Introduction
Worldwide, cervical cancer is the third most common
cancer found in women. There are 529,000 new cases diagnosed each
year (1). In Colombia it has an
incidence of 26.1/100,000 and is the most common cause of death
from cancer in women (2). High-risk
human papillomavirus (hrHPV) are detected in virtually all cervical
carcinomas (3,4). However, other host cell factors are
required for progression of hrHPV-induced precancerous lesions to
invasive cancer.
Telomeres are specialized structures at the end of
linear chromosomes. They consist of non-coding tandem repeated
TTAGGG sequences which are involved in chromosomal stability. In
somatic cells, during each cell division, telomeres are shortened
progressively until reaching cellular senescence (5). Telomerase is a ribonucleoprotein enzyme
complex containing several components, between them a catalytic
subunit composed of hTERT (human Telomerase Reverse Transcriptase)
and a RNA component (hTR). The hTR component acts as a template for
elongation of telomeric DNA (6).
Normal tissues and human somatic cells show low or undetectable
levels of telomerase activity (TA), whereas immortalized cells and
tumour cells from a variety of cancers show highly detectable TA
(7,8).
Telomerase activation, which is very important for cell
immortalization, has been proposed as a critical step for the
development of different types of cancer including cervical cancer
(9).
Identifying molecular markers that will permit
diagnosis and prognosis of women who have cervical lesions as well
as predicting which precursor lesions are likely to become
cancerous will lead to improvements in cervical cancer prevention.
Several studies on women with normal cytology and/or different
grades of cervical lesions have shown increased TA in higher grade
lesions (10–13). Some studies have also shown that
infections with HPV 16 and 18 are associated with increased TA
(14,15). In addition a few studies have shown
that expression of HPV16 E6, along with E6AP can induce hTERT
transcription and TA (16–20). Gain of chromosome 3q, containing the
sequence for the telomerase RNA component (TERC), and gain of
chromosome 5p, containing the TERT gene, have been also associated
with CIN2/CIN3+ in cervical tissue (21–23).
To the best of our knowledge, no previous
epidemiological analysis has been performed on the association
between TA and HPV in cervical scrapings, analyzing the role of TA
as a possible risk factor for HGSIL adjusted for different known
risk factors. Here we report the results of HPV and TA detection in
a nested case control study within a cohort of Colombian women.
Materials and methods
Study population
Between November 1993 and November 1995 the
Colombian National Cervical Cancer Institute conducted a population
census in four health districts in Bogotá. Two thousand women aged
18–85 years were randomly identified and invited to participate in
the cohort study. In order to acquire information on sexually
active adolescents, 200 sexually-active women aged 13–17 years, all
of whom had come to an adolescent clinic for contraceptive
counseling without a doctor's referral, were also invited to
participate. The ethics committee of INC approved the protocol.
Methods of recruitment and data collection have been described
elsewhere (24). Briefly, eligible
women were those residing in Bogota, without history of cervical
neoplasia, conisation or hysterectomy willing to participate and
who gave written informed consent. At study entry and at each
follow-up visit, participants responded to a questionnaire on risk
factors for cervical cancer and underwent a gynaecological
examination with collection of cervical cells for cytology and HPV
DNA detection. Colposcopic examination of the cervix was performed
in all women with repeated cytological diagnosis of Low Grade
Squamous Intraepithelial Lesions (LGSIL) or with cytological
evidence of High Grade Squamous Intraepithelial Lesions (HGSIL).
Colposcopically guided cervical biopsies were performed in women
with cytological or colposcopic evidence of HGSIL. Follow-up visits
were scheduled every 6 months for up to 10 years until March
2004.
The analysis described here is a nested case control
study of the study cohort. Cases were defined as any women with
cytological HGSIL or biopsy-confirmed CIN2/CIN3+ during the first
six years of follow up. Controls were defined as any women that
maintained normal cytology results during the entire follow up
period.
All cases observed during the first six years of
follow up were selected, while controls were randomly chosen from
the participants of the study, but assuring a group age match (±2
years) with cases (at diagnosis). To maximize the statistical power
of the study sample and considering the small number of cases
diagnosed during follow up within the study, a case: control ratio
of 1:4 was used.
From the 38 cases observed during follow up, only 25
cases (3 Invasive cancers, 10 CIN3, 5 CIN2 and 7 HGSIL) had
available sufficient cervical scrape sample to analyze TA and 104
controls were selected with the defined criteria. HPV infections
had previously been tested using GP5+/GP6+ PCR-EIA and genotyped
using a Reverse Line Blot assay (RLB), as described previously
(25). Samples were analyzed for the
presence of 14 high risk HPV types (hrHPV) and 23 low risk HPV
types (lrHPV).
Sample processing for TA. Samples were processed as
described previously (10). Briefly,
samples were centrifuged at 1,200 rpm. Pelleted cells were treated
with trypsin at 37°C for 5 min to separate cell clusters. Cells
were counted with a haemocytometer, washed twice with PBS, and
stored as pellets at −80°C until their use in a TRAP (Telomeric
Repeat Amplification Protocol) assay.
TRAP assay
TA was measured by TRAP assay using a commercially
available kit, Telo TAGGG Telomerase PCR enzyme-linked
immunosorbent assay (ELISA) from Roche Applied Science, Mannheim,
Germany. Assays were performed according to the manufacturer's
specifications. In addition to the kit's positive control, cell
extracts from HeLa cells were used as another positive control for
each assay. Heat-treated cell extracts at 85°C for 10 min from
positive controls and samples were used as negative controls. Final
optical Densities (ODs) of samples were obtained by subtracting ODs
of negative controls from those of the samples. Samples were
regarded as telomerase positive if the difference in absorbance is
higher than 0.2 A450nm-A690nm units.
Statistical analyses
HPV infection, TA and cofactors related to cervical
cancer aetiology (age, parity, smoking status and oral
contraceptive use) were included in the analysis. Fisher's exact
test and chi-squared test were used to compare HPV detection, TA
and cofactors between cases and controls. Association of TA with
HGSIL was evaluated using logistic regression analysis. As hrHPV
infection is a necessary but not sufficient cause of cervical
neoplasia, we also assessed the association of telomerase and HGSIL
among women positive for hrHPV. Odds ratios (OR) and 95% confidence
intervals (CIs) are reported for crude and adjusted analysis. All
statistical analysis was done using STATA software (version 9,
StataCorp, College Station, TX, USA).
Results
Characteristics of the population
Characteristics of the cases and controls are
summarized in Table I. Women included
in this study were aged 14–68 years. While age and education were
similar in both groups and no differences were evident in oral
contraceptive use and smoking status, parity tended to be higher
among cases, but the difference was not statistically
significant.
 | Table I.Main characteristics of cases and
controls. (A) The P-value was calculated from Fisher's exact test.
(B) The P-value was calculated from Pearson's chi-square tests.
hrHPV, high-risk human papillomavirus. |
Table I.
Main characteristics of cases and
controls. (A) The P-value was calculated from Fisher's exact test.
(B) The P-value was calculated from Pearson's chi-square tests.
hrHPV, high-risk human papillomavirus.
|
| Cases | Controls |
|
|---|
|
|
|
|
|
|---|
| Main
characteristics | n=25 | (%) | n=104 | (%) | P-value |
|---|
| Education |
|
None | 1 | (4) | 1 | (1) |
|
|
Primary | 8 | (32) | 19 | (18) |
|
|
Secondary | 14 | (56) | 61 | (59) |
|
|
College/university | 1 | (4) | 10 | (10) | 0.21a |
|
Missing | 1 | (4) | 13 | (12) |
|
| Age (years): |
|
<20 | 1 | (11) | 11 | (11) |
|
|
20–29 | 8 | (24) | 19 | (18) |
|
|
30–44 | 14 | (58) | 69 | (66) |
|
|
45–64 | 1 | (4) | 3 | (3) |
|
|
65+ | 1 | (3) | 2 | (2) | 0.6a |
| Parity: |
|
0–1 | 3 | (12) | 35 | (34) |
|
|
>2 | 21 | (84) | 69 | (66) | 0.07a |
|
Missing | 1 | (4) |
|
|
|
| Oral
contraceptives: |
|
Never | 11 | (44) | 56 | (54) |
|
|
Ever | 14 | (56) | 48 | (46) | 0.49b |
| Smoking status |
|
Never | 18 | (72) | 72 | (69) |
|
|
Ever | 7 | (28) | 32 | (31) | 0.62b |
| HPV |
|
Positive | 21 | (84) | 23 | (22.1) |
|
|
Negative | 4 | (16) | 81 | (77.9) |
<0.0001b |
| hrHPV |
|
Positive | 21 | (84) | 14 | (13.5) |
|
|
Negative | 4 | (16) | 90 | (86.5) |
<0.0001b |
| Telomerase activity
(TA) |
|
Positive | 19 | (76) | 21 | (20.2) |
|
|
Negative | 6 | (24) | 83 | (79.8) |
<0.0001b |
| HPV and TA |
|
Concurrent | 17 | (68) | 8 | (7.7) |
|
| No
concurrent | 8 | (32) | 96 | (92.3) |
<0.0001b |
HPV detection and TA
Among cases, 84% of the samples were HPV positive;
all of these were positive for hrHPV genotypes (100%),
predominantly of the alpha 7 and 9 species (Fig. 1). Among controls, 22.1% of the samples
were HPV positive and 60.8% of these specimens were hrHPV positive.
In this group of women, a mixture of hrHPV and lrHPV types were
detected, predominantly of the alpha 3, 7 and 9 species (Fig. 2). HPV was detected in significantly
more cases than controls (Table
I).
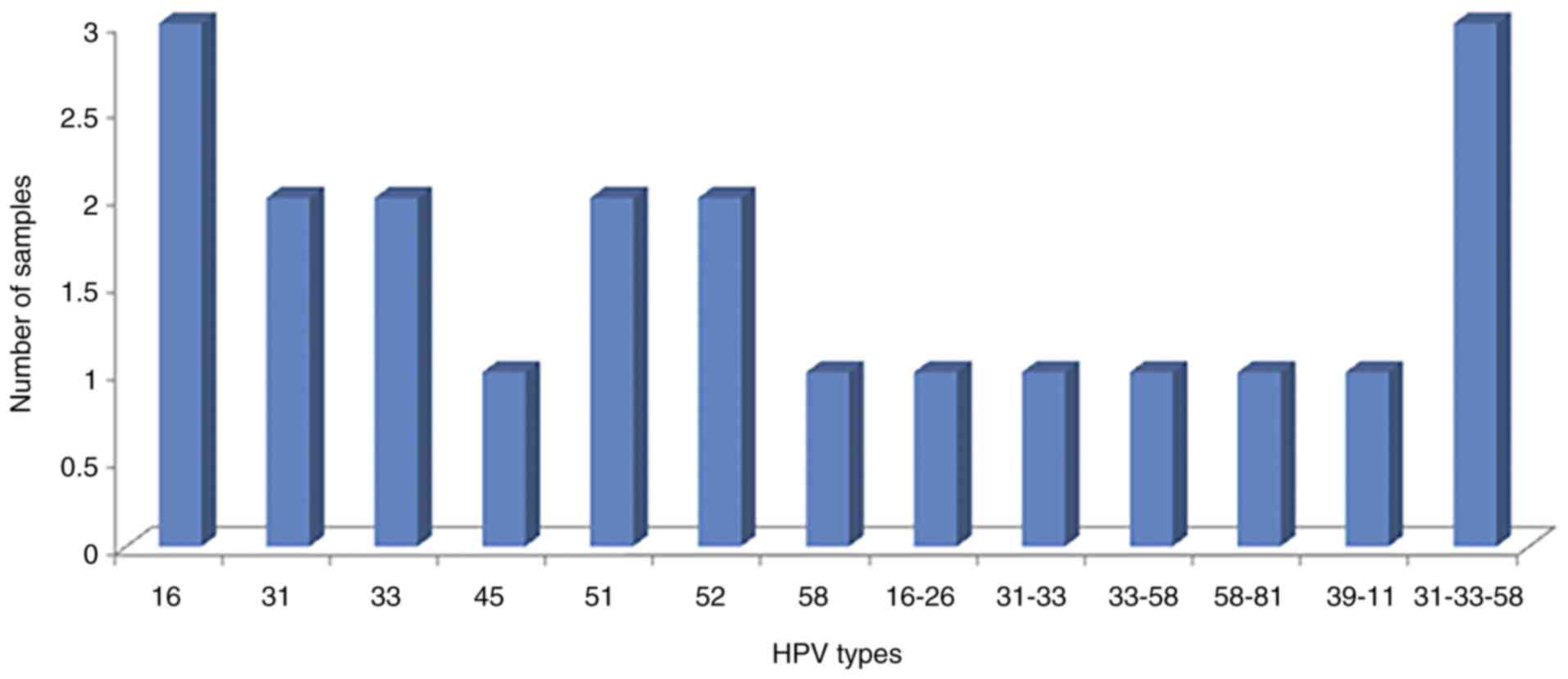 | Figure 1.HPV genotypes detected in cases
(n=25). Alpha 9 species contains the HPV types 16, 31, 33, 35, 52,
58 and 67. Alpha 7 species contains the HPV types 18, 39, 45, 59,
68 and 70. HPV, human papillomavirus. |
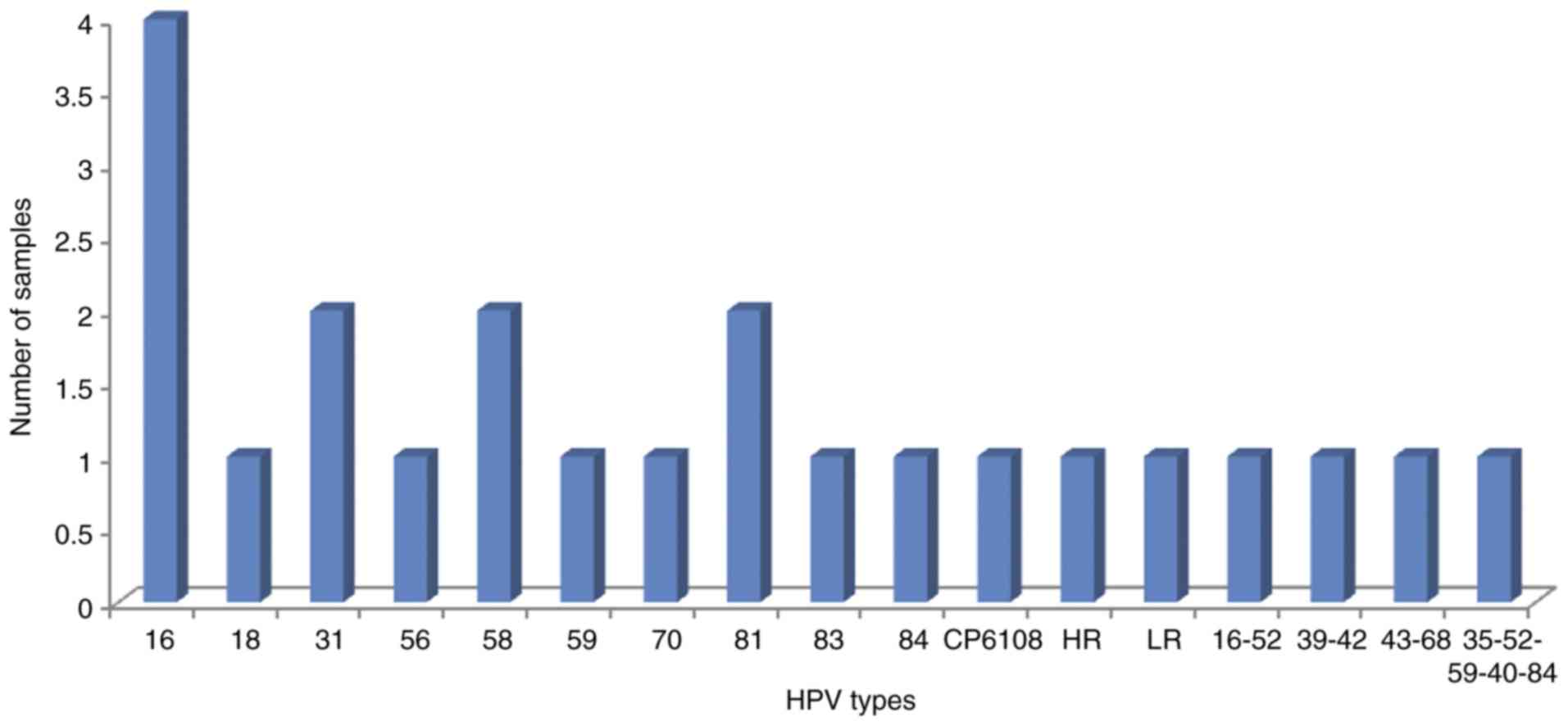 | Figure 2.HPV genotypes detected in controls
(n=104). Alpha 9 species contains the HPV types 16, 31, 33, 35, 52,
58 and 67. Alpha 7 species contains the HPV types 18, 39, 45, 59,
68 and 70. Alpha 3 species contains the HPV types 61, 72, 81, 83,
84. HR, unidentified high risk HPV genotype(s); LR, unidentified
low risk HPV genotype(s); HPV, human papillomavirus. |
TA was positive in 76% of cases, significantly
higher than in controls, of which only 20.2% were positive
(Table I).
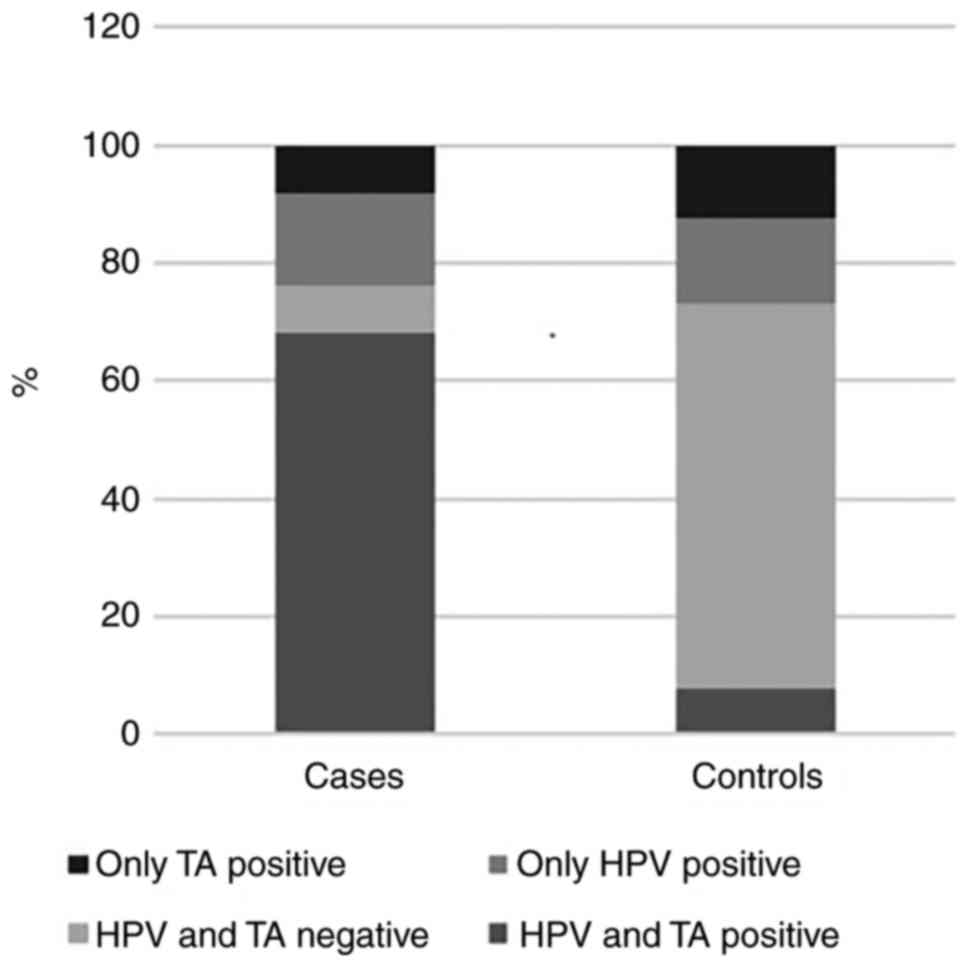
Of the cases, 17 (68%) were positive for TA and HPV
infection concurrently, 2 (8%) were positive only for TA, 4 (16%)
had only HPV infection and 2 (8%) were negative for both. Of the
controls, 8 (7.7%) were positive for TA and HPV infection
simultaneously, 13 (12.5%) were positive only for TA, 15 (14.42%)
had only HPV infection and 68 (65.38%) were negative for both
(Fig. 3). Detection of concurrent TA
and HPV was significantly higher in cases than in controls
(P<0.0001) (Table I).
In the seventeen cases that were positive for HPV
infection and TA concurrently, the presence of hrHPV types in
single and multiples infections was always detected and they were
mainly types belonging to the Alpha 9 species. In the eleven cases
with single HPV infections, type 58 was found in one sample and HPV
16, 31, 33, 51 and 52 were found in two samples each. In the 6
cases with multiple HPV infections, different combinations were
found (HPV 16 and 26, 31 and 33, 58 and 81 in one sample each; and
the combination of HPV 31, 33 and 58 was detected in three
different samples). In the eight controls that were positive for
HPV and TA simultaneously, high and low risk HPV types were
detected in single and multiple infections. In the five controls
with single HPV infections, HPV 16, 58, 59, 81 and 84 were found in
one sample each. In the three controls with multiple HPV
infections, different combinations were found (HPV 39 and 42; HPV
43 and 68; and HPV 35, 52, 59, 40 and 84, in one sample each).
There was no statistically significant interaction between TA and
hrHPV infection (P=0.76).
Risk factors for HGSIL
Significantly increased odds for HGSIL were observed
in women positive for hrHPV (OR=26.10, 95% CI 9.10–74.86) or TA
(OR=12.51, 95% CI 4.44–35.24), or with multiple parities (OR=3.40,
95% CI 0.92–23.40). These associations decreased, but remained
significant, when adjusting for relevant cofactors; (OR=18.26, 95%
CI 4.43–76.92), (OR=5.52, 95% CI 1.43–21.30), and (OR=3.50, 95% CI
1.03–10.30), respectively (Table
II). When considering only women with hrHPV infection, the
association between TA and HGSIL was also significant (OR=7.7, 95%
CI 1.63–35.79). When adjusting for age and other cofactors, this
association remained significant (OR=37.94, 95% CI 1.64–678.1). No
other risk factors were clearly associated with the risk of HGSIL
(Table III).
 | Table II.Crude and adjusted OR and 95% CI of
telomerase activity and relevant risk factors. |
Table II.
Crude and adjusted OR and 95% CI of
telomerase activity and relevant risk factors.
|
|
|
| Crude | Adjusted |
|---|
|
|
|
|
|
|
|---|
| Risk Factors | Cases n=25 | Controls n=104 | OR | (95% CI) | ORa | (95% CI) |
|---|
| High Risk HPV
infection |
| No | 4 | 90 | 1 |
|
|
|
|
Yes | 21 | 14 | 26.1 | (9.10–74.8) | 18.2 | (4.33–76.9) |
| Telomerase
Activity |
| No | 6 | 83 | 1 |
|
|
|
|
Yes | 19 | 21 | 12.5 | (4.44–35.2) | 5.5 | (1.43–21.3) |
| Age (years): |
|
<30 | 9 | 30 | 1 |
|
|
|
|
≥30 | 16 | 74 | 1.8 | (0.70–4.7) | 1.2 | (0.46–3.5) |
| Parity: |
|
0–1 | 3 | 35 | 1 |
|
|
|
|
>2 | 21 | 69 | 3.4 | (0.92–23.4) | 3.5 | (1.03–10.3) |
| Oral
Contraceptives: |
|
Never | 11 | 56 | 1 |
|
|
|
|
Ever | 14 | 48 | 1.5 | (0.69–3.5) | 1.4 | (0.61–3.5) |
| Smoking status |
|
Never | 18 | 72 | 1 |
|
|
|
|
Ever | 7 | 32 | 0.6 | (0.27–1.6) | 0.8 | (0.33–2.3) |
 | Table III.Association of telomerase and main
cofactors with HGSIL, in presence HR HPV infection. |
Table III.
Association of telomerase and main
cofactors with HGSIL, in presence HR HPV infection.
|
|
|
| Crude | Adjusted |
|---|
|
|
|
|
|
|
|---|
| HPV HR
Cofactors | Controls n=14 | Cases n=21 | OR | 95% CI | ORa | 95% CI |
|---|
| Viral load
(tertiles) |
| I | 4 | 1 | 1 |
| 1 |
|
| II | 2 | 2 | 4.0 | (0.56–28.3) | 0.4 | (0.01–20.3) |
|
III | 8 | 18 | 4.8 | (0.84–27.2) | 7.0 | (0.52–95.7) |
| Telomerase
activity |
| No | 9 | 4 | 1 |
| 1 |
|
|
Yes | 5 | 17 | 7.7 | (1.63–35.7) | 37.9 | (1.64–678.1) |
| Parity: |
|
0–1 | 8 | 3 | 1 |
| 1 |
|
|
>2 | 8 | 19 | 5.0 | (1.17–14.6) | 2.3 | (0.12–50.3) |
|
>3 | 2 | 2 | 3.5 | (0.37–32.9) | 2.4 | (0.11–53.0) |
| Oral
contraceptives: |
|
Never | 8 | 8 | 1 |
| 1 |
|
|
Ever | 6 | 13 | 2.2 | (0.54–8.5) | 10.1 | (0.74–137.5) |
| Smoking status |
|
Never | 9 | 16 | 1 |
| 1 |
|
|
Ever | 5 | 5 | 0.8 | (0.16–3.6) | 0.2 | (0.02–2.6) |
Discussion
To the best of our knowledge, no epidemiological
studies have been performed so far analysing association of TA with
HPV in cervical scrapes and its role as a possible risk factor for
HGSIL adjusted for different known risk factors. Our results show
that TA is a risk factor for high grade cervical lesions.
Furthermore, TA was associated with increased odds of high grade
cervical lesions in hrHPV infected women. We propose that TA can be
added to the list of HPV cofactors previously described (26). Moreover, in addition to the mentioned
cofactors, TA can be measured objectively, as can HPV
infection.
TA was detected in 76% of the cases studied, and in
21.1% of the controls. These results are in agreement with previous
studies which have detected TA in cancer and precancerous lesions
while showing undetectable or low TA levels in normal tissue
(12,13). Similar results were observed for the
detection of HPV DNA. As expected, HPV DNA was detected in 84% of
the cases and 22.1% of the controls. Multiple studies have shown a
high prevalence of HPV (particularly with high risk genotypes) in
women with HGSIL and a low prevalence in women without lesions
(27–29).
When both TA and HPV status were considered, we
found a higher percentage of TA and HPV in cases (68%) than in
controls (7.7%). These results are in agreement with previous
studies on cervical biopsies, in which TA and HPV was detected in a
higher percentage of cases with CINII or III than in normal
cervical specimens (7,15).
Our results indicate increased odds of HGSIL in
women positive for hrHPV, in women positive for TA and in women
with more than 2 parities. The epidemiological association of HPV
infection and parity with HGSIL and cervical cancer have been
widely reported (30–33). However epidemiological information on
TA with HGSIL is scanty. A systematic review analyzing the accuracy
of telomerase assay in cervical lesions showed a positive
association between a positive TA test and high grade cervical
lesions with a diagnostic odds ratio (DOR) of 5.8, which is similar
to our results (34). However in that
review the authors did not take into account other important risk
factors, such as HPV infection, of pivotal importance for the
development of the disease. When only women with hrHPV infection
were included in our analysis, TA showed a positive association
with HGSIL in both crude and adjusted models. It was observed that
the odds ratio increases when TA detection is adjusted to
additional cofactors, although it is important to note that the CI
is very wide.
Research into the biological relationship between
HPV infection and TA are ongoing. Some studies have shown a
relationship between the activation of the enzyme and HPV
infection, especially HPV 16 and 18 (14,15).
Telomerase activation by HPV has been principally attributed to
hrHPV E6 oncoprotein because this protein can act as a
transcription factor at the hTERT promoter and upregulate its
expression (16,19,35,36). The
E7 protein and the pRB pathway have been also found to be involved
in the maintenance and induction of TA (37). The fact that amongst cases that were
positive for TA, different HPV types of the alpha 9 species were
detected, i.e. HPV-16, 31, 33, 52, and 58, could suggests that
other hrHPV types can activate telomerase either directly or
indirectly. Recently one in vitro study published by Schutze
et al, 2014 showed that cell lines generated using various hrHPV
types were able to activate telomerase after a period in culture
(38). However more functional assays
are needed to establish the mechanism used by these genotypes.
In the present study, we observed samples with HPV
infection without TA and samples with TA without HPV infection, and
analysis between TA and hrHPV show no statistically significant
interaction. This suggests that there are other factors aside from
HPV infection that can activate the enzyme. Factors including HPV
integration state can influence TA (39). When the viral genome is maintained as
an episome, the E2 protein is expressed and can inhibit expression
of hTERT. In contrast, when HPV is integrated into the host genome,
E6 and E7 expression increases producing the expression of hTERT.
Epigenetic regulation of the hTERT gene could also be important to
evaluate, as some researchers have shown that methylation play a
key role in the regulation of hTERT, although contradictory results
have been reported (40–43). Recently we have published an
association between type specific HPV infections and hTERT
methylation in patients with cervical cancer but additional studies
in patients with HGSIL have to be done (44). In addition, lifestyle aspects
including diet, exposure to stress, and level of physical activity
can also produce changes in TA (45–47).
Unfortunately, we did not assess these factors in this study to
have a more complete picture of the different mechanisms involved
in TA in cases and controls. However our results show clearly that
TA is a risk factor for HGSIL, with the strongest association in
women who are hrHPV positive.
Our study suggests that detection of both HPV
infection and TA at the same time could have a higher predictive
value for HGSIL than either HPV detection or TA detection
independently. The next step of this study is to evaluate TA in a
larger cohort as a diagnostic test in the triage of HPV positive
women.
Limitations of this study include low sample size
resulting in very wide confidence intervals when adjusting for
relevant cofactors.
In summary, this is the first epidemiological study
that shows an independent association of TA with HPV as a risk
factor for HGSIL when adjusted for different risk factors. TA could
be used as an adjunct tool, in addition to HPV DNA detection, for
identifying patients with higher risks of progression to cervical
neoplasia.
Acknowledgements
We thank all study participants, gynaecologists,
nurses and social workers who collaborated to the fieldwork, in
special to the HPV Study Group: Mauricio González, Natasha Ortiz,
Joaquín Luna, Teresa Martinez, Gilberto Martínez, Edmundo Mora,
Gonzalo Pérez, Raul Murillo, José María Fuentes. Constanza Gómez,
Eva Klaus, Constanza Camargo, Cecilia Tobon, Teodolinda Palacio,
Carolina Suárez and Claudia Molina. Also particular thanks to
R.D.M. Steenbergen (VU University Medical Center Amsterdam, The
Netherlands) and D Machalek (The Royal Women's Hospital) for
critical reading of the manuscript. This work was supported by
Colciencias grant No. 331 and funds from the Instituto Nacional de
Cancerología, Bogotá-Colombia.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pardo C and Cendales R: Incidencia
estimada y mortalidad por cancer en Colombia, 2002–2006. Instituto
Nacional de Cancerología; Legis S.A: 2010
|
|
3
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muñoz N: Human papillomavirus and cancer:
The epidemiological evidence. J Clin Virol. 19:1–5. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blackburn EH: Structure and function of
telomeres. Nature. 350:569–573. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cohen SB, Graham ME, Lovrecz GO, Bache N,
Robinson PJ and Reddel RR: Protein composition of catalytically
active human telomerase from immortal cells. Science.
315:1850–1853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Snijders PJ, van Duin M, Walboomers JM,
Steenbergen RD, Risse EK, Helmerhorst TJ, Verheijen RH and Meijer
CJ: Telomerase activity exclusively in cervical carcinomas and a
subset of cervical intraepithelial neoplasia grade III lesions:
Strong association with elevated messenger RNA levels of its
catalytic subunit and high-risk human papillomavirus DNA. Cancer
Res 58: 3812–3818, 1998. Cancer Res 58: 3812–3818, 1998. 58:
3812–3818, 1998:3812-3818, 1998–3818, 1998. 1998.
|
|
8
|
Shay JW and Wright WE: Senescence and
immortalization: Role of telomeres and telomerase. Carcinogenesis.
26:867–874. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holt SE and Shay JW: Role of telomerase in
cellular proliferation and cancer. J Cell Physiol. 180:10–18. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ault KA, Allen HK, Phillips SL, Zimmerman
MB and Klingelhutz AJ: Telomerase activity as a potential
diagnostic marker for triage of abnormal Pap smears. J Low Genit
Tract Dis. 9:93–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma A, Rajappa M, Saxena A and Sharma
M: Telomerase activity as a tumor marker in Indian women with
cervical intraepithelial neoplasia and cervical cancer. Mol Diagn
Ther. 11:193–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Liu S, Wang H, Xie X, Chen X, Zhang
X and Zhang Y: Genomic amplification of the human telomerase gene
(hTERC) associated with human papillomavirus is related to the
progression of uterine cervical dysplasia to invasive cancer. Diagn
Pathol. 7:1472012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Castro-Duque AF, Loango-Chamorro N,
Ruiz-Hoyos BM and Landázuri P: Telomerase activity associated with
progression of cervical lesions in a group of Colombian patients.
Rev Bras Ginecol Obstet. 37:559–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pinto-Tang J, Castro T and Premoli G:
Detection of telomerase activity in cervical lesions by
non-radioactive telomeric repeat amplification protocol (TRAP).
Invest Clin. 46:255–263. 2005.(In Spanish). PubMed/NCBI
|
|
15
|
Kailash U, Soundararajan CC, Lakshmy R,
Arora R, Vivekanandhan S and Das BC: Telomerase activity as an
adjunct to high-risk human papillomavirus types 16 and 18 and
cytology screening in cervical cancer. Br J Cancer. 95:1250–1257.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Veldman T, Liu X, Yuan H and Schlegel R:
Human papillomavirus E6 and Myc proteins associate in vivo and bind
to and cooperatively activate the telomerase reverse transcriptase
promoter. Proc Natl Acad Sci USA. 100:pp. 8211–8216. 2003;
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seo E Jeong, Kim H Jung, Jae Lee C, Tae
Kang H and Hwang E Seong: The role of HPV oncoproteins and cellular
factors in maintenance of hTERT expression in cervical carcinoma
cells. Gynecol Oncol. 94:40–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Yuan H, Fu B, Disbrow GL,
Apolinario T, Tomaic V, Kelley ML, Baker CC, Huibregtse J and
Schlegel R: The E6AP ubiquitin ligase is required for
transactivation of the hTERT promoter by the human papillomavirus
E6 oncoprotein. J Biol Chem. 280:10807–10816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katzenellenbogen RA, Vliet-Gregg P, Xu M
and Galloway DA: NFX1-123 increases hTERT expression and telomerase
activity posttranscriptionally in human papillomavirus type 16 E6
keratinocytes. J Virol. 83:6446–6456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y, Xu F, Tao F, Feng D, Ling B, Qian
L, Yang X, Wang Q, Wang H, Zhao W, et al: GRIM-19 restores cervical
cancer cell senescence by repressing hTERT transcription. J
Interferon Cytokine Res. 36:506–515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koskimaa HM, Kurvinen K, Costa S, Syrjänen
K and Syrjänen S: Molecular markers implicating early malignant
events in cervical carcinogenesis. Cancer Epidemiol Biomarkers
Prev. 19:2003–2012. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li T, Tang L, Bian D, Jia Y, Huang X and
Zhang X: Detection of hTERC and c-MYC genes in cervical epithelial
exfoliated cells for cervical cancer screening. Int J Mol Med.
33:1289–1297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao XY, Cui Y, Jiang SF, Liu KJ, Han HQ,
Liu XS and Li Y: Human telomerase gene and high-risk human
papillomavirus infection are related to cervical intraepithelial
neoplasia. Asian Pac J Cancer Prev. 16:693–697. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Molano M, Posso H, Weiderpass E, van den
Brule AJ, Ronderos M, Franceschi S, Meijer CJ, Arslan A and Munoz
N; HPV Study Group HPV Study, : Prevalence and determinants of HPV
infection among Colombian women with normal cytology. Br J Cancer.
87:324–333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van den Brule AJ, Pol R,
Fransen-Daalmeijer N, Schouls LM, Meijer CJ and Snijders PJ:
GP5+/6+ PCR followed by reverse line blot analysis enables rapid
and high-throughput identification of human papillomavirus
genotypes. J Clin Microbiol. 40:779–787. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Castellsagué X and Muñoz N: Chapter 3:
Cofactors in human papillomavirus carcinogenesis-role of parity,
oral contraceptives, and tobacco smoking. J Natl Cancer Inst
Monogr. 1–28. 2003.PubMed/NCBI
|
|
27
|
Hamlin-Douglas LK, Coutlée F, Roger M,
Franco EL and Brassard P: Prevalence and age distribution of human
papillomavirus infection in a population of Inuit women in Nunavik,
Quebec. Cancer Epidemiol Biomarkers Prev. 17:3141–3149. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berois N, Heard I, Fort Z, Alonso R, Sica
A, Moerzinger P, Rodriguez G, Sancho-Garnier H, Osinaga E and Favre
M: Prevalence of type-specific HPV infection in Uruguay. J Med
Virol. 86:647–652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogembo RK, Gona PN, Seymour AJ, Park HS,
Bain PA, Maranda L and Ogembo JG: Prevalence of human
papillomavirus genotypes among African women with normal cervical
cytology and neoplasia: A systematic review and meta-analysis. PLoS
One. 10:e01224882015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muñoz N, Franceschi S, Bosetti C, Moreno
V, Herrero R, Smith JS, Shah KV, Meijer CJ and Bosch FX;
International Agency for Research on Cancer, ; Multicentric
Cervical Cancer Study Group, : Role of parity and human
papillomavirus in cervical cancer: The IARC multicentric
case-control study. Lancet. 359:1093–1101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Almonte M, Albero G, Molano M, Carcamo C,
García PJ and Pérez G: Risk factors for human papillomavirus
exposure and co-factors for cervical cancer in Latin America and
the Caribbean. Vaccine. 26 Suppl 11:L16–L36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muñoz N, Hernandez-Suarez G, Méndez F,
Molano M, Posso H, Moreno V, Murillo R, Ronderos M, Meijer C and
Muñoz A; Instituto Nacional de Cancerología HPV Study Group, :
Persistence of HPV infection and risk of high-grade cervical
intraepithelial neoplasia in a cohort of Colombian women. Br J
Cancer. 100:1184–1190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roura E, Travier N, Waterboer T, de
Sanjosé S, Bosch FX, Pawlita M, Pala V, Weiderpass E, Margall N,
Dillner J, et al: Correction: The influence of hormonal factors on
the risk of developing cervical cancer and pre-cancer: Results from
the EPIC cohort. PLoS One. 11:e01514272016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rosa MI, Medeiros LR, Bozzetti MC, Fachel
J, Wendland E, Zanini RR, Moraes AB and Rosa DD: Accuracy of
telomerase in cervical lesions: A systematic review. Int J Gynecol
Cancer. 17:1205–1214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klingelhutz AJ, Foster SA and McDougall
JK: Telomerase activation by the E6 gene product of human
papillomavirus type 16. Nature. 380:79–82. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Veldman T, Horikawa I, Barrett JC and
Schlegel R: Transcriptional activation of the telomerase hTERT gene
by human papillomavirus type 16 E6 oncoprotein. J Virol.
75:4467–4472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu X, Roberts J, Dakic A, Zhang Y and
Schlegel R: HPV E7 contributes to the telomerase activity of
immortalized and tumorigenic cells and augments E6-induced hTERT
promoter function. Virology. 375:611–623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schütze DM, Kooter JM, Wilting SM, Meijer
CJ, Quint W, Snijders PJ and Steenbergen RD: Longitudinal
assessment of DNA methylation changes during HPVE6E7-induced
immortalization of primary keratinocytes. Epigenetics. 10:73–81.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee D, Kim HZ, Jeong KW, Shim YS, Horikawa
I, Barrett JC and Choe J: Human papillomavirus E2 down-regulates
the human telomerase reverse transcriptase promoter. J Biol Chem.
277:27748–27756. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kyo S, Takakura M, Fujiwara T and Inoue M:
Understanding and exploiting hTERT promoter regulation for
diagnosis and treatment of human cancers. Cancer Sci. 99:1528–1538.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Wilde J, Kooter JM, Overmeer RM,
Claassen-Kramer D, Meijer CJ, Snijders PJ and Steenbergen RD: hTERT
promoter activity and CpG methylation in HPV-induced
carcinogenesis. BMC Cancer. 10:2712010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang J, Zhao LJ, Zhao C, Zhang G, Zhao Y,
Li JR, Li XP and Wei LH: Hypomethylated CpG around the
transcription start site enables TERT expression and HPV16 E6
regulates TERT methylation in cervical cancer cells. Gynecol Oncol.
124:534–541. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Daniel M, Peek GW and Tollefsbol TO:
Regulation of the human catalytic subunit of telomerase (hTERT).
Gene. 498:135–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Molano M, Moreno-Acosta P, Morales N,
Burgos M, Buitrago L, Gamboa O, Alvarez R, Garland SM, Tabrizi SN,
Steenbergen RD and Mejía JC: Association between type-specific HPV
infections and hTERT DNA methylation in patients with invasive
cervical cancer. Cancer Genomics Proteomics. 13:483–491. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ludlow AT, Zimmerman JB, Witkowski S,
Hearn JW, Hatfield BD and Roth SM: Relationship between physical
activity level, telomere length, and telomerase activity. Med Sci
Sports Exerc. 40:1764–1771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu H, Guo D, Li K, Pedersen-White J,
Stallmann-Jorgensen IS, Huang Y, Parikh S, Liu K and Dong Y:
Increased telomerase activity and vitamin D supplementation in
overweight African Americans. Int J Obes (Lond). 36:805–809. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Deng W, Cheung ST, Tsao SW, Wang XM and
Tiwari AF: Telomerase activity and its association with
psychological stress, mental disorders, lifestyle factors and
interventions: A systematic review. Psychoneuroendocrinology.
64:150–163. 2016. View Article : Google Scholar : PubMed/NCBI
|