Introduction
Prostate cancer is a common disease in the Western
world, with one in five men suffering from prostate cancer in the
United States (1). Early-stage
prostate cancer is usually treated via radical prostatectomy and
radiation therapy; however, there is no effective therapy once
metastatic prostate cancer is diagnosed (2). The US Food and Drug Administration
suggest that docetaxel should be used to treat advanced prostate
cancer, and under this treatment median survival can be improved by
2–4 months (3,4). However, docetaxel causes numerous side
effects, meaning a novel effective and nontoxic treatment is
required.
Whole-tumor cell vaccines have been extensively
studied in melanoma (5) and lung
cancer (6). Various methods have been
developed for antigen preparation, including the use of
γ-irradiated tumor cells, formalin-fixed cells (7,8),
glutaraldehyde-fixed cells (9) and
frozen/thawed cells (10). As
cellular proteins are better preserved in ethanol-fixed tissue
(8,11), ethanol-fixed RM-1 cells were selected
as a source of tumor antigens for immunotherapy.
The RM-1 prostate cancer cell line is derived from
urogenital sinus cells from tumor protein 53-knockout C57BL/6 mice,
and was transformed with Ras proto-oncogene GTPase (Ras) and MYC
proto-oncogene BHLH transcription factor (Myc) (12). It is aggressive, non-immunogenic and
expresses low levels of major histocompatibility complex I (MHCI)
(12). As RM-1 cells express low
levels of MHCI, it is difficult for T cells to mediate an antitumor
response in male C57BL/6 mice. Griffith et al (13) demonstrated that male mice immunized
with γ-irradiated RM-1 cell vaccines failed to clear viable RM-1
cells (13), likely owing to prostate
antigen tolerance following whole-cell vaccination. If this were
the case, females would be well-protected following vaccination, as
females are intolerant to prostate antigen (14). The difference in the immune response
can presumably be attributed to antigen tolerance based on sex
differences. If the sex difference was demonstrated to mediate
immunity in these mice, they may be used to study the effects of
different immunotherapies, including ethanol-fixed cell vaccines
combined with novel adjuvants or cytokines. It is likely that
protection can be improved in males following vaccination. To
investigate the antitumor response, a novel platform was
established based on the unique property of streptavidin (SA) to
bind rapidly and irreversibly to biotin-linked molecules, and the
ability of biotin to be readily incorporated into the proteins on
the cell surface. This allows for the rapid (<2 h), efficient
and durable display of SA-tagged bioactive cytokines on the surface
of biotinylated tumor cells. A granulocyte-macrophage
colony-stimulating factor (GM-CSF)-surface-modified RM-1 cell
vaccine was generated based on this technology, which has
previously been shown to be effective in inducing antitumor
immunity (8,15). In the present study, C57BL/6 female
and male mice were injected with viable RM-1 cells as a novel tumor
model to study the mechanisms of antitumor immunity. This model
makes it possible to identify an effective and convenient method
for studying immunotherapies for prostate cancer.
Materials and methods
Animals and cells
C57BL/6 mice (n, 60; 30 female and 30 male) were
purchased from the Animal Experiment Center of the Southern Medical
University (Guangzhou, China). The mice were housed under specific
pathogen-free conditions. Cages, bedding, food and water were
autoclaved and changed regularly (food and water was added every
morning and evening). The mice were maintained in a 12:12 h
light:dark cycle. All the mice used for the study were at 6–8 weeks
of age. All animal studies were approved by the Experimental Animal
Ethics Committee of the People's Hospital of Yichun (Yichun, China)
and were performed in accordance with the Regulations for the
Administration of Experimental Animals in China published in
1988.
RM-1 prostate cancer cells were provided by the
Southern Medical University, (Guangdong, China). The cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/ml penicillin, and 100 µg/ml
streptomycin. The cells were maintained at 37°C in a humidified
atmosphere of 5% CO2.
Preparation of the
GM-CSF-surface-modified RM-1 cell vaccine
The GM-CSF-surface-modified RM-1 cell vaccine was
prepared as previously described (8,15).
Briefly, RM-1 cancer cells were harvested using trypsin, washed
twice with sterile PBS, incubated with 30% ethanol (v/v) at room
temperature for 1 h, washed twice with PBS, counted using 0.4%
trypan blue and finally resuspended at 2×107 cells/ml in
PBS. Ethanol-fixed RM-1 cells (2×107 cells/ml) were
incubated with 10 mM-fresh EZ-Link Sulfo-NHS-Biotin (Pierce; Thermo
Fisher Scientific, Inc.) at room temperature for 30 min with 4 µg
of 6xHis-L-SA-GM-CSF fusion protein, which was prepared in our
laboratory (8,15). Subsequent to three washes with PBS,
the biological activity of the modified SA-GM-CSF on the cell
surface was assayed by bone marrow cell proliferation as previously
described (8,15).
Tumor model and immunization
C57BL/6 mice were implanted with viable RM-1
prostate cancer cells. C57BL/6 males were injected intradermally
with 2×106 GM-CSF-modified cells (as the vaccine),
ethanol-fixed cells or PBS in the right thigh at weekly intervals
for three consecutive weeks. At 1 week after the final vaccination,
the mice were challenged with a subcutaneous injection of
1×105 viable RM-1 cells suspended in 100 µl of PBS in
the left flank. Tumor growth was measured 2–3 times/week with a
Vernier caliper. The animals were sacrificed when the tumors either
reached a diameter of 20 mm or exhibited ulceration. Male and
female mice were treated equally. All experiments were repeated
three times using groups of 10 mice.
Cytotoxic activity assay
Splenocytes were isolated from the experimental mice
following the second vaccination and 2 weeks after the last
immunization. Red blood cells were lysed with
ammonium-chloride-potassium (ACK) lysis buffer (0.15 M
NH4CL, 1 mM KHCO3, and 0.1 mM NaETDA, pH
7.2). The splenocytes were resuspended in DMEM containing 10% FBS,
following which recombinant human interleukin-2 (IL-2; R&D
Systems China Co., Ltd., Shanghai, China) was added and RM-1 cells
were subjected to 25 µg/ml mitomycin C (Boster Biological
Technology, Pleasanton, CA, USA) incubation for 5 days at room
temperature. Re-stimulated effector T cells were collected using a
discontinuous Ficoll-Hypaque gradient by two centrifugations, each
for 10 min at 289 × g at room temperature, and the concentration
was adjusted to 1×106 cells/ml in DMEM. Target RM-1
cells were seeded at 1×104 cells in 100 µl of medium per
well in 96-well plates. The target RM-1 cells were subsequently
added to effector T cells at various effector-to-target ratios and
cultured for 4 h at 37°C. The supernatant was collected to measure
lactate dehydrogenase activity using the CytoTox 96®
Non-Radioactive Cytotoxicity assay (Promega Corporation, Madison,
WI, USA). The percentage of cytotoxicity was calculated as follows:
100× (experimental-effector spontaneous-target spontaneous)/(target
maximum-target spontaneous).
Purifying cluster of differentiation 8
(CD8)+ T cells
CD8a+ T cells were isolated from murine
splenocytes using the CD8a+ T cell Isolation kit
(Miltenyi Biotec GmbH, Bergisch Gladback, Germany). Splenocytes
were resuspended at 1×107 cells/40 µl of buffer [PBS
containing 0.05% bovine serum albumin (BSA); Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany and 2 mM EDTA, pH 7.2]. A biotin-antibody
cocktail (Miltenyi Biotec GmbH) was added at 10 µl/1×107
total cells, followed by mixing and incubation for 10 min at 4°C.
Next, the cells were cultured with 30 µl of buffer and 20 µl of
anti-biotin microbeads (Miltenyi Biotec GmbH) added to
1×107 cells at 4°C for 15 min, washed once with buffer,
centrifuged at 300 × g for 10 min, and resuspended at
1×108 cells/500 µl of buffer. Finally, the cell
suspension was pipetted onto an MS column (Miltenyi Biotec GmbH),
and CD8a+ T cells were passed through the column.
Interferon-γ (IFN-γ) and IL-4
ELISAs
Splenocytes were isolated from mice prior to
vaccination and 7 days after the last vaccination. The splenocytes
were purified as aforementioned using a CD8a+ T cell
isolation kit and subsequently co-cultured with 20 U/ml recombinant
human IL-2 for 48 h. The culture supernatants were collected, and
the levels of IFN-γ and interleukin IL-4 were measured via ELISA
(R&D Systems China Co., Ltd.).
Splenocyte analysis
Splenocytes were isolated from each experimental
group on day 21 after tumor injection, then added to ACK lysis
buffer to lyse red blood cells, washed twice with PBS with 1% BSA
and incubated with fluorescein isothiocyanate (FITC)-labeled
anti-mCD4, anti-mCD8 and anti-mCD161 antibodies (R&D Systems
China Co., Ltd.) for 1 h at room temperature. CD4+ T
cells, CD8+ T cells, and NK cells were then analyzed via
flow cytometry [BD Biosciences, Franklin Lakes, NJ, USA; FACS
Vantage product with the Cell Quest software system (BD Cell
Quest™ Pro version 6.0; BD Biosciences) was used for
analysis].
Immunohistochemistry
Tumor samples from mice were snap-frozen in liquid
nitrogen in Tissue Tec OCT compound (Boster Biological Technology).
Frozen sections (5–8 µm) were fixed in cold acetone at 4°C for 15
min and then washed with PBS and stained with anti-mCD4 (cat no.
553647; BD Pharmingen; BD Biosciences), anti-mCD8 (cat no. 553027;
BD Pharmingen; BD Biosciences) and anti-mCD161 (cat. no. 566306; BD
Pharmingen; BD Biosciences) overnight at 4°C, according to the
manufacturer's protocol for the HRP detection IHC kit (BD
Biosciences). Dilution of the antibodies was 1:100 for anti-mCD4
and anti-mCD8 and 1:200 for anti-mCD161. The secondary antibody
used was anti-rat IgG SABC kit (cat. no. BA1005; Boster Biological
Technology, Pleasanton, CA, USA). After culture for 30 min at 37°C,
immunoreactivity products were visualized with a chromogenic agent
3,3′-diaminobenzidene, color development was performed for <10
min until the desired color intensity was achieved at room
temperature. Counterstaining was performed with hematoxylin for 1–2
min at room temperature. Positive staining cells were counted in a
blind manner using an inverted microscope (magnification, ×200;
Leica Microsystems GmbH, Wetzlar, Germany). The results were
recorded as the number of immunopositive cells per square
millimeter.
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). All descriptive
statistical data were presented as mean ± standard deviation. The
survival of mice was analyzed using Kaplan-Meier survival analysis
and the log-rank test. For in vitro experiments, significant
differences were determined using the Student's t-test and one-way
analysis of variance, followed by Dunnett's post hoc test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
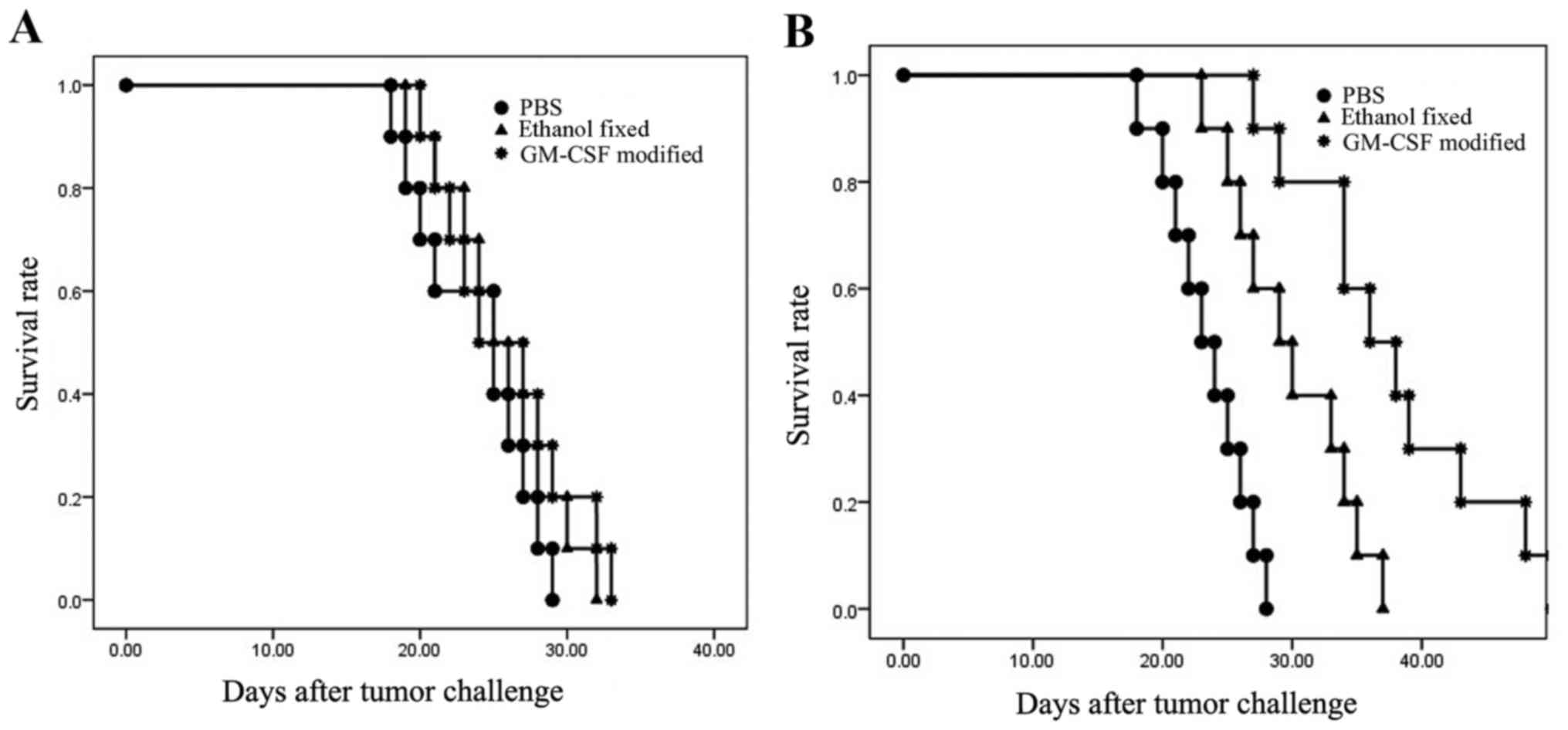
Survival of males and females
Male C57BL/6 mice were administered the
GM-CSF-modified, ethanol-fixed RM-1 cell vaccine or the PBS control
three times at weekly intervals. At 7 days after the final
vaccination, the mice were challenged with 1×105 viable
RM-1 cells. In males, all mice developed tumors within 12 days and
were sacrificed within 35 days. There were no significant
differences between the GM-CSF-modified, ethanol-fixed and PBS
(control) groups (P=0.543; Fig. 1).
However, mice vaccinated with the GM-CSF-modified cells survived
slightly longer compared with the mice from the other groups. In
the females, two out of ten mice that were vaccinated with
GM-CSF-modified cells exhibited tumor-free survival up to 45 days.
All of the control mice formed tumors within 15 days. The survival
curve was significantly different between females that were
administered GM-CSF-modified cells, ethanol-fixed cells and PBS
(P<0.001). Taken together, these results demonstrated that
C57BL/6 males were not protected following vaccination. By
contrast, 20% of females were protected (P<0.05; Fig. 1).
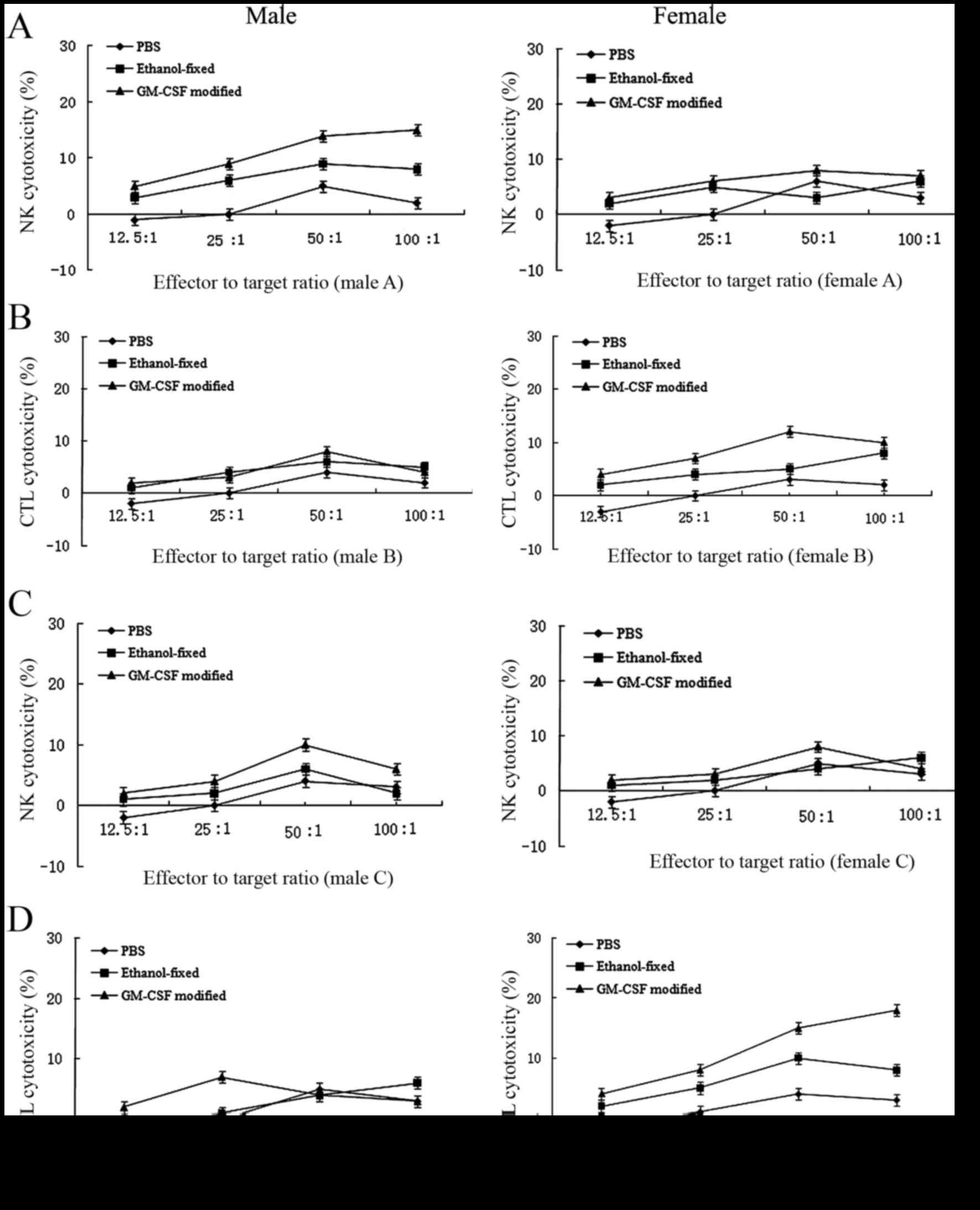
In vitro evaluation of the immune
response
To analyze the antitumor immune response, the
cytotoxicity of splenocytes against RM-1 cells from the
experimental animals was examined using a nonradioactive
cytotoxicity assay (Fig. 2). The
cytotoxic response was collectively caused by NK cell and cytotoxic
T lymphocyte (CTL) lysis. In the early stage (16), cytotoxicity was predominantly due to
NK cell lysis. However, in the late stage, cytotoxicity was
primarily mediated by CTL lysis. In the PBS group, CTL or NK cell
activities were undetectable. In females, the antitumor response
induced by the GM-CSF-modified cell vaccine was primarily mediated
by CTL lysis and, to a lesser extent, by NK cell lysis. By
contrast, males receiving the GM-CSF-modified RM-1 cell vaccine
exhibited minor NK activity during the early stage following
immunization. Additionally, CTL lysis was undetectable. The number
of NK cells was reduced in the late stage, and these cells
exhibited difficulty in mediating an antitumor response, which may
explain the lack of protection afforded by the vaccine observed in
males (Fig. 2).
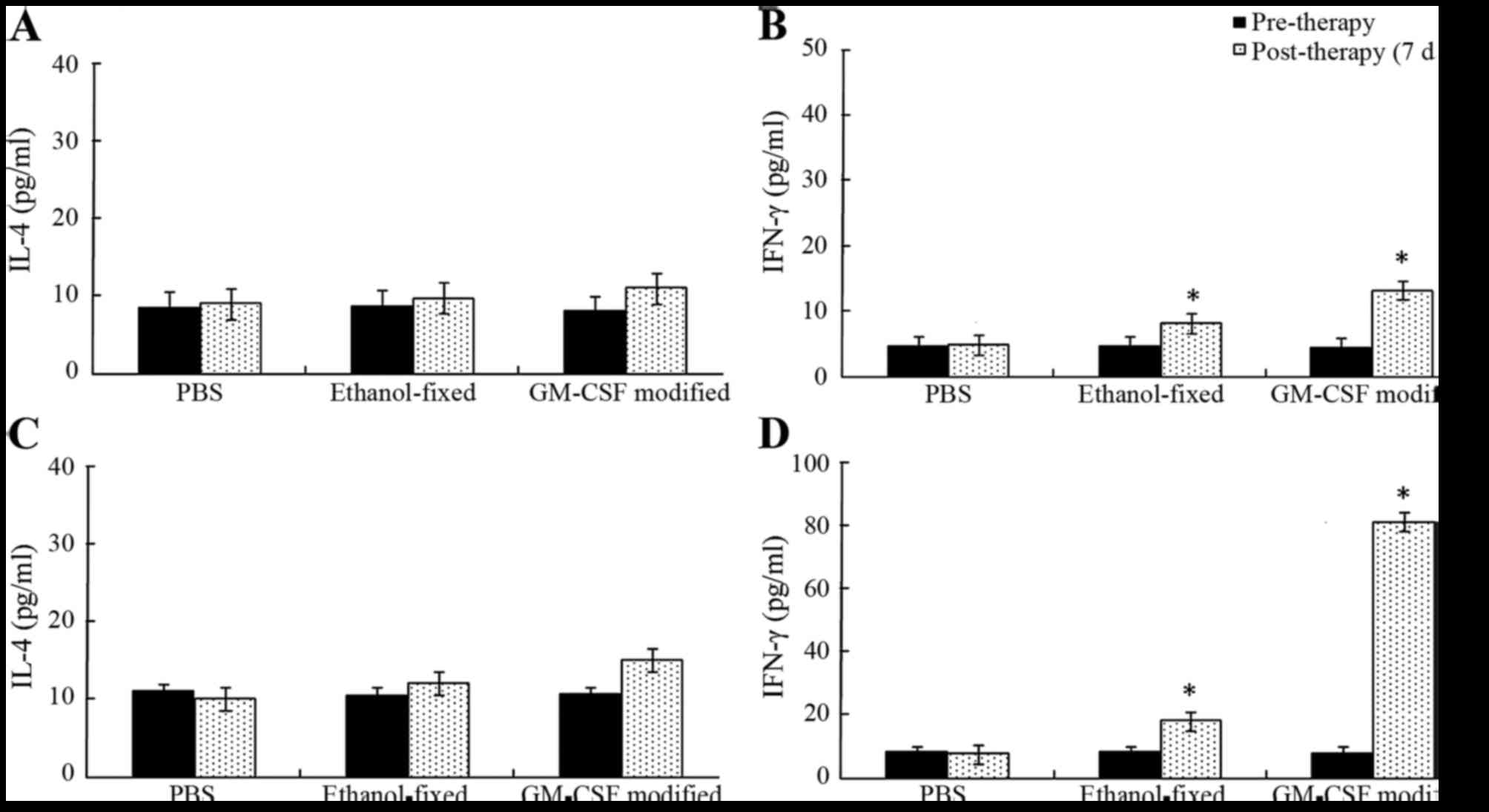
IL-4 and IFN-γ
Splenocytes isolated with the CD8a+ T
cell isolation kit were cultured with recombinant human IL-2 for 48
h. The supernatants were harvested and examined via ELISA (Fig. 3). As expected, the levels of cytokines
IL-4 and IFN-γ in the supernatant were low to undetectable in the
PBS group. The secretion of IFN-γ was significantly increased
following three cycles of immunization in the GM-CSF-modified cell
vaccine group compared with the other groups (P<0.05). However,
the secretion of IL-4 exhibited no significant differences during
the course of vaccine therapy (P>0.05). In males, the levels of
IFN-γ secretion observed in mice receiving GM-CSF-modified cell
vaccine, ethanol-fixed cells and PBS were 12.85±1.01, 8.06±0.49,
and 4.76±0.23 pg/ml, respectively. The supernatant levels of IFN-γ
in the vaccine-treated group were significantly higher compared
with the ethanol-fixed or PBS groups (P<0.05). In females, the
supernatant levels of IFN-γ in mice vaccinated with the
GM-CSF-modified cell vaccine, ethanol-fixed cells and PBS was
80.34±3.01, 17.47±1.51, and 7.47±1.12 pg/ml, respectively. The
secretion of IFN-γ was reduced following vaccination in males
compared with females. These results indicated that the females
presented a Th1 cytokine profile (Fig.
3).
Assessing CD4 T cells, CD8 T cells,
and NK cells following vaccination
Splenocytes were isolated from the experimental mice
on day 21 following the final vaccination and incubated with
FITC-labeled anti-mCD4, anti-mCD8 and anti-mCD161 antibodies for 1
h. The proportions of CD4+ T cells, CD8+ T
cells and NK cells in the spleen were assessed via flow cytometry
(Fig. 4A). The proportions of
CD4+ and CD8+ T cells in the GM-CSF
membrane-modified cell vaccine group were significantly higher
compared with the other groups, with the proportions of
CD4+ T cells and CD8+ T cells being higher in
female spleens compared with in male spleens. However, the number
of NK cells in females was lower compared with that in male mice,
with NK cells not detectable in tumor tissue (Fig. 4A). The infiltration of CD4+
T and CD8+ T lymphocytes in the tumor tissue was
examined via immunohistochemistry. Large numbers of CD4+
T cells and CD8+ T cells were identified in females
administered with the GM-CSF membrane-modified cell vaccine
(Fig. 4B). The data indicated that
the GM-CSF membrane-modified cell vaccine may enhance antitumor
immunity by increasing the numbers of T lymphocytes and NK cells,
and that the observed difference in immunity was largely due to sex
differences.
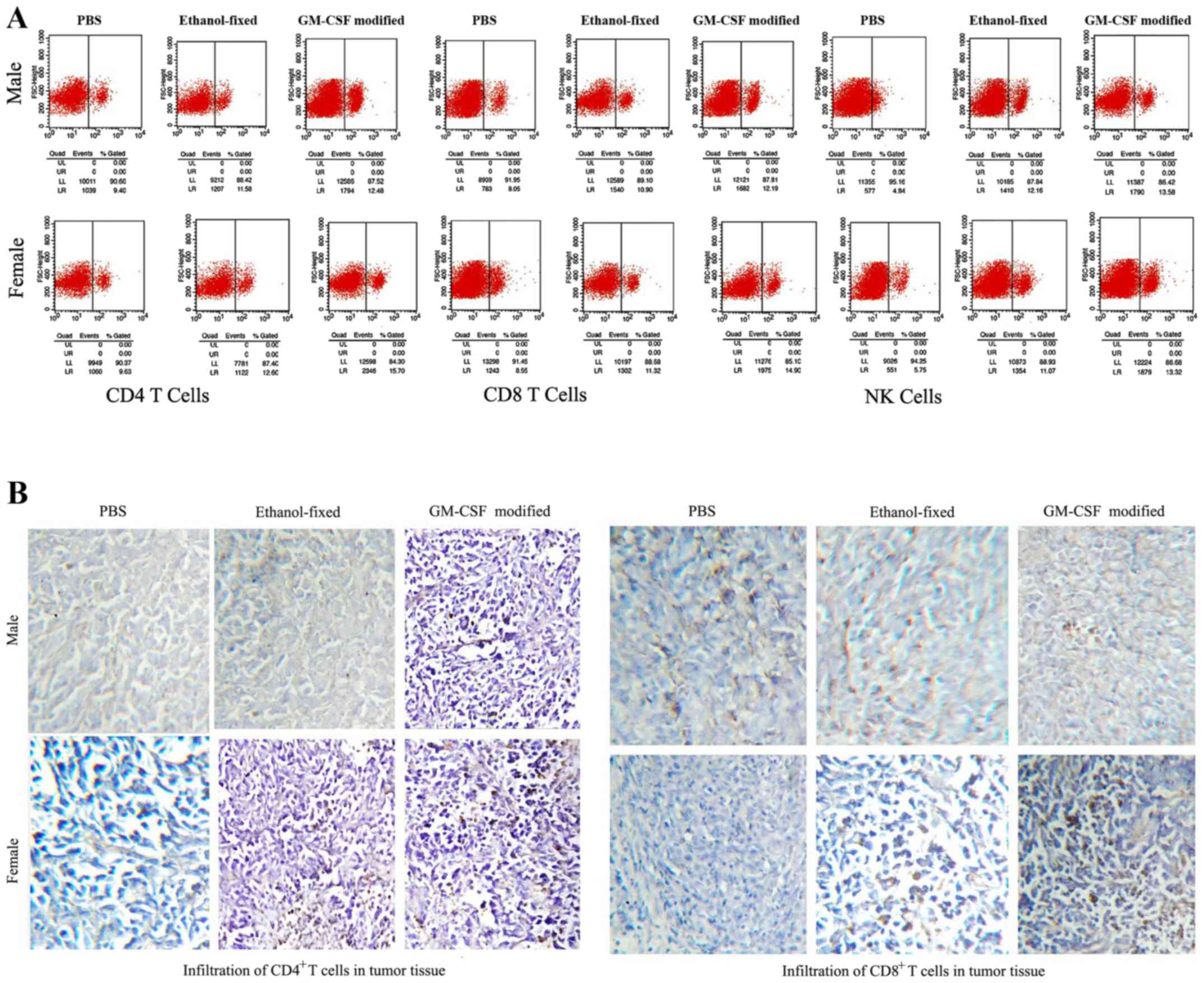 | Figure 4.Analysis of lymphocytes in the spleens
and tumors. (A) Splenoctytes were isolated 7 days after the final
vaccination. Red blood cells were lysed with
ammonia-chloride-potassium lysis buffer. Splenocytes were stained
with FITC-labeled anti-mCD4, anti-mCD8 and anti-mCD161 antibodies
for 1 h. CD4+ T cells, CD8+ T cells, and NK
cells were analyzed using flow cytometry. The number of
CD4+ T cells and CD8+ T cells was highest in
the spleens of female mice administered the GM-CSF-modified cell
vaccine. By contrast, the male GM-CSF-modified cell vaccine groups
exhibited the highest proportion of NK cells in the spleen. (B)
Immunohistochemical investigation of how lymphocytes mediate the
immune response. NK cells were undetectable in the tumor tissue
from all groups. Infiltrating CD4+ T cells and
CD8+ T cells were evaluated based on tumor histology, as
described in the Materials and methods. The proportion of
CD4+ T cells and CD8+ T cells within the
tumors was highest in the female GM-CSF-modified cell vaccine
groups. Magnification, ×200. FITC, fluorescein isothiocyanate; CD4,
cluster of differentiation 4; NK, natural killer; GM-CSF,
granulocyte-macrophage colony-stimulating factor. |
Discussion
Current methods of treatment for advanced prostate
cancer have only limited success, at least in part due to an
incomplete understanding of the immunobiology of human prostate
cancer (17). A model that accurately
mimics the human situation would therefore be useful for
understanding antitumor immune responses in humans, and may offer a
predictive model for therapeutic efficacy. For the past 10 years,
the Transgenic Adenocarcinoma of the Mouse Prostate model has been
used; however, this model is not appropriate to study how
immunogenic viral oncogenes induce tumorigenicity (18–20). There
is currently no effective model for advanced prostate cancer that
can be used to fully explain antitumor immunobiology in humans.
Thus, a novel model of prostate cancer that may be used to
investigate how the tolerance of tumors can be disrupted to
generate a sustained and potent immune response is required. Mice
injected with viable RM-1 cells have been used as an advanced
prostate cancer model as RM-1 cells are aggressive and
non-immunogenic, expressing very low levels of MHCI (12,20). In
the present study, male and female mice injected with viable RM-1
cells were used as a novel animal model to assess immune responses
to vaccination. The results indicated that the tested vaccine
induced a stronger antitumor immune response in females than in
males, and this result may be associated with immune tolerance.
Only males have prostates, and prostate antigens are therefore
recognized as ‘self’ antigens by the male immune system. In fact,
RM-1 cells express the Myc and Ras oncogenes (12), which do not induce tolerance in males.
Tolerance to an antigen is determined by the antigen's expression
level (21). If the level of an
antigen expressed by tumor cells is too low or too high, the immune
system will become tolerant to the tumor cells. RM-1 prostate
cancer cells express extremely low levels of MHCI and exhibit low
immunogenicity, which makes it difficult for antigen-presenting
cells to present tumor antigens to CD8+ T cells via the
MHC I pathway, preventing T cells from inducing an immune response.
In the female mouse model, such tumor tolerance does not exist, and
the mice therefore generate a sustained and potent immune response
against the cancer. This model may aid the acceleration of vaccine
development for the clinic. The results of the present study
revealed that in males, low numbers of NK cells mediated the
antitumor immune response in the absence of CTLs. In vitro
experiments using splenocytes from mice that received the
GM-CSF-modified cell vaccine against RM-1 cells revealed that the
secretion of IFN-γ was lower in males compared with in females.
Additionally, the proportion of CD4+ T cells and
CD8+ T cells within the spleen and tumor tissue was
lower in the male group that received the GM-CSF-modified cell
vaccine group than in the equivalent female group. However, NK
cells were undetectable in the tumor tissue from all experimental
groups. All males were sacrificed within 35 days, and the survival
curves did not differ significantly between each male treatment
group. By contrast, in females, the data indicated that there was a
20% tumor-free survival rate in the group that received the
GM-CSF-modified RM-1 cell vaccine. The difference in the immune
response was largely associated with sex differences. The prostate
antigen was recognized as ‘self’ by the male immune system but as
‘foreign’ by the female immune system. The GM-CSF-modified RM-1
cell vaccine was seen as ‘non-self’ and was therefore recognized by
the female immune system, which induced a strong response to clear
the tumor. This model may be used to evaluate the effects of
cell-based vaccines in females. In this model, female and male mice
injected with viable RM-1 cells were used to identify differences
in the immune response. An advantage of this model is that it may
be used to study sex differences in the immune response, to
identify approaches for improving protection from disease in
males.
Owing to the lack of an effective model, it remains
unclear how a whole-cell vaccine may induce an immune response
against viable RM-1 cells. Several groups have reported that the
antitumor immune response is mediated by NK cells, CD8+
T cells and CD4+ T cells (22,23).
Previous studies have reported that NK cells directly mediate the
immune response, as CD8+ T cells are inhibited by
CD4+ CD25-regulatory T cells (13,19). Data
from the present study revealed that in females, the antitumor
immune response induced by the GM-CSF-modified cell vaccine was
predominantly mediated by CD8+ T cells and, to a lesser
extent, by NK cells. In males, a small number of NK cells were
involved in the cytotoxic immune response in the early stage, while
CTL lysis was undetectable. Additionally, in the two sexes, the
supernatant from in vitro CD8+ T cell cultures
exhibited RM-1-specific IFN-γ production, but little IL-4
production. In short, clearance of the tumors required
CD8+ T cells and NK cells, although CD8+ T
cells predominantly mediated the antitumor response.
The present study demonstrated that between the
sexes, there was a large difference in the immune response, as
female mice were intolerant to prostate antigens. Thus, the model
used in the present study is clinically relevant and may aid
acceleration of the development of whole-cell vaccines. In
addition, the model represents progress in the study of clinical
immunotherapies for prostate cancer.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crawford ED, Blumenstein BA, Goodman PJ,
Davis MA, Eisenberger MA, McLeod DG, Spaulding JT, Benson R and
Dorr FA: Leuprolide with and without flutamide in advanced prostate
cancer. Cancer. 66(5 Suppl): S1039–S1044. 1990. View Article : Google Scholar
|
|
3
|
Quinn DI, Tangen CM, Hussain M, Lara PN
Jr, Goldkorn A, Moinpour CM, Garzotto MG, Mack PC, Carducci MA,
Monk JP, et al: Docetaxel and atrasentan versus docetaxel and
placebo for men with advanced castration-resistant prostate cancer
(SWOG S0421): A randomised phase 3 trial. Lancet Oncol. 14:893–900.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Armstrong AJ and Carducci MA: Chemotherapy
for advanced prostate cancer: Results of new clinical trials and
future studies. Curr Oncol Rep. 7:220–227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haigh PI, Difronzo LA, Gammon G and Morton
DL: Vaccine therapy for patients with melanoma. Oncology (Williston
Park). 13:1561–1574. 1999.PubMed/NCBI
|
|
6
|
Li H, Jiang HJ, Ma MQ, Wei F, An XM and
Ren XB: Vaccination with allogeneic GM-CSF gene-modified lung
cancer cells: Antitumor activity comparing with that induced by
autologous vaccine. Cancer Biother Radiopharm. 22:790–798. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Obata C, Zhang M, Moroi Y, Hisaeda H,
Tanaka K, Murata S, Furue M and Himeno K: Formalin-fixed tumor
cells effectively induce antitumor immunity both in prophylactic
and therapeutic conditions. J Dermatol Sci. 34:209–219. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin W, He Q, Hu Z, Chen Z, Qifeng M,
Zhichun S, Zhihui Q, Xiaoxia N, Li J and Gao J: A novel therapeutic
vaccine of GM-CSF/TNFalpha surface-modified RM-1 cells against the
orthotopic prostatic cancer. Vaccine. 28:4937–4944. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suckow MA, Wolter WR and Pollard M:
Prevention of de novo prostate cancer by immunization with
tumor-derived vaccines. Cancer Immunol Immunother. 54:571–576.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kotera Y, Shimizu K and Mulé JJ:
Comparative analysis of necrotic and apoptotic tumor cells as a
source of antigen(s) in dendritic cell-based immunization. Cancer
Res. 61:8105–8109. 2001.PubMed/NCBI
|
|
11
|
Ahram M, Flaig MJ, Gillespie JW, Duray PH,
Linehan WM, Ornstein DK, Niu S, Zhao Y, Petricoin EF III and
Emmert-Buck MR: Evaluation of ethanol-fixed, paraffin-embedded
tissues for proteomic applications. Proteomics. 3:413–421. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baley PA, Yoshida K, Qian W, Sehgal I and
Thompson TC: Progression to androgen insensitivity in a novel in
vitro mouse model for prostate cancer. J Steroid Biochem Mol Biol.
52:403–413. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Griffith TS, Kawakita M, Tian J, Ritchey
J, Tartaglia J, Sehgal I, Thompson TC, Zhao W and Ratliff TL:
Inhibition of murine prostate tumor growth and activation of
immunoregulatory cells with recombinant canarypox viruses. J Natl
Cancer Inst. 93:998–1007. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Labarthe MC, Theocharous P, Russell N,
Todryk S, Bangma C, Thraves P, Dalgleish AG and Whelan MA: A novel
murine model of allogeneic vaccination against prostate cancer.
Cancer Immunol Immunother. 57:453–465. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He Q, Li J, Yin W, Song Z, Zhang Z, Yi T,
Tang J, Wu D, Lu Y, Wang Z, et al: Low-dose paclitaxel enhances the
anti-tumor efficacy of GM-CSF surface-modified whole-tumor-cell
vaccine in mouse model of prostate cancer. Cancer Immunol
Immunother. 60:715–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cooper MD and Alder MN: The evolution of
adaptive immune systems. Cell. 124:815–822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li M, Davey GM, Sutherland RM, Kurts C,
Lew AM, Hirst C, Carbone FR and Heath WR: Cell-associated ovalbumin
is cross-presented much more efficiently than soluble ovalbumin in
vivo. J Immunol. 166:6099–6103. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gallucci S, Lolkema M and Matzinger P:
Natural adjuvants: Endogenous activators of dendritic cells. Nat
Med. 5:1249–1255. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sauter B, Albert ML, Francisco L, Larsson
M, Somersan S and Bhardwaj N: Consequences of cell death: Exposure
to necrotic tumor cells, but not primary tissue cells or apoptotic
cells, induces the maturation of immunostimulatory dendritic cells.
J Exp Med. 191:423–434. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grant JF, Iwasawa T, Sinn HW, Siemens DR,
Griffith TS, Takacs EB and Ratliff TL: Induction of protective
immunity to RM-1 prostate cancer cells with
ALVAC-IL-2/IL-12/TNF-alpha combination therapy. Int J Cancer.
119:2632–2641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carbone FR, Kurts C, Bennett SR, Miller JF
and Heath WR: Cross-presentation: A general mechanism for CTL
immunity and tolerance. Immunol Today. 19:368–373. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chhikara M, Huang H, Vlachaki MT, Zhu X,
Teh B, Chiu KJ, Woo S, Berner B, Smith EO, Oberg KC, et al:
Enhanced therapeutic effect of HSV-tk+GCV gene therapy and ionizing
radiation for prostate cancer. Mol Ther. 3:536–542. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schroten-Loef C, de Ridder CM, Reneman S,
Crezee M, Dalgleish A, Todryk SM, Bangma CH and Kraaij R: A
prostate cancer vaccine comprising whole cells secreting IL-7,
effective against subcutaneous challenge, requires local GM-CSF for
intra-prostatic efficacy. Cancer Immunol Immunother. 58:373–381.
2009. View Article : Google Scholar : PubMed/NCBI
|