Introduction
Natural killer (NK) cells, which serve critical
roles in cancer immunity, are regarded as the first line of defense
to eliminate transformed or malignant tumor cells (1). Therefore, multiple clinical laboratories
have examined the implications of NK cell-mediated immunity in
cancer and demonstrated that NK cells are functionally impaired in
the majority of forms of cancer (2–4).
Therefore, clinical attempts to boost endogenous NK cell activity,
using biological response modifiers or the adoptive transfer of
in vitro activated NK cells, have been investigated for the
treatment of patients with cancer (5,6). However,
a reliable tool to evaluate NK cell activity on a per-cell basis in
each patient should be described prior to clinical application in
order to design optimal treatment regimens, particularly since the
degree of impaired NK cell activity and its etiology differ from
patient to patient (7–9).
In vitro cytotoxicity assays have been widely
used in clinical laboratories to study NK cell function in patients
with cancer. A feature of this assay system is the co-culture of
effector cells with their specific target cells over a range of
ratios, in which the cytotoxicity of NK cells against their target
cells is measured by arithmetic calculation of the number of target
cells killed during the given reaction. Purified peripheral NK
cells from blood are a favored source of effector cells, but
unfractionated peripheral blood mononuclear cells (PBMCs) can also
suffice in these analyses. PBMCs are typically employed in research
laboratories, owing to the simple preparation procedures in
comparison with NK cell-specific isolations (2,10).
However, the use of pooled PBMCs prepared by a given number of
cells as effector cells has a clear disadvantage: Variations in NK
cell frequency in every pool of PBMCs hinders the accurate
interpretation of a net- or per-cell cytotoxicity for NK cells,
particularly since cytotoxicity, described as the percent (%) of
dead target cells, tends to be strongly influenced by alterations
in the NK cell population size within PBMC preparations (11–13).
Considering these factors, PBMCs in cytotoxicity
assays should be quantified per ml of blood to reflect the natural
fluctuations in the NK cell population within the bloodstream. This
may result in an improved understanding in cellular cytotoxicity,
since these changes in cell number are examined simultaneously.
The present study presents a novel and simple method
of resolving the NK cell frequency effect by measuring NK cell
cytotoxicity in patients with cancer and addressing new technical
terms, including ‘hemacytotoxicity’ and ‘NK lytic index,’ which
describe cytotoxicity of an undetermined number of PBMCs per ml of
blood and the arithmetical per-cell activity of NK cells inferred
from the hemacytotoxicity measure, respectively. Finally, the
present study illustrates a practical way of employing
hemacytotoxicity and the NK lytic index to improve understanding of
NK cell-mediated immunity in patients with cancer.
Materials and methods
Subjects
Blood was drawn from 47 patients (26 males and 21
females; age range, 34~76) with colorectal cancer (CRC) and 45
healthy volunteers (23 males and 22 females; age range, 48~82), and
immediately collected into heparinized tubes for in vitro
cellular functional assays and into potassium-EDTA tubes (Greiner
Bio-One, Kremsmünster, Austria) for the enumeration of lymphocyte
subsets. Informed written consent with a questionnaire to identify
medical history was obtained from all the participants. In case of
the patients, blood was collected at least three times: 3–8 days
prior to surgery, and then 7 days and 1 month after the surgery.
All procedures were performed with the approval of the
institutional review board of Seoul Song Do Colorectal Hospital
(IRB Number 2014–008).
Cytotoxicity assays
NK cell cytotoxicity against K562 cells was tested
following two different PBMC isolation methods as described in our
previous work (12). K562 cells
(KCLBNo. 10243), a human erythromyeloblastoid leukemia cell line,
were obtained from Korean Cell Line Bank (Seoul, Korea) and
maintained in RPMI-1640 medium (Welgene, Inc., Gyeongsang, Korea)
supplemented with 5% (v/v) fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and 1% (v/v)
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in
a humidified incubator (Thermo Fisher Scientific, Inc.) with 5%
CO2 at 37°C. The cell line was characterized utilizing
short tandem repeat profiling. For comparative analysis of two
different assays, ‘conventional cytotoxicity assays’ were performed
with three different effector-to-target cell ratios (E/T):
1.25×105, 2.5×105 and 5×105 PBMCs
against 20,000 K562 cells. Similarly, blood cytotoxicity assays
were simultaneously conducted with three different preparations of
PBMCs from 250, 500, and 1,000 µl of blood against 20,000 K562
cells. Then, a single E/T ratio of 1:25 was used for conventional
cytotoxicity, and PBMCs from 500 µl of blood were tested against
2,000 K562 cells for hemacytotoxicity. Percent cytotoxicity was
measured by using CytoTox 96 non-Radioactive assay kits (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol. The result of conventional cytotoxicity assays was
defined as ‘cytotoxicity’ and that of the per-ml blood cytotoxicity
assay was marked as ‘hemacytotoxicity’ to distinguish between the
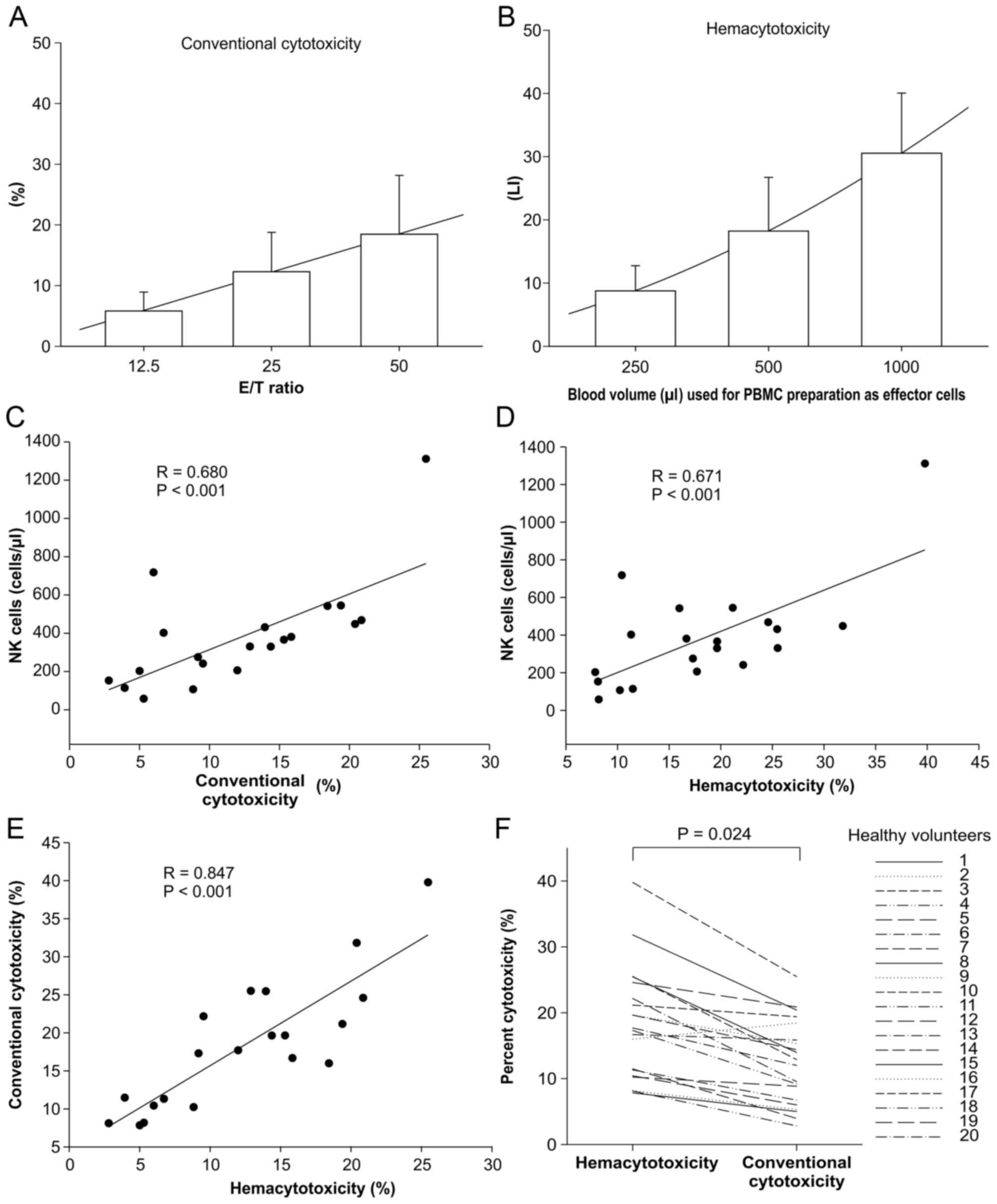
results (Figs. 1A and B). Percent
cytotoxicity was calculated using the following formula:
Cytotoxicity (%)=[(experimental release-effector
spontaneous release-target spontaneous release)/(target maximum
release-target spontaneous release)]x100.
Analysis of blood lymphocyte
subsets
Blood was collected in EDTA-coated vacuum containers
and the percentages of CD3+ T cells,
CD3+CD4+ helper T cells,
CD3+CD8+ cytotoxic T cells, NKG2D-positive
CD3−CD56+ NK cells, and
CD3+CD56+ NKT cells were analyzed in
erythrocyte-lysed whole blood by flow cytometry. Briefly, 50 µl of
blood was aliquoted into 12×75-mm capped polypropylene test tubes
and stained blood cells in a dark room for 15 min at room
temperature using the following mouse anti-human monoclonal
antibodies (MoAbs) purchased from BD Biosciences (San Jose, CA,
USA): Anti-CD3-fluorescein isothiocyanate (FITC; 1:100; cat. no.
555339), anti-CD8-phycoerythrin (PE; 1:100; cat. no. 555635),
anti-CD4-PE-Cy5 (1:100; cat. no. 555348), anti-CD56-PE (1:20; cat.
no. 555516), and anti-CD314-PE-Cy5 (1:20; cat. no. 562365).
Erythrocyte in blood cells were then lysed by adding 450 µl of FACS
lysing solution (BD Biosciences, Franklin Lakes, NJ) for 15 min at
room temperature, and analyzed using flow cytometry (FACSCalibur;
BD Biosciences). Enumeration of lymphocyte subsets was analyzed by
double platform method by calculating absolute counts from the
lymphocyte differentials using an automatic hematology analyzer
(Sysmex Corporation, Kobe, Japan). Data were analyzed using
CellQuest™ Pro software version 6.0 (BD
Biosciences).
Analysis of surface-bound transforming
growth factor (TGF)-β on lymphocyte subsets
Surface-bound TGF-β expression on lymphocyte subsets
was analyzed using flow cytometry with a panel of MoAbs against
FITC/PE/Peridinin chlorophyll protein complex combinations of CD4
(1:100; cat. no. 555346)/CD25 (1:20; cat. no. 555432)/TGF-β1 (1:20;
cat. no. FAB2463C) and CD19 (1:100; cat. no. 555412)/CD25/TGF-β1
for the analysis of CD4+CD25+ regulatory T
cells and CD19+ regulatory B cells, respectively.
Anti-TGF-β1 antibody was purchased from R&D Systems, Inc.
(Minneapolis, MN, USA) and the remaining antibodies were purchased
from BD Biosciences. PBMCs (10 µl) derived from 100 µl blood were
incubated at room temperature with human FC block (1:10; cat. no.
564220; BD Biosciences) for 30 min, labeled with each panel of
antibodies and 40 µl PBS, incubated for 30 min at room temperature
in the dark, washed in PBS, centrifuged at 440 × g for 5 min at
room temperature, and then resuspended in 200 µl PBS. Flow
cytometry was performed using a FACSCalibur™ flow
cytometer (BD Biosciences) and CellQuest™ Pro software
version 6.0 (BD Biosciences).
Statistical analysis
Pearson's and Spearman's correlation tests and the
Student's t-test were used for statistical tests, and all analyses
were carried out using the SPSS statistical package program,
version 18 (SPSS, Inc., Chicago, IL, USA), except for determination
of sensitivity, specificity, positive predictive value (PPV),
negative predictive value (NPV), and area under the curve (AUC)
obtained by Receiver operating characteristic analysis for
identifying patients with CRC. For this, DeLong's test was used and
calculations were performed using MedCalc software, version 9.5.2
(MedCalc Software bvba, Ostend, Belgium). P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinical characteristics of the study
population
A total of 47 patients (26 males and 21 females)
with CRC and 45 healthy volunteers (23 males and 22 females) were
classified by gender and age. There were no age differences between
patients and healthy groups (61±11 years vs. 59±9 years, P=0.538).
The tumor-node-metastasis (TNM) staging system was used for further
classification of the patients (Table
I).
 | Table I.Clinical characteristics of patients
with colorectal cancer and healthy controls. |
Table I.
Clinical characteristics of patients
with colorectal cancer and healthy controls.
|
Characteristics | Patient group
(n=47) | Healthy control
group (n=45) |
|---|
| Gender |
|
Male | 26 | 23 |
|
Female | 21 | 22 |
| Age (yr), mean ±
SD | 61 ± 11 | 59 ± 9 |
| T stage |
| T1 | 1 |
|
| T2 | 4 |
|
| T3 | 35 |
|
| T4 | 7 |
|
| Nodal status |
| N0 | 13 |
|
| N1 | 17 |
|
| N2 | 17 |
|
| Distant
metastasis |
| M0 | 44 |
|
| M1 | 3 |
|
| Stage |
| I | 0 |
|
| II | 13 |
|
|
III | 30 |
|
| IV | 4 |
|
NK cell frequency effect on percent
cytotoxicity
It was anticipated that NK cell frequency in the
pool of PBMCs in the cytotoxicity assay system would affect the
percent cytotoxicity. Therefore the effect of the frequency of NK
cells and other lymphocyte subsets on percent cytotoxicity was
investigated. To begin with, conventional cytotoxicity and
hemacytotoxicity were assessed in two different methods of
preparing PBMCs from 20 healthy volunteers. The mean concentration
of PBMCs was 9.36±3.04×105 cells/ml, and the percent
cytotoxicity for the three different ratios in both methods
demonstrated a linear correlation with the proportion of PBMCs
(r=0.994 for conventional cytotoxicity and 0.996 for
hemacytotoxicity, P<0.001; Fig. 1A and
B). Therefore, a 1:25 ratio (20,000 K562 cells:
5×105 PBMCs) for conventional cytotoxicity, and the
PBMCs in 500 µl of blood against 20,000 K562 cells for
hemacytotoxicity, were selected from the three different ratios.
Cytotoxicity and hemacytotoxicity were correlated with the absolute
number of NK cells in the pooled PBMCs (r=0.680 and r=0.671;
P<0.001, in both cases; Fig. 1C and
D), but not with the other cell types in the lymphocytes (data
not shown). Two different percent cytotoxicities were positively
correlated (r=0.847, P<0.001) where if the percentage of
conventional cytotoxicity were increase, the percentage of
hematocytotoxicity also rise as demonstrated in Fig. 1E; however, the mean ranks of 20
healthy volunteers were significantly different (P=0.024; Fig. 1F). These observations indicated that
NK cell frequency influences percent cytotoxicity regardless of
PBMC preparation method and should be properly adjusted to
accurately determine the cytotoxicity at a single-cell level in the
cytotoxicity assay system.
Compensation of NK cell frequency
effect on percent cytotoxicity
To compensate for the effect of NK cell frequency on
percent cytotoxicity, a novel method was developed for determining
the single cell activity of NK cells. Preparation of PBMCs per ml
of blood enabled the counting of the total number of NK cells in
such a way that it would be possible to calculate the dead target
cell counts per one single NK cell in the reaction. However, it was
technically difficult to determine NK cell numbers in the
conventional cytotoxicity assay system from a given number of
PBMCs. Therefore, by using hemacytotoxicity and absolute numbers of
NK cells in a given volume of blood, a new parameter, NK lytic
index, was devised to assess the inferential single cell activity
of NK cells. The formula for NK lytic index is as follows: NK lytic
index (LI)=dead target cell counts/NK cell counts ×1,000.
Therefore, one NK lytic index (LI) can be defined as
an arithmetical unit of single NK cell activity against target
cells. The lytic index for one of the healthy volunteers was
calculated in the following manner: 2×104 K562 cells
were co-cultured with undetermined numbers of PBMCs derived from
500 µl of whole blood and the consequent hemacytotoxicity was 20%.
Then, the number of lysed K562 cells in the reaction was determined
to be 4×103 cells by calculation of
(2×104initial K562 cells)x(0.2percent
hemacytotoxicity). The absolute number of NK cells was 500
cells/µl and the total number of NK cells in the reaction was
2.5×105 cells by calculation of (500
cells/µlabsolute number of NK cells)x(500 µlinitial
blood volume) in the pool of PBMCs from 500 µl of blood.
Thus, NK lytic index was 16 LI by division of
(4×103dead target cells) by
(2.5×105total NK cells in 500 µl
blood)x1,000. With this process, the NK lytic index of 20
healthy donors was calculated as depicted in Table II.
 | Table II.Data on the NK lytic index of 20
healthy volunteers, used to adjust NK cell frequency in PBMC
preparation. |
Table II.
Data on the NK lytic index of 20
healthy volunteers, used to adjust NK cell frequency in PBMC
preparation.
| Number | Gender | Age | NK
cellsa |
Hemacytotoxicityb | Conventional
cytotoxicityb | NK lytic
indexc |
|---|
| 1 | F | 63 | 448 | 31.82 | 20.40 | 28.41 |
| 2 | M | 58 | 366 | 19.65 | 15.32 | 21.48 |
| 3 | M | 35 | 1,311 | 39.78 | 25.47 | 12.14 |
| 4 | F | 43 | 275 | 17.30 |
9.18 | 25.16 |
| 5 | M | 68 | 718 | 10.42 |
6.00 |
5.81 |
| 6 | M | 60 | 206 | 17.69 | 11.99 | 34.35 |
| 7 | F | 56 | 114 | 11.48 |
3.94 | 40.28 |
| 8 | M | 44 | 431 | 25.46 | 13.96 | 23.63 |
| 9 | F | 51 | 58 |
8.18 |
5.30 | 56.41 |
| 10 | M | 53 | 545 | 21.16 | 19.39 | 15.53 |
| 11 | F | 61 | 153 |
8.11 |
2.81 | 21.20 |
| 12 | M | 66 | 468 | 24.59 | 20.86 | 21.02 |
| 13 | F | 51 | 381 | 16.68 | 15.84 | 17.51 |
| 14 | M | 53 | 330 | 19.64 | 14.38 | 23.81 |
| 15 | F | 50 | 203 |
7.84 |
5.01 | 15.45 |
| 16 | M | 53 | 542 | 15.99 | 18.43 | 11.80 |
| 17 | F | 56 | 331 | 25.51 | 12.89 | 30.83 |
| 18 | F | 24 | 402 | 11.32 |
6.72 | 11.26 |
| 19 | M | 67 | 107 | 10.23 |
8.83 | 38.24 |
| 20 | F | 59 | 241 | 22.17 |
9.53 | 36.80 |
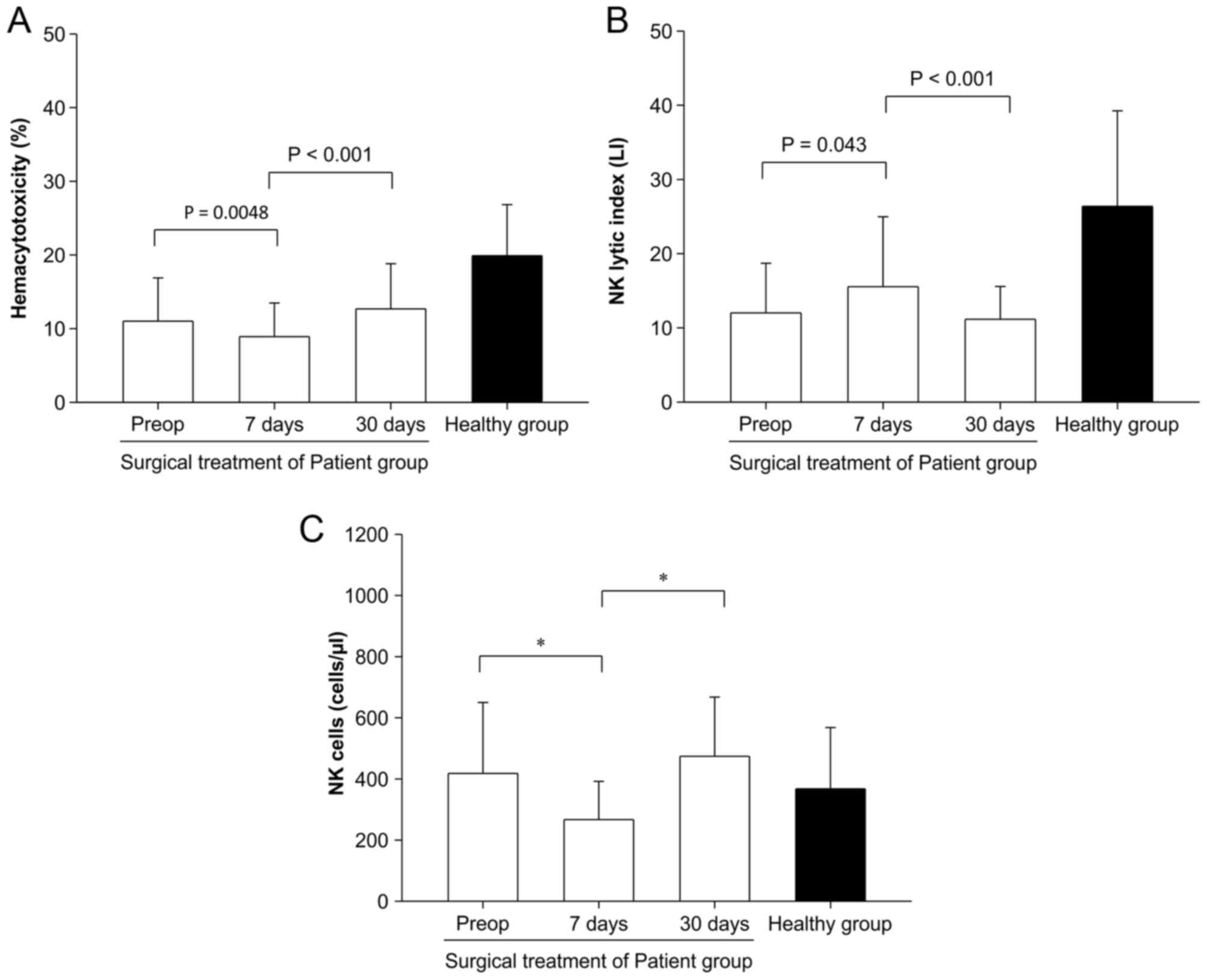
Inconsistent patterns of
hemacytotoxicity and NK lytic index
Next, it was investigated whether the patterns of NK
lytic index (single-cell activity) were consistent with those of
hemacytotoxicity (total-cell activity). To compare these two
parameters, the effect of surgery on hemacytotoxicity and NK lytic
index was investigated in 47 patients with CRC. The
hemacytotoxicity of preoperative (pre-op) patients was reduced
significantly 7 days following surgery (11.01±5.87% vs. 8.91±4.57%,
P=0.0048), but almost recovered 30 days later compared with pre-op
counterparts (8.91±4.51% vs. 12.68±6.14 %, P<0.001) (Fig. 2A). On the contrary, the NK lytic index
in pre-op state was significantly increased 7 days following
surgery (12.02±8.19 LI vs. 15.54±9.51 LI, P=0.043) and decreased
again 30 days following surgery (15.54±9.51 LI vs. 11.16±4.41 LI,
P<0.001) (Fig. 2B). NK cell number
in pre-op state was significantly decreased following surgery
(418±232 cells/µl vs. 267±125 cells/µl, P<0.05); however, that
was restored 30 days following surgery (267±125 cells/µl vs.
474±194 cells/µl, P<0.05) (Fig.
2C).
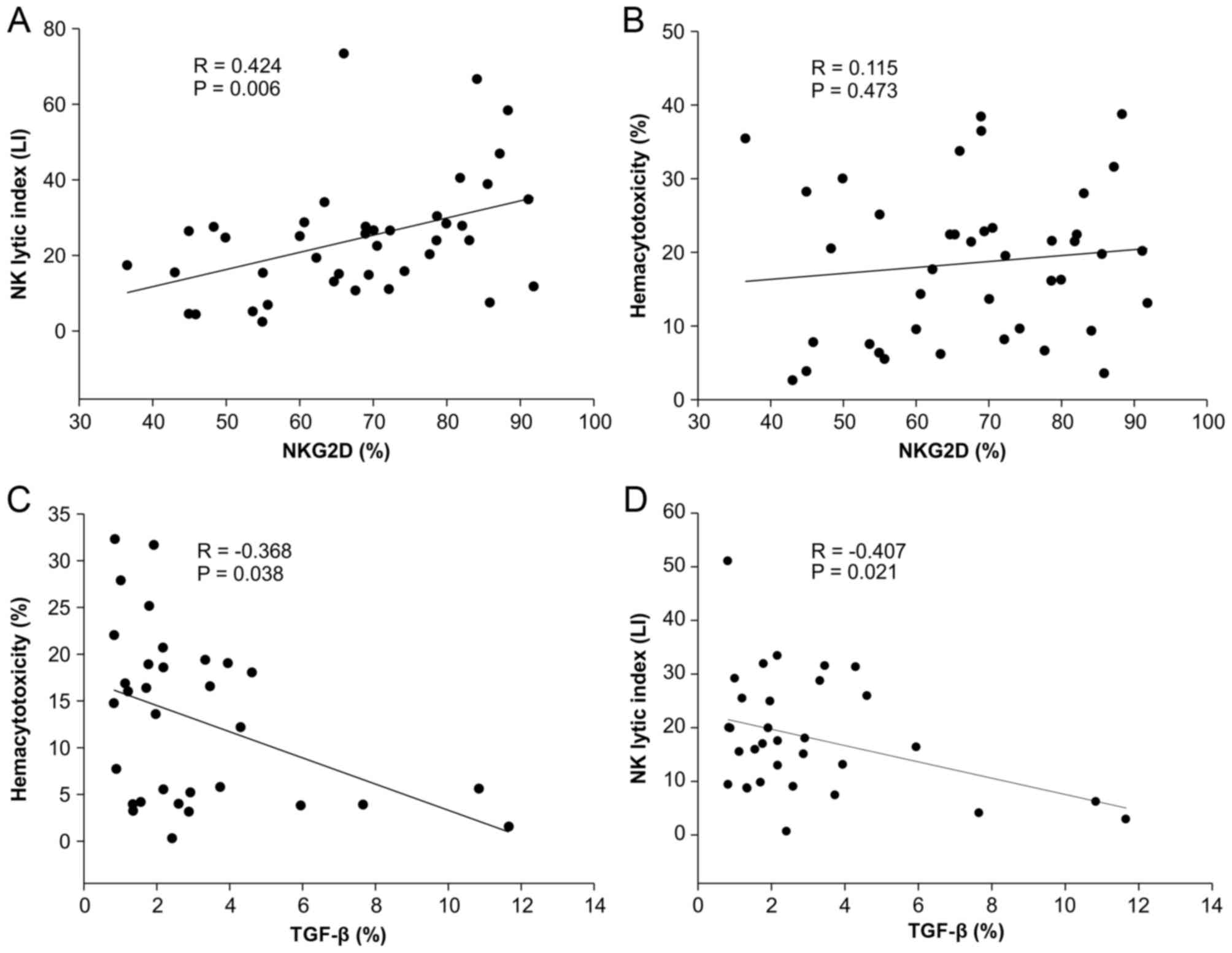
Different impact of NKG2D and TGF-β on
hemacytotoxicity and NK lytic index
On account of the different impacts of surgical
treatment on hemacytotoxicity and NK lytic index, it was examined
whether expression levels of NKG2D on NK cells and surface-bound
TGF-β on CD4+CD25+ regulatory T cells or
CD19+ regulatory B cells affected hemacytotoxicity and
NK lytic index differently. NKG2D expression on
CD3−CD56+ NK cells was positively correlated
with NK lytic index (r=0.424, P=0.006) but not with
hemacytotoxicity (r=0.115, P=0.473) (Fig.
3A and B). However, surface-bound TGF-β on regulatory T cells
and regulatory B cells did not exhibit any correlation with
hemacytotoxicity and NK lytic index (data not shown). The total
TGF-β expressing cell population examined by light scatter gating
using forward vs. side scatter was negatively correlated with
hemacytotoxicity and NK lytic index (r=−0.368 and r=−0.407,
respectively; P<0.05 in both cases) (Fig. 3C and D).
Sensitivity, specificity, and
predictive values of hemacytotoxicity and NK lytic index in
preoperative patients with colorectal cancer
Hemacytotoxicity and NK lytic index from 47
preoperative patients and healthy volunteers were further analyzed
for comparison. In hemacytotoxicity, the optimal cutoff value was
13.92 and value >13.92% had best sensitivity, specificity,
positive predictive value (PPV), and negative predictive value
(NPV). For the NK lytic index, as the cutoff value increased from
14.2 LI to 18.17 LI, the sensitivity and PPV value decreased but
the specificity and NPV value increased. Receiver operating
characteristic (ROC) curve analysis of hemacytotoxicity and NK
lytic index irrespective of cutoff value resulted in 0.831 and
0.843 (P=0.799), respectively (Table
III).
 | Table III.Sensitivity, specificity, PPV, and
NPV for hemacytotoxicity and NK lytic index. |
Table III.
Sensitivity, specificity, PPV, and
NPV for hemacytotoxicity and NK lytic index.
| Variable | Sensitivity (95%
CI) | Specificity (95%
CI) | PPV (95% CI) | NPV (95% CI) | AUC (95% CI) |
|---|
| Hemacytotoxicity
(%) |
|
>13.92 | 84.44
(70.5–93.5) | 70.21
(55.1–82.7) | 73.1
(59.0–84.4) | 82.5
(67.2–92.7) | 0.831
(0.738–0.901) |
| NK lytic index
(LI) |
|
>14.2 | 84.44
(70.5–93.5) | 65.96
(50.7–79.1) | 70.4
(56.4–82.0) | 81.6
(65.7–92.3) |
|
|
>16.69 | 71.11
(55.7–83.6) | 74.47
(59.7–86.1) | 72.7
(57.2–85.0) | 72.9
(58.2–84.7) | 0.843
(0.752–0.911) |
|
>18.17 | 62.22
(46.5–76.2) | 80.85
(66.7–90.9) | 75.7
(58.8–88.2) | 69.1
(55.2–80.9) |
|
Discussion
Despite many advantages, predicting the in
vivo states of biological systems by extrapolating in
vitro experimental results is challenging (14). The in vitro NK cell
cytotoxicity assay using PBMCs is advantageous but also
challenging; many researchers and clinicians have employed this
useful technique for decades to understand the role of NK cells in
various immune-related human diseases (15–17). The
introduction of lymphokine-activated killer cells by Rosenberg and
colleagues in 1982 (18), encouraged
more oncologists to perform this in vitro assay, which has
become a gold standard for screening and diagnostic testing tools
assessing cancer immunity. Thus, the role of NK cells had been
extensively studied by using cytotoxicity assays in terms of
implication or correlation of NK cells on critical matters
associated with cancer development or treatment, including
incidence and prognosis (13,19,20), stage
and metastasis (21,22), and surgery and chemotherapy (23–27).
Cytotoxicity assays are often challenging owing to
their limitations when used as a general diagnostic tool of cancer
immunity in clinical settings. For instance, certain scientists
have argued that NK cell cytotoxicity described in percent specific
lysis of target cells is not suitable for comparative study even
though such comparisons were made at the same E/T ratio in a
previous study (28). Therefore, the
new conceptual parameter, lytic unit, was devised and introduced as
an alternative for using percent cytotoxicity (29). A lytic unit can be defined as the
number of effector cells necessary to cause lysis of a specified
percentage of its target cells (30).
However, certain researchers have raised another objection to using
the lytic unit in comparing the results of cytotoxicity in patients
because the lytic unit inferred from establishing a model of the
dose-response curve data may lead to inaccurate estimation
(28–30).
However, a fundamental limitation for the general
application of the cytotoxicity assay in clinical settings may be
the low significance of the results in predicting the
pathophysiological states of patients with cancer in terms of
cancer immunity; this lack of significance may primarily result
from a lack of knowledge of the effect of NK cell frequency on
percent cytotoxicity. In particular, the lytic unit does not
address the matter of NK cell frequency in the assay system;
rather, it should be regarded as a reasonable proxy for the percent
lysis generated at various E/T ratios. In contrast, an agarose gel
or poly-L-lysine hydrobromide polymer-based cell cytotoxicity assay
system, in which effector cell/target cell conjugates are made and
then the degree of effector cells bound to target cells is measured
as a percentage to determine NK cell activity, may resolve the NK
cell frequency effect (31,32). This method deals with single-cell
activity conceptually equivalent to the lytic index. In fact, this
type of assay system has been widely used and published as a
‘single-cell cytotoxicity assay.’ However, this single-cell
cytotoxicity assay should be performed separately from conventional
cytotoxicity assays, and the resulting requirement for dual assays
may be time-consuming and cumbersome.
In this context, by demonstrating that NK cell
frequency significantly influences the results of the percent
cytotoxicity in evaluating NK cell activity of patients with
cancer, hemacytotoxicity generated from the preparation of PBMCs
per ml of blood should be used in place of the conventional
cytotoxicity assays, as NK cell frequency effect can be compensated
for by adoption of the NK lytic index which simply indicates a net
per-cell cytotoxicity. However, NK lytic index would not be the
same as a per-cell activity of NK cells resulting from the system,
in which isolated pure NK cells are used as effector cells against
its target cells; alternatively, it could imply an actual in
vivo per-cell activity of NK cells in the bloodstream in which
NK cells cross-talk with diverse immune cells like dendritic cells,
helper T cells, regulatory T cells, and NKT cells, and these
reciprocal interactions affect NK cell activity (33–35).
On formulating the NK lytic index, the present study
identified that the order of rank between a paired hemacytotoxicity
and NK lytic index does not correspond as described in Table II. This observation raised the
question of whether the total cell activity represented by
hemacytotoxicity would be equal to the sum of single cell
activities described as NK lytic indexes. To investigate this
matter, the surgical effect on the hemacytotoxicity and NK lytic
index was investigated with an idea that a number of previous
studies demonstrate that surgical stress induces impaired NK cell
activity in the early period following surgery (25,36,37). In
the present study, hemacytotoxicity decreased at 7 days following
surgery but recovered from surgical stress within 30 days; however,
NK lytic index increased in 7 days following surgery and decreased
again 30 days following surgery. In addition, the redistribution of
NK cells corresponded to the changing pattern of hemacytotoxicity.
This observation does not agree with those of previous reports
indicating that impaired NK cell cytotoxicity is mainly caused by
direct toxic effects on NK cells (24,26).
Instead, the present study revealed that impaired NK cell activity
is primarily due to NK cell redistribution caused by surgical
stress, and impaired NK cell total activity may be compensated for
by increasing single cell activity by means of immune homeostatic
regulation; e.g., the phenomenon that decreasing the number of
cytotoxic T cells may be compensated for by increasing the number
of NK cells with ageing (38).
Therefore, the results from the present study imply that total NK
cell activity is tightly regulated by redistribution of NK cell
number and single cell activity.
Similarly, results of further analysis suggested
that all three parameters, namely total NK cell activity, NK cell
number and single cell activity, should be examined in parallel.
Reduced NKG2D expression in NK cells in the majority of patients
with cancer has been reported in a number of studies (39–41), and
modulation of NKG2D is regarded as a promising therapeutic approach
(42). In the present study, NKG2D
expression on NK cells correlated with NK lytic index but not with
hemacytotoxicity. This observation suggests that decreased NKG2D
expression leads to decreased per-cell activity and total cell
activity may not be affected by decreased NKG2D expression, because
increasing NK cell numbers may overcome decreased per-cell
activity. Likewise, TGF-β1 is a critical molecule for regulating NK
cell activity and induces impaired NK cell activity by the
downregulation of NKG2D expression (43–46). The
observation from the present study that surface-bound TGF-β1 on
lymphocytes inversely correlates with hemacytotoxicity and NK lytic
index indicates that suppression of TGF-β1 signaling may be a
critical checkpoint in recovering impaired NK cell function
compared with direct modulation of NKG2D expression.
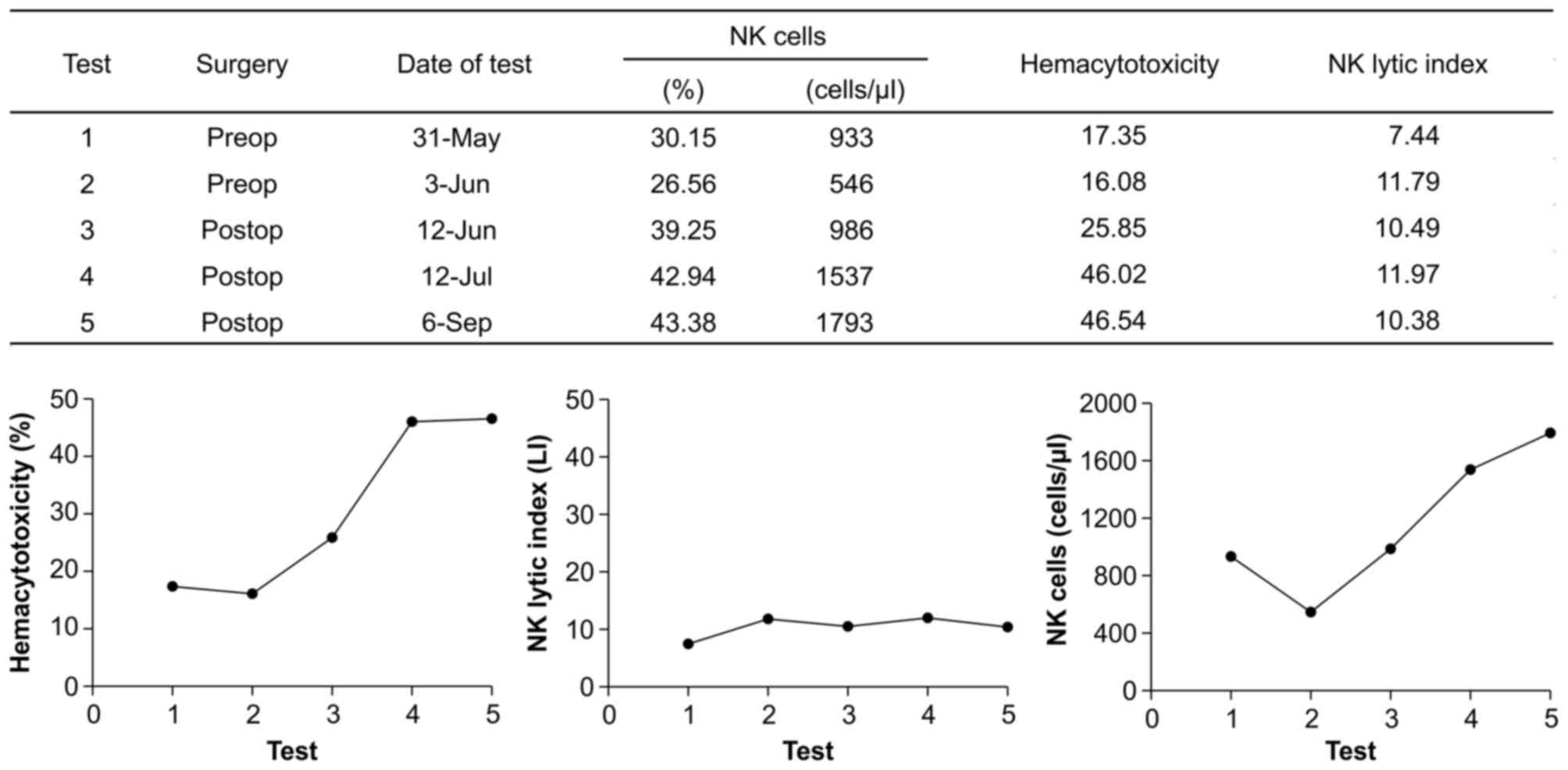
Therefore, parallel analysis of hemacytotoxicity and
NK lytic index can be beneficial in evaluating NK cell immunity in
patients with cancer as a representative case chosen from among 47
patients (Fig. 4). The patient
appeared to be improving in terms of NK cell activity during the
study period. However, the seemingly increased hemacytotoxicity is
induced by increasing the NK cell number. Therefore, it could not
be concluded that the NK cell activity of the patient is improving
following surgery because the low NK lytic index of the patient
remained unchanged. For this reason, a treatment for increasing
single cell activity of NK cells might be first considered in this
patient.
There are certain limitations in the system
considering hemacytotoxicity and NK lytic index. One is a technical
defect in that the absolute counts of NK cells were analyzed in a
double-platform method of flow cytometry by using lymphocyte
differentials resulting from a hematology analyzer. Regarding
immunophenotyping of blood immune cells, a current gold standard is
the single platform method (47–49). For
this reason, using NK cell counts determined by the above-mentioned
method may lower the accuracy of calculating the NK lytic index.
The other limitation is the matter of general standardization for
clinical application. Since hemacytotoxicity and the NK lytic index
also depend on the selected E/T ratio, comparing the results of
patients obtained at different E/T ratios would not be appropriate;
however, this problem could potentially be circumvented by
technical standardization to control for inter- and intra-assay
variation.
In conclusion, the present study demonstrated that
the NK cell frequency effect on the NK cell cytotoxicity assay
using PBMC preparations should be resolved to obtain precise
results by employing the NK lytic index. The results indicated that
total cell activity is not always a sum of single cell activities,
and that surgical treatment exerts different effects on total cell
activity (hemacytotoxicity) and single cell activity (NK lytic
index). The present study suggests that the three parameters,
hemacytotoxicity, NK lytic index, and NK cell number, should be
thoroughly assessed to evaluate NK cell immunity in patients with
cancer.
Acknowledgements
The present study was supported and funded (grant
no. SD2014008) by Seoul Song Do Colorectal Hospital (Seoul,
Republic of Korea).
Glossary
Abbreviations
Abbreviations:
|
AUC
|
area under the curve
|
|
CRC
|
colorectal cancer
|
|
E/T
|
effector-to-target cell ratio
|
|
FITC
|
fluorescein isothiocyanate
|
|
LI
|
lytic index
|
|
NK
|
natural killer
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
PE
|
phycoerythrin
|
References
|
1
|
Smyth MJ, Hayakawa Y, Takeda K and Yagita
H: New aspects of natural-killer-cell surveillance and therapy of
cancer. Nat Rev Cancer. 2:850–861. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levy EM, Roberti MP and Mordoh J: Natural
killer cells in human cancer: From biological functions to clinical
applications. J Biomed Biotechnol. 2011:6761982011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coca S, Perez-Piqueras J, Martinez D,
Colmenarejo A, Saez MA, Vallejo C, Martos JA and Moreno M: The
prognostic significance of intratumoral natural killer cells in
patients with colorectal carcinoma. Cancer. 79:2320–2328. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishigami S, Natsugoe S, Tokuda K, Nakajo
A, Che X, Iwashige H, Aridome K, Hokita S and Aikou T: Prognostic
value of intratumoral natural killer cells in gastric carcinoma.
Cancer. 88:577–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng M, Chen Y, Xiao W, Sun R and Tian Z:
NK cell-based immunotherapy for malignant diseases. Cell Mol
Immunol. 10:230–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Albertsson PA, Basse PH, Hokland M,
Goldfarb RH, Nagelkerke JF, Nannmark U and Kuppen PJ: NK cells and
the tumour microenvironment: Implications for NK-cellfunction and
anti-tumour activity. Trends Immunol. 24:603–609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balch CM, Tilden AB, Dougherty PA and
Cloud GA: Depressed levels of granular lymphocytes with natural
killer (NK) cell function in 247 cancer patients. Ann Surg.
198:192–199. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levy S, Herberman R, Lippman M and
d'Angelo T: Correlation of stress factors with sustained depression
of natural killer cell activity and predicted prognosis in patients
with breast cancer. J Clin Oncol. 5:348–353. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schantz SP, Shillitoe EJ, Brown B and
Campbell B: Natural killer cell activity and head and neck cancer:
A clinical assessment. J Natl Cancer Inst. 77:869–875.
1986.PubMed/NCBI
|
|
10
|
Brunner KT, Mauel J, Cerottini JC and
Chapuis B: Quantitative assay of the lytic action of immune
lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro;
inhibition by isoantibody and by drugs. Immunology. 14:181–196.
1968.PubMed/NCBI
|
|
11
|
Pross HF, Baines MG, Rubin P, Shragge P
and Patterson MS: Spontaneous human lymphocyte-mediated
cytotoxicity against tumor target cells. IX. The quantitation of
natural killer cell activity. J Clin Immunol. 1:51–63. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JC, Choi J, Lee SJ, Lee YA, Jeon YM,
Kang YW and Lee JK: Evaluation of cytolytic activity and phenotypic
changes of circulating blood immune cells in patients with
colorectal cancer by a simple preparation of peripheral blood
mononuclear cells. J Korean Surg Soc. 85:230–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
White D, Jones DB, Cooke T and Kirkham N:
Natural killer (NK) activity in peripheral blood lymphocytes of
patients with benign and malignant breast disease. Br J Cancer.
46:611–616. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hartung T and Daston G: Are in vitro tests
suitable for regulatory use? Toxicol Sci. 111:233–237. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smith C, Jalbert E, de Almeida V, Canniff
J, Lenz LL, Mussi-Pinhata MM, Cohen RA, Yu Q, Amaral FR, Pinto J,
et al: Altered natural killer cell function in HIV-exposed
uninfected infants. Front Immunol. 8:4702017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mhatre S, Madkaikar M, Ghosh K, Desai M,
Pujari V and Gupta M: Rapid flow cytometry based cytotoxicity assay
for evaluation of NK cell function. Indian J Exp Biol. 52:983–988.
2014.PubMed/NCBI
|
|
17
|
Cho D, Shook DR, Shimasaki N, Chang YH,
Fujisaki H and Campana D: Cytotoxicity of activated natural killer
cells against pediatric solid tumors. Clin Cancer Res.
16:3901–3909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grimm EA, Mazumder A, Zhang HZ and
Rosenberg SA: Lymphokine-activated killer cell phenomenon. Lysis of
natural killer-resistant fresh solid tumor cells by interleukin
2-activated autologous human peripheral blood lymphocytes. J Exp
Med. 155:1823–1841. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tartter PI, Steinberg B, Barron DM and
Martinelli G: The prognostic significance of natural killer
cytotoxicity in patients with colorectal cancer. Arch Surg.
122:1264–1268. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin CC, Kuo YC, Huang WC and Lin CY:
Natural killer cell activity in lung cancer patients. Chest.
92:1022–1024. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanna N and Schneider M: Enhancement of
tumor metastasis and suppression of natural killer cell activity by
beta-estradiol treatment. J Immunol. 130:974–980. 1983.PubMed/NCBI
|
|
22
|
Espi A, Arenas J, Garcia-Granero E, Marti
E and Lledó S: Relationship of curative surgery on natural killer
cell activity in colorectal cancer. Dis Colon Rectum. 39:429–434.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beano A, Signorino E, Evangelista A, Brusa
D, Mistrangelo M, Polimeni MA, Spadi R, Donadio M, Ciuffreda L and
Matera L: Correlation between NK function and response to
trastuzumab in metastatic breast cancer patients. J Transl Med.
6:252008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pollock RE, Lotzová E and Stanford SD:
Mechanism of surgical stress impairment of human perioperative
natural killer cell cytotoxicity. Arch Surg. 126:338–342. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lennard TW, Shenton BK, Borzotta A,
Donnelly PK, White M, Gerrie LM, Proud G and Taylor RM: The
influence of surgical operations on components of the human immune
system. Br J Surg. 72:771–776. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pollock RE, Lotzová E and Stanford SD:
Surgical stress impairs natural killer cell programming of tumor
for lysis in patients with sarcomas and other solid tumors. Cancer.
70:2192–2202. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Greenfeld K, Avraham R, Benish M, Goldfarb
Y, Rosenne E, Shapira Y, Rudich T and Ben-Eliyahu S: Immune
suppression while awaiting surgery and following it: Dissociations
between plasma cytokine levels, their induced production, and NK
cell cytotoxicity. Brain Behav Immun. 21:503–513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bryant J, Day R, Whiteside TL and
Herberman RB: Calculation of lytic units for the expression of
cell-mediated cytotoxicity. J Immunol Methods. 146:91–103. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pollock RE, Zimmerman SO, Fuchshuber P and
Lotzová E: Lytic units reconsidered: Pitfalls in calculation and
usage. J Clin Lab Anal. 4:274–282. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bloom ET and Korn EL: Quantification of
natural cytotoxicity by human lymphocyte subpopulations isolated by
density: Heterogeneity of the effector cells. J Immunol Methods.
58:323–335. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Montelli TC, Peraçoli MT, Gabarra RC,
Soares AM and Kurokawa CS: Familial cancer: Depressed NK-cell
cytotoxicity in healthy and cancer affected members. Arq
Neuropsiquiatr. 59:6–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim GS, Youn JK, Kim JD and Kim NH:
Natural killer cell activity in rheumatoid arthritis measured by a
single cell cytotoxicity assay. Yonsei Med J. 29:160–165. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fernandez NC, Lozier A, Flament C,
Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T,
Maraskovsky E and Zitvogel L: Dendritic cells directly trigger NK
cell functions: Cross-talk relevant in innate anti-tumor immune
responses in vivo. Nat Med. 5:405–411. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zingoni A, Sornasse T, Cocks BG, Tanaka Y,
Santoni A and Lanier LL: Cross-talk between activated human NK
cells and CD4+ T cells via OX40-OX40 ligand interactions. J
Immunol. 173:3716–3724. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Terme M, Chaput N, Combadiere B, Ma A,
Ohteki T and Zitvogel L: Regulatory T cells control dendritic
cell/NK cell cross-talk in lymph nodes at the steady state by
inhibiting CD4+ self-reactive T cells. J Immunol. 180:4679–4686.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ogawa K, Hirai M, Katsube T, Murayama M,
Hamaguchi K, Shimakawa T, Naritake Y, Hosokawa T and Kajiwara T:
Suppression of cellular immunity by surgical stress. Surgery.
127:329–336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Na YM, Kim MY, Kim YK, Ha YR and Yoon DS:
Exercise therapy effect on natural killer cell cytotoxic activity
in stomach cancer patients after curative surgery. Arch Phys Med
Rehabil. 81:777–779. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi J, Lee SJ, Lee YA, Maeng HG, Lee JK
and Kang YW: Reference values for peripheral blood lymphocyte
subsets in a healthy korean population. Immune Netw. 14:289–295.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Saito H, Osaki T and Ikeguchi M: Decreased
NKG2D expression on NK cells correlates with impaired NK cell
function in patients with gastric cancer. Gastric Cancer. 15:27–33.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee JC, Lee KM, Kim DW and Heo DS:
Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies
impaired NK cytotoxicity in cancer patients. J Immunol.
172:7335–7340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hilpert J, Grosse-Hovest L, Grünebach F,
Buechele C, Nuebling T, Raum T, Steinle A and Salih HR:
Comprehensive analysis of NKG2D ligand expression and release in
leukemia: Implications for NKG2D-mediated NK cell responses. J
Immunol. 189:1360–1371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Spear P, Wu MR, Sentman ML and Sentman CL:
NKG2D ligands as therapeutic targets. Cancer Immun.
13:82013.PubMed/NCBI
|
|
43
|
Bellone G, Aste-Amezaga M, Trinchieri G
and Rodeck U: Regulation of NK cell functions by TGF-beta 1. J
Immunol. 155:1066–1073. 1995.PubMed/NCBI
|
|
44
|
Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z
and Wei H: TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP
expression on human NK cells contributes to HBV persistence. PLoS
Pathog. 8:e10025942012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Krasagakis K, Thölke D, Farthmann B,
Eberle J, Mansmann U and Orfanos CE: Elevated plasma levels of
transforming growth factor (TGF)-beta1 and TGF-beta2 in patients
with disseminated malignant melanoma. Br J Cancer. 77:1492–1494.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ghiringhelli F, Ménard C, Terme M, Flament
C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, et
al: CD4+CD25+ regulatory T cells inhibit natural killer cell
functions in a transforming growth factor-beta-dependent manner. J
Exp Med. 202:1075–1085. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lima M, Teixeira MA, Queirós ML, Leite M,
Santos AH, Justiça B and Orfão A: Immunophenotypic characterization
of normal blood CD56+lo versus CD56+hi NK-cell subsets and its
impact on the understanding of their tissue distribution and
functional properties. Blood Cells Mol Dis. 27:731–743. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bryceson YT, Fauriat C, Nunes JM, Wood SM,
Björkström NK, Long EO and Ljunggren HG: Functional analysis of
human NK cells by flow cytometry. Methods Mol Biol. 612:335–352.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kane KL, Ashton FA, Schmitz JL and Folds
JD: Determination of natural killer cell function by flow
cytometry. Clin Diagn Lab Immunol. 3:295–300. 1996.PubMed/NCBI
|