Introduction
The biological activity of colorectal cancer (CRC)
is most accurately reflected by histological characteristics at the
invasive front. One parameter of CRC activity is tumor budding,
which represents de-differentiation of epithelial cells into more
invasive phenotypes in a process known as the
epithelial-mesenchymal transition (EMT) (1–3). Tumor
budding at the invasive margin has been demonstrated to involve
molecules such as Laminin-5, CD44, and L1 cell adhesion molecule
(L1CAM) and is associated with poor prognosis in CRC (4–8). Although
an association with hematogeneous metastasis has also been
documented, the underlying molecular mechanism is not known.
Tissue microarray (TMA) is a technique for high
through-put evaluation of protein expression in a large number of
archival tissue blocks used for routine histopathological
diagnosis. A cohort of tissue core specimens obtained from original
tissue blocks are arranged into a single recipient paraffin block
(9). TMA analysis efficiently screens
for molecular alterations in a large number of cases. Using TMA, we
have shown that protein expression levels in CRC are heterogeneous
and that the findings from core specimens taken from the invasive
front most precisely reflect tumor aggressiveness (6).
Sialyl Lewisx (SLX) is known to be a
carbohydrate ligand for endothelial-selectin (E-selectin) expressed
on vascular endothelial cells. SLX expression in cancer cells
promotes their adhesion to vascular endothelial cells, and several
clinical studies show that SLX expression in cancer tissues plays a
role in hematogenous metastasis and cancer prognosis (10–13). In
previous reports, SLX expression was evaluated by microscopic
examination of a slide that was representative of the whole tumor.
SLX immunoexpression is shown to be heterogeneous between different
areas in a tumor and is higher at the invasive front (12,14,15).
Hence, evaluation of tumor cells at the invasive front is
considered to be important for revealing the role of SLX expression
in CRC metastasis.
This study aims to investigate the association
between SLX expression at the invasive front of tumors and the
prognosis of Stage II CRC using TMA analysis. In addition, we
compare the prognostic significance of SLX expression at the
invasive front with that of other tumor regions and examine the
prognostic significance of preoperative serum SLX
concentrations.
Materials and methods
This study was conducted after obtaining approval
from the internal review board of the National Defense Medical
College hospital (Tokorozawa, Japan). A consecutive series of 314
patients with Stage II CRC who underwent a potentially curative
resection between January 1997 and December 2000 was derived from
the files of the Department of Surgery of the National Defense
Medical College. Serum SLX levels were measured preoperatively by
radioimmunoassay (RIA) in the 209 patients who were enrolled in
this study. Serum SLX levels were divided into two categories based
on the upper limit of the normal range (38 U/ml): Normal SLX, any
value ≤38 U/ml; high SLX, any value >38 U/ml.
Clinicopathological features were assessed according to the 2nd
edition of the Japanese classification of CRC (16). We defined a focus of tumor budding as
an isolated single cancer cell or a cluster composed of fewer than
five cancer cells and then classified these cancers based on the
number of foci found in a ×200 microscopic field of hematoxylin and
eosin (H&E) stained section as follows: G1, 0–4 foci; G2, 5–9
foci; G3, 10 or more foci (2). We
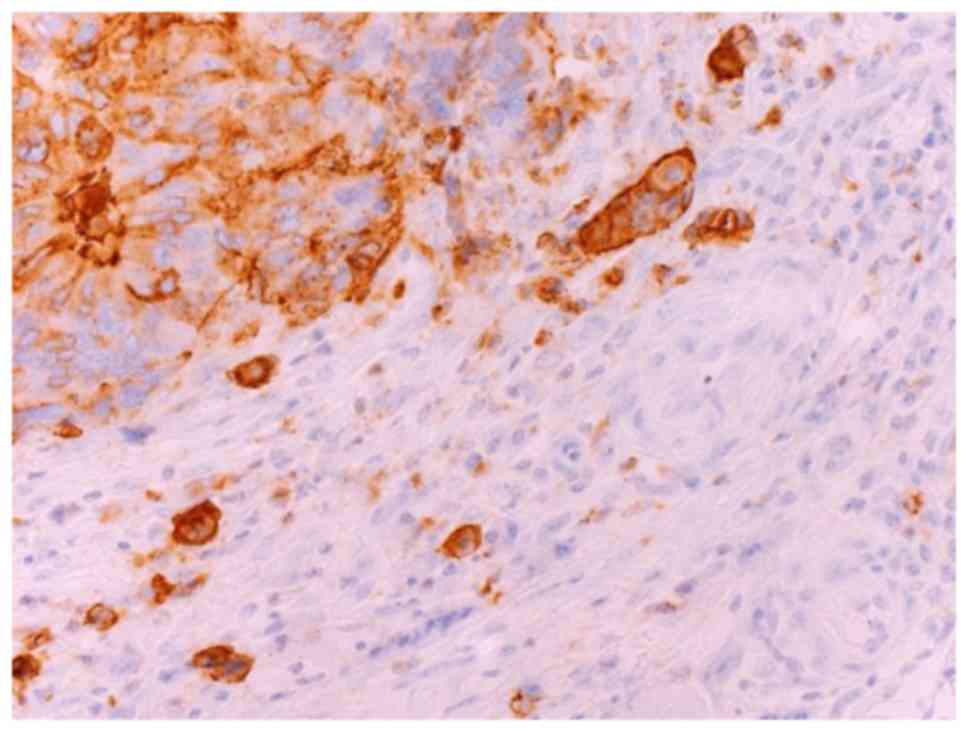
showed tumor budding expressing SLX in Fig. 1. Disease-free survival (DFS) was
defined as the time from surgery to the first event of either
recurrence disease or death. Cancer-specific survival (CSS) was
defined as the time from surgery to death from CRC recurrence. The
word ‘recurrence’ was used in this report to denote metachronous
metastasis at the same site or in another location.
TMA construction and
immunohistochemical staining
We first identified two regions of the invasive
front (submucosal and subserosal) and two regions of non-invasive
frontal lesions (central or superficial tumor area) with viable
cancer cells by referring to an H&E stained whole section
microscopically. To construct a TMA block, a single tissue core
(2-mm diameter) was taken from each region in formalin-fixed
paraffin-embedded CRC tissue blocks (‘donor’ blocks) using a Tissue
Microarrayer (Beecher Instruments, Silver Spring, MD, USA) and was
transferred to the ‘recipient’ blocks (TMA blocks). TMA blocks were
cut (4-mm-thick slices) and deparaffinized using standard
histological techniques. These sections were deparaffinized with
xylene and rehydrated with ethanol. Antigen retrieval was performed
in an autoclave (121°C, 15 min). Endogenous peroxidase activity was
blocked using 5% H2O2. The sections were
incubated in 10% normal goat serum to block nonspecific binding of
the antibody and then incubated with an SLX monoclonal antibody
(clone KM93; dilution 1:100; Kyowa Medics, Tokyo, Japan) as the
primary antibody overnight at 4°C. Subsequently, the sections were
incubated with the secondary antibody for 1 h at room temperature,
immersed in 0.1% diaminobenzidine tetrahydrochloride (DAB) solution
for 5 min, and counterstained with hematoxylin for visualization of
the antigen. The negative control was stained using an identical
procedure without the primary antibody.
Evaluation of SLX expression
We determined the proportion of stained cancer cells
in a whole core of TMA. The distribution of staining was scored as
0 (0–25%), 1 (26–50%), 2 (51–75%), and 3 (76–100%). The sum of the
distribution scores from two invasive fronts was used as the final
staining score (0–6) for SLX immunoexpression. Final scores of 5
and 6 were considered positive (high SLX expression) because
postoperative recurrence and disease-specific mortality rates of
patients with a score of 5–6 were extremely high [postoperative
recurrence rates, 9.6% (patients with score 0–2), 14.0% (3–4), and 22.7%
(5–6);
mortality rates, 3.5% (0–2), 6.0% (3–4), and 13.6%
(5–6)].
The sum of scores from two non-invasive frontal regions was also
used for assessment in the same manner; none of the score ranges
had prognostic significance [postoperative recurrence rates: 12.8%
(0–2), 15.2% (3–4), and 13.2% (5–6); mortality
rates: 11.2% (0–2), 8.7% (3–4), and 7.9% (5–6)]. In
addition, we conducted immunohistochemical staining of SLX in 30
standard sections to confirm the integrity of the TMA data. They
were classified as either high (≥50%) or low (<50%) grade in
terms of the percentage of immunopositive cells among all cancer
cells located at the invasive front (the deepest 3 mm width) of the
tumor. Immunohistochemical staining was independently evaluated by
two observers (MY and ES); in cases of discrepancy, a consensus was
reached after re-evaluation.
Followup
All 209 patients received regular follow-up care at
our outpatient clinic. Physical examination, serum carcinoembryonic
antigen (CEA) levels, and carbohydrate antigen (CA) 19-9 levels
were monitored every 3 months. Contrast computed tomography (CT)
scan was performed every 6 months, and colonoscopy was performed
biannually. Whenever any findings suggestive of cancer relapse did
not appear after 5 years, the follow-up procedure was changed to an
annual physical without any other detailed examinations. At the
date of the last followup, 32 patients had died, with a median time
from surgery to death of 44.5 months (range, 13.5–121.6 months). Of
these, 19 died from CRC recurrence, 5 died from other carcinomas,
and 8 died from other reasons or from unclear causes. The median
follow-up period for the survivors (n=177) was 62.9 months (range,
38.8–133.3 months). Adjuvant chemotherapy was administered to 13%
of all patients (27/209) after curative surgery.
Statistical analysis
Comparisons between groups were performed using the
χ2 test or Fisher's exact method. Survival was analyzed
according to the Kaplan-Meier product limit method. The
significance of differences was determined using the log-rank test.
Covariates with trend-significant effects (P<0.10) on univariate
analysis were selected for multivariate analysis of the factors for
survival using Cox's proportional hazard model and for
postoperative recurrence using a logistic regression model.
Statistical analysis used JMP version 11, and statistical
significance was considered as P<0.05. The degree of
interobserver agreement for the evaluation of immunoreactivity was
measured using the generalized κ test for two or more observers. In
accordance with the criteria of Landis and Koch (17), κ values were assigned a strength of
agreement score of poor (<0.00), slight (0.00–0.20), fair
(0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), and
near perfect (0.81–1.00).
Results
Interobserver agreement
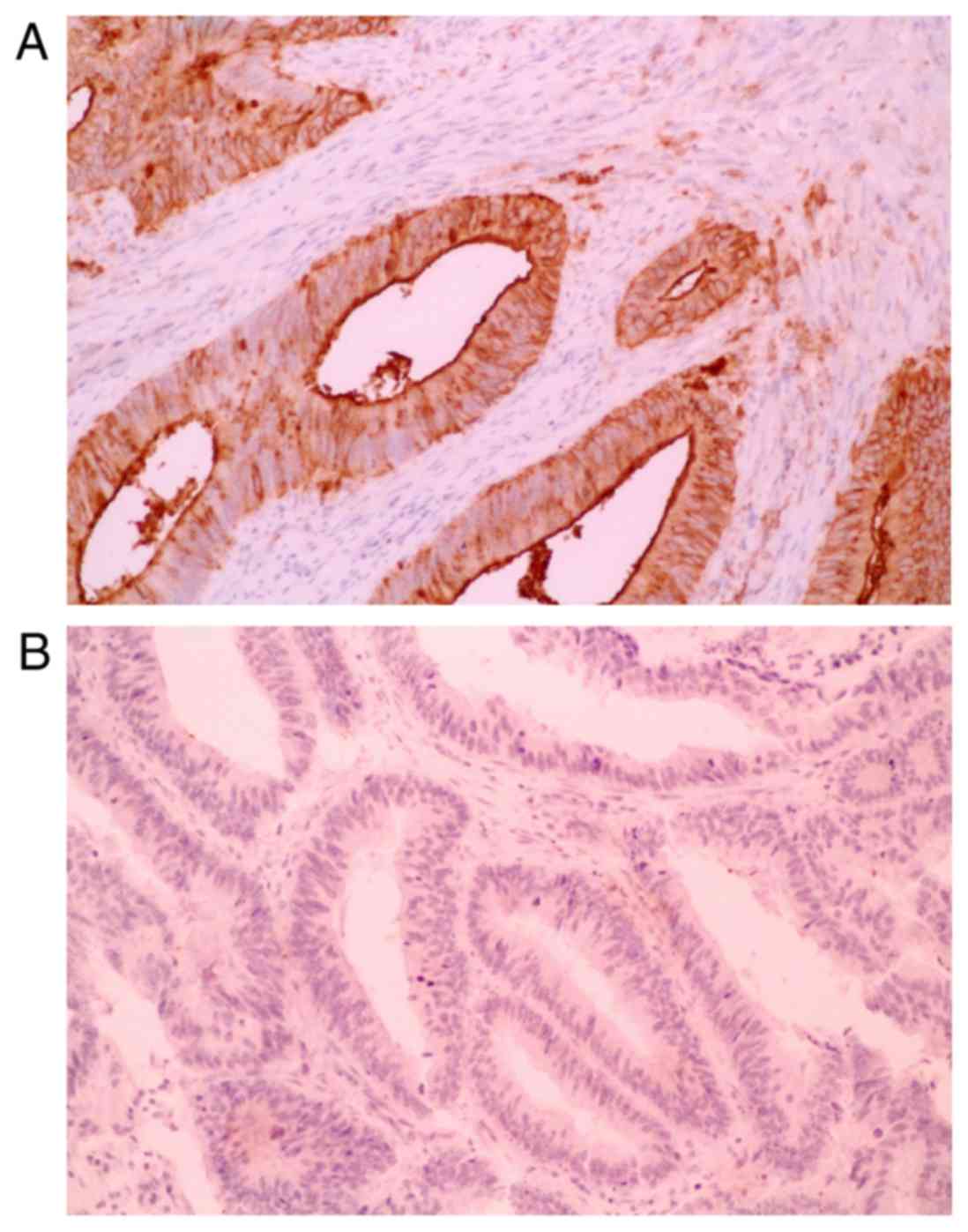
Characteristic microscopic appearances of
SLX-positive and SLX-negative TMA specimens of CRCs are shown in
Fig. 2, respectively. The level of
interobserver agreement for evaluation of SLX immunostaining was
substantial [82.3% (κ=0.63)]. We showed the association of pair
scores of SLX expression at two invasive frontal regions in
Table I. SLX expression had
heterogeneity even at the invasive frontal region. The proportion
of the cases with the gap of 2 or more score was 24%. SLX
expression at the invasive front in TMA was in good agreement with
that in standard section in 26 of 30 cases (87%).
 | Table I.Association of pair scores of Sialyl
Lewisx expression at two invasive frontal regions. |
Table I.
Association of pair scores of Sialyl
Lewisx expression at two invasive frontal regions.
|
| Distribution score
(subserosal) |
|---|
|
|
|
|---|
| Distribution score
(submucosal) | 0 (%) | 1 (%) | 2 (%) | 3 (%) |
|---|
| 0 | 48 (23) | 12 (6) | 10 (5) | 7 (3) |
| 1 | 13 (6) | 7
(3) | 6 (3) | 10 (5) |
| 2 | 6
(3) | 7
(3) | 21 (10) | 2 (1) |
| 3 | 7
(3) | 11 (5) | 3 (2) | 39 (19) |
Relationship to survival
The serum SLX level was high in 9% of all subjects,
whereas high tissue expression of SLX at the invasive front and
non-invasive frontal region were observed in 21 and 18%,
respectively.
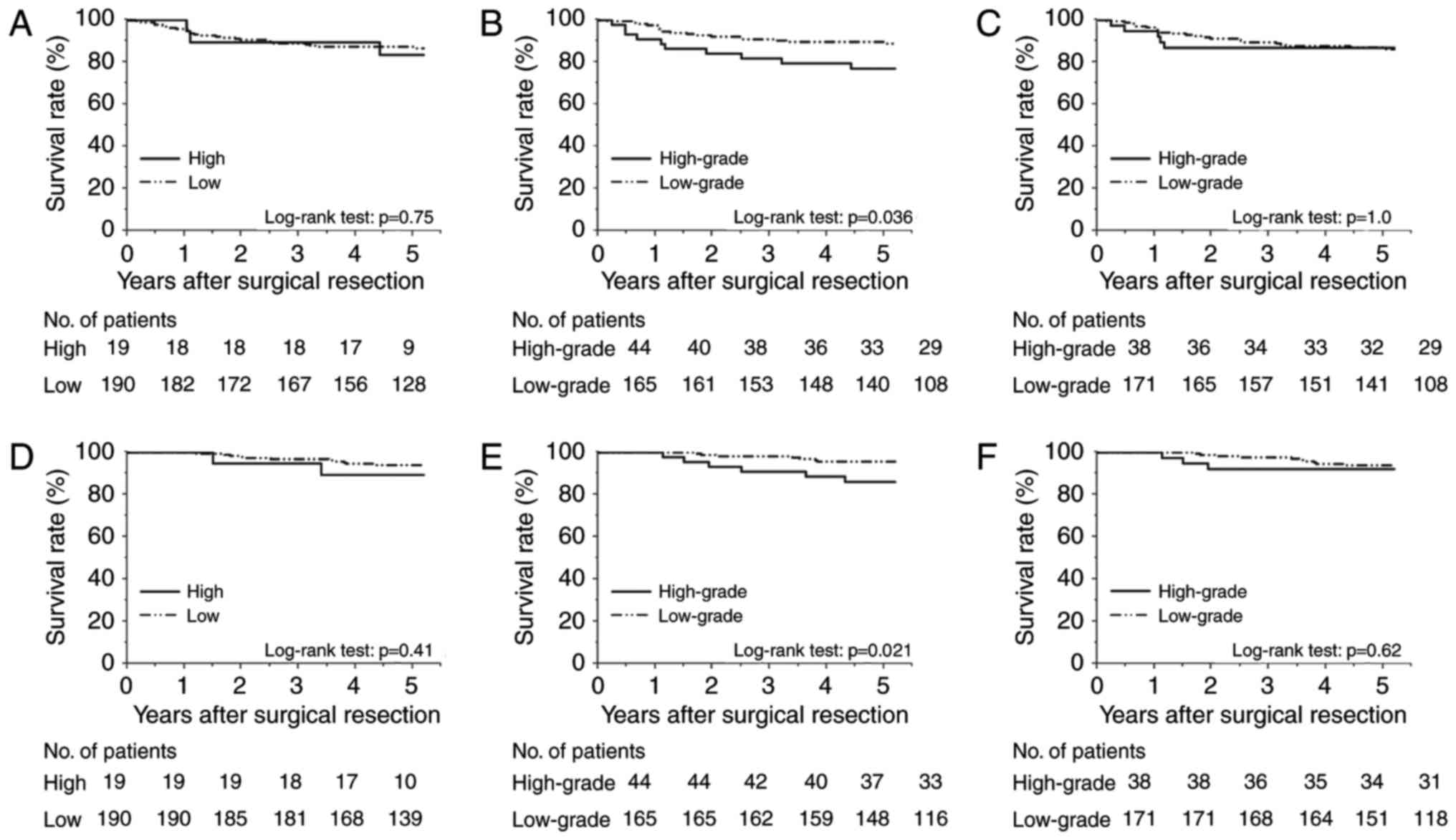
Fig. 3A-C show the
Kaplan-Meier DFS curves, and Fig.
3D-F display the CSS curves according to the preoperative serum
SLX levels, SLX expression levels at the invasive front, and those
at non-invasive frontal regions, respectively. The DFS and CSS of
the high serum-SLX group did not differ significantly from those of
the low serum-SLX group (5-year DFS rates, 83.5 and 87.3%; P=0.75;
5-year CSS rates, 89.5 and 94.0%; P=0.41). In contrast, the group
with high SLX expression at the invasive front had poorer prognoses
than did the low-expression group (5-year DFS rates, 77.0 and
89.7%; P=0.036; 5-year CSS rates, 86.1 and 95.6%; P=0.021). High
SLX expression at non-invasive frontal regions did not correlate
with shorter survivals (5-year DFS rates, 86.8 and 86.2%; P=1.0;
5-year CSS rates, 92.1 and 93.9%; P=0.62). These data indicate the
superiority of the invasive front for clinically relevant
evaluation of SLX expression.
Univariate analyses of DFS indicate that lymphatic
invasion (P<0.001), venous invasion (P=0.023), tumor budding
(P=0.001), serum CEA level (P=0.022), SLX expression at the
invasive front (P=0.036), the number of retrieved lymph nodes
(P=0.073), and the depth of tumor invasion (P=0.090) are
prognostically significant or marginally significant. Using these
factors as variables, multivariate analysis revealed that lymphatic
invasion (hazard ratio [HR] 2.8; P=0.028) and tumor budding (HR
2.6; P=0.021) are independent prognostic factors.
Relationship to postoperative
recurrence
Of the entire cohort, 28 patients (13.4%) had
postoperative recurrence. The overall recurrence rate for the group
with high SLX expression at the invasive front was higher than that
of the group with low SLX expression (22.7 vs. 10.9%; P=0.041)
(Table II). Regarding sites of
recurrence, liver cancer recurrence was more frequent among
patients with high SLX expression at the invasive front than among
those with low SLX expression (15.9 vs. 2.4%; P=0.002). We observed
no statistically significant differences between groups with
respect to primary site, lung, or peritoneal recurrence. Univariate
analyses revealed that liver cancer recurrence is associated with
venous invasion, tumor budding, serum CEA level, and SLX expression
at the invasive front (Table III).
Further, multivariate analysis revealed that venous invasion and
SLX expression at the invasive front are independent factors for
postoperative liver cancer recurrence. Patients with both moderate
to severe venous invasion and high SLX expression at the invasive
front had a significantly higher liver cancer recurrence rate (25.0
vs. 3.6%; P=0.006) and a significantly poorer prognosis (5-year
DFS, 62.5 vs. 89.1%; P<0.001) compared with the other
patients.
 | Table II.Correlation between postoperative
recurrence and degree of SLX staining at the invasive front. |
Table II.
Correlation between postoperative
recurrence and degree of SLX staining at the invasive front.
|
|
| Degree of SLX
staining, no (%) |
|
|---|
|
|
|
|
|
|---|
| Recurrence | Total (n=209) | High expression
(n=44) | Low expression
(n=165) | P-value |
|---|
| Overall | 28 (13.4) | 10 (22.7) | 18 (10.9) | 0.041 |
| Primary recurrence
sitea |
|
Local | 11 (5.3) | 4 (9.1) | 7 (4.2) | 0.25 |
|
Liver | 11 (5.3) | 7 (15.9) | 4 (2.4) | 0.002 |
|
Lung | 10 (4.8) | 4 (9.1) | 6 (3.6) | 0.22 |
|
Peritoneum | 3
(1.4) | 2 (4.6) | 1 (0.6) | 0.11 |
| Other
organs | 3
(1.4) | 1 (2.3) | 2 (1.2) | 0.51 |
 | Table III.Univariate and multivariate analyses
of risk factors influencing liver recurrence in patients with
colorectal cancer. |
Table III.
Univariate and multivariate analyses
of risk factors influencing liver recurrence in patients with
colorectal cancer.
|
|
| Univariate
analysis | Mutivariate
analysis by logistic regression model |
|---|
|
|
|
|
|
|---|
| Parameter | Comparison | Hazard ratio | 95% confidence
interval | P-value | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Depth of tumor
invasion |
| T4 | T3 | 2.49 | 0.62–8.68 | 0.18 |
|
|
|
| Histologic
type |
|
Por/Muca |
Wel/Modb | 0.78 | 0.06–18.15 | 1.00 |
|
|
|
| Lymphatic
invasion |
|
Moderate to severe | No or minimal | 1.87 | 0.27–7.90 | 0.47 |
|
|
|
| Venous
invasion |
|
Moderate to severe | No or minimal | 4.67 | 1.31–16.58 | 0.016 | 4.14 | 1.07–18.15 | 0.040 |
| Tumor
buddingc |
| G3 | G1/G2 | 3.51 | 1.02–12.11 | 0.036 | 1.56 | 0.35–6.27 | 0.54 |
| Serum CEA
level |
| >5.0
ng/ml | ≤5.0 ng/ml | 4.91 | 1.38–17.47 | 0.013 | 3.20 | 0.81–13.94 | 0.097 |
| The number of
sampled lymph nodes |
|
<12 | ≥12 | 2.06 | 0.52–7.15 | 0.28 |
|
|
|
| SLX expression at
the invasive front |
|
High | Low | 7.61 | 2.12–27.37 | 0.002 | 5.26 | 1.39–22.27 | 0.015 |
Relationship to clinicopathological
findings (Table IV)
Tumors from the group with high SLX expression at
the invasive front showed more aggressive properties than did those
from the low SLX-expression group with respect to the depth of
tumor invasion (pT4, 34 vs. 16%; P=0.007), grade of tumor budding
(G3, 32 vs. 18%; P=0.038), and the serum CEA level (CEA >5.0
ng/ml, 50 vs. 22%; P<0.001). A strong correlation was observed
between the serum SLX concentration and the level of tissue SLX
protein expression at the invasive front (P<0.001).
Discussion
This study indicates that SLX expression at the
tumor invasive front is significantly associated with the depth of
tumor invasion, the grade of tumor budding, and the CEA level.
Univariate analysis of DFS revealed that the SLX expression level
is a significant prognostic factor. With respect to the site of
postoperative recurrence, SLX expression level associates strongly
with liver cancer recurrence but not recurrence in the primary
site, lung, or peritoneum. Thus, SLX expression might be a specific
predictive marker of postoperative liver cancer recurrence in Stage
II CRC.
Hematogenous metastasis of CRC is known to be a
multistep process. Membrane-type 1 matrix metalloproteinase
(MT1-MMP) is a membrane-anchored zinc-binding endopeptidase that is
expressed at the leading edge of various invasive carcinomas.
MT1-MMP degrades the basement membrane, and once in direct contact
with the stroma, cleaves pro-MMP-2 made by stromal cells,
converting it to an active protease. Subsequently, the activated
MMP-2 dissolves the collagen I network and creates a channel in
front of the carcinoma cell that allows it to invade more deeply
into the stroma (18). Furthermore,
it has been revealed that cancer cells in the invasive front
acquire the cell motility by activating proteins like Rho-family
and are prone to invade blood vessels (19). Through these steps, the cancer cells
at the invasive front show migration across the stroma and
approaching blood vessels. When cancer cells overexpressing SLX in
the invasive front invade a blood vessel, they circulate to the
liver, lung, and other distant organs. Ligand-receptor interactions
between CAs and cell adhesion molecules of the selectin family are
proposed to play a role in the preparation of metastatic foci at
distant organs. Overexpressed SLX on cancer cells gives rise to
their weak adhesion to selectins on endothelial cells and prepares
their stronger adhesion and transmigration outside the blood vessel
through integrin, CD44, CXCR4, and their receptor systems (20–22).
Accordingly, circulating cancer cells overexpressing SLX increase
the likelihood of forming a metastatic lesion. This study reveals
that SLX expression at the invasive front is an independent risk
factor for postoperative liver cancer recurrence of Stage II CRC.
This finding is similar to that for the venous invasion level,
which well supports the theory that hematogenous metastasis occurs
through the adhesion of cancer-expressing SLX to E-selectin on the
surface of endothelial cells.
Our results are consistent with a previous report by
Akamine et al (11) showing
that CRC patient prognosis is rarely associated with serum SLX
level but significantly associated with SLX expression level in
tumor tissues. Nakagoe et al (23,24)
reported that the level of serum SLX from the drainage vein of a
tumor was significantly higher than that of a peripheral vein, and
the concentration of SLX in the tumor's drainage vein but not the
peripheral vein is associated with CRC prognosis. We propose that
the measurement of SLX through a typical blood test is of little
meaning for predicting postoperative recurrence during the
surveillance period.
A number of meta-analyses indicate that intensive
follow-up examinations are associated with a favorable prognosis in
CRC patients (25). Early detection
and treatment of postoperative recurrences likely contribute to
better survival. As for CRC, liver cancer recurrence occurs
frequently, and 29% of patients will develop liver metastases
within 3 years of diagnosis (26).
Meanwhile, patient survival after curative resection of liver
cancer recurrence has improved dramatically, with 5-year survival
ranging from 25 to 74% (27,28). Consequently, it is worthwhile to
identify the patients at highest risk of postoperative liver cancer
recurrence and to perform intensive examinations during
surveillance of these patients. Increasing evidence indicates that
unfavorable features such as T4 lesions, poorly differentiated
histology, the number of sampled lymph nodes (<12), venous
invasion, lymphatic invasion, perineural invasion, ileus or
perforation as an initial symptom, and high serum CEA level
(>5.0 ng/ml) are risk factors for postoperative recurrence in
Stage II CRC (29–31). Although information on perineural
invasion and initial symptoms could not be collected here, this
study reveals that the levels of SLX expression at the invasive
front and venous invasion are key predictive factors of
postoperative liver cancer recurrence. Thus, we recommend intensive
followup for patients whose tumors show evidence of these two
factors.
This study has several potential limitations. First,
the TMA method is not generally used in routine pathological
diagnosis; thus, it is necessary to determine whether similar
results are obtained from examinations using standard sections.
Second, as this was a retrospective study with limited cases, the
verification of our results requires a large-scale prospective
study. Nonetheless, this is the first report to clearly show that
SLX expression in the invasive front of CRC is a predictor of
hematogeneous metastasis, especially liver recurrence.
In conclusion, the present study demonstrates the
important role of SLX expression at the invasive front as an
independent liver recurrence predictor in Stage II CRC. The level
of SLX expression also correlates with the serum SLX concentration,
the depth of tumor invasion, and the grade of tumor budding. The
clinical application of these findings could identify patients at
high risk for postoperative liver cancer recurrence, allowing for
the selection of patients for whom intensive followup is most
beneficial.
Acknowledgements
This study was supported in part by the Japan
Society for the Promotion of Science KAKENHI (grant nos. 23501302,
25462074, and 26462032).
Glossary
Abbreviations
Abbreviations:
|
SLX
|
Sialyl Lewisx
|
|
E-selectin
|
endothelial selectin
|
|
CRC
|
colorectal cancer
|
|
TMA
|
Tissue microarray
|
|
RIA
|
radioimmunoassay
|
|
H&E
|
hematoxylin and eosin
|
|
DAB
|
diaminobenzidine
tetrahydrochloride
|
|
CEA
|
carcinoembryonic antigen
|
|
CA
|
carbohydrate antigen
|
|
CT
|
computed tomography
|
|
DFS
|
disease-free survival
|
|
CSS
|
cancer-specific survival
|
|
HR
|
hazard ratio
|
|
MT1
|
membrane-type 1
|
|
MMP
|
matrix metalloproteinase
|
References
|
1
|
Ueno H, Price AB, Wilkinson KH, Jass JR,
Mochizuki H and Talbot IC: A new prognostic staging system for
rectal cancer. Ann Surg. 240:832–839. 2004. View Article : Google Scholar
|
|
2
|
Ueno H, Murphy J, Jass JR, Mochizuki H and
Talbot IC: Tumor ‘budding’ as an index to estimate the potential of
aggressiveness in rectal cancer. Histopathology. 40:127–132. 2002.
View Article : Google Scholar
|
|
3
|
Hase K, Shatney C, Johnson D, Trollope M
and Vierra M: Prognostic value of tumor ‘budding℉ in patients with
colorectal cancer. Dis Colon Rectum. 36:627–635. 1993. View Article : Google Scholar
|
|
4
|
Rogers AC, Winter DC, Heeney A, Gibbons D,
Lugli A, Puppa G and Sheahan K: Systematic review and meta-analysis
of the impact of tumour budding in colorectal cancer. Br J Cancer.
115:831–840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukazawa S, Shinto E, Tsuda H, Ueno H,
Shikina A, Kajiwara Y, Yamamoto J and Hase K: Laminin β3 expression
as a prognostic factor and a predictive marker of chemoresistance
in colorectal cancer. Jpn J Clin Oncol. 45:533–540. 2015.PubMed/NCBI
|
|
6
|
Shinto E, Tsuda H, Ueno H, Hashiguchi Y,
Hase K, Tamai S, Mochizuki H, Inazawa J and Matsubara O: Prognostic
implication of laminin-5 gamma 2 chain expression in the invasive
front of colorectal cancers, disclosed by area-specific four-point
tissue microarrays. Lab Invest. 85:257–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masaki T, Goto A, Sugiyama M, Matsuoka H,
Abe N, Sakamoto A and Atomi Y: Possible contribution of CD44
variant 6 and nuclear beta-catenin expression to the formation of
budding tumor cells in patients with T1 colorectal carcinoma.
Cancer. 92:2539–2546. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kajiwara Y, Ueno H, Hashiguchi Y, Shinto
E, Shimazaki H, Mochizuki H and Hase K: Expression of l1 cell
adhesion molecule and morphologic features at the invasive front of
colorectal cancer. Am J Clin Pathol. 136:138–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kononen J, Bubendorf L, Kallioniemi A,
Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G
and Kallioniemi OP: Tissue microarrays for high-throughput
molecular profiling of tumor specimens. Nat Med. 4:844–847. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kannagi R, Izawa M, Koike T, Miyazaki K
and Kimura N: Carbohydrate-mediated cell adhesion in cancer
metastasis and angiogenesis. Cancer Sci. 95:377–384. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akamine S, Nakagoe T, Sawai T, Tsuji T,
Tanaka K, Hidaka S, Shibasaki S, Nanashima A, Yamaguchi H, Nagayasu
T and Yasutake T: Differences in prognosis of colorectal cancer
patients based on the expression of sialyl Lewisa, sialyl Lewisx
and sialyl Tn antigens in serum and tumor tissue. Anticancer Res.
24:2541–2546. 2004.PubMed/NCBI
|
|
12
|
Nakagoe T, Fukushima K, Tanaka K, Sawai T,
Tsuji T, Jibiki M, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H
and Arisawa K: Evaluation of sialyl Lewis(a), sialyl Lewis(x), and
sialyl Tn antigens expression levels as predictors of recurrence
after curative surgery in node-negative colorectal cancer patients.
J Exp Clin Cancer Res. 21:107–113. 2002.PubMed/NCBI
|
|
13
|
Hoff SD, Matsushita Y, Ota DM, Cleary KR,
Yamori T, Hakomori S and Irimura T: Increased expression of
sialyl-dimeric LeX antigen in liver metastases of human colorectal
carcinoma. Cancer Res. 49:6883–6888. 1989.PubMed/NCBI
|
|
14
|
Schiffmann L, Schwarz F, Linnebacher M,
Prall F, Pahnke J, Krentz H, Vollmar B and Klar E: A novel sialyl
Le(X) expression score as a potential prognostic tool in colorectal
cancer. World J Surg Oncol. 10:952012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ono M, Sakamoto M, Ino Y, Moriya Y,
Sugihara K, Muto T and Hirohashi S: Cancer cell morphology at the
invasive front and expression of cell adhesion-related carbohydrate
in the primary lesion of patients with colorectal carcinoma with
liver metastasis. Cancer. 78:1179–1186. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Japanese Society for Cancer of the Colon
and Rectum, . Japanese Classification of Colorectal Carcinoma. 2nd.
Kanehara & Co., Ltd.; Tokyo: 2009
|
|
17
|
Landis JR and Koch GG: The measurement of
observer agreement for categorical data. Biometrics. 33:159–174.
1977. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weinberg RA: The biology of cancer. 2nd.
Garland Science; New York, NY: pp. 685–689. 2014, PubMed/NCBI
|
|
19
|
Leber MF and Efferth T: Molecular
principles of cancer invasion and metastasis (Review). Int J Oncol.
34:881–895. 2009.PubMed/NCBI
|
|
20
|
Takada A, Ohmori K, Yoneda T, Tsuyuoka K,
Hasegawa A, Kiso M and Kannagi R: Contribution of carbohydrate
antigens sialyl Lewis A and sialyl Lewis X to adhesion of human
cancer cells to vascular endothelium. Cancer Res. 53:354–361.
1993.PubMed/NCBI
|
|
21
|
Gassmann P, Haier J, Schlüter K,
Domikowsky B, Wendel C, Wiesner U, Kubitza R, Engers R, Schneider
SW, Homey B and Müller A: CXCR4 regulates the early extravasation
of metastatic tumor cells in vivo. Neoplasia. 11:651–661. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujisaki T, Tanaka Y, Fujii K, Mine S,
Saito K, Yamada S, Yamashita U, Irimura T and Eto S: CD44
stimulation induces integrin-mediated adhesion of colon cancer cell
lines to endothelial cells by up-regulation of integrins and c-Met
and activation of integrins. Cancer Res. 59:4427–4434.
1999.PubMed/NCBI
|
|
23
|
Nakagoe T, Sawai T, Tsuji T, Jibiki M,
Ohbatake M, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H and
Tagawa Y: Differences in release mechanisms and distributions for
sialyl Le(a) and sialyl Le(x) antigens in colorectal cancer. Ann
Surg Oncol. 7:289–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakagoe T, Sawai T, Tsuji T, Jibiki M,
Ohbatake M, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H and
Arisawa K: Prognostic value of serum sialyl Lewis(a), sialyl
Lewis(x) and sialyl Tn antigens in blood from the tumor drainage
vein of colorectal cancer patients. Tumour Biol. 22:115–122. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berman JM, Cheung RJ and Weinberg DS:
Surveillance after colorectal cancer resection. Lancet.
355:395–399. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leporrier J, Maurel J, Chiche L, Bara S,
Segol P and Launoy G: A population-based study of the incidence,
management and prognosis of hepatic metastases from colorectal
cancer. Br J Surg. 93:465–474. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanas GP, Taylor A, Primrose JN, Langeberg
WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA and Poston G:
Survival after liver resection in metastatic colorectal cancer:
Review and meta-analysis of prognostic factors. Clin Epidemiol.
4:283–301. 2012.PubMed/NCBI
|
|
28
|
House MG, Ito H, Gönen M, Fong Y, Allen
PJ, DeMatteo RP, Brennan MF, Blumgart LH, Jarnagin WR and
D'Angelica MI: Survival after hepatic resection for metastatic
colorectal cancer: Trends in outcomes for 1,600 patients during two
decades at a single institution. J Am Coll Surg. 210:744–755. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2010 for the treatment of colorectal cancer. Int J Clin
Oncol. 17:1–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmoll HJ, Van Custem E, Stein A,
Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde
CJ, Balmana J, Regula J, et al: ESMO consensus guidelines for
management of patients with colon and rectal cancer. A personalized
approach to clinical decision making. Ann Oncol. 23:2479–2516.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Benson AB III, Schrag D, Somerfield MR,
Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J,
McAllister P, Van Cutsem E, et al: American Society of Clinical
Oncology recommendations on adjuvant chemotherapy for stage II
colon cancer. J Clin Oncol. 22:3408–3419. 2004. View Article : Google Scholar : PubMed/NCBI
|