Introduction
Cholangiocarcinoma (CCA) is a cancer of the lining
of the bile duct epithelium, the prevalence of which is increasing
worldwide (1–3). CCA is a major public health problem in
the northeast of Thailand where its etiology is strongly associated
with liver fluke (Opisthorchis viverrini) infection that
causes persistent bile duct inflammation (4). The incidence rates of CCA are between 93
and 318 per 100,000 individuals annually (5) with males being more commonly affected
than females, and with an estimated 20,000 mortalities annually
(6). CCA progression is relatively
slow and patients frequently present at the hospital with
late-stage disease in which the cancer has spread to other organs.
Notably, chemotherapy in combination with surgery, rather than
surgery alone, decreases tumor size and prolongs the patients'
survival time (7). Therefore, the
mechanisms, particularly alterations in molecular pathways that
drive tumor cell functions during CCA progression, require further
investigation in order to contribute to the improvement of
guidelines for the treatment of CCA.
The protein 14-3-3ζ belongs to the 14-3-3 protein
family that exhibits an oncogenic potential through its interaction
with target proteins involved in cancer initiation and progression
(8). 14-3-3ζ has been identified in
several types of cancer, including breast (9), oral (10)
and gastric (11) cancer, esophageal
squamous carcinomas (12) and
intrahepatic CCA (13), leading to
apoptosis resistance, cancer recurrence and chemoresistance
(9,14). In contrast, 14-3-3ζ small interfering
RNA (siRNA) treatment of cancer cell lines sensitized cells to
stress-induced apoptosis and effectively decreased the onset and
growth of tumor xenografts (9). In
addition, 14-3-3ζ knockdown by siRNA in lung cancer cells increased
sensitivity to cisplatin in vitro and in vivo
(15).
The molecular mechanisms by which 14-3-3ζ exerts its
functions in cancer cells have been elucidated. Neal et al
(16) reported that 14-3-3ζ
overexpression enhanced protein kinase B (Akt) phosphorylation via
binding to the p85α regulatory subunit of phosphoinositide 3-kinase
(PI3K), an upstream signaling protein of Akt in breast cancer cell
lines, contributing to cancer cell proliferation and survival. The
PI3K/Akt signaling pathway is prominently activated in CCA,
regulating tumor growth and metastasis, and its inhibitors may be
potentially used as a targeted drug for the treatment of CCA
(17). However, to the best of our
knowledge, neither the functions of 14-3-3ζ regulating the PI3K/Akt
signaling pathway nor its chemosensitivity in CCA cells have been
reported previously.
In the present study, it was demonstrated that the
co-expression of 14-3-3ζ and pAkt is associated with a poor
prognosis of patients with CCA. Specific targeting of 14-3-3ζ
inhibited CCA cell proliferation via the suppression of pAkt
activity and enhanced the chemotherapeutic effect of
gemcitabine.
Materials and methods
Human CCA tissues
Tissue specimens from 75 patients with CCA admitted
to Srinagarind Hospital, Khon Kaen University (Khon Kaen, Thailand)
were collected. Of the 75 patients, 50 (67%) were male and 25 (33%)
were female. The median age of patients was 57 years (range, 32-73
years) (Table I). The
paraffin-embedded CCA tissues were obtained from the specimen bank
of the Liver Fluke and Cholangiocarcinoma Research Center, Faculty
of Medicine, Khon Kaen University, between January 1999 and
December 2007. The protocol for collection and the study design
were approved by the Ethics Committee for Human Research, Khon Kaen
University (HE571283) and written informed consent was obtained
from each subject prior to surgery.
 | Table I.Association between the co-expression
of 14-3-3ζ and pAkt (Ser473) with clinicopathological
characteristics of patients with CCA demonstrated by
immunohistochemical staining. |
Table I.
Association between the co-expression
of 14-3-3ζ and pAkt (Ser473) with clinicopathological
characteristics of patients with CCA demonstrated by
immunohistochemical staining.
|
|
| Co-expression of
14-3-3ζ and pAkt (Ser473) |
|
|---|
|
|
|
|
|
|---|
| Factor | No. of patients
(n=75) | High | Low | Others | P-valuea |
|---|
| Age, years |
|
|
|
| 0.527 |
|
<57 | 36 | 18 | 5 | 13 |
|
| ≥57 | 39 | 15 | 5 | 19 |
|
| Sex |
|
|
|
| 0.611 |
|
Female | 25 | 13 | 3 | 9 |
|
| Male | 50 | 20 | 7 | 23 |
|
| Histological
type |
|
|
|
| 0.118 |
|
Papillary | 13 | 4 | 4 | 5 |
|
|
Non-papillary | 62 | 29 | 6 | 27 |
|
| Overall
metastasis |
|
|
|
| 0.006a |
|
Non-metastasis | 28 | 8 | 8 | 12 |
|
|
Metastasis | 47 | 25 | 2 | 20 |
|
CCA cell lines
The human intrahepatic CCA cell lines KKU-M213 and
its derivative KKU-M214 were isolated from Thai patients with CCA
and established in the Liver Fluke and Cholangiocarcinoma Research
Center. Cells were cultured in Gibco® Ham's F-12
nutrient mixture (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% Gibco® heat-inactivated
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 100 mg/ml streptomycin at 37°C in a humidified
incubator containing 5% CO2.
Immunohistochemical analysis
The expression of 14-3-3ζ and pAkt was examined in
CCA tissue sections using immunohistochemical (IHC) staining. The
4-µm-thick tissue sections were deparaffinized and rehydrated in an
aqueous ethanol solution series. Antigen retrieval was performed by
submerging slides in 10 mM citrate buffer (pH 6.0) and heating in a
microwave oven at 100°C for 10 min. The sections were blocked with
0.3% hydrogen peroxide followed by 10% skimmed milk at room
temperature for 30 min for each blocking step. Subsequently, the
sections were incubated with rabbit antibody against 14-3-3ζ
(dilution, 1:1,000; cat. no. ab51129; Abcam, Cambridge, UK) and
pAkt (Ser473; dilution, 1:1,000; cat. no. SAB4300042,
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 4°C overnight.
Subsequently, sections were incubated with horseradish
peroxidase-conjugated EnVision anti-rabbit secondary antibody at
room temperature for 1 h (undiluted; cat. no. K4003; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA). Antigen-antibody
complexes were developed with 3,3′-diaminobenzidine
tetrahydrochloride substrate kit (Vector Laboratories, Inc.,
Burlingame, CA, USA) for 10 min, and were counterstained with
hematoxylin for 2 min. The intensity of staining was scored as
follows: 0, negative staining; 1, weak staining; 2, moderate
staining; and 3, strong staining. The proportion was scored based
on the percentage of stained cells as follows: 0, all with negative
staining, +1, <25% positive, +2, 25–50% positive; and +3,
>50% positive. The final score was assigned by multiplying the
intensity score by the proportion score. The median of the final
score was selected as a threshold value to determine the high and
low expression of 14-3-3ζ and pAkt.
Transient siRNA transfection
RNA interference (RNAi) was performed using a small
fragment duplex of RNA (siRNA) which is specific to 14-3-3ζ mRNA
(SMARTpool ON-TARGETplus siRNA, ID L-003332-00-0005) containing
four specific sequences, 5′-AGAAAGGGAUUGUCGAUCA-3′,
5′-GCAGAUGGCUCGAGAAUAC-3′, 5′-GCCCGUAGGUCAUCUUGGA-3′ and
5′-AAAGACAGCACGCUAAUAA-3′, and control sequences,
5′-UGGUUUACAUGUCGACUAA-3′, 5′-UGGUUUACAUGUUGUGUGA-3′,
5′-UGGUUUACAUGUUUUCUGA-3′ and 5′-UGGUUUACAUGUUUUCCUA-3′ (GE
Healthcare Dharmacon, Inc., Lafayette, CO, USA). The CCA cells,
seeded at a density of 6×104 cells, were transfected
with 50 nM si14-3-3ζ using Lipofectamine RNAiMAX (Invitrogen;
Thermo Fisher Scientific, Inc.) in 6-well plates for 24, 48 and 72
h post-transfection. The protein level was determined using western
blotting to confirm transfection efficiency. Cellular functions
were determined upon siRNA transfection to CCA cells.
Gemcitabine sensitivity testing in CCA
cells
At 12 h post-seeding, gemcitabine (Fresenius Kabi
Oncology Ltd., Haryana, India) was added at different
concentrations (0, 0.0001, 0.001, 0.01, 0.1, 1, 10 and 100 µM) to
CCA cell cultures. The doses of gemcitabine were selected on the
basis of the half-maximal inhibitory concentration
(IC50) of its treatment of CCA cells. A concentration of
1 µM, which provided a time-dependent inhibitory effect, was
selected to treat si14-3-3ζ-transfected cells at 24 h
post-transfection. To determine cell viability, cells with combined
treatments were cultured for additional time periods (24 and 48 h).
Cell viability and apoptosis assays were performed at the
aforementioned time points.
Western blot analysis
Transfected cells were harvested and then lysed with
radioimmunoprecipitation assay cell lysis buffer (150 mM NaCl, 0.5
M Tris-HCl pH 7.4, 1% Tween-20, 1% sodium deoxycholate, 0.1% SDS)
for 10 min on ice. The cell lysates were centrifuged at 4°C at
14,000 × g for 10 min. Protein concentration was determined using a
bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein extracts were solubilized in 4X SDS
buffer containing dithiothreitol and were boiled at 95°C. Protein
was loaded (20 µg/well) and separated on 10% polyacrylamide gel by
SDS-PAGE prior to being transferred onto polyvinylidene fluoride
membranes. The membranes were blocked with 5% skimmed milk at room
temperature for 1 h and probed with each primary antibody at 4°C
overnight, including rabbit anti-human 14-3-3ζ (dilution, 1:1,000;
cat. no. ab51129; Abcam), rabbit anti-human pAkt
(Ser473) (dilution, 1:1,000; cat. no. SAB4300042;
Sigma-Aldrich; Merck KGaA), mouse anti-human PI3K p85α (dilution,
1:2,000; cat. no. ab86714; Abcam), rabbit anti-human Akt (dilution,
1:1,000; cat. no. 9272; Cell Signaling Technology, Inc., Danvers,
MA, USA), mouse anti-human p27 (dilution, 1:1,000; cat. no. 3698S;
Cell Signaling Technology, Inc.). β-actin was used as a loading
control (mouse anti-human β-actin; dilution, 1:10,000; cat. no.
A5441; Sigma-Aldrich; Merck KGaA). Following incubation with
horseradish peroxidase-conjugated secondary antibodies, including
goat anti-rabbit (dilution, 1:2,000; cat. no. G21234) and rabbit
anti-mouse (dilution, 1:4,000; cat. no. A16166; both Invitrogen;
Thermo Fisher Scientific, Inc.) at room temperature for 1 h, the
band intensity was measured using enhanced chemiluminescence Prime
Western Blotting Detection reagent (GE Healthcare, Chicago, IL,
USA). The apparent density of the bands on membranes was captured
by ImageQuant™ Imager (GE Healthcare).
Cell viability and apoptosis
assays
The sulforhodamine B (SRB) assay (cat. no. S1402;
Sigma-Aldrich; Merck KGaA) was used for determining cell viability
in transfected KKU-M213 and KKU-M214 cells. Briefly, cells were
seeded at a density of 2×103 cells in 96-well
flat-bottom microtiter plates and incubated for 24, 48 and 72 h.
Cells were then fixed with 10% trichloroacetic acid and stained
with 0.4% SRB in 1% acetic acid for 45 min. The protein-bound stain
was solubilized with 10 mM Tris base (pH 10.5) and absorbance was
measured at 540 nm using a microplate reader (Sunrise, Tecan Group,
Ltd., Mannedorf, Switzerland). The apoptotic cells were detected
using an Annexin V-FITC and propidium iodide staining kit (Roche
Diagnostics, Basel, Switzerland), according to the manufacturer's
protocol. Stained cells were enumerated using FACSCanto II flow
cytometry and analyzed using BD FACSDiva™ software, version 6.1.3
(BD Biosciences, San Jose, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS Inc., Chicago, IL, USA). A survival
curve was calculated using the Kaplan-Meier estimator method. The
association between 14-3-3ζ and pAkt (Ser473) and
patients' clinicopathological data was analyzed using Fisher's
exact test. The correlation between 14-3-3ζ with pAkt
(Ser473) was performed using a Spearman-rank correlation
test. Statistical comparisons between two different groups were
performed using unpaired Student's t-tests. The significance of the
data compared between siRNA-treated and control groups was analyzed
using one-way analysis of variance, followed by Fisher's least
significant difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Co-expression of 14-3-3ζ and pAkt is
associated with poor prognosis of patients with CCA
Of the 75 patients with intrahepatic CCA from whom
CCA tissue was obtained, 50 (67%) were male and 25 (33%) female.
The age of patients ranged from 32 to 73 years (median age, 57
years) (Table I). The histological
types were classified as papillary in 13 (17%) and non-papillary in
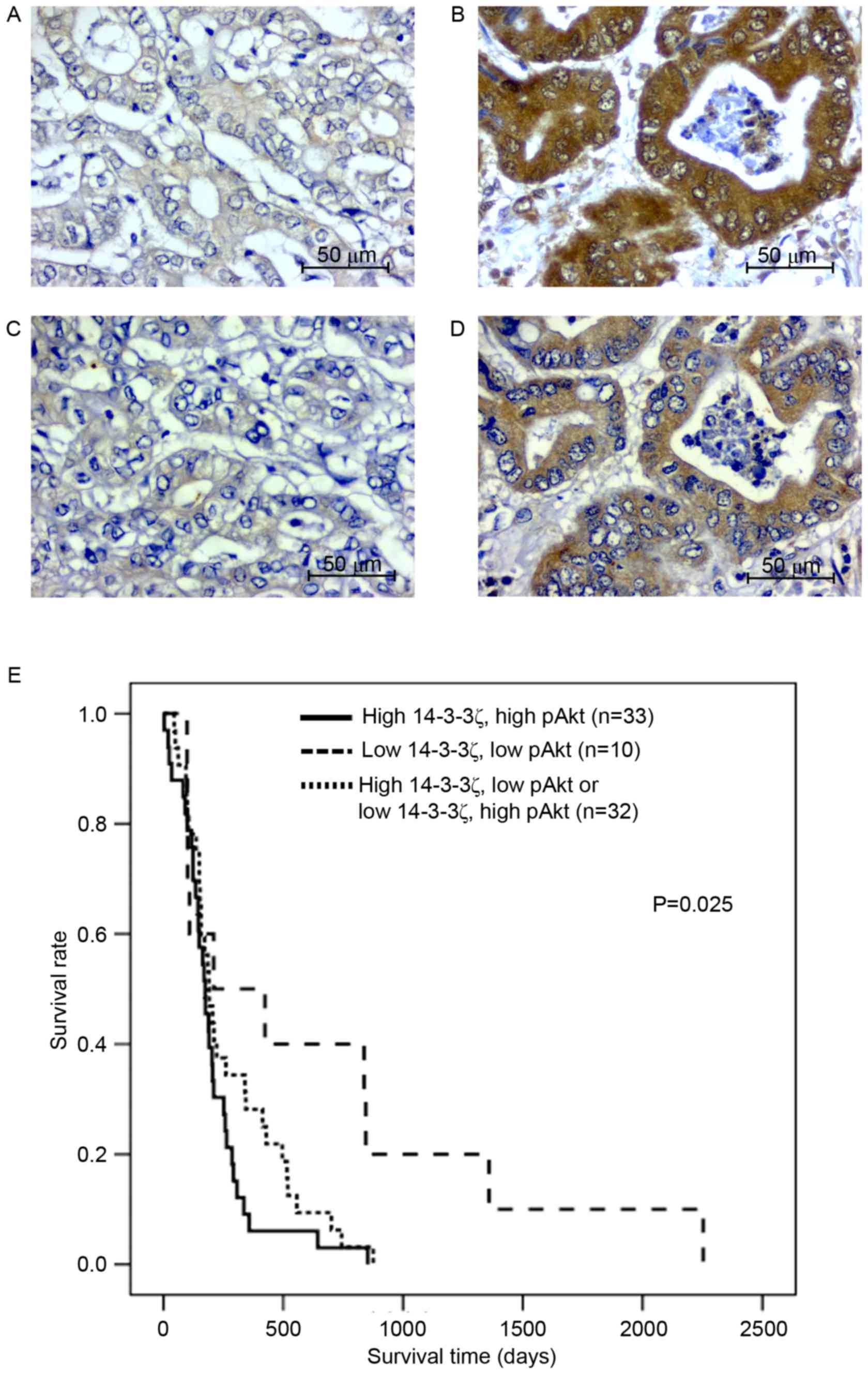
62 (83%) cases. For all 75 CCA cases, positive immunohistochemical
staining for 14-3-3ζ and pAkt was observed in the cytoplasmic
region of the tissue sections (Fig.
1A-D). High co-expression of 14-3-3ζ and pAkt was observed in
33 (44%) cases, whereas low co-expression of 14-3-3ζ and pAkt was
observed in 10 (13%) cases. High expression of 14-3-3z with low
expression of pAkt was observed in 18 (24%) and low expression of
14-3-3ζ with high expression of pAkt was observed in 14 (19%)
cases. The immunohistochemical scores for 14-3-3ζ and pAkt were
analyzed for correlation using bivariate analysis. The results
revealed that 14-3-3ζ expression was positively correlated with
pAkt expression (r=0.287; P=0.013).
There was a high co-expression of 14-3-3ζ and pAkt
associated with overall metastasis (Table
I; P=0.006). Age, sex and histological type were significantly
different between these two groups. The cumulative survival rate,
analyzed using the Kaplan-Meier estimator method, revealed that
patients with CCA with high co-expression of 14-3-3ζ and pAkt
(n=33) had a significantly shorter survival time compared with
those with low co-expression (n=10) or inverse expression of
14-3-3ζ and pAkt (n=33; P=0.025; Fig.
1E).
Knockdown of 14-3-3ζ attenuates the
PI3K/Akt pathway and increases the p27 protein level
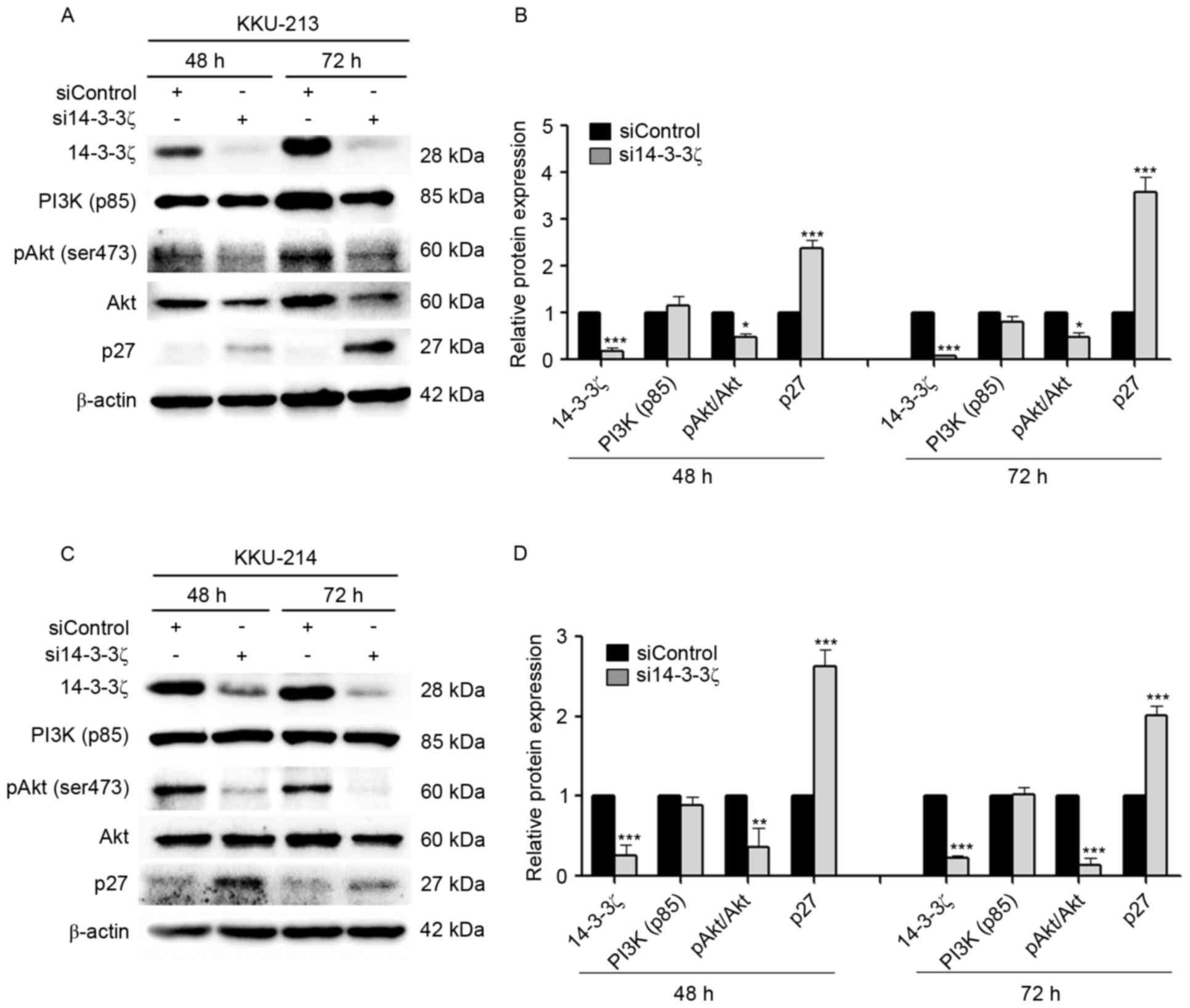
The 14-3-3ζ siRNA (si14-3-3ζ) and the negative
control (siControl) were transfected into KKU-M213 and KKU-M214
cell lines. The successful suppression of 14-3-3ζ expression at 72
h was verified by western blot analysis, which identified
suppression of 91% in KKU-M213 (Fig. 2A
and B) and 76% in KKU-M214 (Fig. 2C
and D) cells. To test the hypothesis that knockdown of 14-3-3ζ
affects the PI3K/Akt signaling pathway resulting in interference of
the CCA cell cycle, the levels of pAkt (Ser473), a
downstream signaling molecule of PI3K, and the cyclin-dependent
inhibitor p27, were assessed by western blotting in KKU-M213
(Fig. 2A and B) and KKU-M214
(Fig. 2C and D) cells. The results
identified that the ratio of pAkt (Ser473) to Akt was
significantly decreased in si14-3-3ζ-transfected CCA cells when
compared with siControl-transfected cells. si14-3-3ζ transfection
did not interfere with the PI3K p85α level. Knockdown of 14-3-3ζ
inhibited cell cycle demonstrated by markedly increasing p27
protein levels was observed for transfected KKU-M213 and KKU-M214
cells at 48 and 72 h post-transfection.
Knockdown of 14-3-3ζ leads to enhanced
chemosensitivity to gemcitabine
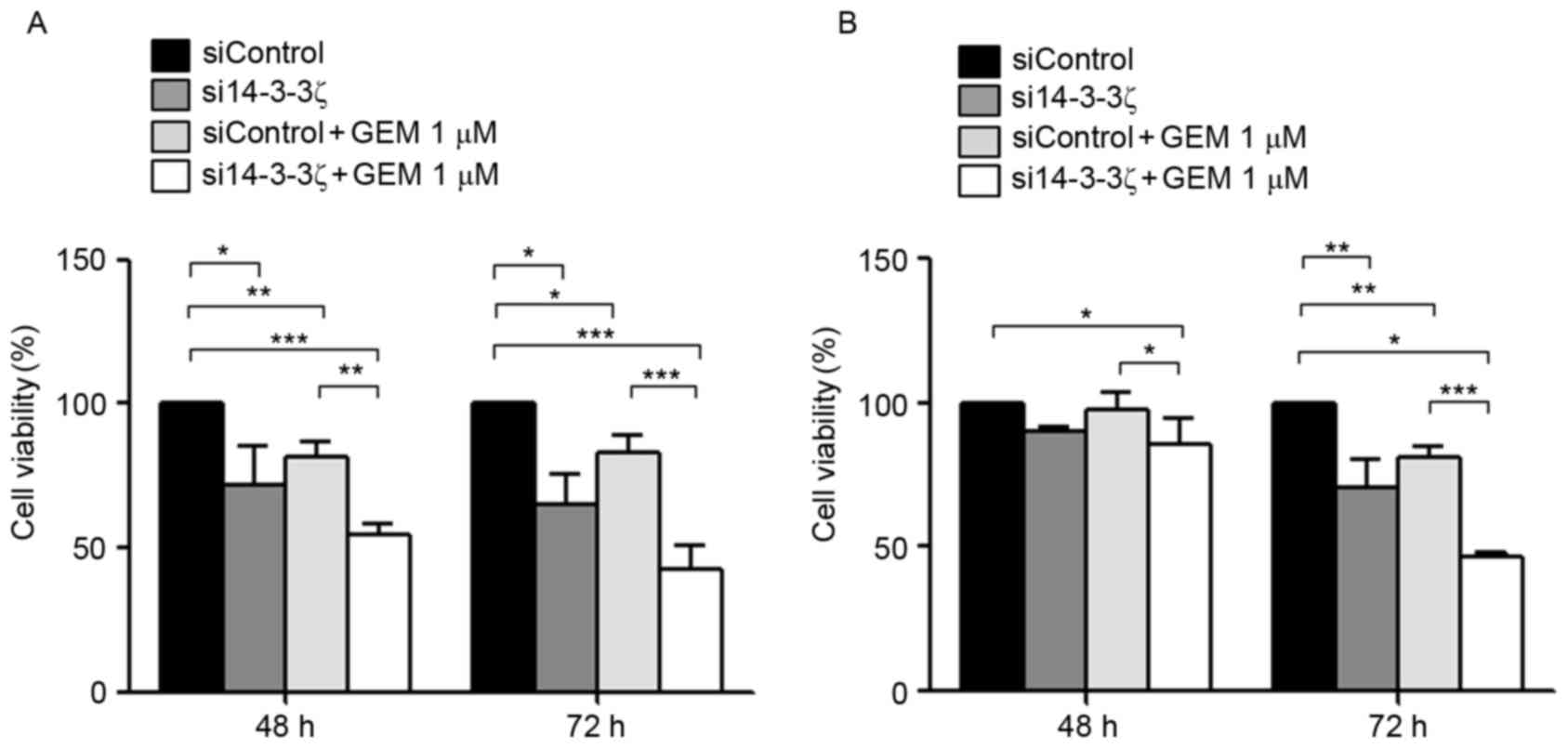
It was investigated further whether knockdown of
14-3-3ζ may enhance the chemosensitivity of gemcitabine in CCA
cells. KKU-M213 and KKU-M214 cells were transfected with si14-3-3ζ
with or without gemcitabine for 48 and 72 h and subjected to cell
viability (Fig. 3) and apoptosis
assays (Fig. 4). si14-3-3ζ (50 nM)
transfection significantly inhibited KKU-M213 cell viability by 10
and 29% at 48 and 72 h post-transfection, respectively, when
compared with control cells (Fig.
3A). si14-3-3ζ transfection inhibited KKU-M214 cell viability
by 28 and 35% at 48 and 72 h post-transfection, respectively,
compared with control cells (Fig.
3B). Furthermore, treatment with 1 µM gemcitabine combined with
50 nM si14-3-3ζ transfection was analyzed, to test whether this
combination was able to enhance the inhibitory effect on viability
in CCA cells. KKU-M213 cell viability was inhibited by 2 and 19% at
48 and 72 h post-transfection, respectively (Fig. 3A), and KKU-M214 by 18 and 17% at 48
and 72 h post-transfection, respectively, when compared with
control cells (Fig. 3B). Gemcitabine
(1 µM) combined with si14-3-3ζ (50 nM) suppressed KKU-M213 cell
viability by 15 and 53% at 48 and 72 h post-transfection,
respectively, when compared with the control cells (Fig. 3A). For KKU-M214 cells, gemcitabine (1
µM) combined with si14-3-3ζ (50 nM) suppressed cell viability by 46
and 57% at 48 and 72 h post-transfection, respectively, when
compared with the control cells (Fig.
3B). These results indicate that the combined effect of
gemcitabine and si14-3-3ζ was greater than the sum of the
individual effects of treatments alone, compared with the control
cells.
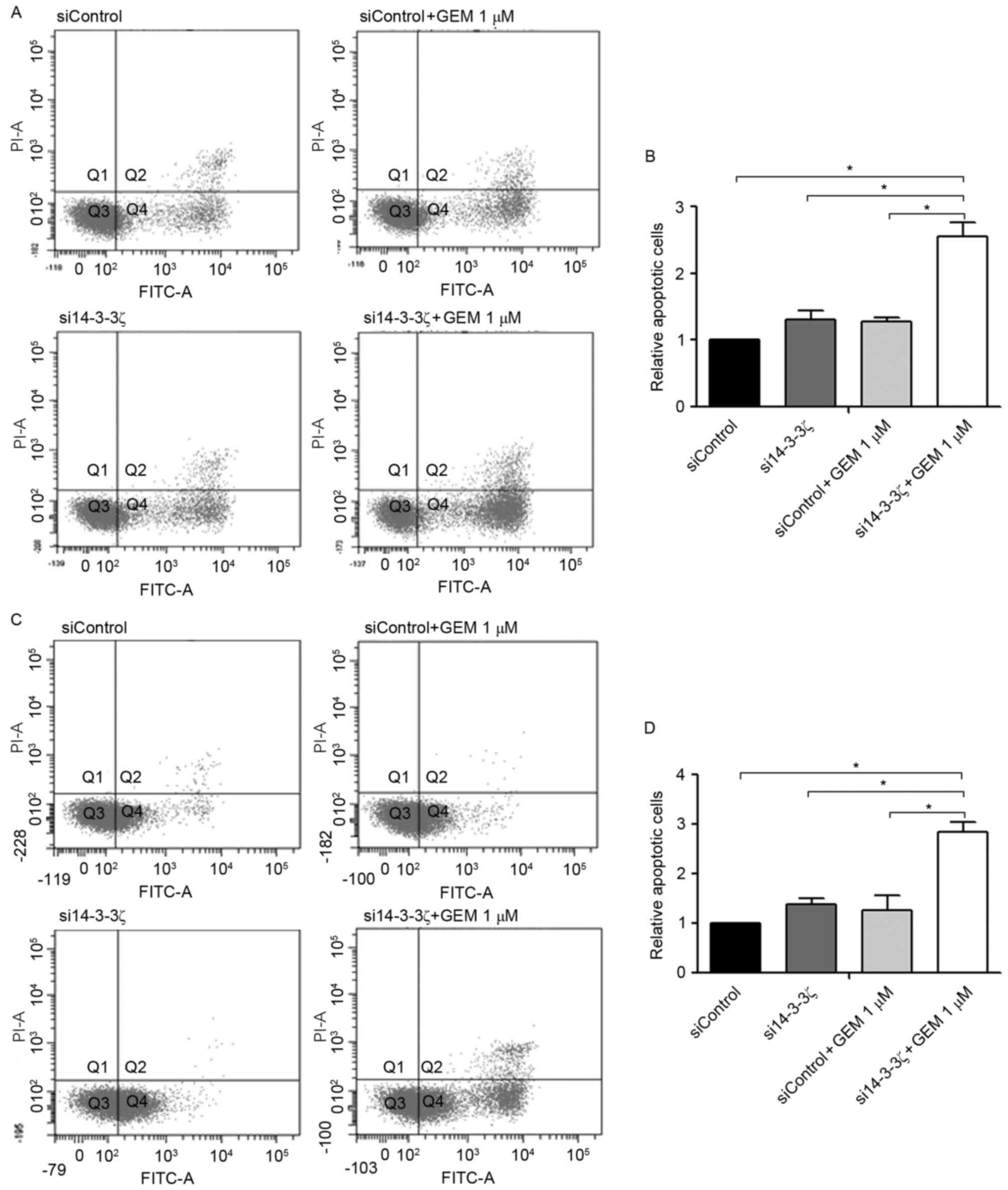
As presented in Fig.
4, KKU-M213 and KKU-M214 cells were transfected with si14-3-3ζ
(50 nM) for 24 h followed by gemcitabine treatment for an
additional 48 h (i.e., 72 h post-transfection). Neither the
si14-3-3ζ (50 nM) transfection nor the gemcitabine treatment alone
induced apoptosis in CCA cell lines at 72 h post-transfection when
compared with the control cells. In contrast, gemcitabine (1 µM)
combined with si14-3-3ζ (50 nM) significantly increased the number
of apoptotic cells 2.8-fold in KKU-M213 (Fig. 4A and B) and 2.6-fold in KKU-M214
(Fig. 4C and D) when compared with
the control cells.
Discussion
14-3-3ζ acts as a regulatory protein that is able to
interact with multiple client proteins that are involved in cancer
initiation, progression and chemoresistance in multiple types of
cancer (18). Several studies have
demonstrated previously that overexpression of 14-3-3ζ in multiple
types of cancer, including breast cancer (9), lung cancer (15) and intrahepatic CCA (13), is associated with poor prognosis of
patients and may potentially serve as a novel molecular target for
cancer treatment (19). The molecular
mechanism by which 14-3-3ζ serves a function in cancer cells has
been investigated previously (16).
It leads to an increase in the activation of the PI3K/Akt signaling
pathway through binding with Ser83 on p85α. This leads
to activation of the PI3K signaling cascade, contributing to
proliferation and survival of human breast cancer cells (16). In addition, suppression of 14-3-3ζ
decreases the proliferative capacity caused by S phase arrest and
promotes cell proliferation by activation of the
extracellular-signal-regulated kinase signaling cascade and
epithelial-mesenchymal transition induction in invasive QBC939 and
RBE CCA cells (13). The results of
the present study revealed that opisthorchiasis-associated CCA, in
which the PI3K/Akt signaling pathway is predominantly activated, is
linked with co-expression of 14-3-3ζ and pAkt in CCA tissues. High
scores for immunostaining of 14-3-3ζ and pAkt were significantly
correlated with a poor prognosis, suggesting that these molecules
may serve as prognostic indicators for patients with CCA. The
results of the present study are similar to those observed for
breast cancer in which overexpression of 14-3-3ζ was associated
with increased Akt phosphorylation and correlated with a shorter
survival time of patients (16).
In the present study, the intracellular function of
14-3-3ζ in CCA cells has been demonstrated. The suppression of
14-3-3ζ attenuated CCA cell proliferation by inhibiting pAkt
activity and inducing p27, leading to cell cycle arrest. However,
suppression of 14-3-3ζ to diminish pAkt activity was not sufficient
to trigger apoptotic cell death. This is similar to a previous
study by Yothaisong et al (17), in which inhibition of PI3 K/Akt using
selective inhibitors was able to suppress CCA cell proliferation,
but not induce apoptosis. Therefore, elimination of CCA cells by
inhibiting this kinase signaling pathway requires multiple
targeting strategies to gain therapeutic effectiveness.
CCA demonstrates an extreme resistance to
therapeutic chemotherapies, targeted agents and radiotherapy. This
intense resistance to a variety of therapies points to altered cell
survival and metabolic pathways in these refractory types of
cancer. Currently, gemcitabine appears to be an efficient
therapeutic agent for patients with CCA (7,20) as it
exerts an inhibitory effect on CCA cell proliferation. Notably, it
was revealed that 14-3-3ζ knockdown synergistically enhanced
gemcitabine sensitivity in CCA cells and induced substantial
apoptotic cell death. The combined effect of gemcitabine and
si14-3-3ζ was greater than the sum of the individual effects on
proliferative inhibition and apoptosis induction. This initial
observation provides a new approach for CCA treatment by increasing
the effectiveness of gemcitabine-based therapy. Further
investigation using drug combinations between 14-3-3 inhibitors and
gemcitabine in CCA cells is required in in vitro and in
vivo studies.
In conclusion, high co-expression of 14-3-3ζ and
pAkt was significantly associated with a poor prognosis for
patients with CCA, indicating that combined 14-3-3ζ and pAkt may
serve as prognostic indicators. Knockdown of 14-3-3ζ inhibited pAkt
(Ser473) activity and increased p27, leading to
suppression of CCA cell proliferation and induction of apoptosis.
Combining siRNA treatment that targets 14-3-3ζ with cytotoxic
drugs, including gemcitabine may provide an effective strategy for
the treatment of CCA.
Acknowledgements
The present study was supported by a Mid-Career
Grant (grant no. RSA5980012), Thailand Research Fund, the Higher
Education Research Promotion and National Research University
Project of Thailand, Office of the Higher Education Commission,
through the Health Cluster (SHeP-GMS) Khon Kaen University, a grant
from Khon Kaen University, and a grant from the Faculty of
Medicine, Khon Kaen University for supporting the M.Sc. program
(grant. no. IN58218).
References
|
1
|
Khan SA, Emadossadaty S, Ladep NG, Thomas
HC, Elliott P, Taylor-Robinson SD and Toledano MB: Rising trends in
cholangiocarcinoma: Is the ICD classification system misleading us?
J Hepatol. 56:848–854. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertuccio P, Bosetti C, Levi F, Decarli A,
Negri E and La Vecchia C: A comparison of trends in mortality from
primary liver cancer and intrahepatic cholangiocarcinoma in Europe.
Ann Oncol. 24:1667–1674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ghouri YA, Mian I and Blechacz B: Cancer
review: Cholangiocarcinoma. J Carcinog. 14:12015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sripa B and Pairojkul C:
Cholangiocarcinoma: Lessons from Thailand. Curr Opin Gastroenterol.
24:349–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sriamporn S, Pisani P, Pipitgool V,
Suwanrungruang K, Kamsa-ard S and Parkin DM: Prevalence of
Opisthorchis viverrini infection and incidence of
cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop Med Int
Health. 9:588–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bundhamcharoen K, Odton P, Phulkerd S and
Tangcharoensathien V: Burden of disease in Thailand: Changes in
health gap between 1999 and 2004. BMC Public Health. 11:532011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Valle JW, Wasan H, Johnson P, Jones E,
Dixon L, Swindell R, Baka S, Maraveyas A, Corrie P, Falk S, et al:
Gemcitabine alone or in combination with cisplatin in patients with
advanced or metastatic cholangiocarcinomas or other biliary tract
tumours: A multicentre randomised phase II study-The UK ABC-01
Study. Br J Cancer. 101:621–627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Freeman AK and Morrison DK: 14-3-3
Proteins: Diverse functions in cell proliferation and cancer
progression. Semin Cell Dev Biol. 22:681–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neal CL, Yao J, Yang W, Zhou X, Nguyen NT,
Lu J, Danes CG, Guo H, Lan KH, Ensor J, et al: 14-3-3zeta
overexpression defines high risk for breast cancer recurrence and
promotes cancer cell survival. Cancer Res. 69:3425–3432. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matta A, Bahadur S, Duggal R, Gupta SD and
Ralhan R: Over-expression of 14-3-3zeta is an early event in oral
cancer. BMC Cancer. 7:1692007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishimura Y, Komatsu S, Ichikawa D, Nagata
H, Hirajima S, Takeshita H, Kawaguchi T, Arita T, Konishi H,
Kashimoto K, et al: Overexpression of YWHAZ relates to tumor cell
proliferation and malignant outcome of gastric carcinoma. Br J
Cancer. 108:1324–1331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bajpai U, Sharma R, Kausar T, Dattagupta
S, Chattopadhayay TK and Ralhan R: Clinical significance of 14-3-3
zeta in human esophageal cancer. Int J Biol Markers. 23:231–237.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C, Liu LX, Dong ZR, Shi GM, Cai JB,
Zhang PF, Ke AW, Yu JX, Zhou J and Fan J: Up-regulation of
14-3-3zeta expression in intrahepatic cholangiocarcinoma and its
clinical implications. Tumour Biol. 36:1781–1789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frasor J, Chang EC, Komm B, Lin CY, Vega
VB, Liu ET, Miller LD, Smeds J, Bergh J and Katzenellenbogen BS:
Gene expression preferentially regulated by tamoxifen in breast
cancer cells and correlations with clinical outcome. Cancer Res.
66:7334–7340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan T, Li R, Todd NW, Qiu Q, Fang HB, Wang
H, Shen J, Zhao RY, Caraway NP, Katz RL, et al: Up-regulation of
14-3-3zeta in lung cancer and its implication as prognostic and
therapeutic target. Cancer Res. 67:7901–7906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neal CL, Xu J, Li P, Mori S, Yang J, Neal
NN, Zhou X, Wyszomierski SL and Yu D: Overexpression of 14-3-3zeta
in cancer cells activates PI3K via binding the p85 regulatory
subunit. Oncogene. 31:897–906. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yothaisong S, Dokduang H, Techasen A,
Namwat N, Yongvanit P, Bhudhisawasdi V, Puapairoj A, Riggins GJ and
Loilome W: Increased activation of PI3K/AKT signaling pathway is
associated with cholangiocarcinoma metastasis and PI3K/mTOR
inhibition presents a possible therapeutic strategy. Tumour Biol.
34:3637–3648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matta A, Siu KW and Ralhan R: 14-3-3zeta
as novel molecular target for cancer therapy. Expert Opin Ther
Targets. 16:515–523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matta A, DeSouza LV, Shukla NK, Gupta SD,
Ralhan R and Siu KW: Prognostic significance of head-and-neck
cancer biomarkers previously discovered and identified using
iTRAQ-labeling and multidimensional liquid chromatography-tandem
mass spectrometry. J Proteome Res. 7:2078–2087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan SA, Thomas HC, Davidson BR and
Taylor-Robinson SD: Cholangiocarcinoma. Lancet. 366:1303–1314.
2005. View Article : Google Scholar : PubMed/NCBI
|