Introduction
E26 transformation-specific (ETS) domain-containing
protein Elk-3 (ELK3) is a ternary complex factor belonging to the
ETS transcription factor family. ELK3 was first identified as a
transcriptional repressor of c-Fos, but it is able to function as a
transcriptional activator once phosphorylated by the
Ras/mitogen-activated protein kinase signaling pathway (1–3). The
transcriptional activity of ELK3 has been linked to vasculogenesis
and wound healing in cell lines and in mice (4–6). ELK3
regulates vascular integrity through early growth response protein
1 (4), and regulates angiogenesis
through the control of vascular endothelial growth factor (VEGF)
expression (6). Mice lacking ELK3
protein develop smaller tumors, which are not able to become
vascularized and oxygenated, indicating that ELK3 serves a major
role in tumorigenecity (6). In
addition to phosphorylation, the transcriptional activity of ELK3
is regulated by nuclear-cytoplasmic shuttling in response to
specific signaling pathways (7). ELK3
has two nuclear localization signals and it is primarily localized
to the nucleus under physiological conditions (7). ELK3 also has a nuclear export signal,
and the nuclear exclusion of ELK3 occurs via specific signaling
pathways, including the c-Jun kinase pathway (7).
The tumor and organ microenvironment is important
for cancer growth and metastasis. Diverse cells residing in the
microenvironment influence cancer cells through secreted factors
and their corresponding signaling pathways (8). These cell-to-cell communications
contribute to cancer progression, angiogenesis, invasion,
epithelial-to-mesenchymal transition phenotypes and antitumor drug
resistance (9–11). Therefore, cells within the tumor
microenvironment have emerged as attractive targets to effectively
inhibit cancer. Lymphatic vessels (LV) are major components of the
tumor microenvironment and are composed of lymphatic endothelial
cells (LEC) (12). As LVs are more
permeable than blood vessels, they are particularly important for
tumor dissemination and metastasis (12). Despite the importance of LV in tumor
progression, the factors secreted by LEC and the signals through
which they influence the behavior of the tumor remain to be
elucidated.
The present study demonstrated that ELK3 regulates
cell migration, tube formation and cell permeability of LEC. These
results indicate that ELK3 is a major regulator of
lymphangiogenesis in LEC.
Materials and methods
Cell culture
LEC and human umbilical vein endothelial cells
(HUVEC) were purchased from Modern Cell & Tissue Technologies
(Seoul, Korea) and maintained in endothelial cell growth medium-2
supplemented with 5% fetal calf serum (FCS, #CC-3202; Clonetics;
Lonza, Basel, Switzerland) on surfaces coated with 2 µg/ml bovine
fibronectin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
MDA-MB-231 and MCF7 breast cancer cell lines were cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Small interfering RNA (siRNA)
transfection, RNA purification and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The non-specific control siRNA (siNS; #D-001810-10;
Dharmacon, Inc., Chicago, IL, USA) and human ELK3 siRNA (siELK3;
#L-010320-00-0005; Dharmacon, Inc.) were obtained from Dharmacon
(GE Healthcare Life Sciences, Chalfont, UK). The siRNAs (100 nM)
were transfected into LEC using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol, and cells were collected at 48 h following transfection
for further analysis. Total cellular RNA was extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.,
Invitrogen, Carlsbad, CA, USA) following the manufacturer's
protocol. Total RNA (2 µg) was used for single-stranded cDNA
synthesis with OmniScript® reverse transcriptase (Qiagen
GmbH, Hilden, Germany). RT-qPCR was performed using the CFX96
Touch™ Real-Time PCR Detection system (Bio-Rad Laboratories Inc.,
Hercules, CA, USA) using SYBR-Green I (Qiagen Inc., Valencia, CA,
USA). The final volume of PCR mixture was 20 µl, comprising 1 µg
cDNA, 10 pmol forward and reverse primers and 10 µl of 2X SYBR mix.
The reactions were performed for 40 cycles using optimized cycling
conditions (denaturation at 95°C for 1 min, annealing at 55°C for
30 sec and extension at 72°C for 30 sec). RT-qPCR data was
processed based on the 2−ΔΔCq method (13). The primers used in this analysis are
listed in Table I.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primers. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primers.
| Gene | Primer sequence
(5′-3′) |
|---|
| GAPDH | F-ACC ACA GTC CAT GCC
ATC AC |
|
| R-TCC ACC ACC CTG TTG
CTG TA |
| ELK3 | F-ACC CAA AGG CTT GGA
AAT CT |
|
| R-TGT ATG CTG GAG GAC
AGT GG |
| VE-cadherin | F-TCC TCT GCA TCC TCA
CTA TCA CA |
|
| R-GTA AGT GAC CAA CTG
CTC GTG AAT |
| LYVE1 | F-ACT TCC ATC TGG ACC
ACG AG |
|
| R-AGC CTA CAG GCC TCC
TTA GC |
| PROX1 | F-CAG CCC GAA AAG AAC
AGA AG |
|
| R-GGG TCT AGC TCG CAC
ATC TC |
| VEGFR1 |
F-GCTGTGCGCGCTGCTT |
|
|
R-AACTCAGTTCAGGACCTTTTAATTTTGA |
|
VEGFR2 | F-GTG ACC AAC ATG GAG
TCG TG |
|
| R-TGC TTC ACA GAA GAC
CAT GC |
| VEGFR3 | F-GAG ACA AGG ACA GCG
AGG AC |
|
| R-TCA CGA ACA CGT AGG
AGC TG |
| VEGFA | F-GAG GAT CAA ACC TCA
CCA AG |
|
| R-CCG CCT CGG CTT GTC
ACA T |
| VEGFB | F-CCC TTG ACT GTG GAG
CTC AT |
|
| R-GGC TTC ACA GCA CTG
TCC TT |
| VEGFC | F-ACC AAA CAA GGA GCT
GGA TG |
|
| R-ATT TCT GGG GCA GGT
TCT TT |
| VEGFD | F-AGG ACT GGA AGC
TGT GGA GA |
|
| R-ATC GGA ACA CGT
TCA CAC AA |
Protein extraction and western
blotting
Total protein was isolated using Cell Lysis buffer
(#9803; Cell Signaling Technology Inc., Danvers, MA, USA) according
to the manufacturer's protocol. A bovine serum albumin (BSA)
standard curve was used to estimate the protein concentration. A
total of 8 BSA standards were made by dilating 2 µg/µl albumin
standard (#23209; Thermo Fisher Scientific, Inc.) into cell lysis
buffer, and they were then incubated at 37°C for 30 min. A standard
curve was produced based on the absorbance values of BSA solution
in plate reader (Epoch microplate spectrophotometer; Biotek
Instruments, Inc., Winooski, VT, USA) at 562 nm. Total protein (20
µg) was separated by 10% SDS-PAGE and transferred to a
polyvinylidene difluoride (PVDF) membrane (EMD Millipore,
Billerica, MA, USA). The membrane was blocked by 5% skim milk
solution at room temperature for 30 min and then incubated with an
anti-ELK3 antibody (#sc-17860, 1:1,000 dilution; Santa Cruz
Biotechnology Inc., Dallas, TX, USA), an antibody recognizing
active β-catenin (unphosphorylated at Ser33/37/Thr41, #4270S), an
anti-phospho-β-catenin antibody (phosphorylated at Ser33/37/Thr41,
#9561S) (both from Cell Signaling Technology, Inc.), an anti-lamin
B antibody (#sc-6216) or an anti-β-actin antibody (#sc-47778) (both
from Santa Cruz Biotechnology, Inc.) overnight at 4°C, followed by
incubation with a secondary antibody (#sc-2005, 1:2,000 dilution;
Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The
membrane was washed three times in TBST for 5 min each time.
Immunoreactive proteins were detected using the SuperSignal West
Pico Chemiluminescent Substrate Western Blot Detection system
(catalog no. 34080; Thermo Fisher Scientific, Inc.) according to
the protocol of the manufacturer. All experiments were repeated at
least three times.
2D migration assay
The migration potential of LEC was assessed using a
wound healing assay. LEC, following 24 h transfection with siNS or
siELK3, were cultured on 26×76 mm glass coverslips in endothelial
cell growth medium-2 supplemented with 5% FCS and wounded using a
micropipette tip when the cells were fully confluent. The cells
were incubated for 24 h at 37°C and then observed for migration
using a light microscope (CKX41SF; Olympus, Tokyo, Japan).
In vitro tube formation assay
The ability of LEC to form capillary-like structures
(tubes) was assessed using a tube formation assay (14). LEC were harvested and cultured in
media with or without recombinant VEGF-C for 30 min at room
temperature. A total of 1.5×104 LEC cells were seeded on
slides coated with Matrigel (#356231; Corning Inc., Corning, NY,
USA) supplemented with vitronectin (SPR3186; Sigma-Aldrich; Merck
KGaA) (5 mg/ml). Cells were incubated for 24 h in the presence of
5% CO2 at 37°C for further analysis.
Cell proliferation assay
Cell proliferation was analyzed using the MTT assay.
Briefly, 3×103 cells were seeded onto 96-well plates and
cultured for 24, 48 and 72 h at 37°C. MTT solution was added to
each well at a final concentration of 0.5 mg/ml and incubated for 4
h at 37°C. The resulting formazan crystals were dissolved in 150 µl
dimethyl sulfoxide per well. The absorbance was measured at a
wavelength of 570 nm using an automated plate reader (Thermo Fisher
Scientific, Inc.).
Immunocytochemical staining
Immunocytochemical staining was performed as
described previously (15), using a
rabbit anti-human VE-cadherin antibody (#sc-9989; dilution, 1:200;
Santa Cruz Biotechnology, Inc.) and an Alexa Fluor®
488-conjugated secondary antibody (#A21206; 1:300 dilution; Thermo
Fisher Scientific, Inc.) at room temperature. Nuclei were stained
by incubating cells with 5 µg/ml DAPI labeling solution (#ab104140;
Abcam, Cambridge, UK) for 2-5 min in the dark at room
temperature.
Analysis of VEGF-C protein in the
culture media
The amount of VEGF-C in the culture media was
measured by VEGF-C Quantikine ELISA kit (R&D Systems, Inc.,
Minneapolis, USA; #DVEC00) according to the protocol of the
manufacturer.
Statistical analysis
Graphical data are presented as the means ± standard
deviation. P<0.05 and P<0.01 were considered to indicate
significant and highly significant results based on Student's
t-test analyses, respectively. Statistical analyses were performed
using the SAS statistical package v.9.13 (SAS Institute, Cary, NC,
USA; http://www.sas.com/).
Results
ELK3 regulates the proliferation of
LEC
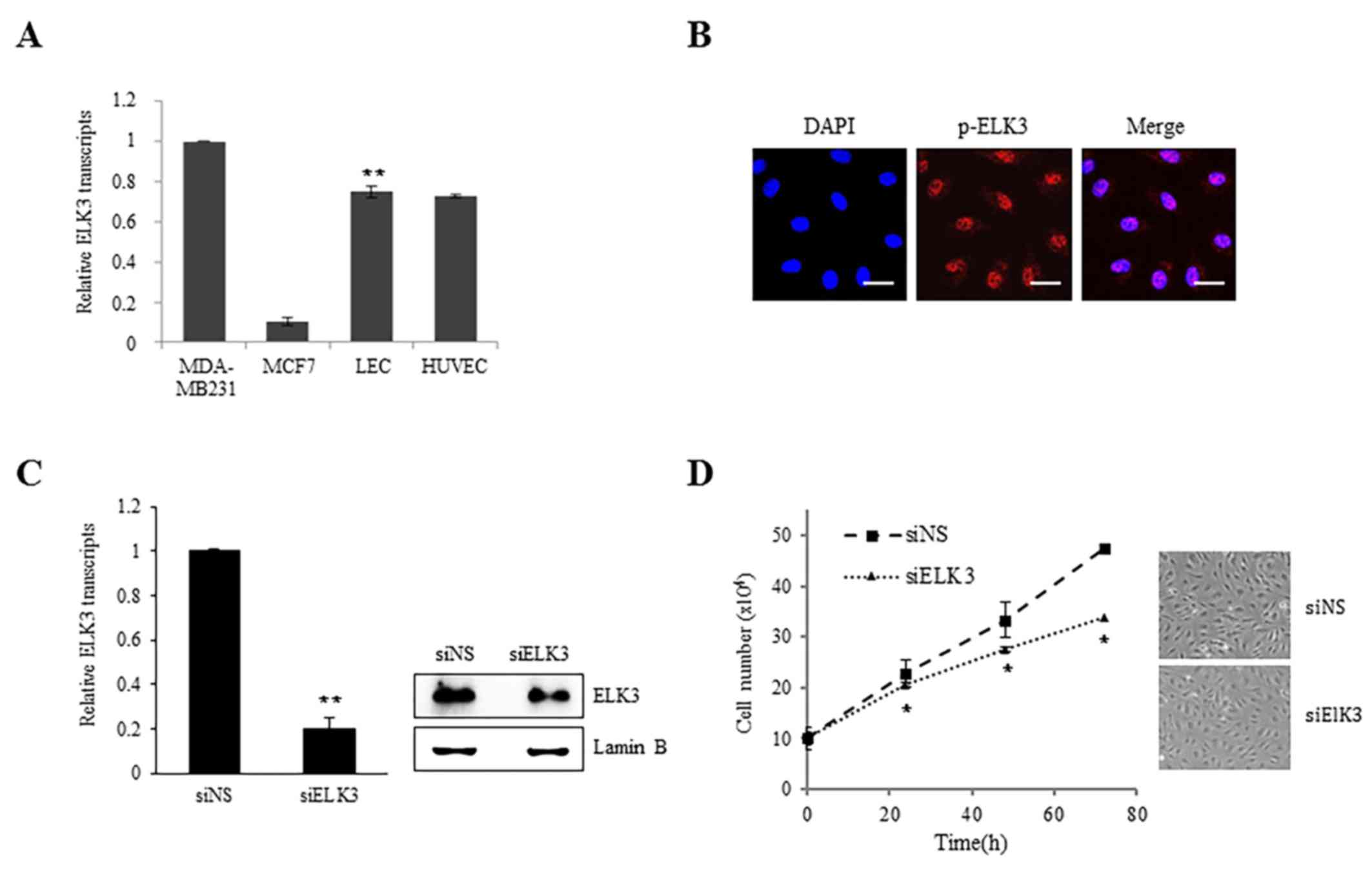
ELK3 is highly expressed in the LV of mice (13). The expression of ELK3 in LEC was
examined to elucidate the role of ELK3 in lymphangiogenesis. The
expression levels of ELK3 in LEC were comparable to those in HUVEC
cells and in the MDA-MB-231 and MCF-7 breast cancer cell line, in
which ELK3 has been established to be important in angiogenesis
(Fig. 1A). ELK3 is primarily
localized to the nucleus unless it is actively exported to the
cytoplasm via specific signaling molecules, including c-Jun
(16). As presented in Fig. 1B, the phosphorylated form of ELK3 was
detected in the nucleus, indicating that ELK3 may function as an
active transcription factor in LEC. To examine the role of ELK3 in
LEC, the protein expression of ELK3 was suppressed using siRNA
(Fig. 1C). Suppression of ELK3
protein expression did not exhibit any effect on LEC morphology but
did result in a decreased proliferation rate (P<0.05; Fig. 1D).
ELK3 regulates migration and tube
formation of LEC
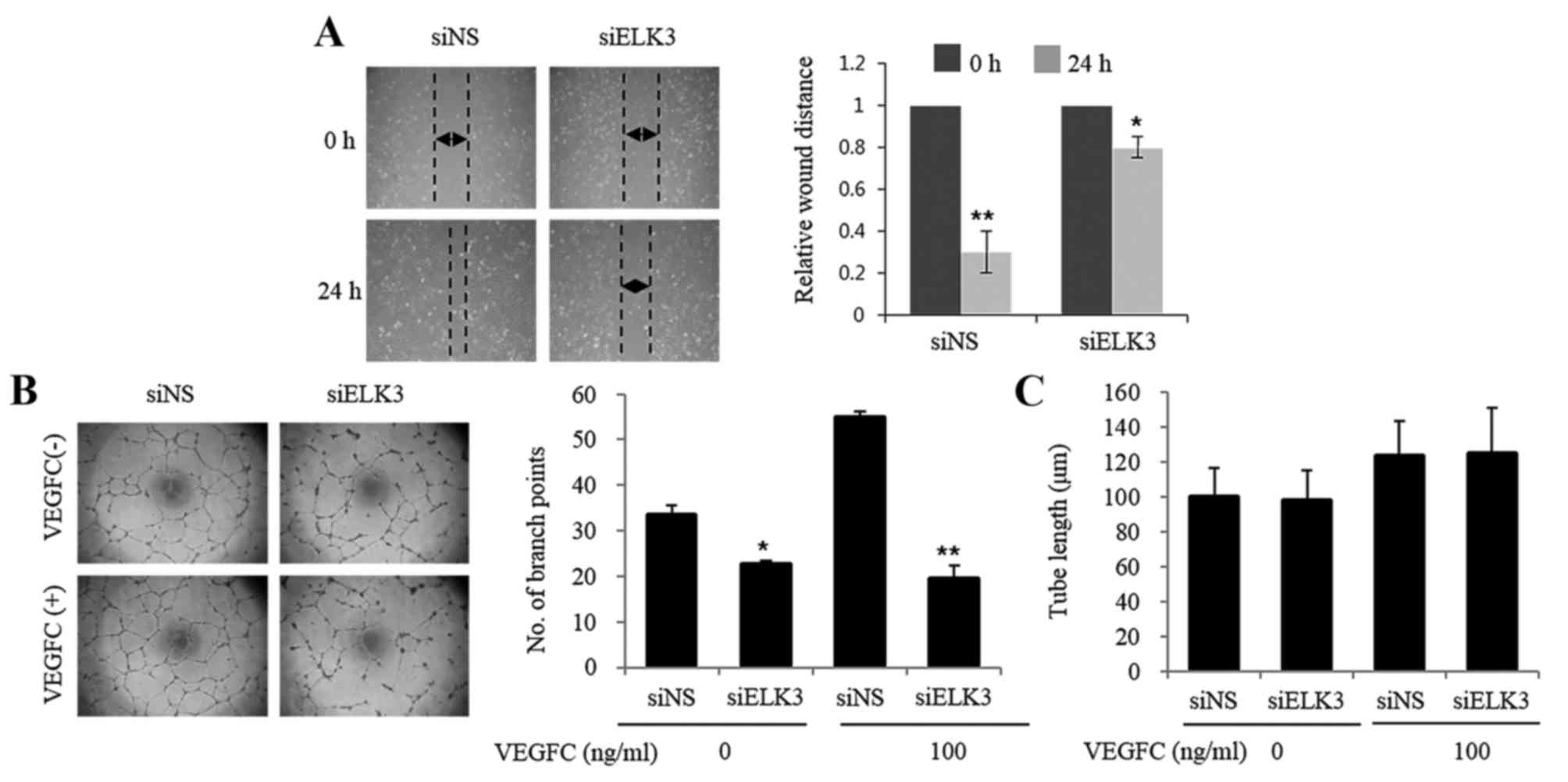
An in vitro scratch assay that mimics cell
migration into an artificial wound produced on a cell monolayer was
used to investigate the role of ELK3 in LEC migration (17). The healing ability of LEC was
inhibited at 24 h following wounding in cells transfected with
siELK3 (Fig. 2A). The effect of ELK3
suppression on the vascular behavior of LEC was evaluated using a
tube formation assay. As presented in Fig. 2B, LEC transfected with siELK3 formed
fewer branch points compared with those in the siNS controls in the
presence and absence of VEGF-C (*P<0.05, **P<0.01). However,
tube length was not affected by the silencing of ELK3 (Fig. 2C). These results suggest that ELK3 may
regulate the vasculogenic activity of LEC.
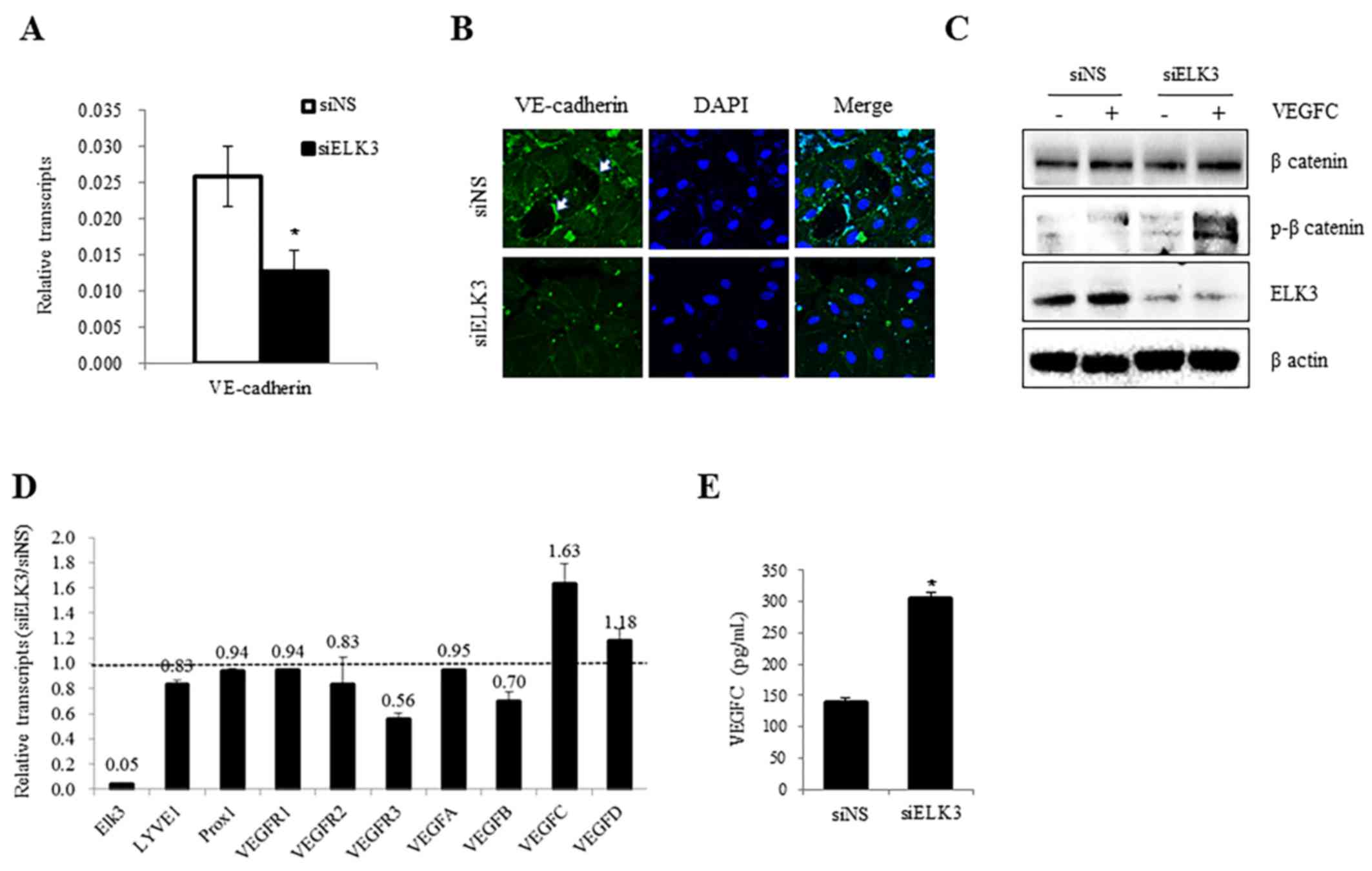
Expression of VE-cadherin and VEGFR-3
and the phosphorylation of β-catenin, are regulated by ELK3
Endothelial permeability is closely associated with
the dissemination of cancer cells and is, therefore, particularly
important in cancer biology (18). As
the expression of VE-cadherin regulates endothelial permeability
and mediates cell-to-cell contact (19), the expression levels of VE-cadherin
were analyzed in the presence and absence of siELK3. As presented
in Fig. 3A, the expression of
VE-cadherin mRNA was decreased following transfection with siELK3
(P<0.05). The accumulation of VE-cadherin near the membrane and
at points of cell-to-cell contact was lower in siELK3-transfected
LEC compared with siNS control cells (P<0.05, Fig. 3B). These results suggest that ELK3 may
function as a positive regulator of VE-cadherin expression levels
in LEC. In addition to the expression of VE-cadherin, the
phosphorylation of β-catenin has also been implicated in
VE-cadherin-mediated cell adhesion (20–22). As
the phosphorylation of β-catenin correlates with the loss of
VE-cadherin function (23), the
phosphorylation of β-catenin was compared in siELK3-transfected and
control LEC in the presence and absence of VEGF-C. Notably, ELK3
suppression was correlated with the phosphorylation of β-catenin in
the presence and absence of VEGF-C (Fig.
3C). These results suggest that ELK3 may regulate the
transcriptional expression of VE-cadherin as well as the
phosphorylation of β-catenin.
In the tumor microenvironment, particularly in
breast cancer, LV is a predominant route of tumor dissemination
(24). Tumor lymphangiogenesis is
driven by growth factors, including VEGF-C and VEGF-D, which are
secreted from tumor cells (25). LEC
in the LV also secrete factors into the tumor microenvironment that
facilitate tumor dissemination (24).
Therefore, the effect of ELK3 suppression on the expression levels
of angiogenic factors was analyzed by RT-qPCR. VEGFR-3, which
serves an important role in lymphangiogenesis as the receptor for
VEGF-C, was significantly downregulated, whereas the expression of
VECF-C was upregulated in siELK3-transfected LEC (Fig. 3D). Consistent with the mRNA levels,
the quantity of VEGF-C protein in the culture media of
siELK3-transfected LEC was higher compared with the controls
(P<0.05, Fig. 3E). These results
demonstrate that ELK3 suppression may make LEC insensitive to
VEGF-C stimulation, possibly via the reduced expression of
VEGFR-3.
Discussion
The present study demonstrated that ELK3 may be
involved in the proliferation, migration and tube formation by LEC,
activities that are required for lymphangiogenesis (14). In particular, the results suggested
that ELK3 positively regulates VEGFR-3 expression in LEC. Other ETS
family transcription factors possess the ability to induce
lymphangiogenesis by regulating VEGFR-3 (26). However, the current study, to the best
of our knowledge, is the first report that ELK3 may regulate
VEGFR-3 expression levels.
The lymphatic endothelium functions as a selective
permeable barrier controlling transfer between vessel and tissues
(27). Impaired endothelium
permeability results in persistent vascular leakage, and is
implicated in diverse pathological conditions (28). Mice that express a mutant version of
ELK3 lacking the ETS DNA-binding domain develop dilated LV
(4). This is concordant with the
results of the current study, which suggest that ELK3 may be
implicated in LEC permeability. It is also important to evaluate
whether VEGFR-3 downstream signaling is regulated by ELK3, as the
binding of ligands including VEGF-C to VEGFR-3 activates the
phosphoinositide 3-kinase/protein kinase B (PI3-K/Akt) signaling
pathway, and the physical interaction between VEGFR-3 and PI3 K is
associated with lymph node metastasis (29).
In conclusion, it is therefore important for future
studies to elucidate whether alterations in ELK3 expression are
associated with disturbances in PI3-K/Akt signaling. As the
VEGFR-3/PI3-K signaling pathway is implicated in lymphangiogenesis
in the LEC, the ELK3-VEFGR3-PI3K axis may be a novel therapeutic
target to inhibit tumor dissemination through the lymphatic
system.
Acknowledgements
The present study was supported by the Korea Science
and Engineering Foundation of the Korean government (grant no.
2015R1A2A2A01003498). Ministry of Education, Science, and
Technology (NRF-2017-M3A9B4031169).
Glossary
Abbreviations
Abbreviations:
|
ELK3
|
E26 transformation-specific
domain-containing protein Elk-3
|
|
VEGFR-3
|
vascular endothelial growth factor
receptor-3
|
|
LEC
|
lymphatic endothelial cells
|
|
LV
|
lymphatic vessels
|
|
ETS
|
E26 transformation-specific
|
|
HUVEC
|
human umbilical vein endothelial
cells
|
References
|
1
|
Criqui-Filipe P, Ducret C, Maira SM and
Wasylyk B: Net, a negative Ras-switchable TCF, contains a second
inhibition domain, the CID, that mediates repression through
interactions with CtBP and de-acetylation. EMBO J. 18:3392–3403.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giovane A, Pintzas A, Maira SM,
Sobieszczuk P and Wasylyk B: Net, a new ets transcription factor
that is activated by Ras. Genes Dev. 8:1502–1513. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maira SM, Wurtz JM and Wasylyk B: Net
(ERP/SAP2) one of the Ras-inducible TCFs, has a novel inhibitory
domain with resemblance to the helix-loop-helix motif. EMBO J.
15:5849–5865. 1996.PubMed/NCBI
|
|
4
|
Ayadi A, Zheng H, Sobieszczuk P,
Buchwalter G, Moerman P, Alitalo K and Wasylyk B: Net-targeted
mutant mice develop a vascular phenotype and up-regulate egr-1.
EMBO J. 20:5139–5152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nozaki M, Onishi Y, Kanno N, Ono Y and
Fujimura Y: Molecular cloning of Elk-3, a new member of the Ets
family expressed during mouse embryogenesis and analysis of its
transcriptional repression activity. DNA Cell Biol. 15:855–862.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng H, Wasylyk C, Ayadi A, Abecassis J,
Schalken JA, Rogatsch H, Wernert N, Maira SM, Multon MC and Wasylyk
B: The transcription factor Net regulates the angiogenic switch.
Genes Dev. 17:2283–2297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ducret C, Maira SM, Dierich A and Wasylyk
B: The net repressor is regulated by nuclear export in response to
anisomycin, UV, and heat shock. Mol Cell Biol. 19:7076–7087. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen ST, Pan TL, Juan HF, Chen TY, Lin YS
and Huang CM: Breast tumor microenvironment: Proteomics highlights
the treatments targeting secretome. J Proteome Res. 7:1379–1387.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Funasaka T and Raz A: The role of
autocrine motility factor in tumor and tumor microenvironment.
Cancer Metastasis Rev. 26:725–735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tredan O, Galmarini CM, Patel K and
Tannock IF: Drug resistance and the solid tumor microenvironment. J
Natl Cancer Inst. 99:1441–1454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yong LC and Jones BE: A comparative study
of cultured vascular and lymphatic endothelium. Exp Pathol.
42:11–25. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heo SH and Cho JY: ELK3 suppresses
angiogenesis by inhibiting the transcriptional activity of ETS-1 on
MT1-MMP. Int J Biol Sci. 10:438–447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park KS, Cha Y, Kim CH, Ahn HJ, Kim D, Ko
S, Kim KH, Chang MY, Ko JH, Noh YS, et al: Transcription elongation
factor Tcea3 regulates the pluripotent differentiation potential of
mouse embryonic stem cells via the Lefty1-Nodal-Smad2 pathway. Stem
Cells. 31:282–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson GL and Nakamura K: The c-jun
kinase/stress-activated pathway: Regulation, function, and role in
human disease. Biochim Biophys Acta. 1773:1341–1348. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishida N, Yano H, Nishida T, Kamura T and
Kojiro M: Angiogenesis in cancer. Vasc Health Risk Manag.
2:213–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gavard J: Endothelial permeability and
VE-cadherin: A wacky comradeship. Cell Adh Migr. 8:158–164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lilien J and Balsamo J: The regulation of
cadherin-mediated adhesion by tyrosine
phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell
Biol. 17:459–465. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monaghan-Benson E and Burridge K: The
regulation of vascular endothelial growth factor-induced
microvascular permeability requires Rac and reactive oxygen
species. J Biol Chem. 284:25602–25611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Winter MC, Shasby S and Shasby DM:
Compromised E-cadherin adhesion and epithelial barrier function
with activation of G protein-coupled receptors is rescued by Y-to-F
mutations in beta-catenin. Am J Physiol Lung Cell Mol Physiol.
294:L442–L448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Timmerman I, Hoogenboezem M, Bennett AM,
Geerts D, Hordijk PL and van Buul JD: The tyrosine phosphatase SHP2
regulates recovery of endothelial adherens junctions through
control of β-catenin phosphorylation. Mol Biol Cell. 23:4212–4225.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shayan R, Achen MG and Stacker SA:
Lymphatic vessels in cancer metastasis: Bridging the gaps.
Carcinogenesis. 27:1729–1738. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao Y: Opinion: Emerging mechanisms of
tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer.
5:735–743. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshimatsu Y, Yamazaki T, Mihira H, Itoh
T, Suehiro J, Yuki K, Harada K, Morikawa M, Iwata C, Minami T, et
al: Ets family members induce lymphangiogenesis through physical
and functional interaction with Prox1. J Cell Sci. 124:2753–2762.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sukriti S, Tauseef M, Yazbeck P and Mehta
D: Mechanisms regulating endothelial permeability. Pulm Circ.
4:535–551. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arima M, Cui D, Kimura T, Sonoda KH,
Ishibashi T, Matsuda S and Ikeda E: Basigin can be a therapeutic
target to restore the retinal vascular barrier function in the
mouse model of diabetic retinopathy. Sci Rep. 6:384452016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coso S, Zeng Y, Opeskin K and Williams ED:
Vascular endothelial growth factor receptor-3 directly interacts
with phosphatidylinositol 3-kinase to regulate lymphangiogenesis.
PLoS One. 7:e395582012. View Article : Google Scholar : PubMed/NCBI
|