Introduction
Uveal melanoma is a rare but life-threatening
disease (1). The annual incidence of
choroidal melanoma is between 4 and 7 cases per million individuals
and this rate has remained stable over the last twenty years
(2,3).
The 15-year melanoma-associated mortality rate in the US is between
40 and 50%, primarily due to liver metastasis (4). Eye-sparing treatments have replaced
enucleation as the most common initial therapy for small and
medium-sized choroidal melanomas. Radiotherapy serves an important
role in organ conservation when treating patients with intraocular
tumors and retained visual function. Plaque brachytherapy,
stereotactic radiotherapy and proton beam radiotherapy are the most
common treatments (5,6).
Gamma knife stereotactic radiosurgery (GK-SRS) was
initially designed to treat brain targets with the skull rigidly
fixed to the stereotactic coordinate frame. When these targets are
around the eyes, the positioning and mobility of the eyes result in
the use of GK-SRS to precisely position the eye for treatment
challenging. Different approaches include the use of suction
devices, retro- or parabulbar anesthesia and active fixation on a
light point by the patient (7,8).
The present case study analyzes the use of a novel
GK-SRS protocol and endo diode laser thermotherapy to treat a
patient with intraocular melanoma at Taipei Medical
University-Shuang Ho Hospital (New Taipei City, Taiwan, R.O.C).
Furthermore, the precision and safety of GK-SRS as a primary
treatment for uveal melanoma was evaluated. The patient provided
informed written consent to participate in the present study and
for the publication of patient data and photographs.
Case report
History
A 57-year-old female without a history of underlying
disease presented with increasing deviation of the right eye with
initial pain in October, 2011. On physical examination, the
patient's visual acuity was 0.8 in the right and left eye, and
intraocular pressure was 15 mmHg in the right eye, within the
normal range. A fundoscopic examination revealed a choroidal
melanoma near the macula, surrounding the optic nerve fovea of the
right eye. A B-scan demonstrated that the internal reflectivity was
consistent with a uveal melanoma. An orbital magnetic resonance
imaging (MRI) scan was performed, which revealed a small nodular
lesion in the right choroid near the macula without extraorbital
extension. The basal dimensions of the tumor were 9.5×8.9 mm and
the thickness was 4.1 mm. A computed tomography (CT) scan was
performed that demonstrated no evidence of lung or liver
metastasis. Following the tumor survey, a right eye stage IIA
(cT2aN0M0; according to the 7th edition of the American Joint
Committee on Cancer staging manual) (9) choroidal melanoma with retained visual
function was diagnosed through the aforementioned eye examinations
and imaging tests (ophthalmoscopy, ultrasound, orbital MRI and CT).
Subsequently, the patient underwent Leksell Gamma Knife
Perfexion® stereotactic radiosurgery at Taipei Medical
University-Shuang Ho Hospital.
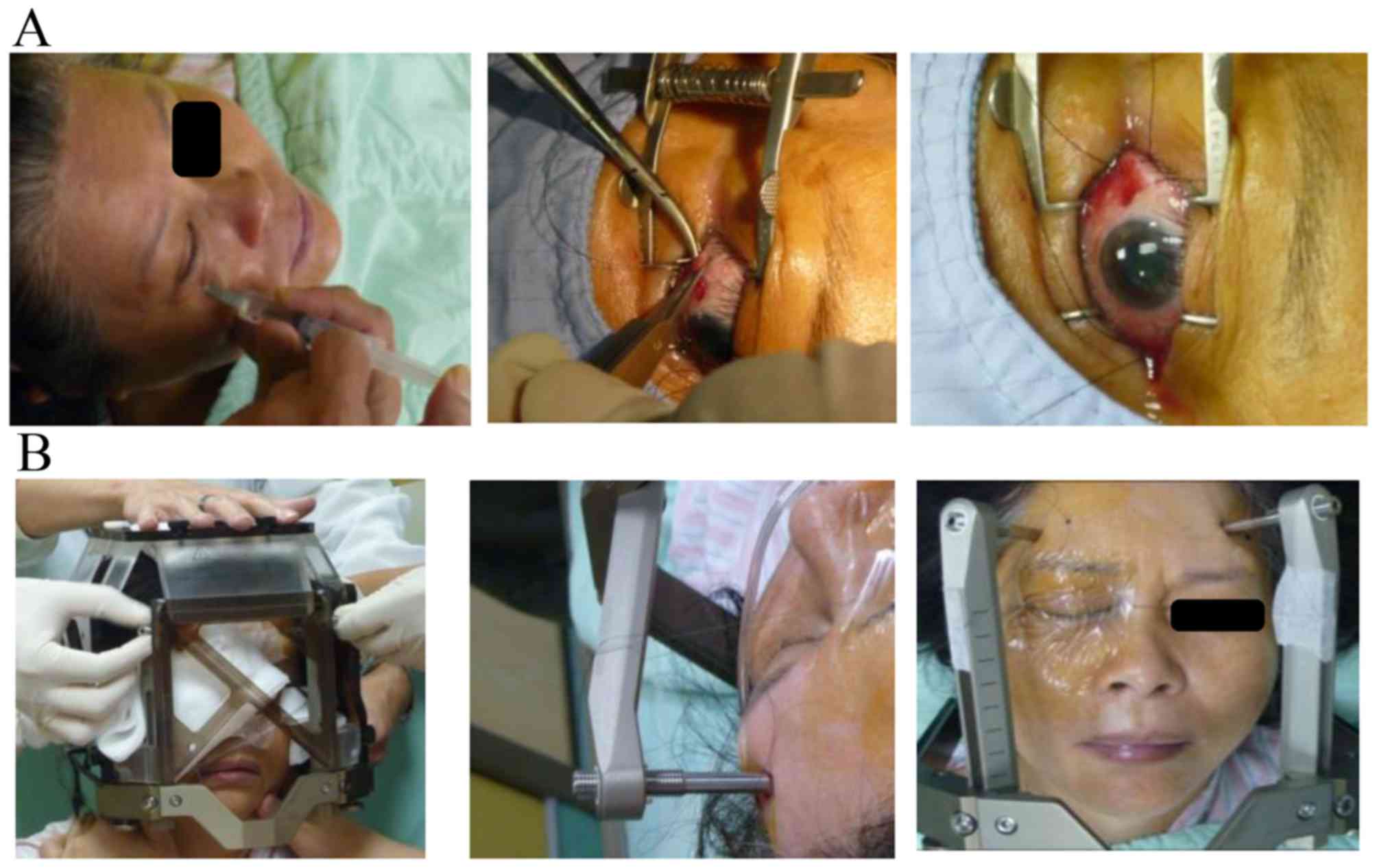
Eyeball fixation
A prophylactic Levofloxacin ophthalmic solution was
administered to prevent infection. Following the patient receiving
retrobulbar anesthesia with long-acting marcaine (0.5%, 20 ml) to
produce complete akinesia, the ophthalmologist sutured two
extraocular muscles, on the basis of the tumor location (the medial
rectus and lateral rectus).
Stereotactic frame fixation
A Leksell G stereotactic frame (Elekta Instrument
AB, Stockholm, Sweden) was fixed to the patient's head using four
pins. The frame provided the coordinate system for target
localization. The midline of the stereotactic system was placed
close to the eye to be treated. Subsequently, the threads of the
two sutured muscles were fixed to the stereotactic frame to
immobilize the eye throughout the procedure (Fig. 1).
Imaging
The stereotactic frame, with MRI and CT N-rod
localizer boxes, was used to obtain MRI and CT data.
High-resolution contrast-enhanced MRI scans (Signa HDx 1.5T MRI
with a 1-mm slice interval) of the brain were acquired and the
images were loaded to the gamma knife treatment planning system for
Gamma Plan dose planning (Leksell GammaPlan® 9.0; Elekta
Instrument AB). CT images were acquired using a Philips 16-slice CT
scanner (Brilliance Big Bore; Philips Healthcare, Cleveland, OH,
USA) with a 1-mm slice thickness (Fig.
2).
Dose planning
The gross volume of the melanoma was defined using
the MRI scan sections in three-dimensional reconstructions: Axial,
sagittal and coronal. A conformation treatment planning technique
was used to apply conformal irradiation to the tumor and healthy
tissue (the lens and optic nerve). The plan was designed to
encompass the entire tumor volume with the prescribed dose to the
periphery of the tumor. The tumor volume was 270.3 mm3.
The dose to the tumor margin was 30 Gy at the 55% isodose line
(Figs. 2D and 3).
Treatment
During the treatment, the patient was maintained in
a supine position, instead of the traditional prone position
(Fig. 2G). The treatment was
performed in one session. The beam-on time was 108.2 min. Following
GK-SRS treatment, the stereotactic frame and the sutures were
removed from the patient. Ophthalmic Betamethasone (2 mg/g) and
Neomycin (3.5 mg/g) ointments were prescribed to cover treated eye
for symptom control twice daily. The patient was discharged on the
following day.
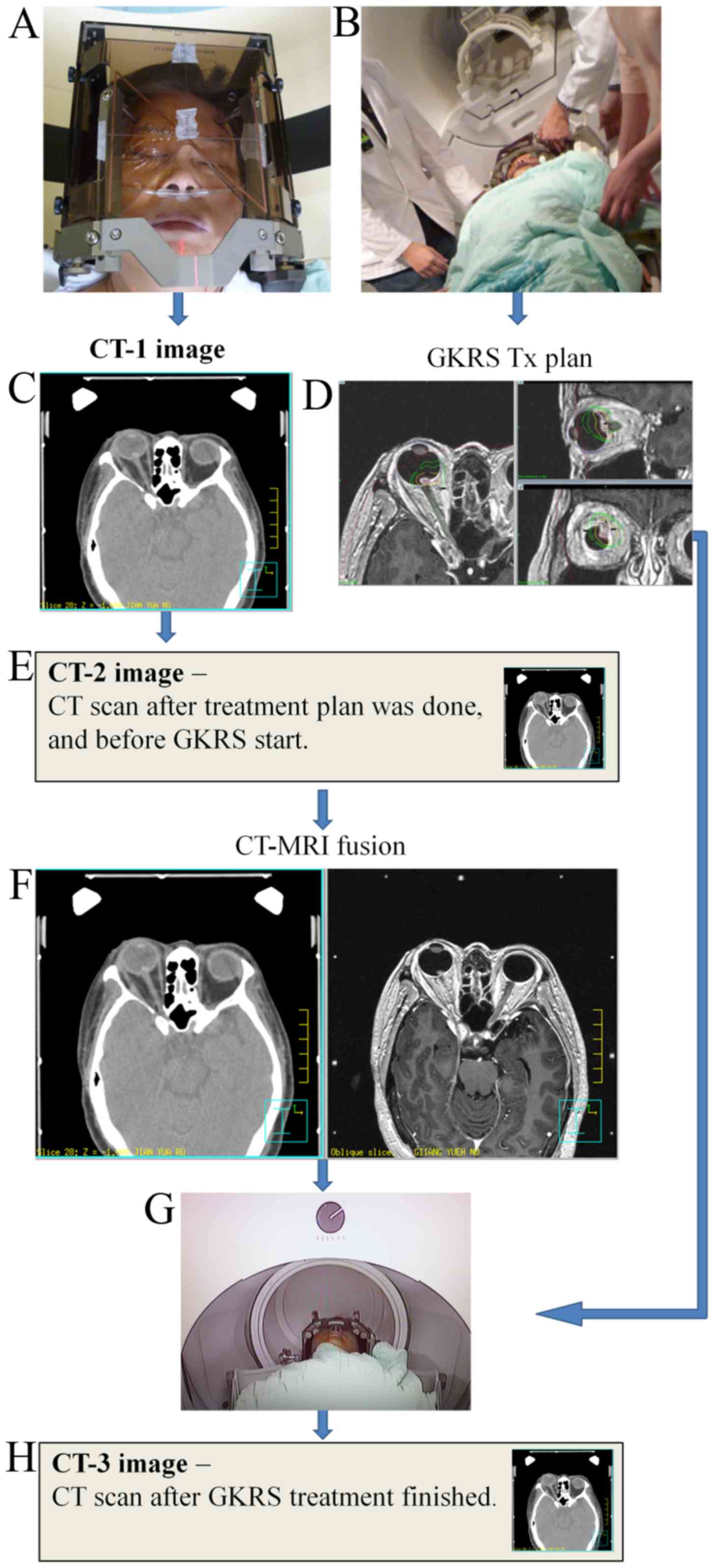
Tumor localization precision
To determine the quality of the eye fixation
technique and the localization precision of the orbital tumor, 3
sets of CT scans were performed on the patient. The first CT scan
(CT-1) was performed immediately following eye fixation and
stereotactic frame immobilization. The second CT scan (CT-2) was
performed following completion of the treatment planning, but prior
to the GK-SRS procedure (Fig. 2E).
The third CT scan (CT-3) was performed immediately following the
completion of the GK-SRS procedure (Fig.
2H). The three sets of CT images were fused on the basis of the
stereotactic frame localizer position.
Owing to the time limitation on the treatment day,
the CT-2 and MRI images were analyzed side by side by using
Pinnacle3 Auto-Planning software version 9.2 (Philips
Healthcare), and evaluated from the axial, sagittal and coronal
planes to determine the reproducibility of the eye position, and
whether the frame and the head anatomy matched (Fig. 2F).
To additionally evaluate the accuracy of the eye
fixation protocol, all 3 sets of CT scans were loaded into the
gamma knife treatment planning system. The tumors, lens and optic
nerves were contoured for all CT images. CT-2 and CT-3 were
individually fused with CT-1, on the basis of on the localizer
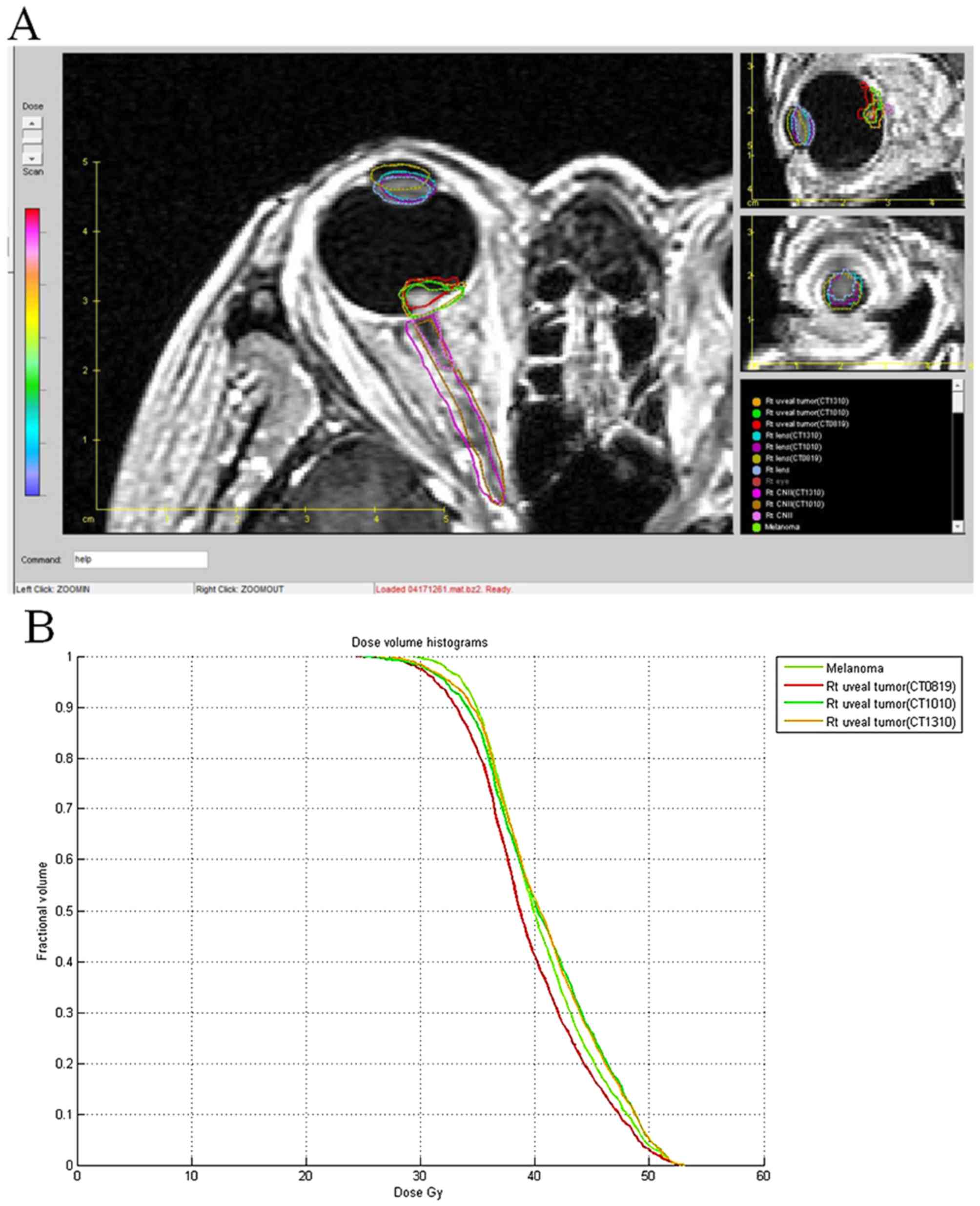
position. The fused images (Fig. 3A)
and the raw data were exported from GK-SRS to the Computational
Environment for Radiotherapy Research (CERR) treatment planning
software (10), to compare the
positioning error, dose volume histogram of the tumor and critical
organs (Fig. 3B). The tumor volume
and location deviation of the tumor, lens and optic nerves were
analyzed. The MRI scans with the GK-SRS treatment planning dose
distribution were fused with a different CT set. The targets were
contoured by an experienced radiation oncologist for all MRI and CT
sets and tumor coverage, referring to the proportion of target
coverage by the prescription dose was calculated for all CT sets
using the following formula: Coverage (%)=(TVPIV/TV)
×100%. TV is the target volume, PIV is the prescription isodose
volume, and TVPIV is the TV covered by the PIV.
Statistical analyses
On the basis of evaluation, targets were small and
oval shaped following treatment. The gravity center point (GCP) was
used to represent the evaluation point. The formula is as follows:
r⇀cg=Σr⇀imimtotal,
where r⇀cg
indicates GCP, r⇀i
indicates each point position, mi indicates each point
mass, and mtotal indicates the total mass.
To determine the precision of the eye position used
in the present case study, the 3 sets of CT images and the MRI
images were fused according to the stereotactic frame. On the basis
of the fused images, the position differences of the GCPs of the
evaluation objects were calculated.
The deviations between CT-1 and CT-2 (Δ1) and
between CT-1 and CT-3 (Δ2) were calculated using the following
formula: Δ=Δx2+Δy2+Δz2,
where Δx, Δy and Δz represent the left-right,
up-down and forward-back deviations, respectively.
The Digital Imaging and Communications in
Medicine-radiation therapy data for each patient in the GK
treatment planning system was transferred to the CERR radiation
therapy treatment planning software for plan evaluation (10). Tumor volume, tumor location deviation,
lens deviation and the dose volume histogram of the tumor, on the
basis of the MRI scan and the 3 sets of CT images, were
analyzed.
Eye fixation analysis
The treatment plan and tumor volumes measured for
the 3 sets of CT and MRI scans are listed in Tables I and II. Table
III presents the GCP coordinates and the deviations for tumor
and lens between the different sets of CT images. On the basis of
the GCP position differences, the deviations of the tumor and lens
were <0.110 mm in the CT-1, CT-2 and CT-3 images.
 | Table I.Summary of the GK-SRS treatment plan
for patient with choroidal melanoma. |
Table I.
Summary of the GK-SRS treatment plan
for patient with choroidal melanoma.
| Tumor volume,
mm3 | Marginal dose,
Gy | Maximal dose,
Gy | Prescribed isodose
line, % | Beam-on time,
min | Optic nerve maximal
dose, Gy |
|---|
| 270.5 | 30.0 | 54.5 | 55 | 108.2 | 38.9 |
 | Table II.Summary of tumor volume according to
MRI and 3 sets of CT scans. |
Table II.
Summary of tumor volume according to
MRI and 3 sets of CT scans.
| MRI,
mm3 | CT-1,
mm3 | CT-2,
mm3 | CT-3,
mm3 |
|---|
| 270.5 | 266.1 | 262.3 | 266.9 |
 | Table III.GCP coordinates and the deviations
between the tumor and lens across the different CT image sets. |
Table III.
GCP coordinates and the deviations
between the tumor and lens across the different CT image sets.
|
| CT-1 (x, y,
z) | CT-2 (x, y,
z) | CT-3 (x, y,
z) | Δ1, mm | Δ2, mm |
|---|
| Tumor | (−2.109, 4.398,
−1.964) | (−2.047, 4.271,
−1.933) | (−2.076, 4.282,
−1.891) | 0.099 | 0.109 |
| Lens | (−2.528, 5.904,
−1.459) | (−2.449, 5.764,
−1.460) | (−2.444, 5.805,
−1.479) | 0.107 | 0.089 |
Dose distributions and tumor coverage with the
prescribed dose were calculated and evaluated for the 3 sets of CT
images. The tumor coverage is listed in Table IV. The analysis of the dosimetric
consequences in these cases revealed that the tumor was covered
with isodose curves >95%.
 | Table IV.Summary of the tumor coverage at the
prescribed isodose. |
Table IV.
Summary of the tumor coverage at the
prescribed isodose.
| Prescription dose,
Gy | MRI, % | CT-1, % | CT-2, % | CT-3, % |
|---|
| 30 | 99.7 | 97.4 | 98.1 | 98.3 |
The tumor deviations between CT-1 and CT-2, and
between CT-1 and CT-3 were 0.099 mm and 0.109 mm, respectively. The
lens deviation between CT-1 and CT-2, and between CT-1 and CT-3
were 0.107 mm and 0.089 mm, respectively. The dose distributions
and the coverage of the tumor with the prescribed dose were
calculated. The tumor coverage, on the basis of the MRI, CT-1, CT-2
and CT-3 scans were 99.7, 97.4, 98.1 and 98.3%, respectively.
Postoperative course
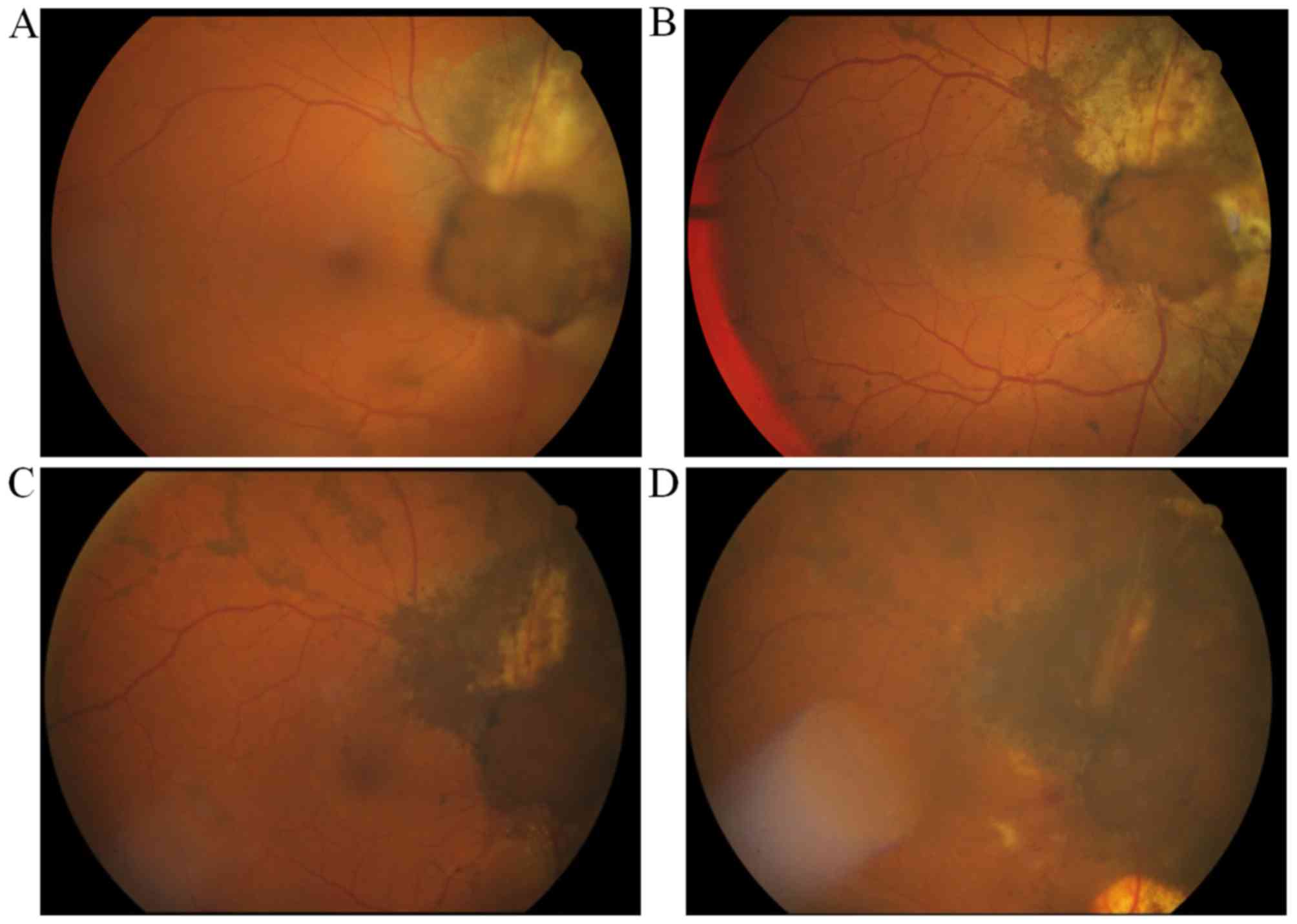
The patient tolerated the procedure well. The
follow-up fundoscopic examination, performed 1 month after GK-SRS,
revealed hyperpigmentation of the tumor surface, distinct borders
of the tumor margins and tumor shrinkage (Fig. 4). However, 3 months after the gamma
knife procedure, the patient complained of decreased vision in the
right eye with a visual acuity of 0.03. Subsequently, a B-scan and
mydriatic fundus examination revealed persistent right eye vitreous
hemorrhage. The patient underwent a trans pars plana vitrectomy for
a dense non-clearing vitreous opacity of right eye. During this
surgery, the ophthalmologist applied endo diode laser thermotherapy
(wavelength, 810 nm) to a choroid tumor that involved the optic
nerve and achieved safe margins (2 mm/500-1,000 mW/spot; 1
min/spot; total, 30 spots), until the tumor turned from white to
gray. Samples taken by needle aspiration from the vitreous fluid
were fixed in 10% neutral buffered formalin for 24–48 h at room
temperature, embedded in paraffin and sectioned to 4–5-µm. The
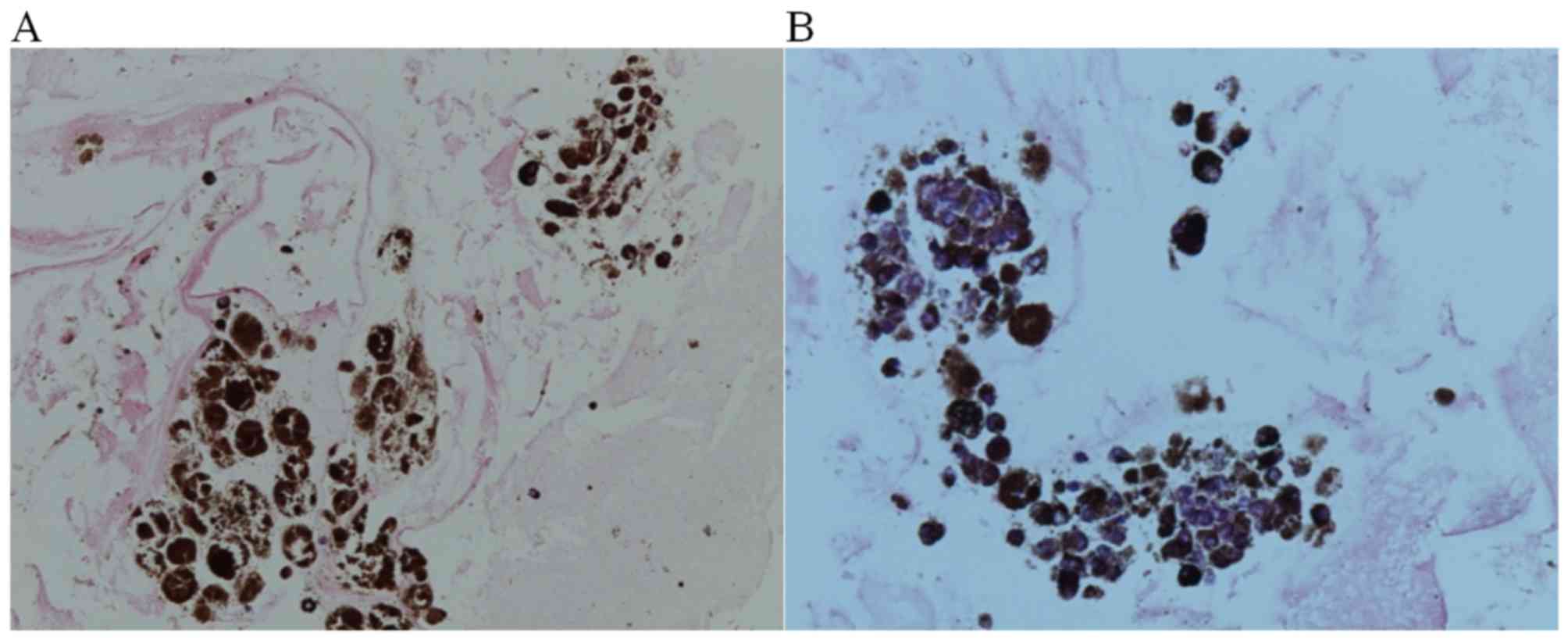
immunohistochemical staining of the cell block cytology revealed
positive staining for HMB45, a monoclonal antibody used to confirm
melanoma, using an anti-HMB45 primary antibody (cat. no. 282M-95;
dilution, 1:20; Thermo Fisher Scientific. Inc.), incubating the
samples with this primary antibody for 2 h at 36°C. The ultraView
Universal Alkaline Phosphatase Red Detection kit (Ventana Medical
Systems, Inc., Tucson, AZ, USA) was used to detect the anti-HMB45
primary antibody. The immunohistochemical staining result was also
positive for Ki67, which is a cellular marker for proliferation,
using an anti-Ki67 antibody (cat. no. RM-9106-S; dilution, 1:200;
Thermo Fisher Scientific, Inc.), incubating samples with this
antibody for 48 min at 36°C, The ultraView Universal DAB Detection
kit (Ventana Medical Systems, Inc.) was used to detect the
anti-Ki67 primary antibody. An Olympus BX41 light microscope
(Olympus Corporation, Tokyo, Japan) was used to visualize results
at a magnification of ×400 (Fig. 5),
demonstrated sparse malignant cell nests with rich melanin-pigment
compatible with malignant melanoma.
Follow-up assessment
This patient underwent a complete ophthalmological
diagnostic investigation and follow-up evaluation following GK-SRS
that included visual acuity testing (Snellen charts), indirect
ophthalmoscopy and color fundus photos every 3 months after
treatment for 3 years. A head MRI was performed every 3 months for
1 year, and then every 6 months for 2 years for tumor evaluation.
Furthermore, liver ultrasonography and chest X-ray were conducted
every 6 months for 3 years to detect early metastasis.
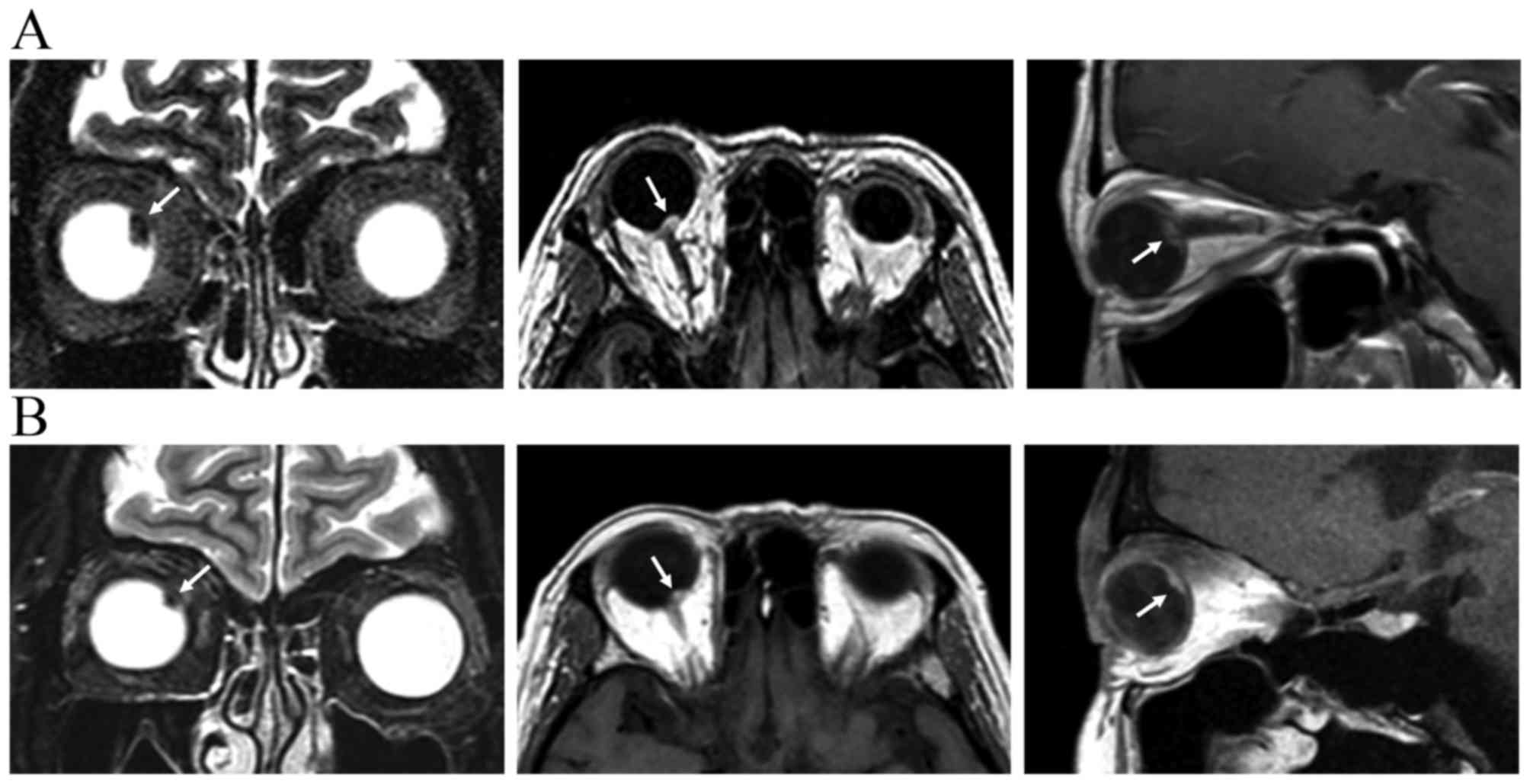
At 37 months after LGK-SRS and 32 months after
vitrectomy, follow-up MRI scans revealed tumor shrinkage (9.5×8.9
to 7.2×6.4 mm) and a decrease in tumor thickness (4.1–3.0 mm)
(Fig. 6). The patient remained free
of metastatic disease. The best-corrected visual acuity decreased
from 1.0, at the time of uveal melanoma diagnosis, to 0.03, prior
to vitrectomy, and 0.2, following vitrectomy.
Discussion
Ocular melanoma typically affects Europeans and
rarely occurs among Asian, African-American or Hispanic populations
(1,11). Cheng and Hsu (12) identified that the incidence of ocular
melanoma in Taiwan was 0.39/million. Furthermore, Chinese patients
with uveal melanoma were typically younger compared with Western
patients (12). The choroid is the
most common site of involvement, followed by the ciliary body and
the iris (13). In spite of the low
incidence of uveal melanoma, the management of patients with this
condition remains a therapeutic challenge for oncologists and
surgeons (13).
Historically, uveal melanoma was treated with
enucleation; however, enucleation was hypothesized to promote tumor
cells that metastasize due to elevated intraocular pressure and
tumor disruption (14). Novel
techniques have been developed for orbital preservation including
transpupillary thermotherapy, brachytherapy, local tumor resection,
proton beam irradiation and stereotactic radiosurgery (5,6,15); however, the use of endo diode laser
thermotherapy has rarely been studied. The Collaborative Ocular
Melanoma Study randomly assigned 1,317 patients with medium-sized
choroidal melanomas (apical height, between 2.5 and 10.0 mm; basal
diameter, between 5 and 16 mm) to receive either enucleation or
iodine-125 brachytherapy. No difference was identified with regard
to the 5-year survival rates between enucleation and brachytherapy
(81 and 82%, respectively; P=0.48) (16). Plaque brachytherapy has evolved into a
promising alternative to enucleation because it provides successful
local control with only a moderate decrease in visual acuity
(16).
GK-SRS was developed by the Swedish neurosurgeon
Lars Leksell for intracranial lesion treatment (17). This technique is engineered to deliver
a single dose of ionizing radiation to a small target with a steep
dose fall-off at the margins. These characteristics are crucial for
irradiating intraocular tumors while sparing normal peritumoral
tissue (17). In 1987, Rand et
al (18) used GK-SRS with a
single dose of 60–90 Gy delivered at 90% isodose to treat six
rabbits with eye melanoma and an overall regression was
demonstrated. Long-term SRS results regarding uveal melanoma via
GK-SRS reveal satisfying tumor control with rates of approximately
90% (19–21).
To ensure the treatment precision of GK-SRS, eyeball
immobilization and tumor imaging are two important steps in the
treatment protocol. Different eye-immobilization systems for eye
melanoma have been described previously (22,23).
Mueller et al (24) used a
retrobulbar injection of a long-acting anesthetic agent to achieve
complete akinesia during treatment and performed a second MRI scan
to validate post-treatment tumor position. Langmann et al
(21) and Modorati et al
(19) immobilized the globe using
retrobulbar anesthesia, and by suturing the extraocular muscles.
Due to the application and subsequent resorption of the anesthetic
liquid, whether the eye would be displaced within the orbit during
the treatment was investigated. Based on the present case study, by
retrospectively evaluating the tumor and lens deviations in the
present case on the basis of the CT images obtained during
treatment, the accuracy of the eye positioning was analyzed. The
deviations between the pre- and post-treatment positions were ≤0.1
mm for all data points investigated. The analysis of the dosimetric
consequences in these cases revealed that the tumor remained
covered by >97% isodose curves. Therefore, retrobulbar
anesthesia and two extraocular muscle sutures are feasible and
efficacious methods for orbital tumor radiosurgery.
Logani et al (20) demonstrated that ocular melanoma cell
lines are radioresistant in vitro, particularly at lower
doses; however, ocular melanoma cell lines may be responsive to a
single high dose delivered using stereotactic radiosurgery or
brachytherapy. As such, their initial report revealed that higher
doses ranging between 90 and 50 Gy at the tumor margins were
prescribed (18). However, secondary
side effects including neovascular glaucoma, radiation retinopathy,
optic neuropathy and maculopathy were common; therefore, the
irradiation dose was decreased over time (19,24,25).
Langmann et al (25)
demonstrated that if the mean margin dose was decreased to between
52.1 and 41.5 Gy, a lower complication rate of neovascular glucoma
was noted with the similar local control rate. Modorati et
al (19) demonstrated that a
decrease in dose (to between 50 and 35 Gy) at the tumor margin was
not significantly associated with survival probability, but was
associated with decreased radiation-induced side effects. Mueller
et al (24) demonstrated that
the current treatment for uveal melanoma was performed using 25 Gy
at a 50% isodose. Previous studies have revealed decreased rates of
radiogenic side effects due to the lowering of the total
irradiation dose to ~40 Gy and 35 Gy at 50% isodose (19,25). In
the present case study, 30 Gy at the 55% isodose line was
administered to the tumor margin.
In the present case study, as the tumor was located
close to the optic nerve, the maximal dose to the optic nerve was
38.9 Gy. During the 15-month follow-up period, ophthalmoscopy
revealed tumor shrinkage and serous retinal detachment
disappearance. However, vitreous hemorrhage occurred 5 months after
treatment. The patient subsequently received trans pars plana
vitrectomy for dense non-clearing vitreous opacity of the right
eye. The management of the vitreous hemorrhage using this technique
in eyes with previously irradiated uveal melanoma appears to be
safe, without increased risk of intraocular, local, orbital or
systemic dissemination of the tumor (26).
Enucleation and fine-needle lesion biopsy are direct
ways of acquiring tissue for additional analysis; however, the
former method sacrifices the affected eye and the latter may lead
to tumor metastasis. Fine-needle biopsy of the vitreous humor is a
relatively safe procedure, but it has decreased success rates
(27). In the present case study,
vitreous fluid was selected during the pars plana vitrectomy and
the pathological examination revealed sparse malignant cell nests
with rich melanin-pigment compatible with malignant melanoma.
Thermotherapy uses the principle of
intermediate-level hyperthermia between 45 and 60°C (28). Nuijs-Beems et al (28) demonstrated that the temperature range
was optimal for the destruction of cells throughout the full
thickness of the tumor. Intermediate-level hyperthermia differs
from photocoagulation, in that the latter heats the tissue to
temperatures >60°C with resultant destruction of the cells in
the outer layers of the tumor. The outer layer cells form a
reflective surface and do not allow additional penetration of the
heat into the mass. In thermotherapy, the spot size is larger (≤6
vs. <1 mm) and the duration of exposure is increased (1–10 min
vs. 0.5–1 sec) compared with that of photocoagulation (28). To achieve improved local tumor control
in the present case study, endo diode laser thermotherapy was
initially administered to the choroid tumor, which involved the
optic nerve, and achieved safe margins (28). At the regular follow-up assessment, an
MRI scan revealed tumor shrinkage and no metastatic disease.
GK-SRS, used in the present case study, is a
relatively non-invasive, organ-conserving and brief single fraction
treatment for intraocular tumor, compared with enucleation and
plaque brachytherapy. The present study hypothesizes that eye
fixation with retrobulbar anesthesia and two extraocular muscles
suture is a feasible and efficacious method for radiosurgery within
6 h. Furthermore, endo diode laser thermotherapy is a safe and
effective procedure for patients with uveal melanoma. On the basis
of the present case report, additional clinical trials and studies
are required.
References
|
1
|
Devesa SS, Silverman DT, Young JL Jr,
Pollack ES, Brown CC, Horm JW, Percy CL, Myers MH, McKay FW and
Fraumeni JF Jr: Cancer incidence and mortality trends among whites
in the United States, 1947–84. J Natl Cancer Inst. 79:701–770.
1987.PubMed/NCBI
|
|
2
|
Singh AD and Topham A: Incidence of uveal
melanoma in the United States: 1973–1997. Ophthalmology.
110:956–961. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh AD, Turell ME and Topham AK: Uveal
melanoma: Trends in incidence, treatment and survival.
Ophthalmology. 118:1881–1885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh AD and Topham A: Survival rates with
uveal melanoma in the United States: 1973–1997. Ophthalmology.
110:962–965. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finger PT: Radiation therapy for choroidal
melanoma. Surv Ophthalmol. 42:215–232. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Daftari IK, Petti PL, Shrieve DC and
Phillips TL: Newer radiation modalities for choroidal tumors. Int
Ophthalmol Clin. 46:69–79. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dieckmann K, Bogner J, Georg D, Zehetmayer
M, Kren G and Pötter R: A linac-based stereotactic irradiation
technique of uveal melanoma. Radiother Oncol. 61:49–56. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bogner J, Petersch B, Georg D, Dieckmann
K, Zehetmayer M and Pötter R: A noninvasive eye fixation and
computer-aided eye monitoring system for linear accelerator-based
stereotactic radiotherapy of uveal melanoma. Int J Radiat Oncol
Biol Phys. 56:1128–1136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deasy JO, Blanco AI and Clark VH: CERR: A
computational environment for radiotherapy research. Med Phys.
30:979–985. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hudson HL, Valluri S and Rao NA: Choroidal
melanomas in Hispanic patients. Am J Ophthalmol. 118:57–62. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng CY and Hsu WM: Incidence of eye
cancer in Taiwan: An 18-year review. Eye (Lond). 18:152–158. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peyman GA, Sanders DR and Goldberg MF:
Principles and practice of ophthalmology. Saunders. 1980.
|
|
14
|
Zimmerman LE, McLean IW and Foster WD:
Does enucleation of the eye containing a malignant melanoma prevent
or accelerate the dissemination of tumour cells. Br J Ophthalmol.
62:420–425. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuisting B and Richard G: Transpupillary
thermotherapy (TTT)-Review of the clinical indication spectrum. Med
Laser Appl. 25:214–222. 2010. View Article : Google Scholar
|
|
16
|
Diener-West M, Earle JD, Fine SL, Hawkins
BS, Moy CS, Reynolds SM, Schachat AP and Straatsma BR;
Collaborative Ocular Melanoma Study Group, : The COMS randomized
trial of iodine 125 brachytherapy for choroidal melanoma, III:
Initial mortality findings. COMS Report No. 18. Arch Ophthalmol.
119:969–982. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leksell L: The stereotaxic method and
radiosurgery of the brain. Acta Chir Scand. 102:316–319.
1951.PubMed/NCBI
|
|
18
|
Rand RW, Khonsary A, Brown WJ, Winter J
and Snow HD: Leksell stereotactic radiosurgery in the treatment of
eye melanoma. Neurol Res. 9:142–146. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Modorati G, Miserocchi E, Galli L, Picozzi
P and Rama P: Gamma knife radiosurgery for uveal melanoma: 12 years
of experience. Br J Ophthalmol. 93:40–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Logani S, Cho AS, Ali BH, Withers HR,
McBride WH, Kozlov KL, Hall MO, Lee DA and Straatsma BR:
Single-dose compared with fractionated-dose radiation of the OM431
choroidal melanoma cell line. Am J Ophthalmol. 120:506–510. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Langmann G, Pendl G, Klaus Müllner,
Papaefthymiou G and Guss H: Gamma knife radiosurgery for uveal
melanomas: An 8-year experience. J Neurosurg. 93 Suppl 3:S184–S188.
2000.
|
|
22
|
Tokuuye K, Akine Y, Sumi M, Kagami Y,
Ikeda H and Kaneko A: Fractionated stereotactic radiotherapy for
choroidal melanoma. Radiother Oncol. 43:87–91. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zehetmayer M, Menapace R, Kitz K and Ertl
A: Suction attachment for stereotactic radiosurgery of intraocular
malignancies. Ophthalmologica. 208:119–121. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mueller AJ, Talies S, Schaller UC,
Horstmann G, Wowra B and Kampik A: Stereotactic radiosurgery of
large uveal melanomas with the gamma-knife. Ophthalmology.
107:1381–1387, 1387–1388. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Langmann G, Pendl G, Müllner K,
Feichtinger KH and Papaefthymiouaf G: High-compared with low-dose
radiosurgery for uveal melanomas. J Neurosurg. 97(5 Suppl):
S640–S643. 2002.
|
|
26
|
Bansal AS, Bianciotto CG, Maguire JI,
Regillo CD, Shields JA and Shields CL: Safety of pars plana
vitrectomy in eyes with plaque-irradiated posterior uveal melanoma.
Arch Ophthalmol. 130:1285–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh AD and Biscotti CV: Fine needle
aspiration biopsy of ophthalmic tumors. Saudi J Ophthalmol.
26:117–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nuijs-Beems EM, Oosterhuis JA, Verburg-van
der Marel EH, de Wolff-Rouendaal D, van Delft JL and van Best JA:
Tumor destruction by intermediate level hyperthermia. Curr Eye Res.
9:771–780. 1990. View Article : Google Scholar : PubMed/NCBI
|