Introduction
Cholangiocarcinoma (CCA) is a malignant tumor
originating from biliary epithelial cells. In total, >50% of
CCAs are referred to as perihilar CCAs (pCCAs) or Klatskin tumors.
pCCAs occur at the junction between the cystic duct and
common/second degree bile ducts (1).
Tumor invasion and lymph node metastasis are the primary factors,
which need to be considered prior to pCCA treatment as this may
affect overall prognosis and treatment options (2). Surgical resection is considered the
preferred option for pCCA treatment; however, it is difficult to
perform and often ineffective, which may explain the increasing
mortality rates observed worldwide (3). Therefore, it is important to research
and identify more effective molecular targets for pCCA therapy.
pCCA is highly desmoplastic in nature, and is
surrounded by a large amount of stroma containing several activated
myofibroblasts termed cancer-associated fibroblasts (CAFs)
(4). Vimentin, fibroblast-specific
protein 1 (FSP1) and α-smooth muscle actin (α-SMA) are collectively
considered as specific biomarkers for CAFs (5). CAFs affect the biological behavior (s)
of tumors, including tumor proliferation, invasion and metastasis.
During cancer progression, invasive cancer cells are able to pass
through the basement membrane into the vascular or lymphatic system
(6). Lymphatic vessel density (LVD),
which is defined by the number of lymphatic vessels in a given
area, may contribute to lymph node metastasis and increase the
possibility of invasion (7). In the
present study, clinical data revealed that the expression of
podoplanin in CAFs was associated with lymph node metastasis.
Lymphangiogenesis serves a crucial role in tumor progression as it
may promote metastasis (8). Several
previous studies also reported the association between LVD and
lymph node metastasis, and the associated unfavorable overall
prognosis (9,10). Therefore, the expression of podoplanin
in CAFs, and its significance in lymphangiogenesis requires further
investigation.
Podoplanin, a 38 kDa type I transmembrane
glycoprotein, is expressed in several types of malignant tumor
cells, including epithelial cells and CAFs (11–15). Cell
migration and invasion are initiated by the protrusion of the cell
membrane, which is physically mediated by the actin cytoskeleton
(16). A previous study demonstrated
that podoplanin is present in extracellular and intracellular
regions (17). Furthermore, its
intracellular binding with ezrin, radixin and moesin (ERM) proteins
is considered to lead to morphological changes and cytoskeletal
reorganization in cells (18).
Cofilin-1 is an important actin-binding protein, and is able to
modulate the cytoskeleton that affects actin polymerization,
generation of protrusions and the direction of cell migration
(19). Maintenance and functional
activity of CAFs is dependent on a high level of actomyosin
contractility. Actomyosin contractility, which may lead to matrix
remodeling, is generated by phosphorylation of myosin light-chain 2
(MLC-2) (20). It is hypothesized
that podoplanin expression in CAFs may influence actomyosin
contractility and modulation of the cytoskeleton in order to
enhance the migration ability of CAFs.
In the present study, CAF podoplanin expression
levels in 42 patients with pCCA were analyzed, and the effect of
podoplanin-positive CAFs in pCCA progression is discussed.
Materials and methods
Patients and tissue samples
Paired paraffin-embedded tumor and para-tumor
tissues were obtained from the Department of Pathology of The
Affiliated Drum Tower Hospital of Nanjing University Medical School
(Nanjing, China) with the approval of the Ethics Committee of the
Nanjing Drum Tower Hospital (Nanjing, China). A total of 42 samples
from patients with pCCA who also underwent surgical resection
between September 2000 and December 2012 were analyzed. Para-tumor
tissue was confined to tissue that was ~2 cm from the tumor margin.
Clinicopathological details of patients and tumors were retrieved
from medical records and are presented in Table I.
 | Table I.Associations between podoplanin
expression in cancer-associated fibroblasts and clinicopathological
characteristics of perihilar cholangiocarcinoma. |
Table I.
Associations between podoplanin
expression in cancer-associated fibroblasts and clinicopathological
characteristics of perihilar cholangiocarcinoma.
|
|
| Tumor tissue |
|---|
|
|
|
|
|---|
| Parameter | n | Positive | Negative | P-value |
|---|
| Sex |
|
|
| 0.051 |
|
Male | 25 | 15 | 10 |
|
|
Female | 17 | 5 | 12 |
|
| Age, years |
|
|
| 0.067 |
|
≤55 | 19 | 12 | 7 |
|
|
>55 | 23 | 8 | 15 |
|
| Tumor size, cm |
|
|
| 0.536 |
|
<3 | 21 | 11 | 10 |
|
| ≥3 | 21 | 9 | 12 |
|
|
Differentiation |
|
|
| 0.871 |
| Well
(G1)-moderate (G2) | 33 | 16 | 17 |
|
| Poor
(G3) | 9 | 4 | 5 |
|
| TNM stage |
|
|
| 0.002a |
|
I+II | 21 | 5 | 16 |
|
|
III+IV | 21 | 15 | 6 |
|
| Chronic
cholangitis |
|
|
| 0.406 |
|
Yes | 30 | 16 | 14 |
|
| No | 12 | 4 | 8 |
|
| Neural
invasion |
|
|
| 0.489 |
|
Positive | 35 | 18 | 17 |
|
|
Negative | 7 | 2 | 5 |
|
| Vascular
invasion |
|
|
| 0.569 |
|
Positive | 17 | 9 | 8 |
|
|
Negative | 25 | 11 | 14 |
|
| Cancer embolus |
|
|
| 0.126 |
|
Positive | 14 | 9 | 5 |
|
|
Negative | 28 | 11 | 17 |
|
| Lymph node
metastasis |
|
|
| 0.014a |
|
Present | 19 | 13 | 6 |
|
|
Absent | 23 | 7 | 16 |
|
Hematoxylin and eosin (H&E)
staining, and immunohistochemical (IHC) evaluation
Tissues were fixed in 10% buffered formalin for 8 h
at 4°C and embedded in paraffin at room temperature. Sections were
cut (5 µm thick) for H&E staining and IHC evaluation. All
sections were deparaffinized and rehydrated with serial dilutions
of ethanol. Tissue sections were immunostained with primary
antibodies against α-SMA (mouse anti-human monoclonal antibody;
1:500; cat. no. ab119952; Abcam, Cambridge, MA, USA), vimentin
(rabbit monoclonal antibody; 1:900; cat. no. ab92547; Abcam) and
podoplanin (rabbit polyclonal anti-human; 1:500; cat. no.
sc-134482; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
overnight at 4°C. The following day, sections were incubated with
goat anti-rabbit immunoglobulin G (IgG) secondary antibody (cat.
no. TA130015; 0.5 µg/ml; OriGene Technologies, Inc., Beijing,
China) or goat anti-mouse IgG secondary antibody (cat. no.
TA130070; 0.5 µg/ml; OriGene Technologies, Inc., Beijing, China) at
37°C for 30 min. Sections were then stained with
3,3′-diaminobenzidine and counterstained with hematoxylin. A
distribution score that reflected the distribution of positive
signals among stromal cells was determined as 0 (0%), 1 (1–50%) or
2 (5–100%). Scores reflected the percentage of positive staining of
stromal cells within the same tissue section. The signal intensity
score was evaluated as 0 (no signal or weak), 1 (moderate) or 2
(strong) in accordance with the method of Fukuoka et al
(21). The sum of the distribution
and intensity scores (range, 0–4) was used as a total score (TS): 0
(sum, 0), 1 (sum, 1), 2 (sum, 2) and 3 (sum, 3 or 4). A TS of 0 and
1 was considered negative, whereas a TS of 2 and 3 was considered
positive. In the situation where there was a discrepancy in scores
between duplicated cores from the same patient, the higher score
was assigned as the final score. Quantitative analysis of lymphatic
vessels was also performed, with podoplanin-labeled lymphatic
endothelial cells with brownish yellow staining considered a
positive standard. Three optical fields with the most vascularized
areas were selected at low magnification (×40) for each sample
using a light microscope (Olympus Corporation, Tokyo, Japan).
Lymphatic vessels were counted at high magnification (×200). LVD
was analyzed according to a protocol described in a previous study
(22).
Cell culture
CCA cell line QBC939 was purchased from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China). Human
dermal lymphatic endothelial cells (HDLECs) were purchased from
Scien Cell Research Laboratories (Carlsbad, CA, USA). QBC939 cells
were cultured in RPMI-1640 medium with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin/streptomycin. HDLECs were cultured in endothelial cell
medium with 1% endothelial cell growth supplement, 5% FBS and 1%
penicillin/streptomycin. HDLECs which were passaged between 2 and 7
times were used for later experiments. Cells were grown at 37°C in
a humidified incubator with 5% CO2.
Isolation of CAFs from CCA tumor
xenograft
Pathogen-free BALB/C nude mice (n=5) aged 4–5 weeks
(weight, 20 g; male) were obtained from the Animal Center of
Nanjing Drum Tower Hospital (Nanjing, China). The National Research
Council Guide for the Care and Use of Laboratory Animals (23) was followed (12-h light/12-h dark
cycle; temperature, 24°C; humidity, 65%) and ethical approval was
obtained from the Ethics Committee of The Nanjing Drum Tower
Hospital. The mice were allowed free access to food and water.
QBC939 CCA cells were injected subcutaneously into the right flanks
of the mice (106 cells/mouse). After 4 weeks, all mice
were sacrificed and the xenograft tumors were harvested. Tumor
tissues were cut into small fragments, placed in digestion solution
of 0.1% type IV collagenase (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and
incubated at 37°C in a humidified 5% CO2 incubator for 6
h. Cells were separated from the digested tissue and filtered
through a 70 µm cell strainer. Following centrifugation (at 700 × g
for 5 min at 20°C), adherent cells were collected and CAFs were
purified by repeated brief exposure (within 3 min) to 0.25%
trypsin-EDTA (Gibco; Thermo Fisher Scientific, Inc.), also termed
differential trypsinization. The medium was changed after 30 min
(differential adhesion) (24). CAFs
were grown at 37°C in a humidified 5% CO2 forced-air
incubator.
Adenovirus transfection
CAFs were seeded at 5×104 cells/well into
24-well plates for 24 h, and then transfected with adenovirus
containing either the podoplanin gene (Ad-podoplanin) or no
podoplanin gene (Ad-vector) at a multiplicity of infection of 50.
The culture medium was replaced with fresh medium 8 h later, and
cells were cultured overnight. The expression levels of podoplanin
protein were determined using western blotting.
Dual immunofluorescence staining
Frozen tissue sections were fixed in acetone for 20
min at 4°C and blocked with 5% bovine serum albumin/PBS for 1 h and
incubated with anti-α-SMA antibody (mouse anti-human monoclonal;
1:500; cat. no. ab119952; Abcam) and anti-podoplanin antibody
(rabbit anti-human; polyclonal 1:200; cat. no. sc-134482; Santa
Cruz Biotechnology, Inc.) overnight at 4°C. Secondary antibodies
including Alexa Fluor 488-conjugated goat anti-mouse IgG (1:500;
cat. no. ab150113; Abcam) and cyanine (Cy) 3-conjugated goat
anti-rabbit IgG (1:2,000; cat. ab970075; Abcam) were applied for 1
h, and counterstained with DAPI (Merck KGaA), and washed three
times with PBS. The same protocol was used for dual
immunofluorescence staining of cultured CAFs grown in chamber
slides. Slides were reacted with anti-α-SMA monoclonal antibody
(mouse anti-human; monoclonal; 1:500; cat. no. ab119952; Abcam),
rabbit anti-FSP1 polyclonal antibody (1:100; cat. no. ab27957;
Abcam) and rabbit anti-vimentin monoclonal antibody (1:1,000; cat.
no. ab92547; Abcam) overnight at 4°C. This was followed by
incubation with Alexa Fluor 488-conjugated goat anti-mouse IgG
(1:500; cat. no. ab150113; Abcam) and Cy3-conjugated goat
anti-rabbit IgG (1:1,000; cat. no. ab97075; Abcam) for 1 h, and
counterstained with DAPI (Merck KGaA). Once slides were mounted
with mounting medium (cat. no. ab128982; Abcam), slides were
examined using laser-scanning confocal microscopy.
Flow cytometry
Isolated CAFs were trypsinized and centrifuged at
200 × g for 5 min, fixed with 80% methanol for 5 min and
permeabilized with 0.1% PBS/Triton X-100 for 15 min. Following
washing twice with PBS, cells were incubated with antibodies
against vimentin (rabbit monoclonal anti-human; 1:50; cat. no.
ab92547; Abcam), α-SMA (mouse monoclonal anti-human; 1:200; cat.
no. ab119952; Abcam) and anti-FSP1 (rabbit polyclonal anti-human;
1:100; cat. no. ab27957; Abcam) for 1 h at room temperature.
Following incubation, Alexa Fluor 488-conjugated goat anti-mouse
IgG (1:250; cat. no. ab150113; Abcam) and Cy3-conjugated goat
anti-rabbit IgG (1:500; cat. no. ab97075; Abcam) were applied for
30 min at room temperature. Anti-mouse podoplanin (1:100; cat. no.
12-5381; BD Biosciences, San Jose, CA, USA) was used for the
detection of podoplanin-positive cells. Flow cytometry data were
analyzed using FlowJo software (version 7.6; FlowJo LLC, Ashland,
OR, USA).
Western blotting
The tissue was homogenized in
radioimmunoprecipitation assay lysis buffer (cat. no. P0013B;
Beyotime Biotechnology, Shanghai, China) and incubated on ice for
30 min. The supernatant following centrifugation (at 15,000 × g for
15 min at 4°C) was used for western blotting. Western blotting was
performed as described previously (25). Blots were incubated overnight with the
following primary antibodies: Anti-podoplanin (rabbit polyclonal
antibody; 1:300; cat. no. 251419; Abbiotec, San Diego, CA, USA),
anti-β-tubulin (mouse monoclonal antibody; 1:5,000; cat. no.
AT0003; CMCTAG, Inc., Milwaukee, WI, USA) and anti-cofilin (rabbit
monoclonal antibody; 1:1,000; cat. no. 5175, Cell Signaling
Technology, Danvers, MA, USA), anti-phospho-cofilin (rabbit
monoclonal antibody; 1:1,000; cat. no. 3313; Cell Signaling
Technology), anti-ERM (rabbit monoclonal antibody; 1:1,000; cat.
no. 3142; Cell Signaling Technology), anti-phospho-ERM (rabbit
monoclonal antibody; 1:1,000; cat. no. 3726; Cell Signaling
Technology), anti-MLC-2 (rabbit monoclonal antibody; 1:1,000; cat.
no. 8505; Cell Signaling Technology) and anti-phospho-MLC-2
(1:1,000; cat. no. 3671; Cell Signaling Technology). Western blots
were visualized using an electrophoretic gel imaging system
(Shanghai, China).
Migration assay
Cell migration was determined using a modified
two-chamber migration assay (Corning Incorporated, Corning, NY,
USA) with a pore size of 8 µm. Cells (Ad-podoplanin CAFs and
Ad-vector CAFs) were seeded in 1% FBS/Dulbecco's modified Eagle's
medium (DMEM) in the upper chamber at a concentration of
2×105 cells/ml, and the lower chamber was filled with
10% FBS/DMEM. After 24 h of incubation at 37°C, cells within the
upper chamber were removed with a cotton swab. Cells which had
migrated across the membrane were fixed in 4% paraformaldehyde at
37°C for 30 min, stained with crystal violet (0.5% in 20% methanol)
at room temperature for 30 min. Stained cells were counted in five
randomly selected fields using a light microscope (magnification,
×100; Olympus Corporation).
HDLEC tube-formation assay
Matrigel matrix (BD Biosciences) was added to
96-well plates (50 µl to each chamber) and allowed to polymerize
for 30 min at 37°C. HDLECs were diluted with the supernatant of
Ad-podoplanin CAFs and Ad-vector CAFs at 5×104 cells/ml.
For the vector group, 10% FBS medium was used. Tube formation was
observed after 6 h.
Statistical analysis
Results are presented as the mean ± standard
deviation. Comparisons between two groups were made using Student's
t-test. A χ2 test was used to analyze associations
between immunohistochemistry staining of podoplanin in CAFs, and
clinicopathological characteristics. Survival curves were
constructed using the Kaplan-Meier estimator curves method and
compared using a log-rank test. All statistical analyses were
performed using SPSS (version 22; IBM Corp., Armonk, NY, USA)
software and GraphPad Prism (version 5; GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Observation of CAFs in the pCCA tumor
microenvironment
Immunofluorescence staining of patient samples
demonstrated numerous spindle-shaped stromal cells stained with
α-SMA, which were recognized as CAFs (Fig. 1A). A portion of the CCA epithelium was
also positive for α-SMA. Dual immunolabeling indicated that certain
CAFs in the tumor microenvironment were podoplanin-negative,
whereas others were podoplanin-positive (Fig. 1B). In addition, certain epithelial
cells were positive for podoplanin. Therefore, podoplanin
expression in the CAFs may serve a role in pCCA development.
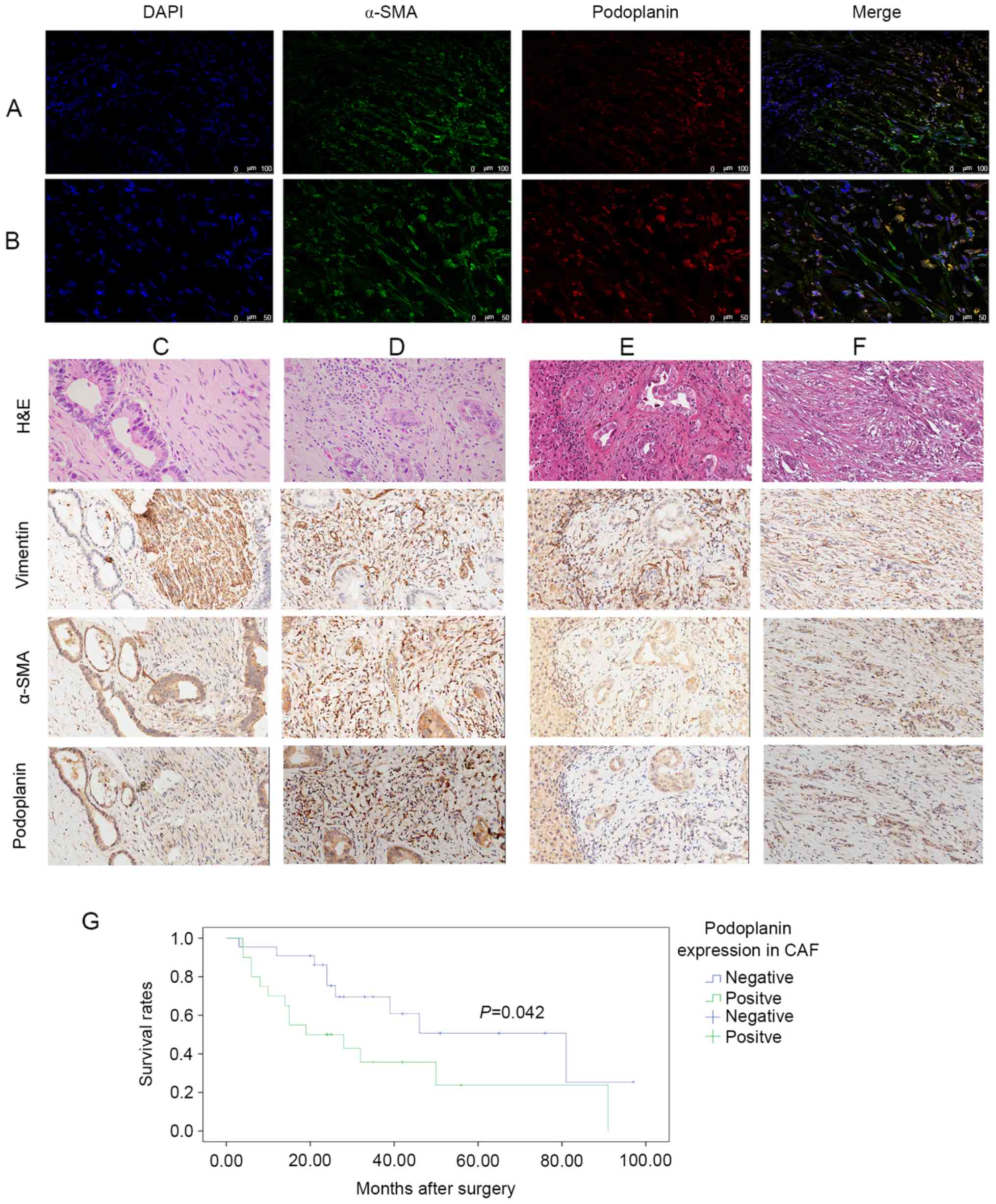 | Figure 1.Podoplanin expression levels in CAFs.
Multicolor images of immunofluorescence labeling for α-SMA
(fluorescein isothiocyanate; FITC) and podoplanin (Cy3). Nuclear
staining with DAPI demonstrated that podoplanin-negative and
podoplanin-positive CAFs are present in pCCA. Original
magnification (A) ×200, (B) ×400. H&E and IHC staining were
performed on pCCA and para-tumor sections. Four sections were
stained with H&E, and α-SMA, vimentin and podoplanin were
immunolabeled in each group. H&E, vimentin and α-SMA were used
for CAF recognition. Magnification ×200. Representative (C)
podoplanin-negative and (D) podoplanin-positive CAFs in tumor
tissue. Representative (E) podoplanin-negative and (F)
podoplanin-positive CAFs in para-tumor tissue. (G) Kaplan-Meier
estimator curves demonstrating overall survival for 42 patients
with pCCA; a significant difference in cumulative overall survival
was observed between patients who were positive or negative for
podoplanin expression in CAFs. (P=0.042, log-rank test). CAF,
cancer-associated fibroblast; Cy, cyanine; pCCA, perihilar
cholangiocarcinoma; H&E, hematoxylin and eosin; α-SMA, α-smooth
muscle actin. |
Association between podoplanin
expression in CAFs and pathological parameters
Podoplanin expression levels in pCCA tumor tissue
was observed in lymphatic vessel endothelium, tumor epithelium and
tumor stroma. CAFs were the primary component of the tumor
microenvironment, and stromal podoplanin was expressed primarily
within these cells. CAFs were identified as large spindle-shaped
cells using H&E and IHC staining. Stroma were identified
through H&E staining and by cell shape, and IHC staining for
α-SMA and vimentin was used to confirm the presence of CAFs.
Expression levels of podoplanin in pCCA tumor (Fig. 1C and D) and para-tumor (Fig. 1E and F) tissues were investigated. The
proportion of podoplanin-positive CAFs in the para-tumor tissue was
decreased and the cells exhibited less marked staining compared
with in the tumor tissue. The ratio of podoplanin-positive CAFs to
podoplanin-negative CAFs in the para-tumor tissue was 8:42, and all
podoplanin-positive CAFs were stained weakly to moderately. In
comparison, the ratio of podoplanin-positive CAFs in tumor tissue
was 20:42, and stained strongly. In addition, podoplanin expression
levels in CAFs significantly differed between paired tumor and
para-tumor tissues (P<0.05).
The clinicopathological characteristics of 42
patients with pCCA and their association with podoplanin expression
levels in CAFs are summarized in Table
I. Out of the 42 specimens, 20 had podoplanin-positive CAFs in
the tumor tissue. Compared with the podoplanin-negative CAF group,
the podoplanin-positive CAF group was significantly associated with
lymph node metastasis and tumor-node-metastasis (TNM) staging.
Survival analysis indicated that the median survival time was 31.59
months, and the 1-, 3- and 5-year survival rates were 90, 66 and
53% in the podoplanin-negative CAF groups, and 57, 37 and 26% in
the podoplanin-positive CAF groups. The overall survival rate in
the podoplanin-negative CAF group were significantly increased
compared with the podoplanin-positive CAF group (P=0.042, log-rank
test, Fig. 1G). This indicated that
podoplanin-positive CAFs in the tumor microenvironment may lead to
more frequent lymph node metastases and promote tumor
progression.
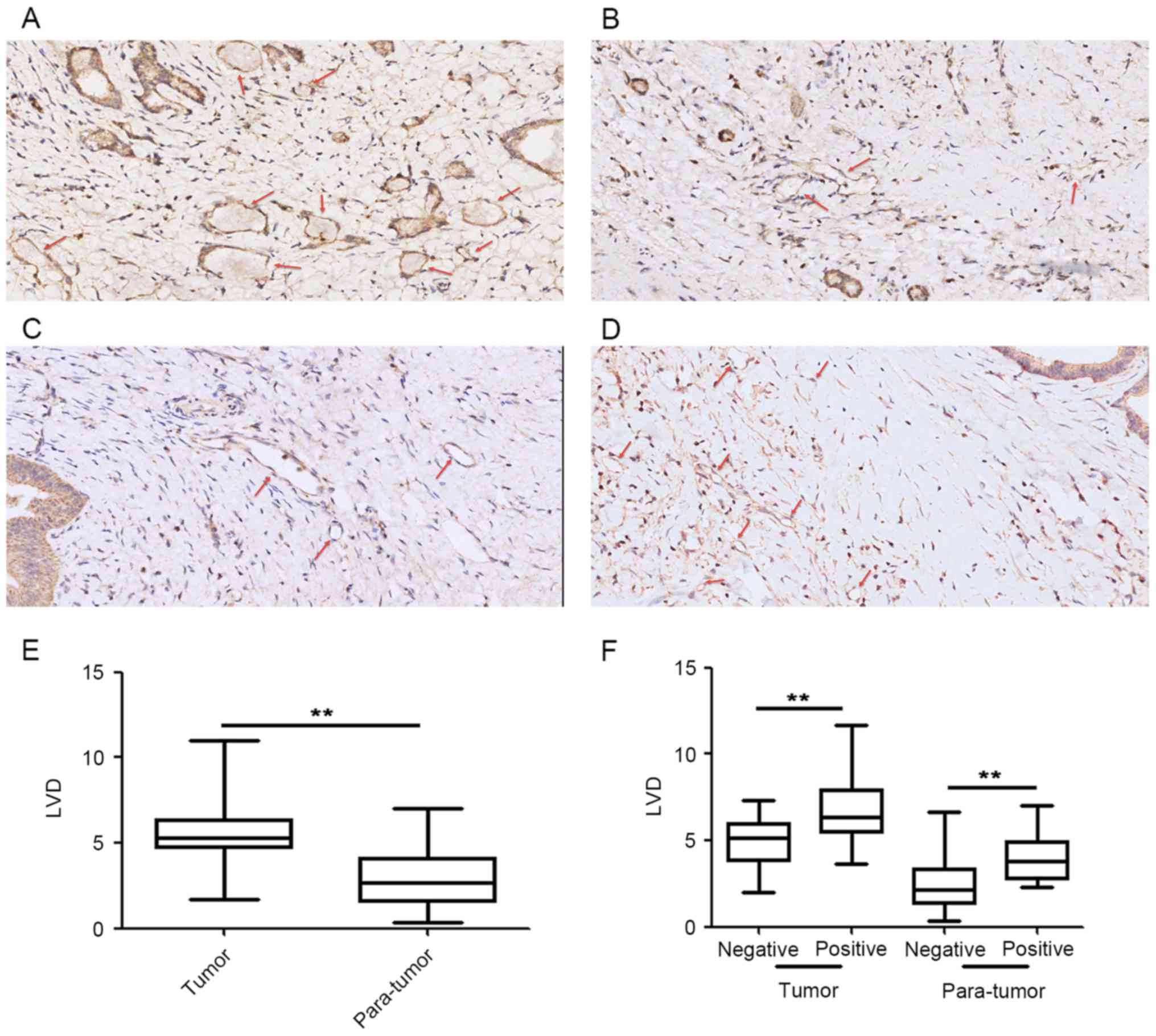
Association between lymphatic vessel
density and podoplanin expression levels in CAFs
A total of 42 paired tumor and para-tumor samples
were used to count lymphatic vessels (Fig. 2), which were visualized using IHC
staining (Fig. 2A and B). Results
demonstrated an increase in the number of lymphatic vessels in the
tumor tissue compared with the para-tumor tissue (Fig. 2E). Tumor tissue from patients with
podoplanin-positive CAFs (Fig. 2C)
exhibited increased LVD compared with patients with
podoplanin-negative CAFs (Fig. 2D).
There were significant differences between LVD and podoplanin
expression levels in CAFs of tumor and para-tumor tissue (Fig. 2F).
Podoplanin overexpression in CAFs
enhances migration ability and does not affect tube formation
In podoplanin-positive CAFs, there was a significant
association between TNM stage and lymph node metastasis.
Quantification of the association between LVD in the tumor and
para-tumor tissue, the presence of podoplanin-positive CAFs and the
occurrence of lymph node metastasis indicated that podoplanin may
serve an important role in tumorigenesis and metastasis. To test
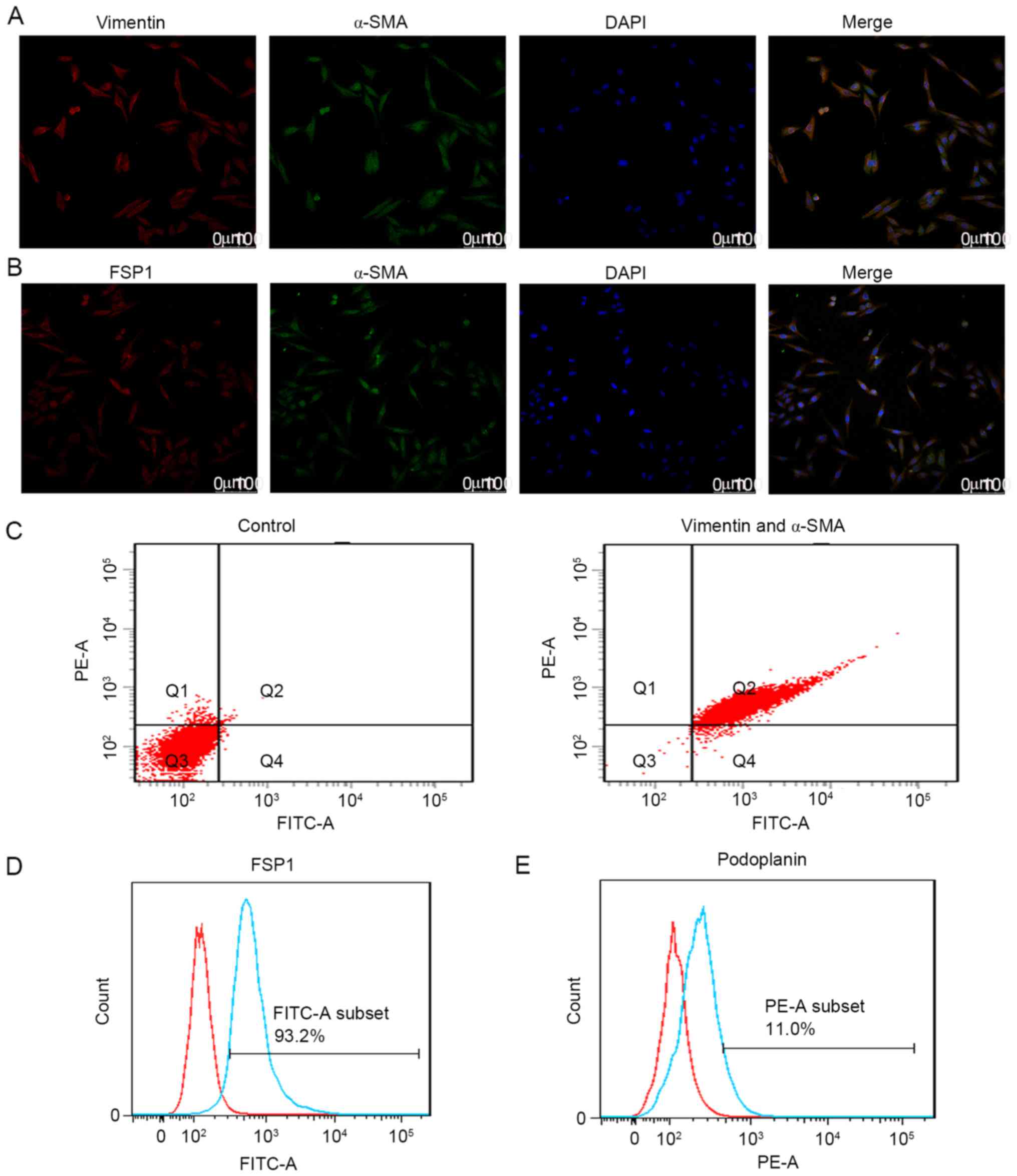
this hypothesis, CAFs were isolated from pCCA tumor xenografts in
the present study. Characterization of cells using dual
immunofluorescence staining (Fig. 3A and
B) with antibodies against α-SMA, vimentin and FSP1 were
performed. Analysis of the proportion of CAFs using double-staining
flow cytometry was also performed. Results demonstrated that 97.6%
of purified CAFs were positive for α-SMA and vimentin (Fig. 3C), and 93.2% of these CAFs were
positive for FSP1 (Fig. 3D). In
addition, flow cytometry results demonstrated that 11% of these
CAFs were positive for podoplanin (Fig.
3E). To understand the function of podoplanin in the CAFs, CAFs
were transfected with Ad-podoplanin adenovirus and examined using
western blotting (Fig. 4A).
Determination of whether podoplanin overexpression affected the
migration ability of CAFs was performed (Fig. 4B), and the number of Ad-podoplanin
CAFs which passed through the membrane into the lower chamber was
significantly increased compared with the Ad-vector CAFs
(P<0.01). Additionally, ERM, MLC and cofilin were phosphorylated
in Ad-podoplanin CAFs (Fig. 4A). The
results indicate that podoplanin has an important function in CAF
migration.
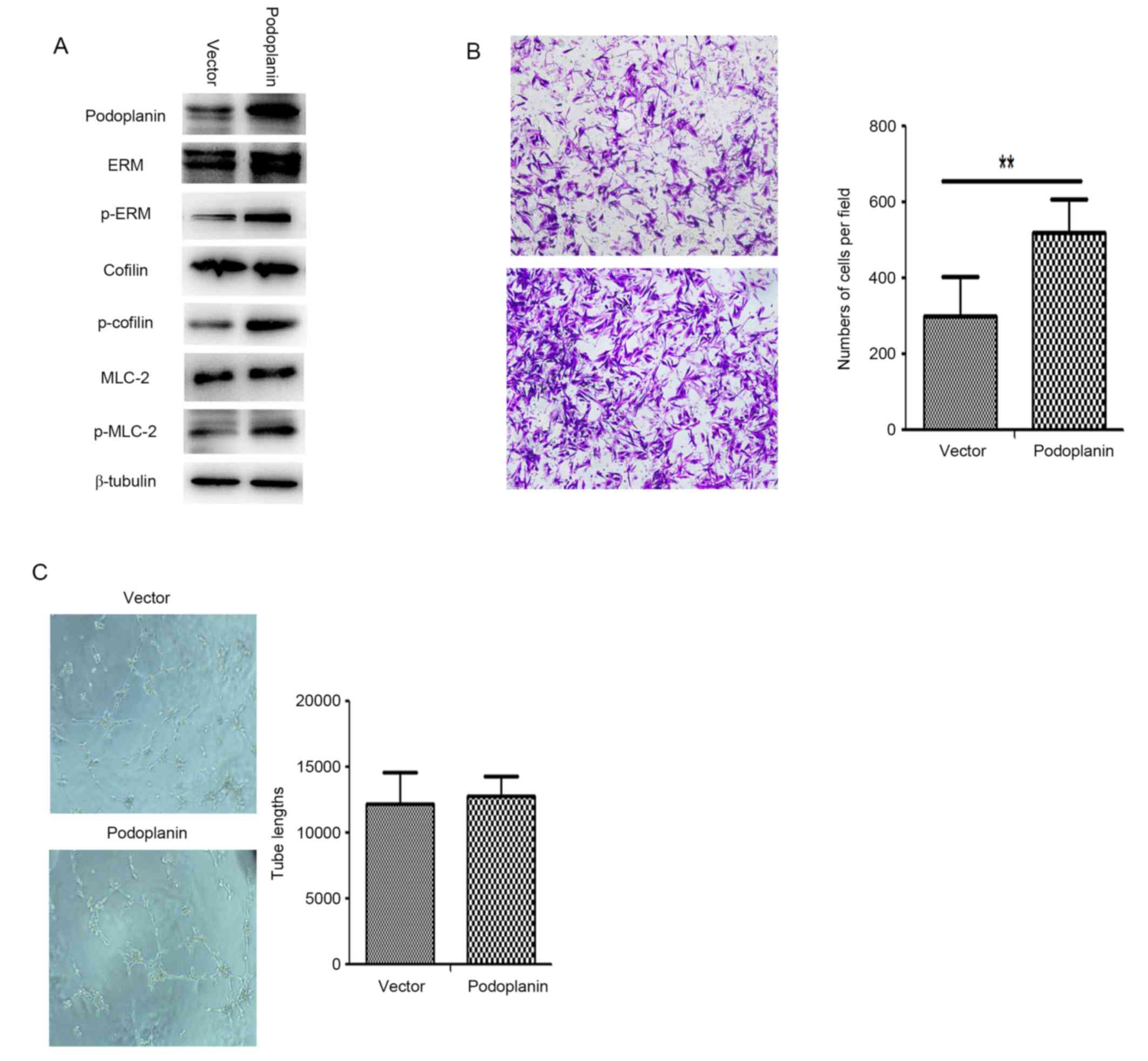 | Figure 4.Podoplanin overexpression enhances
the migration ability of CAFs. (A) Following transfection of CAFs
with a podoplanin plasmid, western blotting demonstrated
significant phosphorylation of ERM, cofilin and MLC compared with
the vector group. (B) Cells were stained with crystal violet and
observed using light microscopy (magnification, ×100). Transwell
migration assays demonstrated differences in migratory cells
between podoplanin-positive and podoplanin-negative CAFs
(P<0.01). (C) Conditioned medium from podoplanin-positive CAFs
had no influence on tube formation in HDLECs. Representative
pictures of tube formation are exhibited. Quantitative analysis of
the lack of tube formation by HDLECs induced by conditioned medium;
mean ± standard deviation; no significant differences were
demonstrated (n = 5). In (B) and (C), all data are representative
of three independent experiments. **P< 0.01. p-, phosphorylated;
ERM, ezrin, radixin and moesin; MLC-2, myosin light chain 2; CAF,
cancer-associated fibroblast; HDLEC, human dermal lymphatic
endothelial cell. |
The effects of transfection with the podoplanin
expression vector and control vector on CAF tube formation are
presented in Fig. 4C. Podoplanin
overexpression in CAFs did not significantly affect the
tube-formation ability of lymphatic endothelial cells. Therefore,
overexpression of podoplanin in CAFs may enhance migration and
adhesion; however, it does not directly influence tube formation in
lymphatic endothelial cells.
Discussion
Cholangiocytes possess different morphologies and
phenotypes at various anatomical levels of the biliary tract; these
differences may reflect various clinicopathological features of CCA
(26). Patients with pCCA exhibit an
increase in lymph node metastasis rate compared with patients with
hepatocellular carcinoma. Lymph node metastasis is an important
prognostic factor for the survival rate of patients with pCCA
following resection (27,28). Therefore, it may be of importance to
study the lymphatic system in pCCA further.
Podoplanin has emerged as a potential therapeutic
target in tumor cells (29). Previous
research has primarily focused on the tumor microenvironment, which
may have a major impact on the progression and dissemination of
tumor cells (30). In particular,
CAFs, which are the primary constituent of the tumor
microenvironment, are able to affect tumor epithelial cells and
other cell types through secretion of cytokines and growth factors
(31). Results from the present study
demonstrated that podoplanin was expressed in a group of CAFs and
epithelial tumor cells in pCCA specimens, and demonstrated that
several clinicopathological characteristics, including TNM stage
and lymph node metastasis, were associated with podoplanin
expression. Survival analysis also demonstrated that increased
expression levels of podoplanin in CAFs were associated with poor
patient outcome. Previous studies have reported high podoplanin
expression in several squamous cell carcinomas, including
esophageal, oral and lung carcinomas, which were associated with a
decreased survival rate and an increased incidence of lymph node
metastasis (12,17,32,33).
However, in certain types of cancer, such as squamous non-small
cell lung cancer and colorectal carcinoma, increased levels of
podoplanin expression is associated with a favorable prognosis.
Therefore, podoplanin serves as a good prognostic marker for
different types of tumor or tumors presenting at different
stages.
Tumor metastasis is a complex and multistep process.
First, tumor cells separate from the primary tumor mass, and then
degrade and penetrate the extracellular matrix and enter the
bloodstream or lymphatic system. Following activation in the tumor
microenvironment, CAFs may facilitate invasion and migration of
cancer cells by remodeling the extracellular matrix (34). The results of the present study
demonstrated that podoplanin overexpression significantly increased
the migration ability of CAFs. Western blotting indicated that
phosphorylation of ERM, MLC-2 and cofilin was upregulated in
podoplanin-overexpressing CAFs. Previous studies have reported that
podoplanin regulates Rho activity in lymphatic endothelial and
fibroblastic reticular cells (35,36). In
addition, podoplanin is able to affect the cytoskeleton by binding
and activating members of the ERM family. Cofilin is an
actin-remodeling protein that is able to generate significant
differences in migration, invasion and metastatic potential in
human cancer cells (37). MLC is the
key regulator of actin-myosin contractility. Phosphorylation of
cofilin and MLC-mediated remodeling of the actin network serve
important roles in cancer cell migration and invasion (38). Results demonstrated that podoplanin
overexpression in CAFs enhances migration ability by regulating the
actin network.
Ochoa-Alvarez et al (39) demonstrated that a
podoplanin-expressing tumor xenograft established using a human
oral cancer cell line was able to induce podoplanin expression in
infiltrating mouse CAFs. In the present study, isolation of CAFs
from a CCA tumor xenograft identified that 11% of these CAFs
expressed podoplanin using flow cytometry analysis. Podoplanin
expression in tumor xenograft stromal cells may depend on the human
oral cancer cell line and tumorigenesis time following injection.
Tumor xenograft models have been widely used in pharmacokinetic and
bioavailability studies. Yoshida et al (40) reported that podoplanin-positive CAFs
served an important role in primary resistance to epidermal growth
factor receptor tyrosine kinase inhibitors. Previous studies also
reported that podoplanin-mediated epithelial-mesenchymal transition
(EMT) may contribute to tumor epithelial cell invasion (17,41). EMT
is considered a dispensable pathway for metastasis; however,
recently it has also been demonstrated to contribute to
chemoresistance (42). These results
collectively suggest that the effect of chemotherapy may depend on
cancer cells and the surrounding tumor microenvironment. The
mechanism of podoplanin-mediated EMT and the association between
podoplanin expression in CAFs, and chemoresistance in the tumor
microenvironment are not fully understood. Further elucidation of
how podoplanin affects tumor migration, invasion and
chemoresistance in CCA will be essential to develop means to limit
excessive podoplanin expression in CAFs, and inhibit the
podoplanin-mediated signaling pathways in CCA; this may offer new
therapeutic strategies for treating pCCA. Further investigation
into accurate prognostic information regarding pCCA is
required.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81670566 and
81500478).
References
|
1
|
Razumilava N and Gores GJ:
Cholangiocarcinoma. Lancet. 383:2168–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng X and Tao H: Diagnostic and
prognostic serum marker of cholangiocarcinoma (Review). Oncol Lett.
9:3–8. 2015.PubMed/NCBI
|
|
3
|
Rizvi S and Gores GJ: Pathogenesis,
diagnosis, and management of cholangiocarcinoma. Gastroenterology.
145:1215–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rizvi S and Gores GJ: Molecular
pathogenesis of cholangiocarcinoma. Dig Dis. 32:564–569. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujiwara M, Kanayama K, Hirokawa YS and
Shiraishi T: ASF-4-1 fibroblast-rich culture increases
chemoresistance and mTOR expression of pancreatic cancer BxPC-3
cells at the invasive front in vitro, and promotes tumor growth and
invasion in vivo. Oncol Lett. 11:2773–2779. 2016.PubMed/NCBI
|
|
6
|
Kodama J Hasengaowa, Kusumoto T, Shinyo Y,
Seki N, Nakamura K, Hongo A and Hiramatsu Y: Loss of basement
membrane heparan sulfate expression is associated with tumor
progression in endometrial cancer. Eur J Gynaecol Oncol.
26:403–406. 2005.PubMed/NCBI
|
|
7
|
Paduch R: The role of lymphangiogenesis
and angiogenesis in tumor metastasis. Cell Oncol (Dordr).
39:397–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Christiansen A and Detmar M:
Lymphangiogenesis and cancer. Genes Cancer. 2:1146–1158. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura Y, Yasuoka H, Tsujimoto M, Imabun
S, Nakahara M, Nakao K, Nakamura M, Mori I and Kakudo K: Lymph
vessel density correlates with nodal status, VEGF-C expression, and
prognosis in breast cancer. Breast Cancer Res Treat. 91:125–132.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al-Shareef H, Hiraoka SI, Tanaka N, Shogen
Y, Lee AD, Bakhshishayan S and Kogo M: Use of NRP1, a novel
biomarker, along with VEGF-C, VEGFR-3, CCR7 and SEMA3E, to predict
lymph node metastasis in squamous cell carcinoma of the tongue.
Oncol Rep. 36:2444–2454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schoppmann SF, Berghoff A, Dinhof C,
Jakesz R, Gnant M, Dubsky P, Jesch B, Heinzl H and Birner P:
Podoplanin-expressing cancer-associated fibroblasts are associated
with poor prognosis in invasive breast cancer. Breast Cancer Res
Treat. 134:237–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan P, Temam S, El-Naggar A, Zhou X, Liu
DD, Lee JJ and Mao L: Overexpression of podoplanin in oral cancer
and its association with poor clinical outcome. Cancer.
107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi A, Ishii G, Neri S, Yoshida T,
Hashimoto H, Suzuki S, Umemura S, Matsumoto S, Yoh K, Niho S, et
al: Podoplanin-expressing cancer-associated fibroblasts inhibit
small cell lung cancer growth. Oncotarget. 6:9531–9541. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ono S, Ishii G, Nagai K, Takuwa T, Yoshida
J, Nishimura M, Hishida T, Aokage K, Fujii S, Ikeda N and Ochiai A:
Podoplanin-positive cancer-associated fibroblasts could have
prognostic value independent of cancer cell phenotype in stage I
lung squamous cell carci: Usefulness of combining analysis of both
cancer cell phenotype and cancer-associated fibroblast phenotype.
Chest. 143:963–970. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi SY, Sung R, Lee SJ, Lee TG, Kim N,
Yoon SM, Lee EJ, Chae HB, Youn SJ and Park SM: Podoplanin, α-smooth
muscle actin or S100A4 expressing cancer-associated fibroblasts are
associated with different prognosis in colorectal cancers. J Korean
Med Sci. 28:1293–1301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noren NK, Liu BP, Burridge K and Kreft B:
p120 catenin regulates the actin cytoskeleton via Rho family
GTPases. J Cell Biol. 150:567–580. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martin-Villar E, Scholl FG, Gamallo C,
Yurrita MM, Muñoz-Guerra M, Cruces J and Quintanilla M:
Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a
small membrane mucin induced in oral squamous cell carcinomas. Int
J Cancer. 113:899–910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith SM and Melrose J: Podoplanin is
expressed by a sub-population of human foetal rib and knee joint
rudiment chondrocytes. Tissue Cell. 43:39–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghosh M, Song X, Mouneimne G, Sidani M,
Lawrence DS and Condeelis JS: Cofilin promotes actin polymerization
and defines the direction of cell motility. Science. 304:743–746.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chronopoulos A, Robinson B, Sarper M,
Cortes E, Auernheimer V, Lachowski D, Attwood S, García R, Ghassemi
S, Fabry B, et al: ATRA mechanically reprograms pancreatic stellate
cells to suppress matrix remodelling and inhibit cancer cell
invasion. Nat Commun. 7:126302016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukuoka J, Dracheva T, Shih JH, Hewitt SM,
Fujii T, Kishor A, Mann F, Shilo K, Franks TJ, Travis WD and Jen J:
Desmoglein 3 as a prognostic factor in lung cancer. Hum Pathol.
38:276–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kyzas PA, Geleff S, Batistatou A, Agnantis
NJ and Stefanou D: Evidence for lymphangiogenesis and its
prognostic implications in head and neck squamous cell carcinoma. J
Pathol. 206:170–177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
The National Academies Collection, .
Reports funded by National Institutes of Health: National Research
Council (US) Committee for the Update of the Guide for the Care and
Use of Laboratory AnimalsGuide for the Care and Use of Laboratory
Animals. National Academy of Sciences. 8th. Washington (DC):
National Academies Press (US); 2011
|
|
24
|
Wang M, Wu CP, Pan JY, Zheng WW, Cao XJ
and Fan GK: Cancer-associated fibroblasts in a human HEp-2
established laryngeal xenografted tumor are not derived from cancer
cells through epithelial-mesenchymal transition, phenotypically
activated but karyotypically normal. PLoS One. 10:e01174052015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou F, Xu Y, Shi J, Lan X, Zou X, Wang L
and Huang Q: Expression profile of E-cadherin, estrogen receptors,
and P53 in early-onset gastric cancers. Cancer Med. 5:3403–3411.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gandou C, Harada K, Sato Y, Igarashi S,
Sasaki M, Ikeda H and Nakanuma Y: Hilar cholangiocarcinoma and
pancreatic ductal adenocarcinoma share similar histopathologies,
immunophenotypes, and development-related molecules. Hum Pathol.
44:811–821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DeOliveira ML, Cunningham SC, Cameron JL,
Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ and Schulick
RD: Cholangiocarcinoma: Thirty-one-year experience with 564
patients at a single institution. Ann Surg. 245:755–762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nimura Y, Kamiya J, Kondo S, Nagino M,
Uesaka K, Oda K, Sano T, Yamamoto H and Hayakawa N: Aggressive
preoperative management and extended surgery for hilar
cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat
Surg. 7:155–162. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wicki A and Christofori G: The potential
role of podoplanin in tumour invasion. Br J Cancer. 96:1–5. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pietras K and Ostman A: Hallmarks of
cancer: Interactions with the tumor stroma. Exp Cell Res.
316:1324–1331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rajaram M, Li J, Egeblad M and Powers RS:
System-wide analysis reveals a complex network of tumor-fibroblast
interactions involved in tumorigenicity. PLoS Genet.
9:e10037892013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kadota K, Huang CL, Liu D, Nakashima N,
Yokomise H, Ueno M and Haba R: The clinical significance of the
tumor cell D2-40 immunoreactivity in non-small cell lung cancer.
Lung Cancer. 70:88–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chuang WY, Yeh CJ, Wu YC, Chao YK, Liu YH,
Tseng CK, Chang HK, Liu HP and Hsueh C: Tumor cell expression of
podoplanin correlates with nodal metastasis in esophageal squamous
cell carcinoma. Histol Histopathol. 24:1021–1027. 2009.PubMed/NCBI
|
|
34
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Navarro A, Perez RE, Rezaiekhaligh M,
Mabry SM and Ekekezie II: T1alpha/podoplanin is essential for
capillary morphogenesis in lymphatic endothelial cells. Am J
Physiol Lung Cell Mol Physiol. 295:L543–L551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Astarita JL, Cremasco V, Fu J, Darnell MC,
Peck JR, Nieves-Bonilla JM, Song K, Kondo Y, Woodruff MC, Gogineni
A, et al: The CLEC-2-podoplanin axis controls the contractility of
fibroblastic reticular cells and lymph node microarchitecture. Nat
Immunol. 16:75–84. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Collazo J, Zhu B, Larkin S, Martin SK, Pu
H, Horbinski C, Koochekpour S and Kyprianou N: Cofilin drives
cell-invasive and metastatic responses to TGF-β in prostate cancer.
Cancer Res. 74:2362–2373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Torka R, Thuma F, Herzog V and Kirfel G:
ROCK signaling mediates the adoption of different modes of
migration and invasion in human mammary epithelial tumor cells. Exp
Cell Res. 312:3857–3871. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ochoa-Alvarez JA, Krishnan H, Pastorino
JG, Nevel E, Kephart D, Lee JJ, Retzbach EP, Shen Y, Fatahzadeh M,
Baredes S, et al: Antibody and lectin target podoplanin to inhibit
oral squamous carcinoma cell migration and viability by distinct
mechanisms. Oncotarget. 6:9045–9060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoshida T, Ishii G, Goto K, Neri S,
Hashimoto H, Yoh K, Niho S, Umemura S, Matsumoto S, Ohmatsu H, et
al: Podoplanin-positive cancer-associated fibroblasts in the tumor
microenvironment induce primary resistance to EGFR-TKIs in lung
adenocarcinoma with EGFR mutation. Clin Cancer Res. 21:642–651.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|