB, Munyangaju JE, Wei MS, Lin
M, Wei FG, Foukunang C and Zhu Y: Impact of natural resources and
research on cancer treatment and prevention: A perspective from
Cameroon. Mol Clin Oncol. 1:610–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keung WM and Vallee BL: Kudzu root: An
ancient Chinese source of modern antidipsotropic agents.
Phytochemistry. 47:499–506. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim MH, Kim SH and Yang WM: Mechanisms of
action of phytochemicals from medicinal herbs in the treatment of
Alzheimer's disease. Planta Med. 80:1249–1258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Xiong J, Liu S, Wang L, Huang J,
Liu L, Yang J, Zhang G, Guo K, Zhang Z, et al: Puerarin protects
dopaminergic neurons in Parkinson's disease models. Neuroscience.
280:88–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan Y, Liu M and Wu B: Puerarin for acute
ischaemic stroke. Cochrane Database Syst Rev. 23:CD0049552008.
|
|
16
|
Wang Q, Wu T, Chen XY, Duan X, Zheng J,
Qiao J, Zhou L, Wei J and Ni J: WITHDRAWN: Puerarin injection for
unstable angina pectoris. Cochrane Database Syst Rev: CD004196.
2016. View Article : Google Scholar
|
|
17
|
Liu LJ, Liu LQ, Bo T, Li SJ, Zhu Z, Cui RR
and Mao DA: Puerarin suppress apoptosis of human osteoblasts via
ERK signaling pathway. Int J Endocrinol. 2013:7865742013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Yang ZR, Guo XF, Song J, Zhang JX,
Wang J and Dong WG: Synergistic effects of puerarin combined with
5-fluorouracil on esophageal cancer. Mol Med Rep. 10:2535–2541.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang JA, Li JQ, Shao LM, Yang Q, Liu BH,
Wu TF, Wu P, Yi W and Chen QX: Puerarin inhibits proliferation and
induces apoptosis in human glioblastoma cell lines. Int J Clin Exp
Med. 8:10132–10142. 2015.PubMed/NCBI
|
|
20
|
Guo XF, Yang ZR, Wang J, Lei XF, Lv XG and
Dong WG: Synergistic antitumor effect of puerarin combined with
5-fluorouracil on gastric carcinoma. Mol Med Rep. 11:2562–2568.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu Z and Li W: Induction of apoptosis by
puerarin in colon cancer HT-29 cells. Cancer Lett. 238:53–60. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Toole CM, Povey S, Hepburn P and Franks
LM: Identity of some human bladder cancer cell lines. Nature.
301:429–430. 1983. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ching CB and Hansel DE: Expanding
therapeutic targets in bladder cancer: The PI3K/Akt/mTOR pathway.
Lab Invest. 90:1406–1414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen M, Gu J, Delclos GL, Killary AM, Fan
Z, Hildebrandt MA, Chamberlain RM, Grossman HB, Dinney CP and Wu X:
Genetic variations of the PI3K-AKT-mTOR pathway and clinical
outcome in muscle invasive and metastatic bladder cancer patients.
Carcinogenesis. 31:1387–1391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
To KK, Au-Yeung SC and Ho YP: Differential
nephrotoxicity of cisplatin and a novel series of traditional
Chinese medicine-platinum anticancer agents correlates with their
chemical reactivity towards sulfur-containing nucleophiles.
Anticancer Drugs. 17:673–683. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mehta RG, Murillo G, Naithani R and Peng
X: Cancer chemoprevention by natural products: How far have we
come? Pharm Res. 27:950–961. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chi BH, Kim SJ, Seo HK, Seo HH, Lee SJ,
Kwon JK, Lee TJ and Chang IH: P70S6K and Elf4E dual inhibition is
essential to control bladder tumor growth and progression in
orthotopic mouse non-muscle invasive bladder tumor model. J Korean
Med Sci. 30:308–316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nawroth R, Stellwagen F, Schulz WA, Stoehr
R, Hartmann A, Krause BJ, Gschwend JE and Retz M: S6K1 and 4E-BP1
are independent regulated and control cellular growth in bladder
cancer. PLoS One. 6:e275092011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tavares MR, Pavan IC, Amaral CL,
Meneguello L, Luchessi AD and Simabuco FM: The S6K protein family
in health and disease. Life Sci. 131:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arroyo DS, Gaviglio EA, Ramos JM Peralta,
Bussi C, Rodriguez-Galan MC and Iribarren P: Autophagy in
inflammation, infection, neurodegeneration and cancer. Int
Immunopharmacol. 18:55–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang WG, Liu XF, Meng KW and Hu SY:
Puerarin inhibits growth and induces apoptosis in SMMC-7721
hepatocellular carcinoma cells. Mol Med Rep. 10:2752–2758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin YJ, Hou YC, Lin CH, Hsu YA, Sheu JJ,
Lai CH, Chen BH, Lee Chao PD, Wan L and Tsai FJ: Puerariae radix
isoflavones and their metabolites inhibit growth and induce
apoptosis in breast cancer cells. Biochem Biophys Res Commun.
378:683–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gan M and Yin X: Puerarin induced in
mantle cell lymphoma apoptosis and its possible mechanisms
involving multi-signaling pathway. Cell Biochem Biophys.
71:367–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao Z, Wei B and Qian C: Puerarin
injection for treatment of unstable angina pectoris: A
meta-analysis and systematic review. Int J Clin Exp Med.
8:14577–14594. 2015.PubMed/NCBI
|
|
36
|
Liang F and Xie S: Puerarin prevents tumor
necrosis factor-α-induced apoptosis of PC12 cells via activation of
the PI3K/Akt signaling pathway. Exp Ther Med. 14:813–818. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H, Dong L, Liu Y, Wang G, Wang G and
Qiao Y: Biopharmaceutics classification of puerarin and comparison
of perfusion approaches in rats. Int J Pharm. 466:133–138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu X, Ding Y, Zhao B, Liu Y, Luo S, Wu J,
Li J and Xiang D: In vitro and in vivo evaluation of
puerarin-loaded PEGylated mesoporous silica nanoparticles. Drug Dev
Ind Pharm. 42:2031–2037. 2016. View Article : Google Scholar : PubMed/NCBI
|