Introduction
Lung cancer is a leading cause of cancer mortality
globally, in which the predominant subtype of non-small cell lung
cancer (NSCLC) represents ~80% of all cases (1). Despite advances in therapy, the overall
5-year survival rate is <15% in patients with NSCLC (2). Currently, conventional chemotherapy
remains an important treatment option for patients with NSCLC.
Anti-mitotic agents, including docetaxel and paclitaxel (PTX), are
predominantly used as chemotherapy regimens for NSCLC (3). However, taxane-based therapy is
disadvantaged by the rapid emergence of acquired resistance
(4). Therefore, novel therapeutic
strategies that overcome the resistance to taxane-based therapy are
required.
Nedd8 (neural precursor cell expressed,
developmentally downregulated 8), a 9-kDa small ubiquitin-like
molecule, is involved in protein neddylation initiated by NEDD8
activating enzyme (NAE) (5). Nedd8
serves a role in the activation of the cullin-RING ligases (CRL),
also termed SKP1-cullin-F-box (SCF) E3 ligases for its founding
member (CRL/SCF) (6), which has been
established to be involved in the regulation of multiple DNA
replication and repair pathways (7,8). MLN4924
is a recently identified small molecule inhibitor of NAE and is
currently in Phase I clinical trials (9,10). By
inhibiting neddylation, MLN4924 promotes uncontrolled S-phase DNA
replication as well as in the induction of DNA damage and
subsequent cell death (11–13). Therefore, MLN4924 exhibits potent
antitumor activity in numerous types of cancer (14). Notably, previous studies have
indicated that MLN4924 overcomes platinum resistance in preclinical
models of ovarian cancer (15,16),
suggesting that inhibiting neddylation with MLN4924 may be a novel
strategy to target drug resistance in cancer.
The focus of the present study was to investigate
the effects of MLN4924 on PTX-resistant NSCLC cells. The results
identified that MLN4924 suppresses the growth of PTX-resistant
NSCLC cells by inducing apoptosis and DNA damage.
Materials and methods
Cell lines and cell culture
PTX-resistant H460 (H460/PTX) cells were cultured in
RPMI 1640 (Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA)
containing 70 nM (60 ng/ml) PTX (Zhejiang University, Hangzhou,
China) (17), PTX-resistant A549
(A549/PTX) cells, were kindly provided by Dr Sang Kook Lee (Seoul
National University, Seoul, Korea) (18) and cultured in RPMI 1640 containing 117
nM (100 ng/ml) PTX to maintain resistance at 37°C in an atmosphere
containing 5% CO2. The cells were cultured in complete
media without PTX for 3 days prior to performing experiments.
Antibodies and reagents
The antibody against β-actin was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany, A5316). The antibody
against regulated in development and DNA damage responses 1 (REDD1)
was purchased from ProteinTech Group, Inc. (Chicago, IL, USA; cat.
no. 10638-1-AP). The anti-checkpoint kinase 2 (CHK2; cat. no.
ab109413), anti-Histone H2AX (H2AX) (cat. no. ab124781) and
anti-p21 (cat. no. ab109199) antibodies were obtained from Abcam
(Cambridge, UK). The anti-cullin1 (cat. no. sc-11384) and
anti-chromatin licensing and DNA replication factor 1 (CDT1; cat.
no. sc-36530) antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The following antibodies
were obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA): Phospho-CHK2 (cat. no. 2661), phospho-H2AX (cat. no. 9718),
Wee1 (cat. no. 4936), p27 (cat. no. 3686), caspase-3 (cat. no.
9662) and poly(ADP-ribose) polymerase (PARP; cat. no. 9532). The
following agents were used: MLN4924 (Merck KGaA) and PTX
(Sigma-Aldrich; Merck KGaA). Recombinant human epidermal growth
factor (EGF), fibroblast growth factors (FGF; both from PeproTech,
Inc., Rocky Hill, NJ, USA) and B-27 (Gibco; Thermo Fisher
Scientific, Inc.) were used to culture spheroids. Drugs were
dissolved in dimethyl sulfoxide (DMSO) and stored at −20°C.
Bromodeoxyuridine (BrdU) labeling
H460/PTX and A549/PTX cells were labeled with 10
mmol/l BrdU (Sigma-Aldrich; Merck KGaA) in growth medium for 12 h
at 37°C. BrdU-labeled DNA was detected with mouse monoclonal
anti-BrdU antibody (cat. no. RPN202; 1:50; GE Healthcare, Chicago,
IL, USA), according to the manufacturer's protocol (19).
Colony formation assay
For clonogenic assay, cells were seeded into 6-well
plates (300 cell/well) in triplicate and cultured for 14 days at
37°C in an atmosphere containing 5% CO2. The colonies on
the plates were fixed with 4% paraformaldehyde at room temperature
for 15 min and stained with crystal violet (0.2% in anhydrous
ethanol) at room temperature for 30 min. Colonies with >50 cells
were counted (19). Colonies were
counted by eye and representative results of 3 independent
experiments with similar results are presented.
Spheroid formation
Adherent cells were suspended in serum-free
Dulbecco's modified Eagle's medium/F12 (Gibco; Thermo Fisher
Scientific, Inc.) containing 20 ng/ml FGF, 20 ng/ml EGF and B-27
(B-27 and medium at a 1:50 volume ratio), plated (1×103
cell/well) onto a 96-well clear flat-bottomed ultra-low attachment
micro plate (Corning Incorporated, Corning, NY, USA) at 37°C and 5%
CO2 for 10 days. Spheroids with a diameter of ~50 µm
were counted.
Cell death assay
A549/PTX and H460/PTX cells were suspended in
complete medium for spheroids, and plated (1×103
cell/well) onto a 96-well clear flat-bottomed ultra-low attachment
microplate (Corning Incorporated, Corning, NY, USA) at 37°C and 5%
CO2 for 10 days. The spheroids were treated with 10 µM
MLN4924 for the 24 and 72 h and stained with propidium iodide (PI)
at 10 µg/ml. Subsequently, cells were visualized using fluorescence
microscopy as previously described (20) where red-fluorescing cells were
indicative of cell death.
Western blot analysis
A549/PTX and H460/PTX cells were plated in 60-mm
dishes and treated with DMSO or 2.5, 5, 10, 20 and 0.1, 0.3, 1, 3,
10, 30 µM MLN4924 at 37°C in an atmosphere containing 5%
CO2. After 12, 24 and 48 h, cells were placed on ice,
washed with cold PBS, harvested using a scraper and lysed in lysis
buffer (1% Triton X-100, 50 mmol/l Tris-HCl (pH 7.5), 150 mmol/l
NaCl, 1 mmol/l EDTA, 1 mmol/l EGTA, 5 mmol/l sodium pyrophosphate,
25 mmol/l NaF, 0.5 mmol/l sodium orthovanadate, 1 mmol/l DTT, 1
µg/ml pepstanin, 2 µg/ml leupeptin, 2 µg/ml aprotinin, 0.1 mg/ml
phenylmethylsulfonyl fluoride). Cell lysates were centrifuged at
12,000 × g for 10 min at 4°C and supernatants were subjected to
western blot analysis. Concentration of protein was determined
using a bicinchoninic acid assay kit (cat. no. 23225; Pierce,
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Protein (50 µg per lane) was loaded on SDS-PAGE (8–15%
gels) and transferred onto a nitrocellulose membrane (Applygen
Technologies, Inc., Beijing, China). The membranes were blocked
with 5% non-fat milk in TBST at room temperature for 3 h and
incubated with primary antibodies at 4°C overnight. The antibodies
for β-actin (cat. no. A5316; 1:10,000; Merck KGaA, Darmstadt,
Germany,), REDD1 (1:2,000), CHK2 (1:2,000), H2AX (1:2,000), p21
(1:1,000), cullin1 (1:200), CDT1 (1:1,000), phospho-CHK2 (1:1,000),
phospho-H2AX (1:1,000), Wee1 (1:1,000), p27 (1:1,000), caspase-3
(1:1,000) and PARP (1:1,000) were used. Following washing three
times with TBS-Tween-20, the membranes were incubated at room
temperature for 1 h with corresponding horseradish
peroxidase-conjugated secondary antibodies (goat anti-mouse,
1:1,000, cat. no. 62-6520; goat anti-rabbit, 1:10,000, cat. no.
65-6120; Invitrogen; Thermo Fisher Scientific, Inc.). The blots
were detected using an ECL Western Blot Substrate kit (cat. no.
34580; Thermo Fisher Scientific, Inc.) according to the
manufacturers protocol.
Flow cytometric analysis of
apoptosis
A549/PTX and H460/PTX cells were treated with DMSO
or 1, 5 and 10 µM MLN4924 for 24, 47 and 72 h at 37°C in an
atmosphere containing 5% CO2, and then the cells were
harvested with 0.25% trypsin without EDTA, washed twice with
ice-cold PBS and resuspended in 500 µl binding buffer (10 mM
Hepes/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2).
Subsequently, cells were incubated with 5 µl annexin V-fluorescein
isothiocyanate (40 µg/ml; BD Biosciences, Franklin Lakes, NJ, USA)
and 5 µl PI (40 µg/ml; BD Biosciences) in the dark for 10 min at
room temperature and detected using flow cytometry. The percentage
of apoptotic cells was determined in 3 independent experiments.
Statistical analysis
The results are expressed as means ± standard
deviation. Statistical significance was evaluated using the
Student's t-test. Group comparisons were evaluated using a one-way
analysis of variance. All statistical tests were performed with
Prism software (version 5; GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
MLN4924 exhibits a potent
antiproliferative effect on PTX-resistant NSCLC cells
To investigate the effect of MLN4924 on A549/PTX and
H460/PTXPTX-resistant NSCLC cells, the cells were treated with
MLN4924 and used for several cell-based assays. As presented in
Fig. 1A, MLN4924 induced the loss of
NEDD8-conjugated (neddylated) cullins in A549/PTX and H460/PTX
cells. In accordance with previous studies, cullin neddylation was
substantially blocked in parent A549 and H460 cells following
MLN4924 treatment (Fig. 1A) (21,22). BrdU
assay analysis demonstrated that MLN4924 treatment led to a
reduction in cell viability of A549/PTX and H460/PTX cells in a
dose- and time-dependent manner (Fig.
1B). The half-maximal inhibitory concentration (IC50) values of
A549/PTX were 18.3, 12.0 and 7.3 µM for 24, 48 and 72 h,
respectively, and the IC50 values of H460/PTX were 12.9, 10.6 and
5.0 µM for 24, 48 and 72 h, respectively (Fig. 1B). Additionally, 10 µM MLN4924
significantly decreased the clonogenic survival of A549/PTX and
H460/PTX cells (P=0.0003; Fig. 1C).
Therefore 10 µM MLN4924 was used throughout the current study.
Taken together, these data indicate that MLN4924 potently inhibits
the growth of A549/PTX and H460/PTX cells.
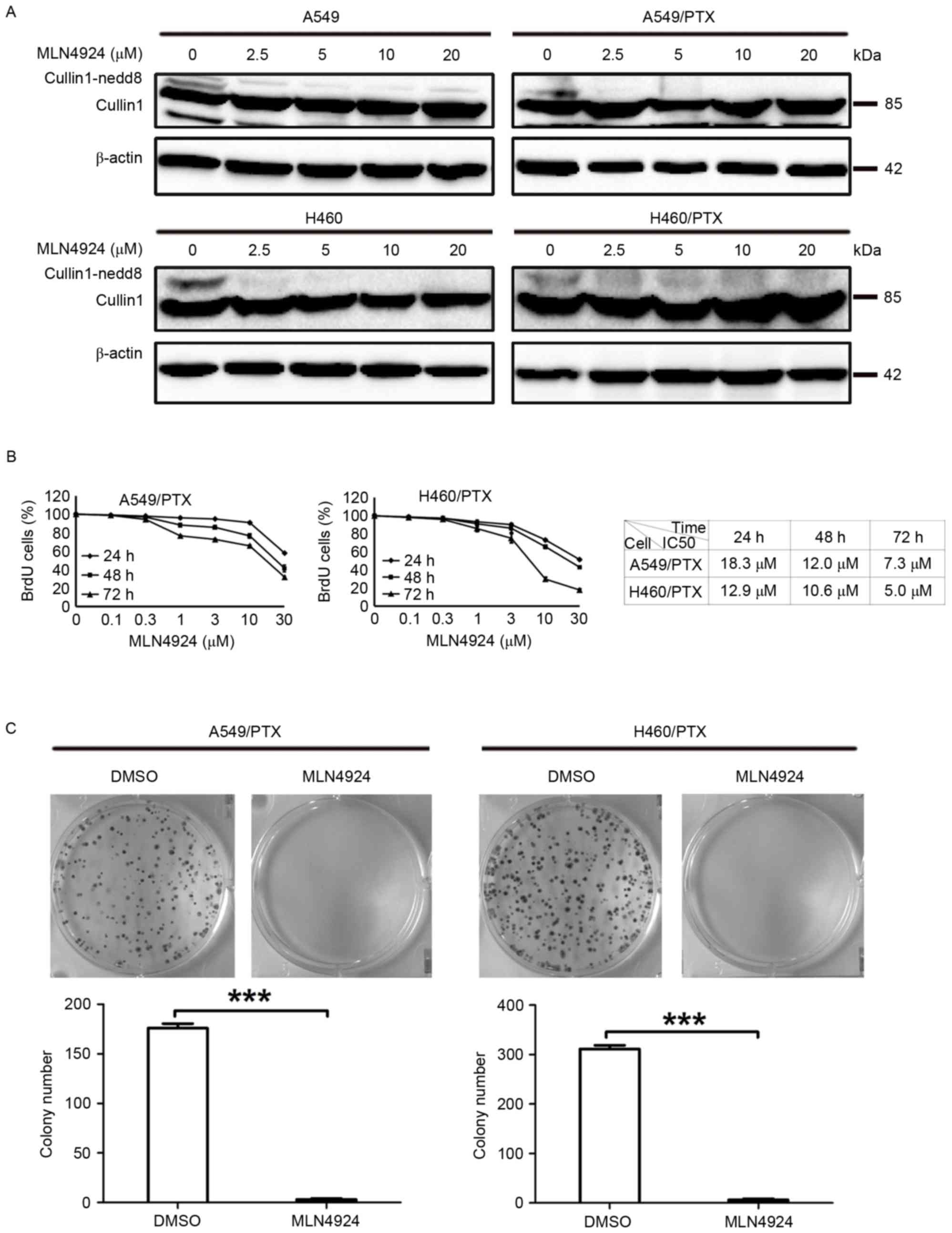 | Figure 1.MLN4924 suppresses the growth of
PTX-resistant NSCLC cells. (A) The A549 and H460 NSCLC cells and
PTX-resistant A549 (A549/PTX) and H460 (H460/PTX) were treated with
vehicle or numerous concentrations (0, 2.5, 5, 10 and 20 µM) of
MLN4924 for 24 h. (A) Cell lysates were examined for cullin1 using
western blot analysis. β-actin was used as a loading control. (B)
The A549/PTX and H460/PTX cells were treated with vehicle or the
indicated concentrations (0, 0.1, 0.3, 1, 3, 10 and 30 µM) of
MLN4924 for 24, 48 and 72 h, the cell viability was determined
using a BrdU assay. The results are presented as the mean ±
standard deviation. (C) The A549/PTX and H460/PTX cells were
treated with vehicle or 10 µM MLN4924 for 14 days in complete
medium and colony formation assay was performed. The number of
colonies was counted and presented as the mean ± standard
deviation. All experiments were repeated 3 times. ***P<0.001.
NSCLC, non-small cell lung cancer; PTX, paclitaxel; BrdU,
bromodeoxyuridine; DMSO, dimethylsulfoxide; IC50, half-maximal
inhibitory concentration. |
MLN4924 promotes apoptosis and DNA
damage in PTX-resistant NSCLC cells
To investigate the underlying mechanism of how
MLN4924 suppresses the growth of PTX-resistant NSCLC cells,
MLN4924-treated A549/PTX and H460/PTX cells were analyzed for the
induction of apoptosis using flow cytometry with annexin V and PI
double staining, as MLN4924 has been established to induce
apoptosis in a number of cancer cells (11,15,22,23).
As presented in Fig. 2A, exposure to
MLN4924 led to a significant increase in apoptosis in A549/PTX and
H460/PTX cells in a time- and dose-dependent manner. Furthermore,
MLN4924 treatment promoted caspase-3 processing and PARP cleavage,
2 hallmarks of apoptosis, in A549/PTX and H460/PTX cells during a
48 h period (Fig. 2B). These results
indicate that apoptosis is induced by MLN4924 in A549/PTX and
H460/PTX cells.
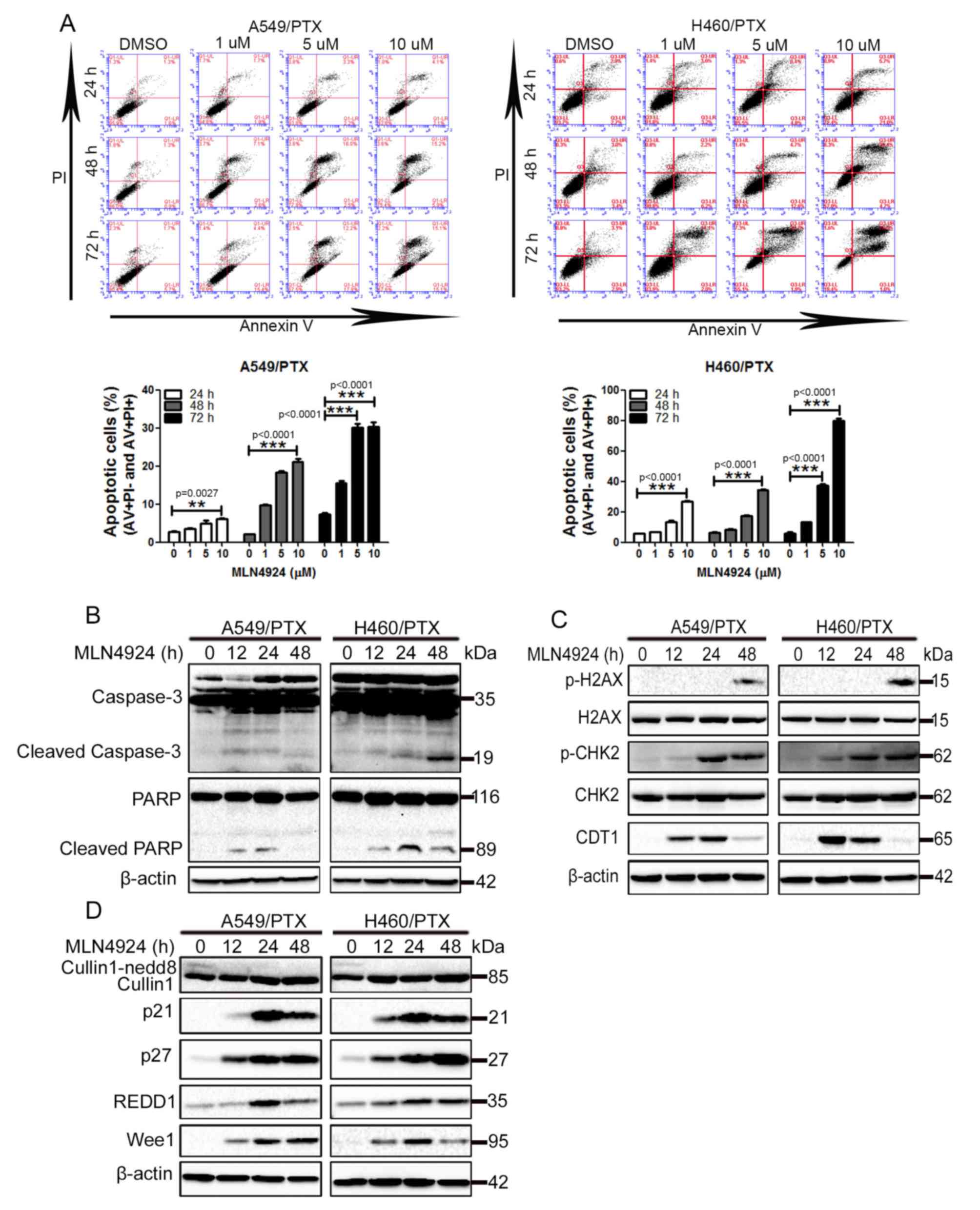 | Figure 2.MLN4924 induces apoptosis and DNA
damage in PTX-resistant non-small cell lung cancer cells. (A)
A549/PTX and H460/PTX cells were treated with DMSO or 1, 5 and 10
µM MLN4924 for 24, 48 and 72 h. The cells were double-stained with
AV and PI, apoptosis was analyzed using flow cytometry. The
percentage of apoptotic cells were presented as the mean ± standard
deviation. The A549/PTX and H460/PTX cells were treated with
vehicle or 10 µM MLN4924 for 12, 24 and 48 h. The cell lysates were
collected and detected using western blot analysis. (B) Western
blot analysis for caspase-3, PARP and β-actin. (C) Western blot
analysis for p-H2AX, H2AX, p-CHK2, CHK2, CDT1 and β-actin. (D)
Western blot analysis for cullin1, p21, p27, REDD1, Wee1 and
β-actin. All experiments were performed 3 times. **P<0.01,
***P<0.001. PI, propidium iodide; AV, annexin V; PTX,
paclitaxel; PARP, poly(ADP-ribose) polymerase; p-, phosphorylated;
H2AX, histone 2AX; CHK2, checkpoint kinase 2; CDT1, chromatin
licensing and DNA replication factor 1; REDD1, regulated in
development and DNA damage responses 1. |
In addition to inducing apoptosis, MLN4924 initiates
the DNA damage response (DDR) in cancer cells (8,11,13,23).
Increased phosphorylation of histone H2AX and CHK2, 2 classical
markers of DDR, was observed in A549/PTX and H460/PTX cells
following MLN4924 treatment (Fig.
2C). In addition, MLN4924-mediated accumulation of the DNA
replication licensing factor, CDT1 in these cells (Fig. 2C). Taken together, these results
indicate that MLN4924 promotes the DDR in PTX-resistant NSCLC
cells.
It has been established that the effect of the
induction of apoptosis and DDR by MLN4924 is due to its
inactivation of CRL/SCF (13,22–24).
Therefore, the levels of numerous CRL/SCF substrates were examined
in MLN4924-treated cells. As presented in Fig. 2D, MLN4924 treatment led to increased
levels of p21, p27 and Wee1 in A549/PTX and H460/PTX cells, whereas
cullin neddylation was inhibited. Increased levels of REDD1 were
observed in MLN4924-treated A549/PTX and H460/PTX cells (Fig. 2D). These data indicate that MLN4924
efficiently inactivates CRL/SCF in A549/PTX and H460/PTX cells.
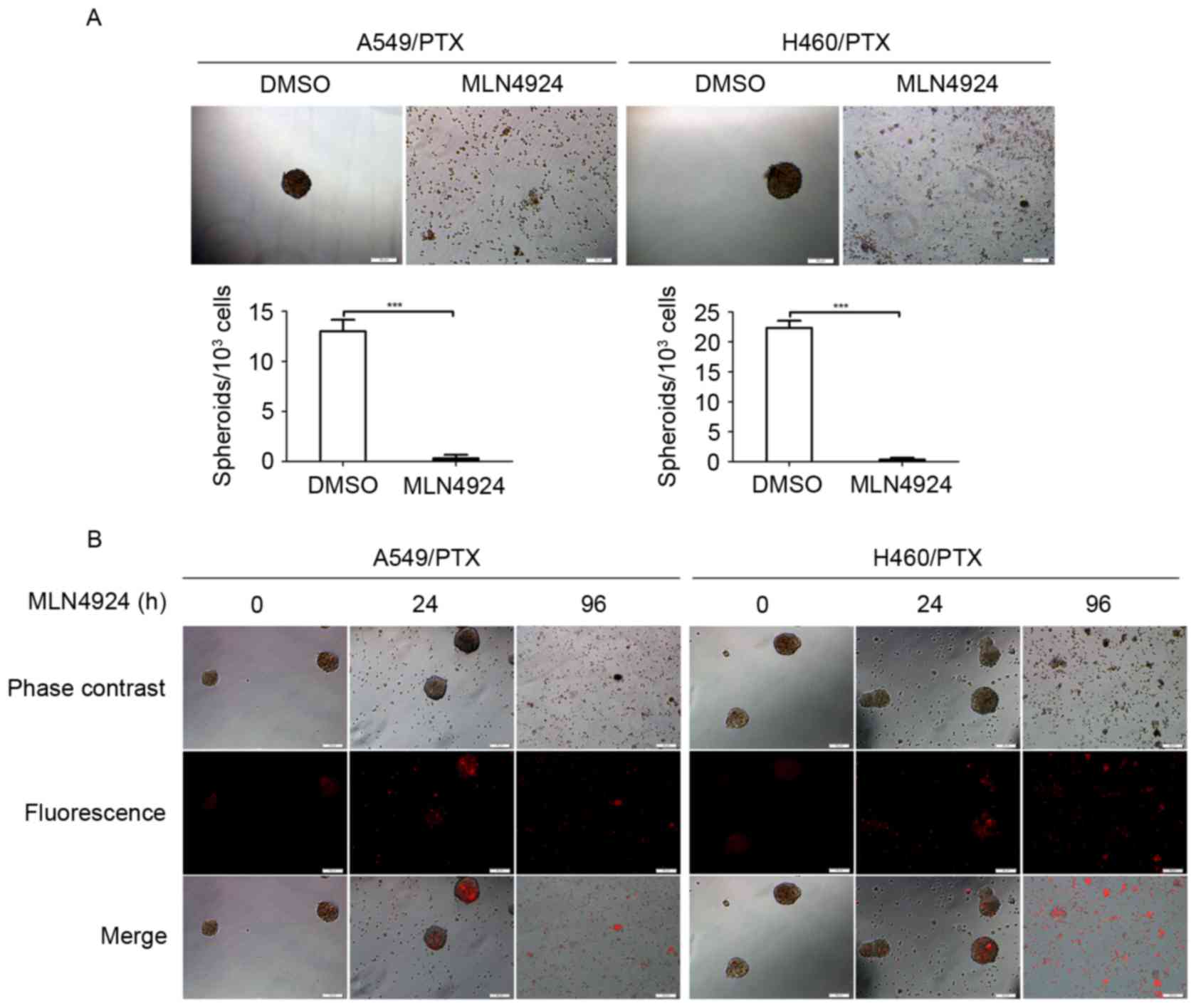
MLN4924 suppresses the growth of
PTX-resistant NSCLC cells in 3-dimensional (3D) cultures
The 3D multicellular tumor spheroids are typically
used as in vitro surrogates of tumorigenesis (25). Therefore, the growth inhibitory effect
of MLN4924 was investigated in PTX-resistant NSCLC cells using 3D
cultures. The A549/PTX and H460/PTX cells formed spheroids (with
diameter of ~50 µm) under appropriate condition for 3D cultures
(Fig. 3A). However, MLN4924-treated
A549/PTX and H460/PTX cells did not form spheroids compared with
the DMSO-treated cells (Fig. 3A),
indicating that MLN4924 has the ability to abrogate the 3D growth
potential of the PTX-resistant NSCLC cells. Furthermore, following
exposure to MLN4924 for 96 h, the A549/PTX and H460/PTX spheroids
had collapsed and there were increased levels of cell debris
(Fig. 3B), indicating that prolonged
treatment of MLN4924 promotes lysis of the A549/PTX and H460/PTX
spheroids.
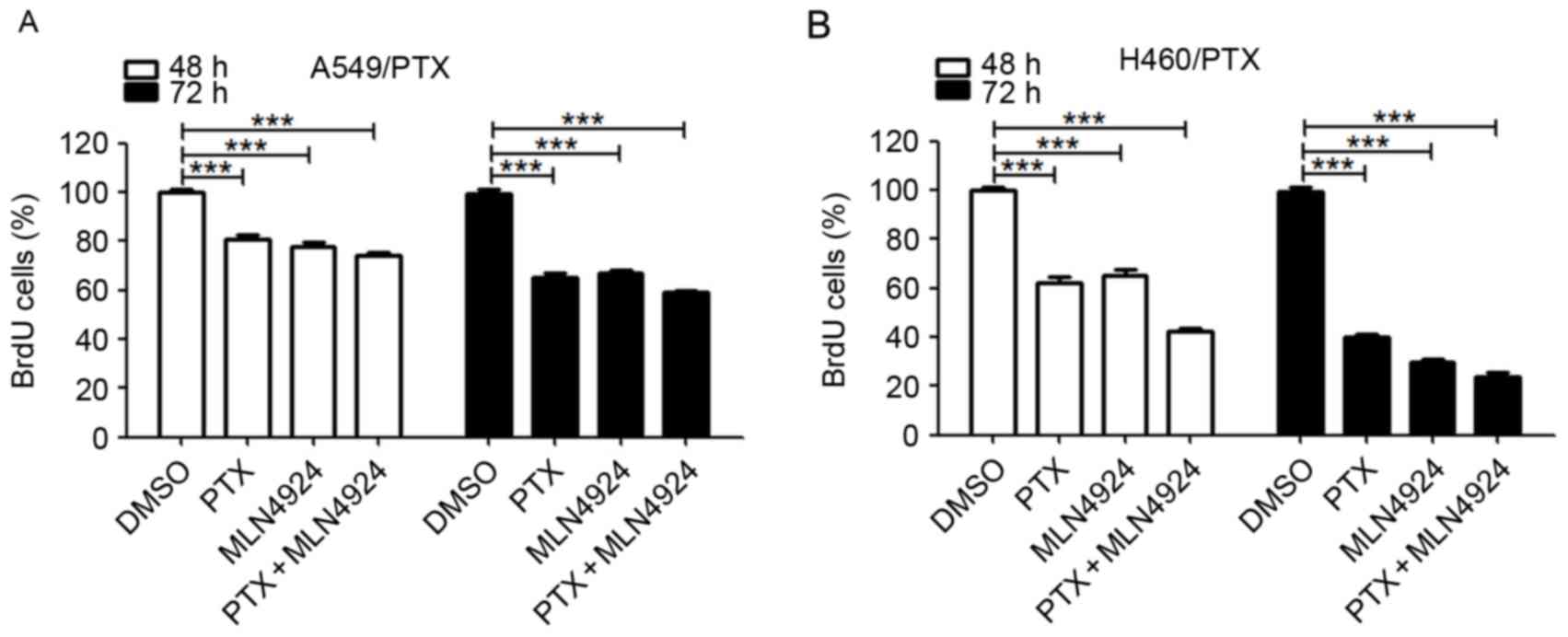
Combining MLN4924 with PTX does not
exhibit synergy in PTX-resistant NSCLC cells
To investigate whether combining MLN4924 treatment
with PTX results in an additive efficacy, the cell viability was
evaluated using the BrdU assay. As presented in Fig. 4A, compared with each drug alone, the
combination of MLN4924 and PTX treatment for numerous durations did
not result in any significant alteration in cell viability in
A549/PTX or H460/PTX cells. These data suggest that there is no
synergistic effect between MLN4924 and PTX in the PTX-resistant
NSCLC cells.
Discussion
Resistance to docetaxel or PTX remains a primary
obstacle in the treatment of NSCLC. The current study demonstrated
that the neddylation inhibitor, MLN4924, potently suppresses the
growth of PTX-resistant NSCLC cells by inducing apoptosis and DNA
damage. Furthermore, in addition to inducing clonogenic and
spheroid formation, MLN4924 promotes the disassembly of
PTX-resistant NSCLC cell spheroids. As MLN4924 is a first-in-class
inhibitor of NAE that is being evaluated in multiple phase I
clinical trials (9,10). Soucy et al (14) identified that MLN4924 suppressed the
growth of human tumor xenografts in mice, suggesting that NAE
inhibitors may have potential as a treatment for cancer. The
results of the present study provide a rationale for the clinical
investigation of protein neddylation inhibition as a novel strategy
for the treatment of PTX-resistant NSCLC.
An increasing number of studies have demonstrated
that MLN4924 promotes a DNA damage response, cell cycle arrest,
apoptosis and senescence in a number of cancer cell types (8,11–13,15,22,23).
In accordance with these previous studies, the current study
observed that MLN4924 promotes apoptosis and the DDR in
PTX-resistant NSCLC cells. Notably, MLN4924 suppresses the growth
of PTX-resistant NSCLC cells in 3D culture. As the multicellular
spheroids are an effective 3D cell culture model that is able to
mimic in vivo microenvironments compared to 2D cell cultures
(26), the results of the present
study suggest a potential in vivo effect of MLN4924 on
PTX-resistant NSCLC cells.
Consistent with previous studies (13,21–24), the
effect of MLN4924 in PTX-resistant NSCLC cells was due to its
inactivation of CRL/SCF demonstrated by the increased levels of a
number of CRL substrates, including p21, p27, REED1 and Wee1. These
data and the previous studies suggest an antineoplastic mechanism
of action for MLN4924.
Previous studies have established that targeting NAE
with MLN4924 effectively overcomes platinum resistance in
preclinical models of ovarian cancer (15,16).
Additionally, a synergic cytotoxic effect between cisplatin and
MLN4924 was observed in platinum-sensitive and -resistant ovarian
cancer cells (15,16). However, in the present study,
combination treatment of MLN4924 and PTX did not result in
synergistic cytotoxicity in the PTX-resistant NSCLC cells.
In conclusion, to the best of our knowledge, the
current study is the first to demonstrate the growth inhibitory
effect of MLN4924 in PTX-resistant NSCLC cells. The results of the
present study support further investigation of NAE targeting with
MLN4924 as an effective strategy for the treatment of PTX-resistant
NSCLC.
Acknowledgements
The authors would like to thank Professor Songshu
Meng (Institute of Cancer Stem Cell, Dalian Medical University of
China, Liaoning, China) for critical discussions and suggestions.
This study was supported in part by grants from the National
Natural Science Foundation of China (grant nos. 81502674 to K.J.
and 81301720 to W.C.).
References
|
1
|
Field JK and Duffy SW: Lung cancer
screening: The way forward. Br J Cancer. 99:557–562. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramalingam SS, Maitland ML, Frankel P,
Argiris AE, Koczywas M, Gitlitz B, Thomas S, Espinoza-Delgado I,
Vokes EE, Gandara DR and Belani CP: Carboplatin and Paclitaxel in
combination with either vorinostat or placebo for first-line
therapy of advanced non-small-cell lung cancer. J Clin Oncol.
28:56–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joshi M, Liu X and Belani CP: Taxanes,
past, present, and future impact on non-small cell lung cancer.
Anticancer Drugs. 25:571–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xirodimas DP: Novel substrates and
functions for the ubiquitin-like molecule NEDD8. Biochem Soc Trans.
36:802–806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng N, Schulman BA, Song L, Miller JJ,
Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, et al:
Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase
complex. Nature. 416:703–709. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia L and Sun Y: SCF E3 ubiquitin ligases
as anticancer targets. Curr Cancer Drug Targets. 11:347–356. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brown JS and Jackson SP: Ubiquitylation,
neddylation and the DNA damage response. Open Biol. 5:1500182015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarantopoulos J, Shapiro GI, Cohen RB,
Clark JW, Kauh JS, Weiss GJ, Cleary JM, Mahalingam D, Pickard MD,
Faessel HM, et al: Phase I study of the investigational
NEDD8-activating enzyme inhibitor pevonedistat (TAK-924/mlN4924) in
patients with advanced solid tumors. Clin Cancer Res. 22:847–857.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nawrocki ST, Griffin P, Kelly KR and Carew
JS: MLN4924: A novel first-in-class inhibitor of NEDD8-activating
enzyme for cancer therapy. Expert Opin Investig Drugs.
21:1563–1573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin JJ, Milhollen MA, Smith PG, Narayanan
U and Dutta A: NEDD8-targeting drug MLN4924 elicits DNA
rereplication by stabilizing Cdt1 in S phase, triggering checkpoint
activation, apoptosis, and senescence in cancer cells. Cancer Res.
70:10310–10320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mackintosh C, Garcia-Dominguez DJ, Ordóñez
JL, Ginel-Picardo A, Smith PG, Sacristán MP and de Álava E: WEE1
accumulation and deregulation of S-phase proteins mediate MLN4924
potent inhibitory effect on Ewing sarcoma cells. Oncogene.
32:1441–1451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blank JL, Liu XJ, Cosmopoulos K, Bouck DC,
Garcia K, Bernard H, Tayber O, Hather G, Liu R, Narayanan U, et al:
Novel DNA damage checkpoints mediating cell death induced by the
NEDD8-activating enzyme inhibitor MLN4924. Cancer Res. 73:225–234.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soucy TA, Smith PG, Milhollen MA, Berger
AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP,
Critchley S, et al: An inhibitor of NEDD8-activating enzyme as a
new approach to treat cancer. Nature. 458:732–736. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jazaeri AA, Shibata E, Park J, Bryant JL,
Conaway MR, Modesitt SC, Smith PG, Milhollen MA, Berger AJ and
Dutta A: Overcoming platinum resistance in preclinical models of
ovarian cancer using the neddylation inhibitor MLN4924. Mol Cancer
Ther. 12:1958–1967. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nawrocki ST, Kelly KR, Smith PG, Espitia
CM, Possemato A, Beausoleil SA, Milhollen M, Blakemore S, Thomas M,
Berger A and Carew JS: Disrupting protein NEDDylation with MLN4924
is a novel strategy to target cisplatin resistance in ovarian
cancer. Clin Cancer Res. 19:3577–3590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lv K, Liu L, Wang L, Yu J, Liu X, Cheng Y,
Dong M, Teng R, Wu L, Fu P, et al: Lin28 mediates paclitaxel
resistance by modulating p21, Rb and Let-7a miRNA in breast cancer
cells. PLoS One. 7:e400082012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim EH, Min HY, Chung HJ, Song J, Park HJ,
Kim S and Lee SK: Anti-proliferative activity and suppression of
P-glycoprotein by (−)-antofine, a natural phenanthroindolizidine
alkaloid, in paclitaxel-resistant human lung cancer cells. Food
Chem Toxicol. 50:1060–1065. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Jiang K, Zhu X, Lin G, Song F, Zhao
Y, Piao Y, Liu J, Cheng W, Bi X, et al: Encorafenib (LGX818), a
potent BRAF inhibitor, induces senescence accompanied by autophagy
in BRAFV600E melanoma cells. Cancer Lett. 370:332–344. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu L, Sun S, Wang T, Li Y, Jiang K, Lin G,
Ma Y, Barr MP, Song F, Zhang G and Meng S: Oncolytic newcastle
disease virus triggers cell death of lung cancer spheroids and is
enhanced by pharmacological inhibition of autophagy. Am J Cancer
Res. 5:3612–3623. 2015.PubMed/NCBI
|
|
21
|
Li H, Tan M, Jia L, Wei D, Zhao Y, Chen G,
Xu J, Zhao L, Thomas D, Beer DG and Sun Y: Inactivation of SAG/RBX2
E3 ubiquitin ligase suppresses KrasG12D-driven lung tumorigenesis.
J Clin Invest. 124:835–846. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L, Wang M, Yu G, Chen P, Li H, Wei D,
Zhu J, Xie L, Jia H, Shi J, et al: Overactivated neddylation
pathway as a therapeutic target in lung cancer. J Natl Cancer Inst.
106:dju0832014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo Z, Yu G, Lee HW, Li L, Wang L, Yang D,
Pan Y, Ding C, Qian J, Wu L, et al: The Nedd8-activating enzyme
inhibitor MLN4924 induces autophagy and apoptosis to suppress liver
cancer cell growth. Cancer Res. 72:3360–3371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu Y, Kaufman JL, Bernal L, Torre C,
Matulis SM, Harvey RD, Chen J, Sun SY, Boise LH and Lonial S:
MLN4924, an NAE inhibitor, suppresses AKT and mTOR signaling via
upregulation of REDD1 in human myeloma cells. Blood. 123:3269–3276.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loessner D, Flegg JA, Byrne HM, Clements
JA and Hutmacher DW: Growth of confined cancer spheroids: A
combined experimental and mathematical modelling approach. Integr
Biol (Camb). 5:597–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pampaloni F, Reynaud EG and Stelzer EH:
The third dimension bridges the gap between cell culture and live
tissue. Nat Rev Mol Cell Biol. 8:839–845. 2007. View Article : Google Scholar : PubMed/NCBI
|