Introduction
Lung cancer is the primary cause of
cancer-associated mortality worldwide, with non-small cell lung
cancer (NSCLC) accounting for ~85% of all lung cancer cases in 2014
(1). The majority of patients present
with advanced disease with a 5-year survival rate of <15% in
2014 (2,3). Although surgery remains the front-line
choice of treatment for localized NSCLC, those with advanced forms
may require chemotherapy (4).
Targeted therapy constitutes a promising treatment strategy for
prolonging the survival of a subset of patients with NSCLC
(5). However, drug resistance
conferred by complex recurrent genetic and epigenetic changes
remains a crucial obstacle for targeted therapies (6–10).
Consequently, there is urgent requirement to understand the
molecular mechanism(s) underlying the development of NSCLC, and
develop new therapies for treating NSCLC.
Transforming growth factor β (TGFβ) regulator 4
(TBRG4), also termed cell cycle progression restoration protein 2
or Fas-activated serine-threonine kinase domain-containing protein
4, encodes a regulator for TGFb (11–14).
TBRG4 (GenBank no. AAH14918.1) is located on chromosome
7p12.3–13, and is duplicated in patients with Sézary syndrome
(15). TBRG4 has previously been
demonstrated to stabilize the expression level of transcription of
cyclin 1 and 2 (12). In 293 and
Jurkat human cell lines, TBRG4 physically interacts with the virus
protein U encoded by the human immunodeficiency virus (16). Furthermore, TBRG4 also interacts with
pleiotrophin protein, and TBRG4 silencing affects the
stability of certain mitochondrial mRNAs (17,18).
However, the contribution of TBRG4 in the tumorigenesis of
NSCLC remains unclear.
In the present study, the effect of TBRG4
silencing on genome-wide gene expression patterns within human
H1299 lung cancer cells was investigated. The expression of
TBRG4 in tumor and adjacent normal tissues was also
evaluated.
Materials and methods
Cell culture
Human H1299 lung cancer cells were purchased from
the Shanghai Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in Dulbecco's modified
Eagle's medium (Corning Life Sciences, Shanghai, China) and
supplemented with 10% fetal bovine serum (Sangon Biotech Co., Ltd.,
Shanghai, China) at 37°C in a humidified atmosphere containing 5%
CO2.
siRNA transduction
The sequence of siRNA (5′-GTTCTTCAGCCTGGTACAT-3′)
was designed by GeneChem Co., Ltd. (Shanghai, China) for targeting
the TBRG4 sequence (GenBank no. NM_004749). The TBRG4
hairpin oligonucleotide was inserted into the pGV115-GFP lentiviral
vector (GeneChem Co., Ltd.) to construct a pGV115-GFP-short hairpin
TBRG4 (shTBRG4) TBRG4-knockdown vector. The negative control
(shCtrl) sequence was previously reported (19,20), and
when incorporated into the lentiviral vector was referred to as
pGV115-GFP-shCtrl. The lentiviral particles were prepared as
previously described (21). For
cellular transduction of shTBRG4 lentiviral or shCtrl lentivirus,
1×105 cells/well were seeded into 6-well plates. The
following day, cells were transduced with validated shTBRG4
lentivirus (5×105 TU/ml, 2 µl) or shCtrl lentivirus
(8×105 TU/ml, 1.25 µl) using 4 µl Lipofectamine 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), according to the manufacturer's protocol. After evaluating
infection efficiency using light and fluorescent microscopy at 72 h
after infection. Cells were harvested and used for subsequent
experiments.
RNA isolation and quantitative
polymerase chain reaction (qPCR)
Total RNA was extracted from the aforementioned
transduced cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and then 2 µg total RNA was reverse-transcribed
using a QuantiTect reverse transcription kit (Qiagen China Co.,
Ltd., Shanghai, China), according to the manufacturer's protocol. A
1 µg amount of cDNA was used as a template for qPCR using a
SYBR® Green PCR Master mix (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The primer sequences
used were as follows: TBRG4 forward,
5′-CAGCTCACCTGGTAAAGCGAT-3′ and reverse,
5′-GGGAGTAGATGCTCGTTCCTTC-3′; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. Results were normalized to GAPDH data
as described previously (22). Data
were analyzed using the method of Pfaffl (23). PCR primers used for validating the
microarray data are listed in Table
I.
 | Table I.Primer sequences used for
quantitative polymerase chain reaction. |
Table I.
Primer sequences used for
quantitative polymerase chain reaction.
| Gene | Primer sequence
5′-3′ |
|---|
| GAPDH
FP |
TGACTTCAACAGCGACACCCA |
| GAPDH
RP |
CACCCTGTTGCTGTAGCCAAA |
| IGF2 FP |
CCTCCAGTTCGTCTGTGGG |
| IGF2 RP |
CACGTCCCTCTCGGACTTG |
| MYBL2
FP |
AGAATAGCACCAGTCTGTCCTT |
| MYBL2
RP |
CCAATGTGTCCTGTTTGTTCCA |
| AURKA
FP |
GCCCTGTCTTACTGTCATTCG |
| AURKA
RP |
AGGTCTCTTGGTATGTGTTTGC |
| CAV1 FP |
CTGAGCGAGAAGCAAGTG |
| CAV1 RP |
AGAGAGAATGGCGAAGTAAATG |
| RAP1A
FP |
CGTGAGTACAAGCTAGTGGTCC |
| RAP1A
RP |
CCAGGATTTCGAGCATACACTG |
| SERPINE1
FP |
GCACCACAGACGCGATCTT |
| SERPINE1
RP |
ACCTCTGAAAAGTCCACTTGC |
| SCD FP |
TACTTGGAAGACGACATTCGC |
| SCD RP |
GGTGTAGAACTTGCAGGTAGGA |
| DDIT3
FP |
CTTCTCTGGCTTGGCTGACTGA |
| DDIT3
RP |
TGACTGGAATCTGGAGAGTGAGG |
| SESN2
FP |
TCTTACCTGGTAGGCTCCCAC |
| SESN2
RP |
AGCAACTTGTTGATCTCGCTG |
| RRM2 FP |
AAGAAACGAGGACTGATGC |
| RRM2 RP |
CTGTCTGCCACAAACTCAA |
| CDC20
FP |
CTTCGGCTCAGTGGAAAA |
| CDC20
RP |
GTCTGGCAGGGAAGGAAT |
| FOXM1
FP |
GCAGCGACAGGTTAAGGTTGAG |
| FOXM1
RP |
GTTGTGGCGGATGGAGTTCTTC |
| IDI1 FP |
TCCATTAAGCAATCCAGCCGA |
| IDI1 RP |
CCCAGATACCATCAGACTGAGC |
| PGK1 FP |
TGGACGTTAAAGGGAAGCGG |
| PGK1 RP |
GCTCATAAGGACTACCGACTTGG |
Gene microarray
The genome-wide effect of TBRG4 knockdown was
studied using a GeneChip® PrimeView™ Human Gene
Expression Array (Affymetrix; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) consisting of 20,000 genes. Three biological
replicates of H1299 cells transduced with shTBRG4 or shCtrl
lentiviruses (for 72 h) were microarrayed. RNA was initially
isolated using TRIzol reagent, and quality was determined using a
NanoDrop 2000 spectrophotometer (NanoDrop; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA) and Agilent Bioanalyzer 2100
(Agilent Technologies, Inc., Santa Clara, CA, USA). Individual
microarrays were used for gene expression profiling of each sample.
Briefly, 500 ng total RNA was reverse-transcribed and labeled with
biotin using the GeneChip® 3′ IVT labeling kit,
according to the manufacturer's protocol. Labeled cDNA was then
hybridized onto the GeneChip® PrimeView™ Human Gene
Expression Array at 60°C overnight. Arrays were performed with
GeneChip® Hybridization Wash and Stain kit using
GeneChip® Fluidics Station 450. All GeneChip®
products were obtained from Affymetrix; Thermo Fisher Scientific,
Inc., and all were used according to the manufacturer's protocol.
The chip array was scanned directly post-hybridization using a
GeneChip® Scanner 3000. Microarray data were analyzed
with GeneSpring software (version 11; Agilent Technologies, Inc.).
Data were normalized using the GeneSpring normalization algorithm.
Finally, genes which were differentially expressed >1.5-fold,
and had a differential score P≤0.05 among test samples, were
identified from normalized data sets.
Ingenuity pathway analysis (IPA)
Datasets representing differentially expressed genes
derived from microarray analyses were imported into the IPA tool
(http://www.ingenuity.com; Ingenuity®
Systems, Redwood City, CA, USA). The ‘core analysis’ function of
the IPA software in the present study was used to interpret the
differentially expressed data, which included ‘Disease and
Functions’, and ‘Molecular Network’. Differentially expressed genes
were mapped onto genetic networks available in the Ingenuity
database and then ranked on the basis of the overlap P-value to
measure enrichment of network-regulated genes in the present
dataset and the activation z-score algorithms computed by IPA
software (24). Analyses performed
within the IPA program include the identification of biological
networks of a particular interesting dataset according to the
purpose of the present study, and its global functions, functional
signaling pathways and downstream target genes.
Patients and tissue samples
A total of 75 patients diagnosed with lung cancer
and who received surgery at The First Affiliated Hospital of Bengbu
Medical College (Anhui, China) between December 2011 and June 2015
were randomly enrolled into the present study. All samples were
obtained following provision of written informed consent and used
with approval from the Review Board of The First Affiliated
Hospital of Bengbu Medical College. The age range of diagnosed
patients was between 35 and 77 years, with a median age of 60
years. Tissue samples were classified as tumor or adjacent
carcinomatous tissue. Baseline characteristics of enrolled patients
included age at time of diagnosis, tissue type, histological grade,
distant metastasis and tumor-node-metastasis (TNM) stage (Table II). All patients were clinically
staged according to the criteria of the American Joint Committee on
Cancer staging system (25).
Histological grades of primary tumors were based on the World
Health Organization recommendations (26).
 | Table II.Association of TBRG4 expression and
clinicopathological characteristics. |
Table II.
Association of TBRG4 expression and
clinicopathological characteristics.
|
|
| TBRG4 |
|
|---|
|
|
|
|
|
|---|
| Variable | n | Low | High | P-value |
|---|
| Sex |
|
|
| 0.808 |
|
Male | 39 | 31 | 8 |
|
|
Female | 35 | 27 | 8 |
|
| Age, years |
|
|
| 0.348 |
|
≤60 | 34 | 28 | 6 |
|
|
>60 | 41 | 30 | 11 |
|
| Tumor size, cm |
|
|
| 0.698 |
| ≤4 | 50 | 38 | 12 |
|
|
>4 | 25 | 20 | 5 |
|
| Distant
metastasis |
|
|
| 0.269 |
| M0 | 71 | 54 | 17 |
|
| M1 | 4 | 4 | 0 |
|
| TNM stage |
|
|
| 0.014a |
|
TNM1 | 37 | 24 | 13 |
|
|
TNM2 | 18 | 16 | 2 |
|
|
TNM3 | 16 | 14 | 2 |
|
|
TNM4 | 4 | 4 | 0 |
|
| Tumor grade |
|
|
|
|
|
I/II | 53 | 40 | 13 | 0.553 |
|
III | 22 | 18 | 4 |
|
Immunohistochemistry
Tissues were fixed in 4% neutral-buffered formalin
at room temperature overnight and then embedded in paraffin.
Sections of formalin-fixed paraffin-embedded sections (5 µm thick)
were dehydrated and deparaffinized in xylene 2–3 times at room
temperature and rehydrated with a gradient alcohol series. Antigen
retrieval was performed using 0.1 mol/l citrate buffer (pH 6.0) at
95°C for 30 min. Endogenous peroxidase was quenched for 10 min with
3% (v/v) H2O2 at room temperature. Slides
were then washed three times with PBS and blocked for 30 min with
10% (w/v) normal goat serum (Sangon Biotech Co., Ltd., Shanghai,
China) in 1% (w/v) bovine serum albumin (Sangon Biotech Co., Ltd.)
in PBS. Slides were incubated with a 1:1,000 dilution of anti-TBRG4
monoclonal antibody (cat. no. D154006; Sangon Biotech Co., Ltd.) at
37°C for 30 min. Following incubation for 1 h at room temperature
with horseradish peroxidase-labeled streptavidin secondary antibody
(dilution 1:1,000; cat. no. D111054; Sangon Biotech Co., Ltd.), the
development reaction was detected by exposure to
3,3′-diaminobenzidine (Sangon Biotech Co., Ltd.) for 5 min at room
temperature. Immunostained slides were digitized using the
ScanScope XT (Aperio, Cista, CA, USA) and scored independently by
two pathologists according to the Allred scoring system (27). The scoring was based on staining
intensity: Negative, 0; weak, 1; intermediate, 2; and strong, 3. A
proportionate score was assigned to represent the estimated
percentage of positively stained cells (0, <1%; 1, 1–25%; 2,
26–50%; 3, 51–75%; 4, ≥76%). The multiplication of the two
parameters was performed to obtain a total score ranging from
negative (0–1) to positive (>2).
Statistical analysis
Numerical data were expressed as the mean ± standard
deviation, and analyzed using Student's t-test. All categorical
data were expressed as a frequency. The Mann-Whitney U test was
used to analyze the clinicopathological and TBRG4 gene
expression data. Analysis was performed with SPSS (version 16.0;
SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Lentivirus-mediated TBRG4
knockdown
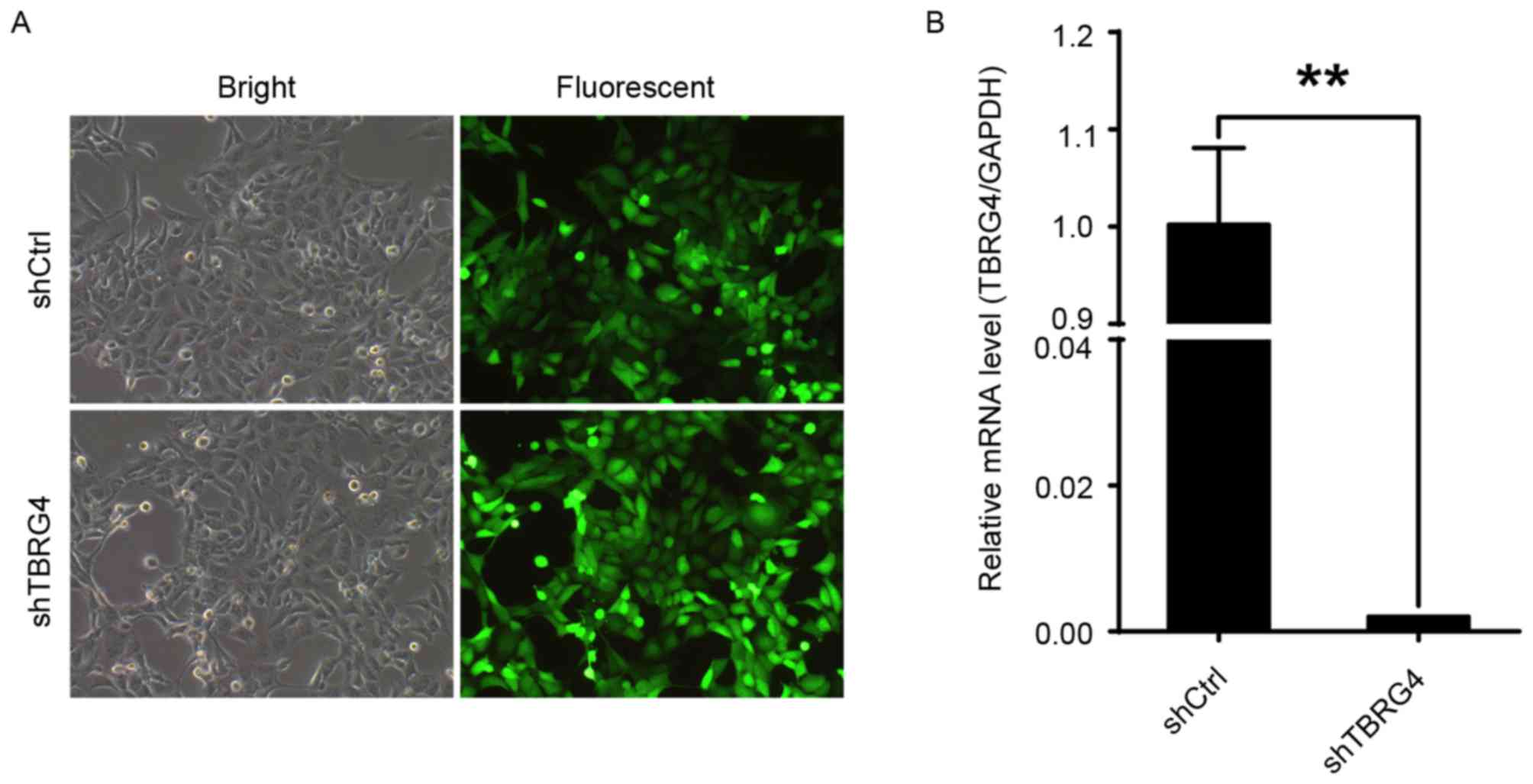
Efficacy of TBRG4-specific siRNA in
downregulating the expression of TBRG4 in H1299 lung cancer
cells was evaluated with RNA expression data generated by qPCR. The
proportions of infected H1299 cells transduced with either shCtrl
or shTBRG4 lentiviruses were >70% at 72 h post-infection
(Fig. 1A). The level of TBRG4
mRNA expression in cells transduced with shTBRG4 was significantly
decreased at 72 h post infection compared with those transduced
with a shCtrl lentivirus (P<0.01; Fig.
1B). Thus, shTBRG4 lentivirus was specific and effective in
knocking down TBRG4.
Genome-wide effects of TBRG4
knockdown
In total, 20,000 genes were microarrayed to
determine the influence that TBRG4 knockdown has on
downstream gene expression. The gene expression profiles of H1299
cells transduced with shTBRG4 or shCtrl-payload lentiviruses were
determined using a GeneChip® PrimeView™ Human Gene
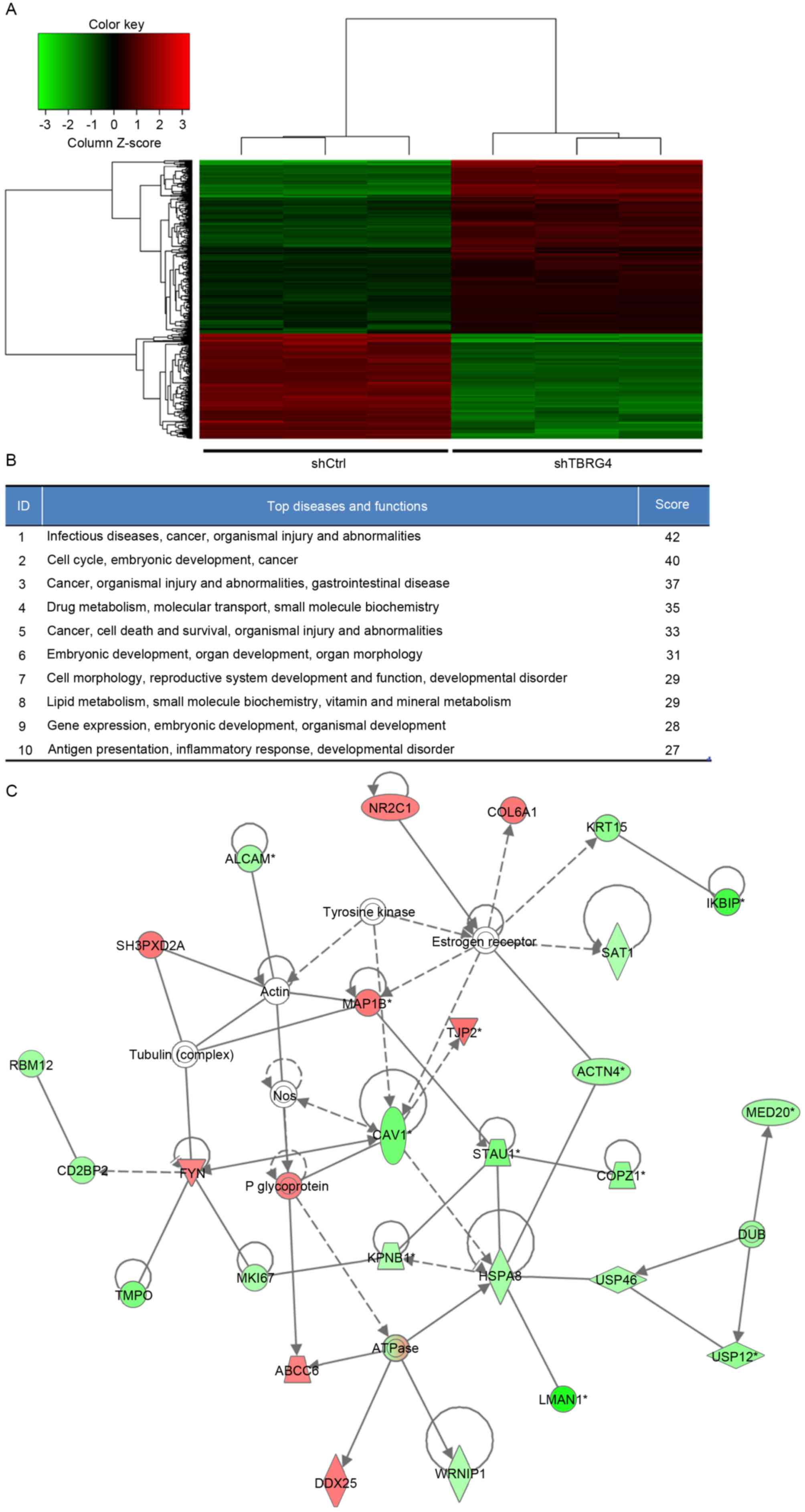
Expression Array using three biological replicates. A total of 586
differentially expressed genes were identified, of which 357 were
downregulated and 229 were upregulated (Fig. 2A).
Elucidation of pathways and
interactions among differentially expressed genes
Biological interactions were identified in the 586
differently regulated genes using the IPA program. This output
highlighted 25 significant networks (data not shown), of which IPA
identified a list of the top 10 networks (Fig. 2B). Of these networks, those associated
with infectious diseases, cancer, organismal injury and
abnormalities were the highest ranked networks, with 27
focus molecules and a significance score of 42 (Fig. 2C). In particular, Fig. 2C presents that TBRG4 is located
upstream of all focus genes.
TBRG4 knockdown markedly upregulates
DDIT3 and downregulates CAV1 and RRM2
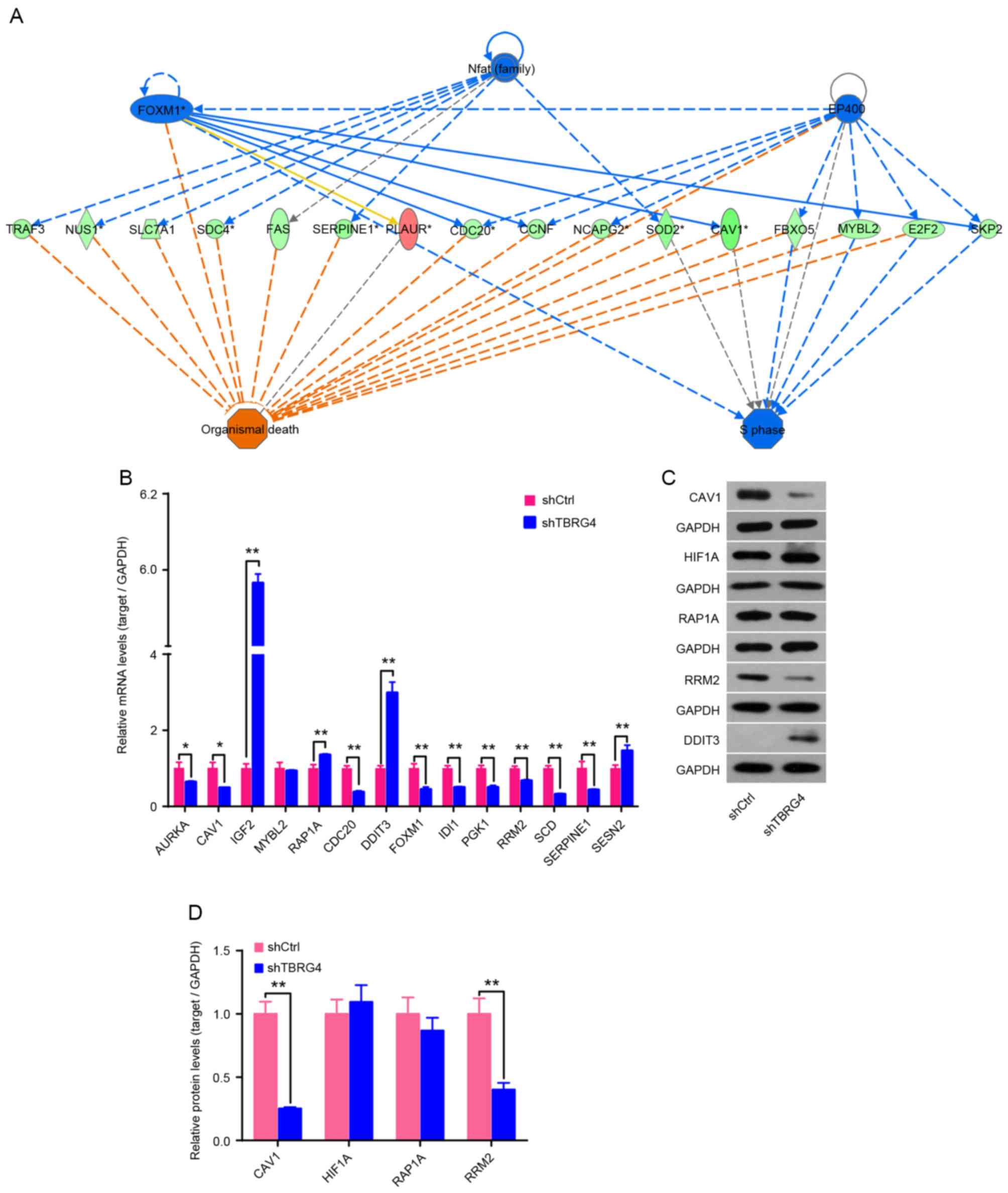
Expression of mRNA for 14 downstream genes of
TBRG4 identified using IPA was analyzed using qPCR,
including AURKA, CAV1, IGF2, MYBL2,
RAP1A, CDC20, DDIT3, FOXM1,
IDI1, PGK1, RRM3, SCD, SERPINE1
and SESN2 (Fig. 3A). Results
demonstrated that IGF2, RAP1A, DDIT3 and
SESN2 were upregulated following TBRG4 knockdown, whereas
the remaining 10 genes were downregulated (Fig. 3B). Western blotting also demonstrated
that knockdown of TBRG4 increased the level of DDIT3
expression and decreased the levels of CAV1 and RRM2 (Fig. 3C and D).
Association of TBRG4 expression in
lung cancer tissues and clinicopathological characteristics
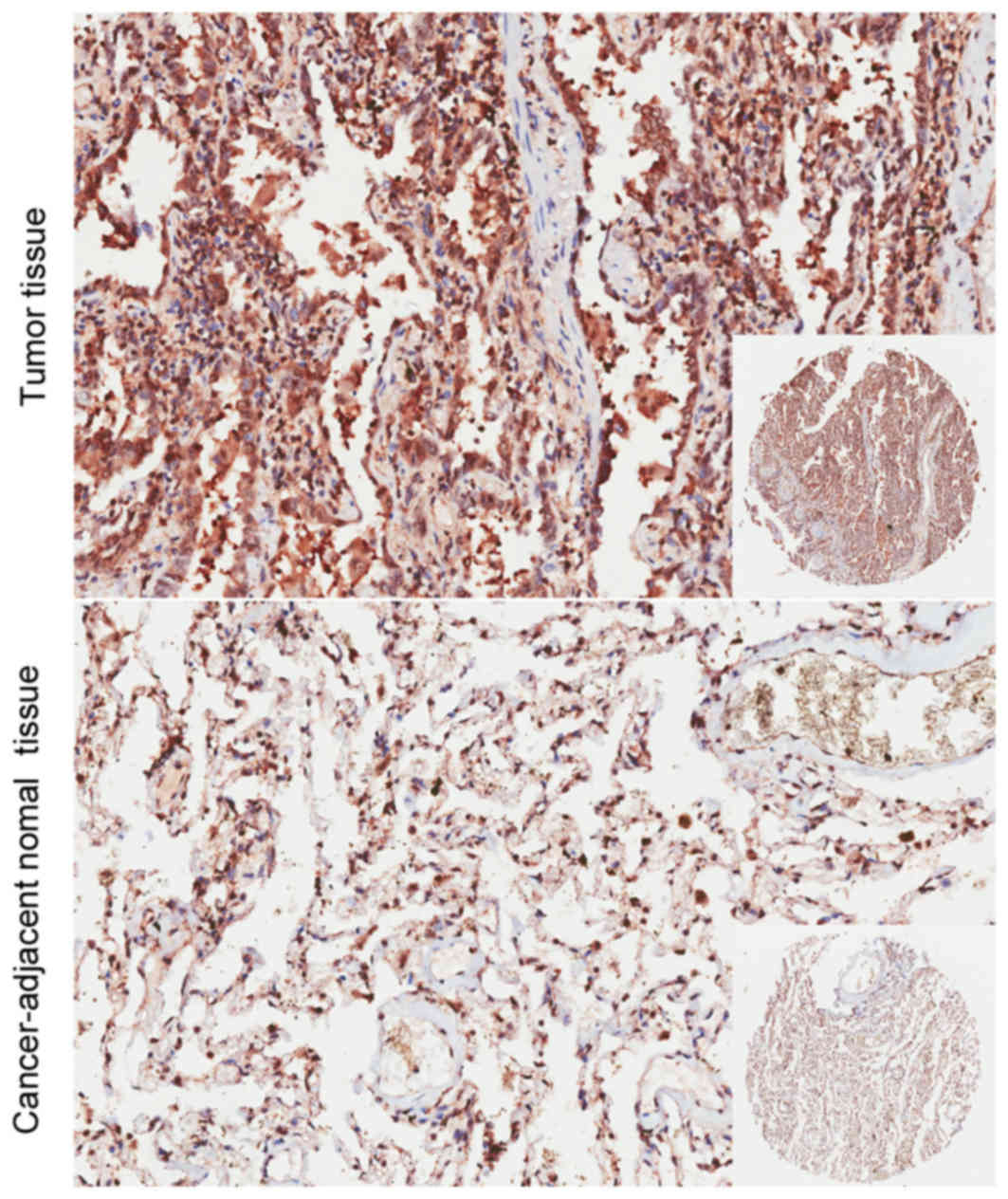
The present study immunostained the TBRG4 protein
expressed within lung cancer and adjacent normal tissues. The
immunohistochemical data revealed that the expression of TBRG4 was
markedly increased within lung cancer tissues compared with normal
tissues retrieved from the adjacent epithelium (Fig. 4). There was no significant difference
identified in TBRG4 expression between the sexes, neither was it
significantly associated with age, tumor size, distant metastasis,
TNM stage or tumor grade (P>0.05; Table II).
Discussion
TBRG4 is implicated in a number of human
malignancies; however, its contribution to lung cancer has not yet
been evaluated. To the best of our knowledge, the present study is
the first to investigate the expression of TBRG4 within lung
carcinomas. The results of the present study demonstrate that there
is a markedly increased expression of TBRG4 in lung cancer
in vitro and ex vivo. As previously identified,
TBRG4 is a regulator of TGFb located on chromosome 7p12.7–13
(15). Chromosomal abnormity,
including breakpoint and duplication at the 7p13 locus, has
previously been reported in a range of hematological malignancies
(28). Previously, it was reported
that this region where TBRG4 is located is disrupted in
patients with Sézary syndrome, a leukemic form of cutaneous T-cell
lymphoma, with chromosomal abnormalities and mutations in certain
genes that are relevant to this disease (14,29). The
region is also amplified in 30% of cell lines derived from patients
with head and neck squamous cell carcinoma (30). Furthermore, recent research has
demonstrated that TBRG4 exhibits significant changes in
extramedullary relapse of multiple myelomas (14). This evidence suggests that
TBRG4 serves an important role in several types of human
cancer.
In order to assess the contribution of TBRG4
to lung cancer, a shTBRG4 lentiviral vector was initially
constructed, which specifically silenced the expression of
TBRG4 in H1299 lung cancer cells. The shTBRG4-infected cells
demonstrated a decrease in expression of TBRG4 compared with
those transduced with a shCtrl control lentivirus. In order to
obtain an understanding of the downstream biological alterations,
the transcriptome of H1299 cells transduced with either shTBRG4 or
shCtrl lentiviruses was investigated with an Affymetrix microarray
of 20,000 genes. Gene expression profiling and network analysis
revealed a set of 586 differentially expressed genes, a number of
which were categorized into infectious diseases, cancer, organismal
injury and abnormalities. A key observation in the present study
was the identification of the set of genes, which were altered
downstream due to the knockdown of TBRG4. Results of the
present study demonstrated a significant increase in the level of
DDIT3, and a decrease in the levels of CAV1 and
RRM2 genes at the transcriptional (qPCR) and translational
(western blotting) levels. DDIT3 is a transcription factor
which is considered as a marker of commitment of endoplasmic
reticulum stress-mediated apoptosis via induction of B-cell
lymphoma 2 downregulation and death receptor 5 activation (31,32).
Caveolin-1 is a multifunctional molecule typically expressed in
membranous structures. The wild-type form acts as a tumor
suppressor protein through contact inhibition of signaling
molecules; however, its loss in cancer cells promotes tumor growth,
motility, vascularization and metastasis, and decreases survival
outcomes (33–36). The ribonucleotide reductase subunit M2
(RRM2) is reported to regulate several oncogenes that control
malignant potential, and therefore may serve a major role in tumor
progression (37,38). RRM2 serves as a prognostic biomarker
for several types of cancer (39–41), and
knockdown is reported to lead to apoptosis in NSCLC (38). Immunohistochemistry results validated
the increased levels of TBRG4 in tumor tissue; therefore,
TBRG4 knockdown complicated the tumorigenesis of H1299 cells
by upregulating DDIT3 and downregulating CAV1 and
RRM2. Further study is currently ongoing in order to
validate the precise role of TBRG4 knockdown.
In conclusion, the results of the present study
highlighted the tumorigenic roles of TBRG4 by silencing its
expression in human H1299 lung cancer cells. This present study has
provided evidence to further the understanding of the precise role
of TBRG4 in the tumorigenesis of human lung cancer. However,
the exact underlying molecular mechanisms by which TBRG4 influences
the biological behaviors of lung cancer cells are yet to be
determined.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reungwetwattana T and Dy GK: Targeted
therapies in development for non-small cell lung cancer. J
Carcinog. 12:222013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumarakulasinghe NB, van Zanwijk N and Soo
RA: Molecular targeted therapy in the treatment of advanced stage
non-small cell lung cancer (NSCLC). Respirology. 20:370–378. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang JW, Wei NC, Su HJ, Huang JL, Chen
TC, Wu YC, Yu CT, Hou MM, Hsieh CH, Hsieh JJ, et al: Comparison of
genomic signatures of non-small cell lung cancer recurrence between
two microarray platforms. Anticancer Res. 32:1259–1265.
2012.PubMed/NCBI
|
|
5
|
Gridelli C and Felip E: Targeted therapies
development in the treatment of advanced nonsmall cell lung cancer.
J Biomed Biotechnol. 2011:4156412011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bergethon K, Shaw AT, Ou SH, Katayama R,
Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang
R, et al: ROS1 rearrangements define a unique molecular class of
lung cancers. J Clin Oncol. 30:863–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt
A, Zhou W, Brace LE, Woods BA, Lin W, Zhang J, et al: Mutations in
the DDR2 kinase gene identify a novel therapeutic target in
squamous cell lung cancer. Cancer Discov. 1:78–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding L, Getz G, Wheeler DA, Mardis ER,
McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan
MB, et al: Somatic mutations affect key pathways in lung
adenocarcinoma. Nature. 455:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peifer M, Fernandez-Cuesta L, Sos ML,
George J, Seidel D, Kasper LH, Plenker D, Leenders F, Sun R, Zander
T, et al: Integrative genome analyses identify key somatic driver
mutations of small-cell lung cancer. Nat Genet. 44:1104–1110. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wangari-Talbot J and Hopper-Borge E: Drug
resistance mechanisms in non-small cell lung carcinoma. J Can Res
Updates. 2:265–282. 2013.PubMed/NCBI
|
|
11
|
Strausberg RL, Feingold EA, Grouse LH,
Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler
GD, Altschul SF, et al: Generation and initial analysis of more
than 15,000 full-length human and mouse cDNA sequences. Proc Natl
Acad Sci USA. 99:pp. 16899–16903. 2002; PubMed/NCBI
|
|
12
|
Edwards MC, Liegeois N, Horecka J, DePinho
RA, Sprague GF Jr, Tyers M and Elledge SJ: Human CPR (cell cycle
progression restoration) genes impart a Far-phenotype on yeast
cells. Genetics. 147:1063–1076. 1997.PubMed/NCBI
|
|
13
|
Simarro M, Gimenez-Cassina A, Kedersha N,
Lazaro JB, Adelmant GO, Marto JA, Rhee K, Tisdale S, Danial N,
Benarafa C, et al: Fast kinase domain-containing protein 3 is a
mitochondrial protein essential for cellular respiration. Biochem
Biophys Res Commun. 401:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sevcikova S, Paszekova H, Besse L,
Sedlarikova L, Kubaczkova V, Almasi M, Pour L and Hajek R:
Extramedullary relapse of multiple myeloma defined as the highest
risk group based on deregulated gene expression data. Biomed Pap
Med Fac Univ Palacky Olomouc Czech Repub. 159:288–293.
2015.PubMed/NCBI
|
|
15
|
Prasad A, Rabionet R, Espinet B, Zapata L,
Puiggros A, Melero C, Puig A, Sarria-Trujillo Y, Ossowski S,
Garcia-Muret MP, et al: Identification of gene mutations and fusion
genes in patients with sezary syndrome. J Invest Dermatol.
136:1490–1499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jager S, Cimermancic P, Gulbahce N,
Johnson JR, McGovern KE, Clarke SC, Shales M, Mercenne G, Pache L,
Li K, et al: Global landscape of HIV-human protein complexes.
Nature. 481:365–370. 2012.
|
|
17
|
Rolland T, Tasan M, Charloteaux B, Pevzner
SJ, Zhong Q, Sahni N, Yi S, Lemmens I, Fontanillo C, Mosca R, et
al: A proteome-scale map of the human interactome network. Cell.
159:1212–1226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolf AR and Mootha VK: Functional genomic
analysis of human mitochondrial RNA processing. Cell Rep.
7:918–931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu H, Liang S, Yang X, Ji Z, Zhao W, Ye X
and Rui J: RNAi-mediated RPL34 knockdown suppresses the growth of
human gastric cancer cells. Oncol Rep. 34:2267–2272. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li LH, He J, Hua D, Guo ZJ and Gao Q:
Lentivirus-mediated inhibition of Med19 suppresses growth of breast
cancer cells in vitro. Cancer Chemother Pharmacol. 68:207–215.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lois C, Hong EJ, Pease S, Brown EJ and
Baltimore D: Germline transmission and tissue-specific expression
of transgenes delivered by lentiviral vectors. Science.
295:868–872. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pfaffl MW, Horgan GW and Dempfle L:
Relative expression software tool (REST) for group-wise comparison
and statistical analysis of relative expression results in
real-time PCR. Nucleic Acids Res. 30:e362002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dubey R, Chhabra R and Saini N: Small
interfering RNA against transcription factor STAT6 leads to
increased cholesterol synthesis in lung cancer cell lines. PLoS
One. 6:e285092011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harvey JM, Clark GM, Osborne CK and Allred
DC: Estrogen receptor status by immunohistochemistry is superior to
the ligand-binding assay for predicting response to adjuvant
endocrine therapy in breast cancer. J Clin Oncol. 17:1474–1481.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dyer MJ, Nacheva E, Fischer P, Heward JM,
Labastide W and Karpas A: A new human T-cell lymphoma cell line
(Karpas 384) of the T-cell receptor gamma/delta lineage with
translocation t(7:14) (p13;q11.2). Leukemia. 7:1047–1053.
1993.PubMed/NCBI
|
|
29
|
Liu H, Krizek J and Bretscher A:
Construction of a GAL1-regulated yeast cDNA expression library and
its application to the identification of genes whose overexpression
causes lethality in yeast. Genetics. 132:665–673. 1992.PubMed/NCBI
|
|
30
|
Carey TE, Van Dyke DL and Worsham MJ:
Nonrandom chromosome aberrations and clonal populations in head and
neck cancer. Anticancer Res. 13:2561–2567. 1993.PubMed/NCBI
|
|
31
|
Han J, Murthy R, Wood B, Song B, Wang S,
Sun B, Malhi H and Kaufman RJ: ER stress signalling through eIF2α
and CHOP, but not IRE1α, attenuates adipogenesis in mice.
Diabetologia. 56:911–924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Flocke LS, Trondl R, Jakupec MA and
Keppler BK: Molecular mode of action of NKP-1339-a clinically
investigated ruthenium-based drug-involves ER- and ROS-related
effects in colon carcinoma cell lines. Invest New Drugs.
34:261–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Engelman JA, Chu C, Lin A, Jo H, Ikezu T,
Okamoto T, Kohtz DS and Lisanti MP: Caveolin-mediated regulation of
signaling along the p42/44 MAP kinase cascade in vivo. A role for
the caveolin-scaffolding domain. FEBS Lett. 428:205–211. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Podar K, Tai YT, Cole CE, Hideshima T,
Sattler M, Hamblin A, Mitsiades N, Schlossman RL, Davies FE, Morgan
GJ, et al: Essential role of caveolae in interleukin-6- and
insulin-like growth factor I-triggered Akt-1-mediated survival of
multiple myeloma cells. J Biol Chem. 278:5794–5801. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Quest AF, Gutierrez-Pajares JL and Torres
VA: Caveolin-1: An ambiguous partner in cell signalling and cancer.
J Cell Mol Med. 12:1130–1150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li M, Chen H, Diao L, Zhang Y, Xia C and
Yang F: Caveolin-1 and VEGF-C promote lymph node metastasis in the
absence of intratumoral lymphangiogenesis in non-small cell lung
cancer. Tumori. 96:734–743. 2010.PubMed/NCBI
|
|
37
|
Fan H, Villegas C and Wright JA:
Ribonucleotide reductase R2 component is a novel malignancy
determinant that cooperates with activated oncogenes to determine
transformation and malignant potential. Proc Natl Acad Sci USA.
93:pp. 14036–14040. 1996; View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rahman MA, Amin AR, Wang D, Koenig L,
Nannapaneni S, Chen Z, Wang Z, Sica G, Deng X, Chen ZG and Shin DM:
RRM2 regulates Bcl-2 in head and neck and lung cancers: A potential
target for cancer therapy. Clin Cancer Res. 19:3416–3428. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mah V, Alavi M, Márquez-Garbán DC, Maresh
EL, Kim SR, Horvath S, Bagryanova L, Huerta-Yepez S, Chia D,
Pietras R and Goodglick L: Ribonucleotide reductase subunit M2
predicts survival in subgroups of patients with non-small cell lung
carcinoma: Effects of gender and smoking status. PLoS One.
10:e01276002015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang H, Liu X, Warden CD, Huang Y, Loera
S, Xue L, Zhang S, Chu P, Zheng S and Yen Y: Prognostic and
therapeutic significance of ribonucleotide reductase small subunit
M2 in estrogen-negative breast cancers. BMC Cancer. 14:6642014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu X, Zhang H, Lai L, Wang X, Loera S,
Xue L, He H, Zhang K, Hu S, Huang Y, et al: Ribonucleotide
reductase small subunit M2 serves as a prognostic biomarker and
predicts poor survival of colorectal cancers. Clin Sci (Lond).
124:567–578. 2013. View Article : Google Scholar : PubMed/NCBI
|