Introduction
Malignant glioma is the most frequent and aggressive
type of primary brain tumor worldwide (1). According to the 2007 World Health
Organization (WHO) classification, malignant gliomas are classified
as grade II (diffuse astrocytoma), grade III (anaplastic
astrocytoma) or grade IV (glioblastoma; GBM) (2,3). The
mechanisms of glioma progression are heterogeneous, including the
activation of oncogenes, the silencing of tumor suppressor genes
and the development of glioma stem cells (4). Therefore, identifying genetic and
molecular lesions underlying glioma progression is required in
order to improve the current understanding of the precise mechanism
underlying malignant glioma, and thus improve therapeutic
methods.
Wnt pathway has widespread functions in a myriad of
cell biological and developmental processes. There are 19 Wnt
ligands, and >15 receptors and co-receptors distributed in >7
protein families in mammals (5). Wnt
molecules are secreted glycoproteins that regulate canonical or
noncanonical signaling pathways (6,7). In the
canonical pathway, in the absence of Wnt ligands, cytoplasmic
β-catenin is phosphorylated by a multiprotein destruction complex,
which is composed of the scaffolding protein AXIN, glycogen
synthase kinase 3 (GSK3), casein kinase 1 and the tumor suppressor,
adenomatous polyposis coli (APC) gene product, resulting in
β-catenin recognition by β-transducin repeat containing E3
ubiquitin protein ligase, an E3 ubiquitin ligase subunit.
Subsequent to ubiquitination, β-catenin is degraded by the
proteasome. Once bound by Wnt, the frizzled (FZD)/low-density
lipoprotein receptor-related protein co-receptor complex activates
the canonical signaling pathway, which inhibits β-catenin
phosphorylation by sequestrating GSK3 into multivesicular
compartments, thus leading to β-catenin stabilization and nucleus
translocation to form a nuclear complex with the transcription
factors T-cell factor (TCF)/lymphoid enhancer binding factor (LEF).
Finally, the target genes of Wnt, including cyclin D1 and AXIN2,
are transcribed to regulate cell proliferation and differentiation
(7,8).
At present, more than thirty years after the
identification of Wnt molecules, the understanding of the
complexity of the function of Wnt pathways in tumor initiation and
progression has increased (9).
Extensive studies, as reviewed in Zhang et al (10), have demonstrated that aberrant Wnt
signaling serves a vital function in the progression of gliomas,
including in cell proliferation, apoptosis, migration and invasion
in vitro. Of these, the best understood is the Wnt canonical
pathway, which contributes to tumorigenesis and cancer progression
in a context-dependent manner. For example, different types of
cancer are sensitive to different Wnt ligands, the stabilization of
β-catenin and the activation of TCF/LEF family of transcription
factors (7,11). Analysis using The Cancer Genome Atlas
(TCGA) database reveals that Wnt3 (12) and Wnt5a (13) are upregulated in human GBM. However,
there remains a lack of studies focusing on the expression pattern
of the core components of Wnt signaling in glioma tissues. In the
present study, the expression of core Wnt signaling molecules at
mRNA and protein levels were examined using a reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis. Furthermore, the clinical implication of key
Wnt signaling components was analyzed using data from the R2
Genomics Analysis and Visualization Platform.
Materials and methods
Tissue samples
Fresh glioma samples were collected from the Brain
Hospital of the Affiliated Hospital of Xuzhou Medical University
(Xuzhou, China) between February 2013 and May 2016. Surgically
removed tissues were immediately stored in liquid nitrogen. The
clinical stage of glioma was determined according to the 2007 WHO
classification (2). There were 12
grade II cases, 11 grade III cases and 14 grade IV cases. A total
of 11 non-tumorous brain tissue samples obtained from the internal
decompression of patients with cerebral injuries were used as the
control. Written informed consent was obtained from all patients
prior to the study, and the present study was approved by the
Research Ethics Committee of the Affiliated Hospital of Xuzhou
Medical University.
Antibodies
The following monoclonal antibodies were used in the
present study: Rabbit anti-Wnt2b (cat. no. ab178418; 1:1,000;
Abcam, Cambridge, UK), rabbit monoclonal anti-GSK3β (cat. no.
9315S; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
rabbit anti-GAPDH (cat. no. 2118S; 1:1,000; Cell Signaling
Technology, Inc.), mouse anti-Wnt3a (cat. no. MAB1324; 1:250;
R&D Systems, Inc., Minneapolis, MN, USA), anti-Wnt5a (cat. no.
MAB645; 1:250; R&D Systems, Inc.), rabbit anti-β-catenin (cat.
no. 9562S;, 1:1,000; Cell Signaling Technology, Inc.) and mouse
anti-cyclin D1 (cat. no. sc-450; 1:1,000, Santa Cruz Biotechnology,
Inc., Dallas, TX, USA).
RT-qPCR
Total RNA was extracted from tissue samples using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and reverse-transcribed using a First-Strand cDNA
kit (Roche Diagnostics, Indianapolis, IN, USA) to cDNA, according
to the manufacturers' protocols. For qPCR, cDNA products were
amplified using the FastStart Universal SYBR-Green Master (ROX;
Roche Diagnostics) according to the manufacturer's protocol. The
cycling conditions were as follows: 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 60 sec. The
2−∆∆Cq method was used to quantify gene expression
(14). All values were normalized
against the means of the non-tumorous group. All primers for the
human samples are listed in Table I.
β-catenin was designed by the authors of the present study, or
obtained from previous studies, including Wnt2b, 3, 3a, 5a, 5b, 7b,
9, 11, FZD, 6, 7 and β-actin (15),
GSK3β (16), APC, AXIN1, AXIN2 and
cyclin D1 (17).
 | Table I.Primers used for quantitative
polymerase chain reaction. |
Table I.
Primers used for quantitative
polymerase chain reaction.
| Gene | Length, bp | Direction | Primer
sequence |
|---|
| Wnt2b | 102 | Forward |
5′-GCCGTGTCATGCTCAGAA-3′ |
|
|
| Reverse |
5′-GTGGACTACCCCTGCTGATG-3′ |
| Wnt3 | 104 | Forward |
5′-CTCGCTGGCTACCCAATTT-3′ |
|
|
| Reverse |
5′-GCCCAGAGATGTGTACTGCTG-3′ |
| Wnt3a | 218 | Forward |
5′-CATGAACCGCCACAACAAC-3′ |
|
|
| Reverse |
5′-TGGCACTTGCACTTGAGGT-3′ |
| Wnt5a | 200 | Forward |
5′-ATTGTACTGCAGGTGTACCTTAAAAC-3′ |
|
|
| Reverse |
5′-CCCCCTTATAAATGCAACTGTTC-3′ |
| Wnt5b | 175 | Forward |
5′-CTGCTGCTGCTGTTCACG-3′ |
|
|
| Reverse |
5′-CACCGGGTTCAAAGCTAATG-3′ |
| Wnt7b | 159 | Forward |
5′-CGCCTCATGAACCTGCATA-3′ |
|
|
| Reverse |
5′-GCTGCATCCGGTCCTCTA-3′ |
| Wnt11 | 137 | Forward |
5′-TGTGCTATGGCATCAAGTGG-3′ |
|
|
| Reverse |
5′-CAGTGTTGCGTCTGGTTCAG-3′ |
| β-actin | 147 | Forward |
5′-CCAACCGCGAGAAGATGA-3′ |
|
|
| Reverse |
5′-CCAGAGGCGTACAGGGATAG-3′ |
| FZD2 | 157 | Forward |
5′-GGTGTCGGTGGCCTACAT-3′ |
|
|
| Reverse |
5′-GAGAAGCGCTCGTTGCAC-3′ |
| FZD6 | 201 | Forward |
5′-TGGGTTGGAAGCAAAAAGAC-3′ |
|
|
| Reverse |
5′-TCTTCGACTTTCACTGATTGGA-3′ |
| FZD7 | 210 | Forward |
5′-GCCAGCTTGTGCCTAATAGAA-3′ |
|
|
| Reverse |
5′-AGCCGGGAGAAACTCACAG-3′ |
| β-catenin | 204 | Forward |
5′-CTTACACCCACCATCCCACT-3′ |
|
|
| Reverse |
5′-CCTCCACAAATTGCTGCTGT-3′ |
| APC | 209 | Forward |
5′-GCCCCTGACCAAAAAGGAAC-3′ |
|
|
| Reverse |
5′-TGGCAGCAACAGTCCCACTA-3′ |
| GSK3β | 221 | Forward |
5′-CAAGCCAAACTTTGTGACTCAG-3′ |
|
|
| Reverse |
5′-TATCAGGATCCAGCAAGAGGTT-3′ |
| Axin1 | 130 | Forward |
5′-AGCCGTGTCGGACATGGA-3′ |
|
|
| Reverse |
5′-AAGTAGTACGCCACAACGATGCT-3′ |
| Axin2 | 210 | Forward |
5′-TGTGAGGTCCACGGAAACTG-3′ |
|
|
| Reverse |
5′-CGTCAGCGCATCACTGGATA-3′ |
| Cyclin D1 | 166 | Forward |
5′-TCAAATGTGTGCAGAAGGAGGT-3′ |
|
|
| Reverse |
5′-GACAGGAAGCGGTCCAGGTA-3′ |
Western blot analysis
Protein lysates were extracted from non-tumorous and
brain glioma tissues by using lysis buffer (containing 1 µM
pepstatin, 100 µM leupeptin, 2 µg/ml aprotinin, 1 mM
dithiothreitol, 1 mM benzamidine, 1 mM
Na3VO4, 1 mM NaF and 200 µM
phenylmethylsulfonyl fluoride). Following incubation for 30 min on
ice, cell lysates were centrifuged for 12,000 × g for 30 min, at
4°C. Protein lysates were measured using the Bicinchoninic Acid
Protein Assay kit (Tiangen Biotech Co., Beijing, China) and an
equal amount (40 µg) of protein lysates were subjected to SDS-PAGE
(10% gel). Protein was transferred onto polyvinylidene difluoride
membranes with a 0.45 µm pore size (EMD Millipore, Billerica, MA,
USA), which were blocked with 3% bovine serum albumin at room
temperature for 2 h and probed with primary antibodies at 4°C
overnight, and secondary antibodies [goat anti-rabbit
immunoglobulin G (IgG)-horseradish peroxidase (HRP); cat. no.
AP132P; 1:4,000; goat anti-mouse IgG-HRP; cat. no. AP124P; 1:4,000;
both EMD Millipore] at room temperature for 2 h. Bound antibodies
were detected using the Pierce ECL Plus Western Blotting Substrate
(Thermo Fisher Scientific, Inc.) and exposed to X-ray film. Band
densities were quantified by using Image-Pro Plus Software (version
6.0; Media Cybernetics, Inc., Rockville, MD, USA) and the
densitometric results were presented. All values were normalized to
GAPDH expression.
Survival analysis
Survival analysis within the glioma dataset was
performed using the glioma microarray dataset (Tumor Glioma
French-284-MAS5.0-u133p2) from the R2: Genomics Analysis and
Visualization Platform (http://r2.amc.nl).
Kaplan-Meier analysis was conducted online, and P-values were
calculated using the log-rank test. A cutoff method ‘Kaplan scan’
provided on the R2 platform was used to separate high and low
expression groups of genes [Expression cutoff: Wnt2B (238512_at),
15.1; Wnt5A (205990_s_at), 133.3; FZD2 (210220_at), 58.3; FZD6
(203987_at), 50.6; FZD7 (203706_at), 249.2). These cutoff gene
values belong to the high expression group. The Kaplan scan
generates a Kaplan-Meier plot to illustrate the association between
overall survival and a certain gene expression.
Statistical analysis
Results were presented as the mean ± standard error
of the mean. Statistical comparisons were performed using one-way
analysis of variance, with post-hoc analysis performed using
Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using Microsoft Office Excel 2007 (Microsoft Corporation,
Redmond, WA, USA), SPSS software (version 20.0; IBM Corp., Armonk,
NY, USA) or GraphPad Prism (version 5; GraphPad Software Inc., La
Jolla, CA, USA).
Results
Wnt signaling mRNA expression levels
in glioma
Although the expression of Wnt/β-catenin signaling
pathway members in glioma has been demonstrated previously
(15,18–20), there
is a lack of studies that have systematically investigated the
expression of Wnt signaling. In order to comprehensively
characterize the expression of Wnt signaling in glioma tissues, the
R2: Genomics Analysis and Visualization Platform database was
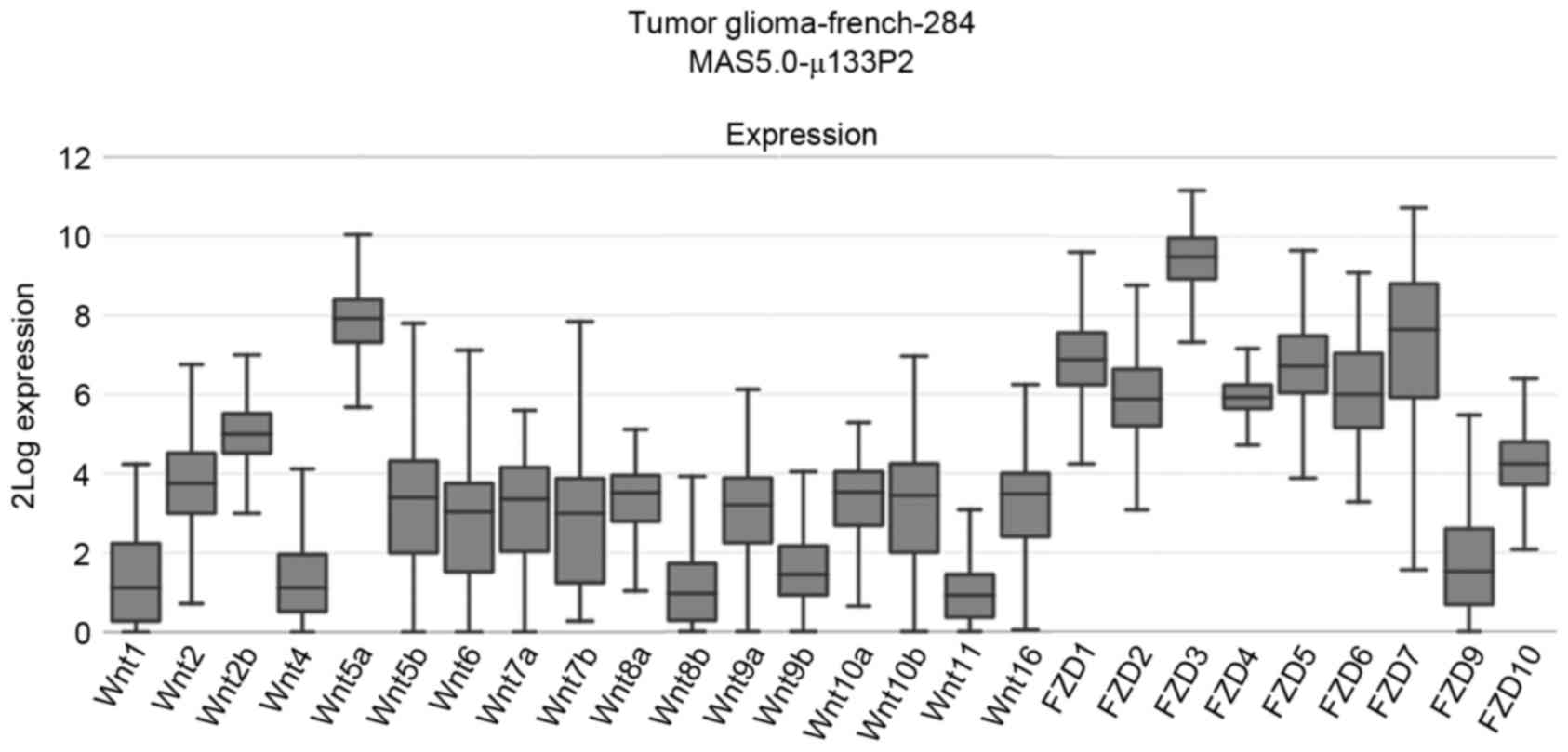
interrogated, which revealed that, in patients with glioma, the
median mRNA expression levels of Wnt2b, Wnt5a, FZD1-7 were
increased compared with other Wnt pathway members (Fig. 1). Thus, the mRNA expression levels of
Wnt ligands, receptors, β-catenin destruction complex and its
target genes cyclin D1 and AXIN2 were determined using qPCR. Based
on previous studies, which detected the Wnt ligands in glioma cells
and tissues (15,18), eight members of the Wnt family (Wnt2b,
3, 3a, 5a, 5b, 7b, 9b and 11) were selected for further
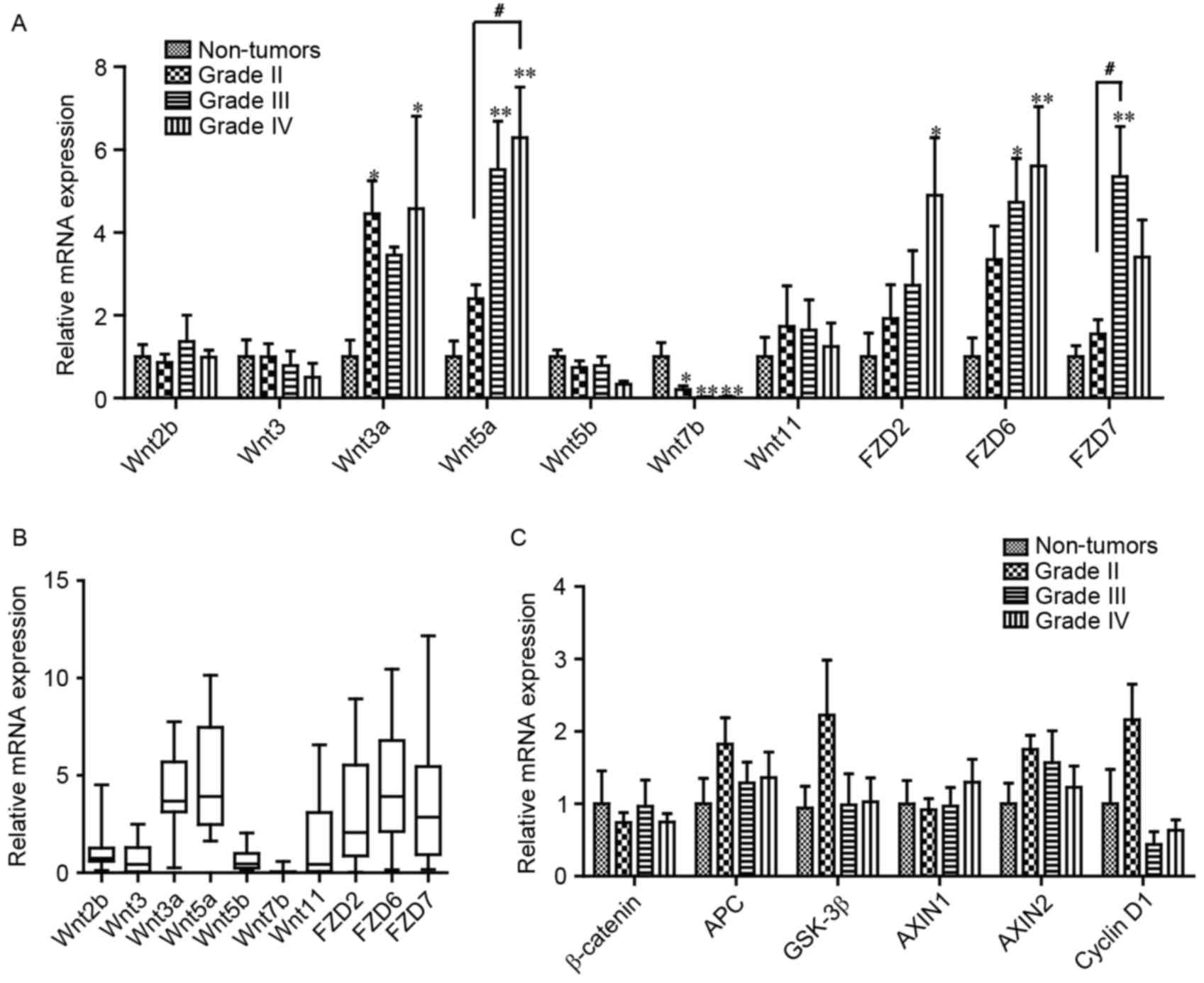
investigation. As presented in Fig.
2A, compared with non-tumor tissues, the mRNA expression level
of Wnt3a and 5a in grade IV gliomas increased significantly, 4.57-
and 5.40-fold, respectively. However, the mRNA expression level of
Wnt7b in grade IV gliomas was significantly decreased, to 3% of
that of non-tumor tissues (Fig. 2A).
In addition, Wnt2b, 3, 5b and 11 demonstrated no difference in mRNA
expression levels between glioma and non-tumor tissue.
Furthermore, the mRNA expression levels of the Wnt
receptors FZD2, 6 and 7 were detected, which serve critical
functions in glioma (10,15). The mRNA expression levels of FZD2 and
FZD6 were significantly increased 4.90- and 5.61-fold in grade IV
gliomas, respectively (Fig. 2A).
Compared with non-tumor tissue, the mRNA expression level of FZD7
was significantly increased 5.35-fold in grade III gliomas
(Fig. 2A). Furthermore, as presented
in Fig. 2B, the mRNA expression level
of the aforementioned molecules was presented as quartiles, which
revealed that the median mRNA expression levels of Wnt3a, Wnt5a,
FZD2, 6 and 7 were increased relative to the other molecules, which
was similar to the results from the R2 platform and those of TCGA
database (13). However, significant
changes were not detected in the mRNA expression levels of
β-catenin and its degradation complex, including APC, GSK 3β,
AXIN1, cyclin D1 or AXIN2, in the glioma samples (Fig. 2C).
Wnt signaling pathway protein
expression levels in glioma
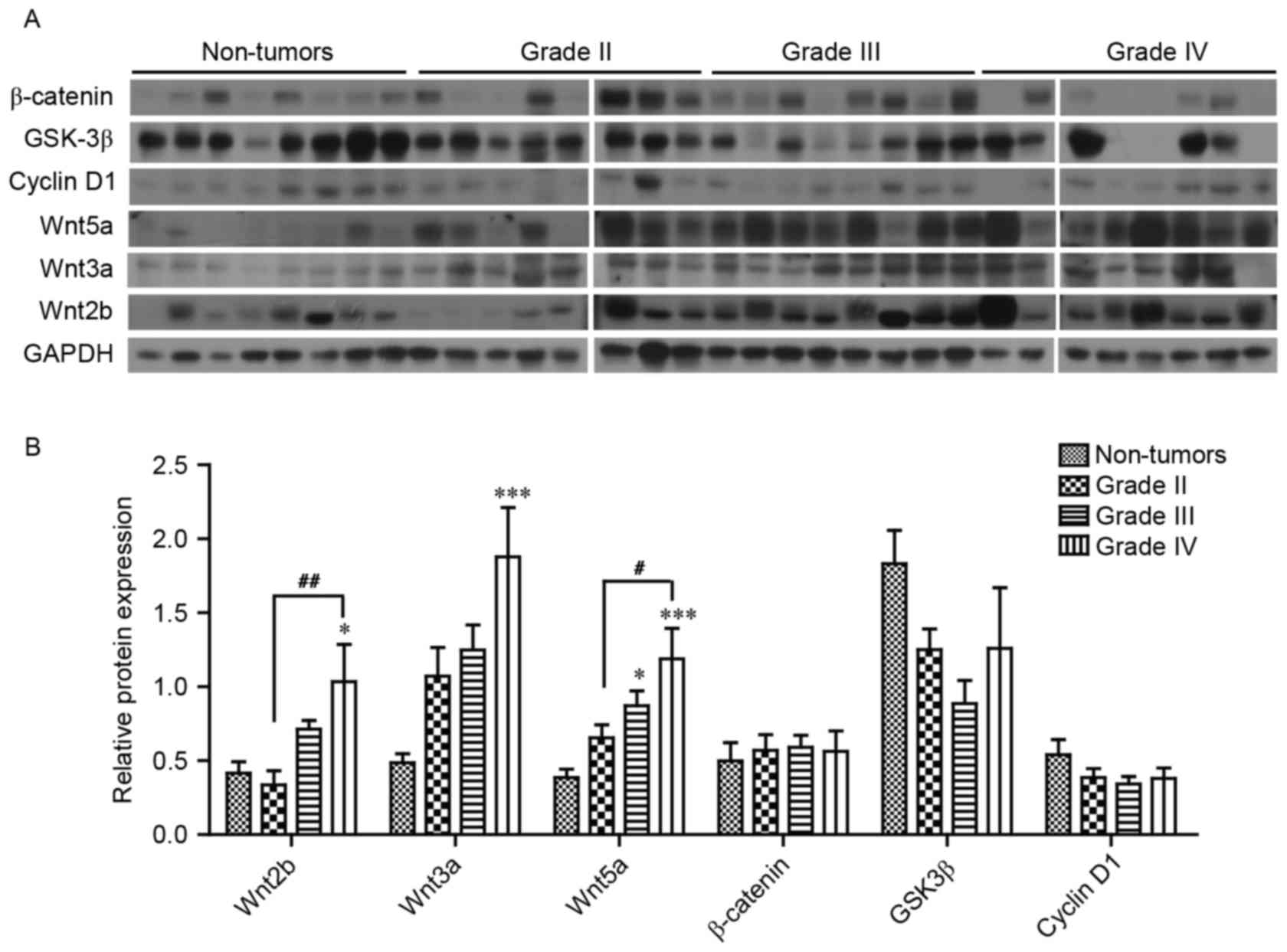
The protein expression levels of a number of the
core components of Wnt signaling were analyzed; it was revealed
that Wnt2b, 3a and 5a expression levels were significantly
increased, 2.49-, 3.86- and 3.08-fold in grade IV gliomas,
respectively, compared with non-tumor brain tissue (Fig. 3A and B). However, no significant
difference in the protein expression levels of β-catenin, GSK3β and
cyclin D1 were observed in glioma tissue compared with non-tumor
brain tissue (Fig. 3B).
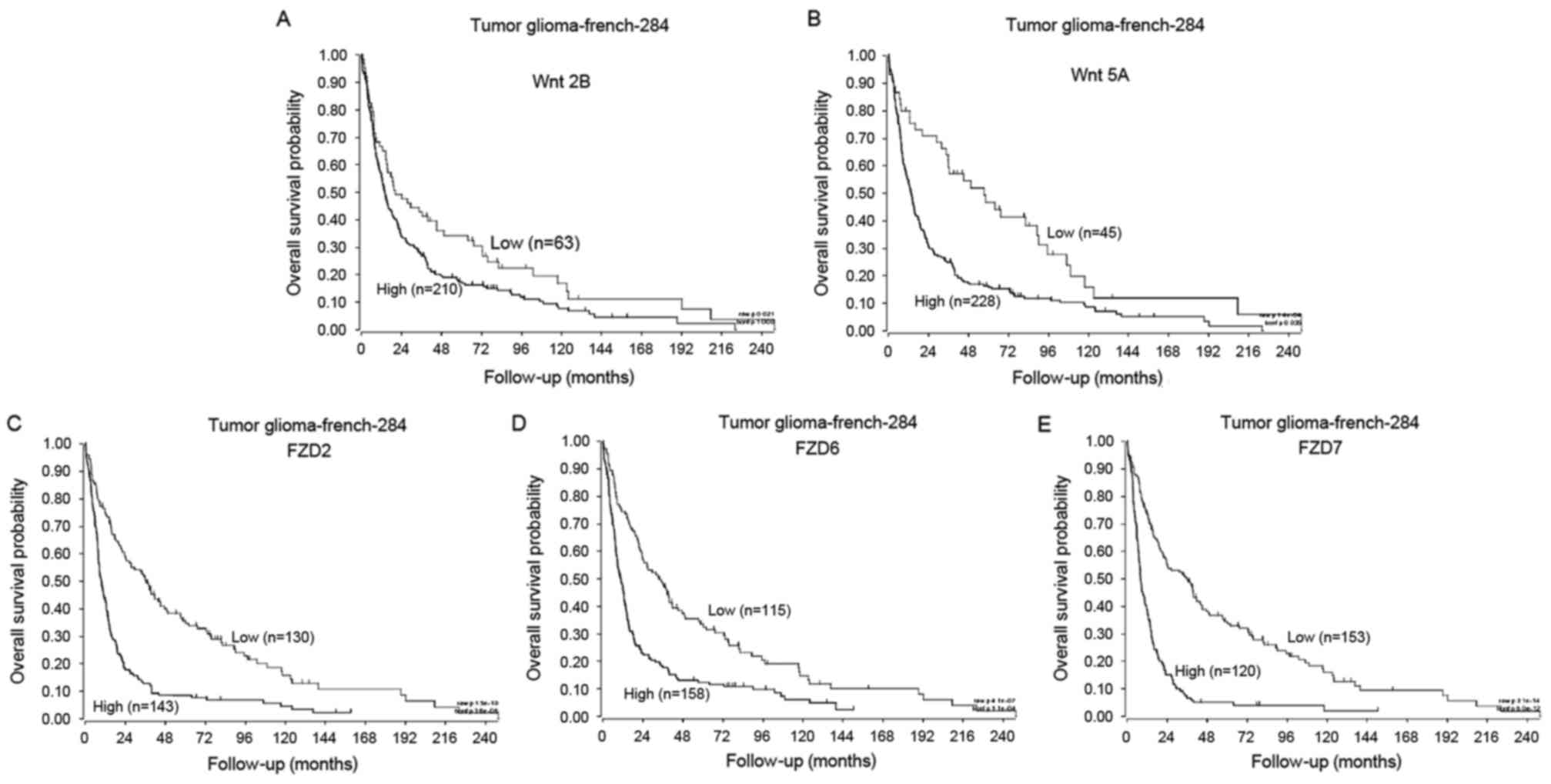
Using data from the R2: Genomics Analysis and
Visualization Platform, the association between the clinical
outcome of patients with glioma and the expression of Wnt molecules
was analyzed using a log-rank test. During the follow-up period,
patients with the high expression of Wnt2b had significantly
reduced survival times compared with patients with low expression
(Fig. 4A; Wnt2b high, n=210, Wnt2b
low, n=63; P<0.05). Similarly, patients with low Wnt5a
expression had a significantly longer overall survival than those
with high Wnt5a expression (Fig. 4B;
Wnt5a high, n=228, Wnt5a low, n=45; P<0.01). Additionally, the
overall survival rates for patients with the high expression of
FZD2, 6 or 7 was significantly reduced than those with low FZD2, 6
and 7 expression (Fig. 4C-E; FZD2
high, n=143, FZD2 low, n=130; FZD6 high, n=158, FZD6 low, n=115;
FZD7 high, n=120, FZD7 low, n=135; P<0.01).
Discussion
Accumulating studies have demonstrated that
Wnt/β-catenin signaling components are aberrantly expressed in
numerous types of human tumor, which contribute to tumor initiation
and development (9). In the present
study, it was revealed that the mRNA expression levels of Wnt3a, 5a
and the receptors FZD2, 6 and 7 were upregulated, whereas Wnt7b was
decreased. However, no significant changes were observed in
β-catenin, APC, AXIN1/2, GSK3β, and cyclin D1 mRNA expression
levels. Similarly, at the protein level, the expression levels of
Wnt2b, 3a and 5a increased, whereas those of β-catenin, GSK3β and
cyclin D1 did not exhibit any changes.
Known as the classical canonical Wnt molecules,
Wnt2, 2b, 3a and 7b have been widely detected in various types of
cancer, including esophageal cancer (21), glioblastoma (12) and prostate cancer (22). Specifically, Wnt2 secreted by tumor
fibroblast promotes the translocation of β-catenin, which
contributes to cell motility and invasiveness in esophageal cancer
(21). Pu et al (18) revealed that the mRNA expression of
Wnt2 was increased in gliomas. Additionally, anti-Wnt2 monoclonal
antibodies induced apoptosis in the treatment of non-small cell
lung cancer (NSCLC) (23), melanoma
(24) and mesothelioma cells
(25). Wnt2b, a known homolog of Wnt2
(26), has rarely been studied in
glioma. In 2012, Liu et al (27) reported that Wnt2b was upregulated in
gastric cancer, hepatocellular carcinoma and NSCLC, which was
demonstrated to be negatively associated with the prognosis of
patients with NSCLC. The study also identified that Wnt2b may
promote the expression of c-Myc and survivin via canonical
signaling pathways. Wnt7b, identified as a direct target gene of
androgen receptors, is markedly upregulated in castration-resistant
prostate cancer cells (22).
Transcriptional profiling also revealed an increased expression of
Wnt7b in pancreatic adenocarcinoma cell lines (28). TCGA database analysis previously
revealed that 52/53 human breast cancer cell lines demonstrated the
substantial upregulation of Wnt7b (29). Kaur et al (12) reported that Wnt3a was upregulated in
glioma cells, primary cultures and glioma stem cells in addition to
tumor tissues, whereas further TCGA database analysis revealed the
high expression of Wnt3 in 55% of all glioblastoma samples.
Regarding β-catenin, Liu et al (19) and Shi et al (20) demonstrated that mRNA and protein
levels increased with an increase in astrocytic glioma pathological
grade. In contrast, Pu et al (18) reported that the mRNA expression of
β-catenin in gliomas analyzed using RT-qPCR did not exhibit any
change, which is consistent with the results of the present study.
This previous study additionally reported that the protein level of
β-catenin was significantly higher in grade III and IV gliomas
compared with grade I and II gliomas, as assessed by
immunohistochemistry, and was implicated in cell proliferation and
glioma growth. However, in the present study, from the western blot
analysis of 37 glioma and 11 non-tumor brain tissues, it was
revealed that β-catenin protein levels varied between different
glioma grades, whereas no significant change was observed between
non-tumor tissues and glioma samples. It is reported that the Wnt
receptors FZD2, 6 and 7 are upregulated in human malignant glioma
cell lines (15). However, Salsano
et al (30) identified that
although FZD2 is overexpressed in medulloblastomas, FZD7 is
underexpressed in certain cases of the medulloblastomas. In the
present study, it was revealed that these Wnt receptors were also
overexpressed in glioma tissue, indicating that they may serve
functions in glioma progression. As for why the mRNA expression of
FZD7 was higher in grade III gliomas compared with non-tumor
tissues, while grade IV gliomas did not exhibit any difference,
this may be due to one of the following reasons: Firstly, only 8
samples of each grade were used in the present study for qPCR
analysis. The lack of variation caused by the limited number of
samples may result in no significant differences between non-tumor
tissues and grade IV glioma tissue being identified. Secondly, it
has been reported that a number of molecules exhibit expression
differences in different glioma cell types. However, in the present
study, samples were selected only according to the WHO grade and
not the cell type, which may be the cause of this difference.
Wnt5a, one of the most highly investigated
non-canonical Wnt molecules, has been revealed to be upregulated in
multiple types of cancer, including melanoma (31), breast carcinoma (32), pancreatic cancer (33), prostate carcinoma (34) and human glioblastoma (13). Notably, Wnt5a is the most upregulated
Wnt member in glioma cells and tissues, which promotes cell
proliferation, migration and tumorigenesis both in vitro and
in vivo (15,35). In addition, Wnt5a is associated with
the antagonization of the canonical Wnt/β-catenin activity in
mammalian cells and Xenopus embryos (36–38). Topol
et al (39) subsequently
uncovered that Wnt5a inhibited canonical Wnt/β-catenin signaling by
modulating β-catenin degradation via a GSK3-independent mechanism.
Similar inhibition mechanisms of Wnt5a have been identified in
hematopoietic stem cells (40),
hepatocyte proliferation (41),
dermal papilla cells and non-melanoma skin cancer (42). Therefore, Wnt5a functions as an
antagonist of the canonical Wnt/β-catenin pathway, and may account
for the unusual results in the present study concerning
Wnt/β-catenin signaling in gliomas.
It has been reported that key components of the Wnt
pathway affect the survival of patients, including effects on
metastasis, chemoresistance and cell proliferation. For example,
the upregulation of Wnt5a may enhance, whilst the downregulation
may reduce, the proliferation and tumorigenesis of GBM-05 and U87MG
cell lines (35). The knockdown of
Wnt5a suppresses the migration, invasion and infiltrative capacity
of glioma cells primarily via a matrix metallopeptidase-2-dependent
mechanism (15). It was revealed that
FZD2 expression enhances epithelial to mesenchymal transition, and
cell migration and invasion through FYN proto-oncogene, Src family
tyrosine kinase and signal transducer and activator of
transcription 3. Specific antibody targeting of FZD2 may decrease
tumor development and metastasis in xenograft mouse models of
colorectal carcinoma (43). In the
circulating tumor cells (CTCs) of pancreatic carcinoma, the
expression of Wnt2 may promote anchorage-independent sphere
formation and metastatic ability (44). Additionally, the β-catenin-independent
Wnt pathway has been demonstrated to be upregulated in the CTCs of
prostate carcinoma cell lines that demonstrate resistance to
androgen receptor inhibition (45).
In summary, increasing evidence has revealed that
β-catenin-dependent and -independent Wnt signaling may promote
tumorigenesis in a tissue-specific manner (46). Furthermore, the proliferation of
glioma cells may be inhibited by targeting specific molecules using
Wnt2 and β-catenin small interfering RNAs (18,47,48).
β-catenin expression demonstrates an association with the poor
prognosis of patients with GBM, including shorter survival times
(19,49). The aforementioned previous studies are
in line with the data presented in the R2 Platform, in which
patients with a high expression of Wnt5a, Wnt2b, FZD2, FZD6 and
FZD7 exhibited a worse overall survival time.
In the present study, only western blot and RT-qPCR
analyses were used to illustrate the expression of Wnt ligands, the
Wnt/β-catenin pathway and its target genes in the context of
gliomas. The results of the present study clearly indicate that
β-catenin is not upregulated in gliomas, and Wnt molecules may
serve important functions in glioma progression via the
noncanonical pathway. Another potential explanation is that the
effect of the Wnt/β-catenin canonical signaling on glioma is
antagonized by Wnt5a upregulation. Thus, future studies should aim
to explain the effect of Wnt/β-catenin on glioma cells in
vitro. Furthermore, the results of the present study offer a
potential opportunity to further explore the precise function of
Wnt2b and Wnt7b in the context of gliomas.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos., 81372699 and
81472345), the 333 Talent Project of the Jiangsu Province (grant
no. BRA2015394) and the Six Major Talent Summit of the Jiangsu
Province (grant no. WSW-039).
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Willert K and Nusse R: Wnt proteins. Cold
Spring Harb Perspect Biol. 4:a0078642012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu J and Virshup DM: Updating the Wnt
pathways. Biosci Rep. 34:pii: e001422014. View Article : Google Scholar
|
|
7
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baarsma HA, Königshoff M and Gosens R: The
WNT signaling pathway from ligand secretion to gene transcription:
Molecular mechanisms and pharmacological targets. Pharmacol Ther.
138:66–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polakis P: Wnt signaling in cancer. Cold
Spring Harb Perspect Biol. 4:pii: a0080522012. View Article : Google Scholar
|
|
10
|
Zhang K, Zhang J, Han L, Pu P and Kang C:
Wnt/beta-catenin signaling in glioma. J Neuroimmune Pharmacol.
7:740–749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaur N, Chettiar S, Rathod S, Rath P,
Muzumdar D, Shaikh ML and Shiras A: Wnt3a mediated activation of
Wnt/β-catenin signaling promotes tumor progression in glioblastoma.
Mol Cell Neurosci. 54:44–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reis M, Czupalla CJ, Ziegler N, Devraj K,
Zinke J, Seidel S, Heck R, Thom S, Macas J, Bockamp E, et al:
Endothelial Wnt/β-catenin signaling inhibits glioma angiogenesis
and normalizes tumor blood vessels by inducing PDGF-B expression. J
Exp Med. 209:1611–1627. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamino M, Kishida M, Kibe T, Ikoma K,
Iijima M, Hirano H, Tokudome M, Chen L, Koriyama C, Yamada K, et
al: Wnt-5a signaling is correlated with infiltrative activity in
human glioma by inducing cellular migration and MMP-2. Cancer Sci.
102:540–548. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klein D, Demory A, Peyre F, Kroll J,
Augustin HG, Helfrich W, Kzhyshkowska J, Schledzewski K, Arnold B
and Goerdt S: Wnt2 acts as a cell type-specific, autocrine growth
factor in rat hepatic sinusoidal endothelial cells
cross-stimulating the VEGF pathway. Hepatology. 47:1018–1031. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Azzolin L, Zanconato F, Bresolin S,
Forcato M, Basso G, Bicciato S, Cordenonsi M and Piccolo S: Role of
TAZ as mediator of Wnt signaling. Cell. 151:1443–1456. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pu P, Zhang Z, Kang C, Jiang R, Jia Z,
Wang G and Jiang H: Downregulation of Wnt2 and beta-catenin by
siRNA suppresses malignant glioma cell growth. Cancer Gene Ther.
16:351–361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Wang L, Zhao S, Ji X, Luo Y and
Ling F: β-Catenin overexpression in malignant glioma and its role
in proliferation and apoptosis in glioblastma cells. Med Oncol.
28:608–614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi Z, Qian X, Li L, Zhang J, Zhu S, Zhu
J, Chen L, Zhang K, Han L, Yu S, et al: Nuclear translocation of
β-catenin is essential for glioma cell survival. J Neuroimmune
Pharmacol. 7:892–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu L, Zhang C, Zhang LY, Dong SS, Lu LH,
Chen J, Dai Y, Li Y, Kong KL, Kwong DL and Guan XY: Wnt2 secreted
by tumour fibroblasts promotes tumour progression in oesophageal
cancer by activation of the Wnt/β-catenin signalling pathway. Gut.
60:1635–1643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng D, Decker KF, Zhou T, Chen J, Qi Z,
Jacobs K, Weilbaecher KN, Corey E, Long F and Jia L: Role of
WNT7B-induced noncanonical pathway in advanced prostate cancer. Mol
Cancer Res. 11:482–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
You L, He B, Xu Z, Uematsu K, Mazieres J,
Mikami I, Reguart N, Moody TW, Kitajewski J, McCormick F and
Jablons DM: Inhibition of Wnt-2-mediated signaling induces
programmed cell death in non-small-cell lung cancer cells.
Oncogene. 23:6170–6174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mazieres J, You L, He B, Xu Z, Twogood S,
Lee AY, Reguart N, Batra S, Mikami I and Jablons DM: Wnt2 as a new
therapeutic target in malignant pleural mesothelioma. Int J Cancer.
117:326–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You L, He B, Xu Z, Uematsu K, Mazieres J,
Fujii N, Mikami I, Reguart N, McIntosh JK, Kashani-Sabet M, et al:
An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant
melanoma cells and inhibits tumor growth. Cancer Res. 64:5385–5389.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katoh M: WNT2B: Comparative integromics
and clinical applications (Review). Int J Mol Med. 16:1103–1108.
2005.PubMed/NCBI
|
|
27
|
Liu D, Kadota K, Ueno M, Nakashima N,
Yokomise H and Huang Cl: Adenoviral vector expressing short hairpin
RNA targeting Wnt2B has an effective antitumour activity against
Wnt2B2-overexpressing tumours. Eur J Cancer. 48:1208–1218. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arensman MD, Kovochich AN, Kulikauskas RM,
Lay AR, Yang PT, Li X, Donahue T, Major MB, Moon RT, Chien AJ and
Dawson DW: WNT7B mediates autocrine Wnt/β-catenin signaling and
anchorage-independent growth in pancreatic adenocarcinoma.
Oncogene. 33:899–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeo EJ, Cassetta L, Qian BZ, Lewkowich I,
Li JF, Stefater JA III, Smith AN, Wiechmann LS, Wang Y, Pollard JW
and Lang RA: Myeloid WNT7b mediates the angiogenic switch and
metastasis in breast cancer. Cancer Res. 74:2962–2973. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salsano E, Paterra R, Figus M, Menghi F,
Maderna E, Pollo B, Solero CL, Massimi L and Finocchiaro G:
Expression profile of frizzled receptors in human medulloblastomas.
J Neurooncol. 106:271–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Da Forno PD, Pringle JH, Hutchinson P,
Osborn J, Huang Q, Potter L, Hancox RA, Fletcher A and Saldanha GS:
WNT5A expression increases during melanoma progression and
correlates with outcome. Clin Cancer Res. 14:5825–5832. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fernandez-Cobo M, Zammarchi F, Mandeli J,
Holland JF and Pogo BG: Expression of Wnt5A and Wnt10B in
non-immortalized breast cancer cells. Oncol Rep. 17:903–907.
2007.PubMed/NCBI
|
|
33
|
Ripka S, König A, Buchholz M, Wagner M,
Sipos B, Klöppel G, Downward J, Gress T and Michl P: WNT5A-target
of CUTL1 and potent modulator of tumor cell migration and invasion
in pancreatic cancer. Carcinogenesis. 28:1178–1187. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Q, Williamson M, Bott S,
Brookman-Amissah N, Freeman A, Nariculam J, Hubank MJ, Ahmed A and
Masters JR: Hypomethylation of WNT5A, CRIP1 and S100P in prostate
cancer. Oncogene. 26:6560–6565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu JM, Jun ES and Jung JS, Suh SY, Han JY,
Kim JY, Kim KW and Jung JS: Role of Wnt5a in the proliferation of
human glioblastoma cells. Cancer Lett. 257:172–181. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Torres MA, Yang-Snyder JA, Purcell SM,
DeMarais AA, McGrew LL and Moon RT: Activities of the Wnt-1 class
of secreted signaling factors are antagonized by the Wnt-5A class
and by a dominant negative cadherin in early Xenopus development. J
Cell Biol. 133:1123–1137. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Olson DJ and Gibo DM: Antisense wnt-5a
mimics wnt-1-mediated C57MG mammary epithelial cell transformation.
Exp Cell Res. 241:134–141. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ishitani T, Kishida S, Hyodo-Miura J, Ueno
N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J and
Matsumoto K: The TAK1-NLK mitogen-activated protein kinase cascade
functions in the Wnt-5a/Ca(2+) pathway to antagonize
Wnt/beta-catenin signaling. Mol Cell Biol. 23:131–139. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Topol L, Jiang X, Choi H, Garrett-Beal L,
Carolan PJ and Yang Y: Wnt-5a inhibits the canonical Wnt pathway by
promoting GSK-3-independent beta-catenin degradation. J Cell Biol.
162:899–908. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nemeth MJ, Topol L, Anderson SM, Yang Y
and Bodine DM: Wnt5a inhibits canonical Wnt signaling in
hematopoietic stem cells and enhances repopulation. Proc Natl Acad
Sci USA. 104:pp. 15436–15441. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yuzugullu H, Benhaj K, Ozturk N, Senturk
S, Celik E, Toylu A, Tasdemir N, Yilmaz M, Erdal E, Akcali KC, et
al: Canonical Wnt signaling is antagonized by noncanonical Wnt5a in
hepatocellular carcinoma cells. Mol Cancer. 8:902009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pourreyron C, Reilly L, Proby C,
Panteleyev A, Fleming C, McLean K, South AP and Foerster J: Wnt5a
is strongly expressed at the leading edge in non-melanoma skin
cancer, forming active gradients, while canonical Wnt signalling is
repressed. PLoS One. 7:e318272012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gujral TS, Chan M, Peshkin L, Sorger PK,
Kirschner MW and MacBeath G: A noncanonical Frizzled2 pathway
regulates epithelial-mesenchymal transition and metastasis. Cell.
159:844–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu M, Ting DT, Stott SL, Wittner BS,
Ozsolak F, Paul S, Ciciliano JC, Smas ME, Winokur D, Gilman AJ, et
al: RNA sequencing of pancreatic circulating tumour cells
implicates WNT signalling in metastasis. Nature. 487:510–513. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miyamoto DT, Zheng Y, Wittner BS, Lee RJ3,
Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J, et
al: RNA-Seq of single prostate CTCs implicates noncanonical Wnt
signaling in antiandrogen resistance. Science. 349:1351–1356. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yano H, Hara A, Shinoda J, Takenaka K,
Yoshimi N, Mori H and Sakai N: Immunohistochemical analysis of
beta-catenin in N-ethyl-N-nitrosourea-induced rat gliomas:
Implications in regulation of angiogenesis. Neurol Res. 22:527–532.
2000.PubMed/NCBI
|
|
48
|
Wang Z and Chen Q: β-catenin knockdown
inhibits the proliferation of human glioma cells in vitro and in
vivo. Exp Ther Med. 11:1059–1064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rossi M, Magnoni L, Miracco C, Mori E,
Tosi P, Pirtoli L, Tini P, Oliveri G, Cosci E and Bakker A:
β-catenin and Gli1 are prognostic markers in glioblastoma. Cancer
Biol Ther. 11:753–761. 2011. View Article : Google Scholar : PubMed/NCBI
|