Introduction
Human cancer remains a leading cause of mortality
worldwide despite recent advances in therapeutic methods (1). Incidences of thyroid cancer have
steadily increased, and the disease has become the most prevalent
endocrine malignancy worldwide (2).
Thyroid cancer has an age-standardized incidence distribution and
is estimated to occur in 9.1 per 100,000 females and 2.9 per
100,000 males in developed countries (3) According to the most recent World Health
Organization classification of thyroid tumors, the disease
classification includes papillary thyroid cancer (PTC), follicular
thyroid cancer (FTC), poorly differentiated thyroid cancer, and
anaplastic thyroid cancer (4). PTC
and FTC are differentiated thyroid cancers and account for >90%
of all thyroid malignancies (5). The
classic treatment of thyroid cancer is a total thyroidectomy
followed by radioiodine treatment (5). Chemotherapy and biochemotherapy have
also been used in the treatment of advanced melanoma. However,
these treatments lack sufficient efficacy, and their toxicity and
significant side effects greatly restrict their clinical
application (6). Therefore, it is
necessary to find an efficient and low-toxic anticancer drug for
patients with thyroid cancer.
Antimicrobial peptides (AMPs) are natural peptides
found in a wide range of organisms, including prokaryotes, insects,
fish, amphibians and mammals (7).
AMPs are cationic and amphiphilic molecules and have a wide range
of characteristics based on peptide sequences, sizes, structural
motifs and the presence of disulfide bonds (8). The molecules possess broad antimicrobial
activity by binding to target bacteria to disrupt the membrane
structure or by inhibiting fundamental bacterial metabolism
(9). In addition to the antimicrobial
activity, previous studies have also demonstrated that AMPs can
selectively kill cancer cells because the membrane proteins of
cancer cells are negatively charged due to glycosylation (10).
Melittin, a major peptide component of bee venom
(Apis mellifera), is a cationic peptide comprising a small
linear peptide of 26 amino acid residues (11,12).
Melittin was demonstrated to exert broad antimicrobial activity
against bacteria by intercalating into cell membranes and causing
changes in the membrane properties (13). Previous studies demonstrated that
melittin also has in vitro anticancer activities against
several cancerous cells, including renal, lung, breast and bladder
cells (14,15). However, high synthesis cost, high
cytotoxicity and low stability have prevented the development of
melittin as a promising anticancer agent (16,17).
TT-1 is a mutant of melittin generated by a
reduction of the peptide chain length and replacing glycine
residues with lysine residues. The peptide sequence was changed
from ‘GIG AVL KVL TTG LPA LIS WIK RKR QQ’ to ‘KIK AVL KVL TT’,
which contained only 11 amino acids. The TT-1 mutant retained the
amino-terminal active site region of melittin, has an increased
hydrophobicity and a decreased net charge, which indicates a higher
stability and lower toxicity compared with melittin (18,19). The
present study investigated the activity and the mechanism of TT-1
in the treatment of human thyroid cancer TT cells. The results
revealed that TT-1 suppressed the proliferation of TT cells by
inducing apoptosis via upregulation of Bax, downregulation of
B-cell lymphoma-2 (Bcl-2) and the activation of caspase-3 and −9 at
transcriptional and translational levels. These interferences
further inhibited TT tumor growth in nude mice. These results
highlighted the therapeutic potential of TT-1 in thyroid
cancer.
Materials and methods
Peptide synthesis
TT-1 (KIKAVLKVLTT) was synthesized by GL Biochem
(Shanghai, China) via a stepwise solid phase methodology.
The resulting peptide was purified via a Sephadex gel column and
high-performance liquid chromatography to achieve a >98%
homogeneity of the purified peptide. The peptide information was
analyzed using the antimicrobial peptide database (http://aps.unmc.edu/AP/main.html). After entering
the home page of the website, ‘Calculation & Prediction’ was
chosen and the amino acid sequence of the peptide was entered in
the newly opened web page, which then displays information on the
peptide.
Cell lines and regents
The human thyroid cancer cell line TT and a normal
human thyroid follicular epithelial cell line Nthy-ori3-1, obtained
from the American Type Culture Collection (Manassas, VA, USA), were
maintained as previously described (20). Briefly, TT and Nthy-ori3-1 were
cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
provided by Gibco (Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin, and 100 U/ml streptomycin. The cells were cultured at
37°C with 5% CO2. MTT, sodium pyruvate and dimethyl
sulfoxide (DMSO) were obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Annexin V-fluorescein (AV) and propidium
iodide (PI) were purchased from Invitrogen (Thermo Fisher
Scientific, Inc.). Fluorometric assay kits for measuring the
activities of caspase-3 and 9 were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Monoclonal antibodies against
Bax (cat. no. sc-52895; dilution: 1:1,000) and Bcl-2 (cat. no.
sc-509; dilution: 1:1,000) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The easy Plus Mini kit,
iScript Select cDNA Synthesis kit, SyberGreen qPCR primer and
iCycleriQ™ multicolor real time PCR detection system were purchased
from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
Cell viability assay
To evaluate the effects of TT-1 on TT cells and
Nthy-ori3-1 cells, the MTT assay was conducted as previously
described (21). Briefly, the cells
were cultured in 96-well plates at a density of 5×103
cells/well and allowed to attach for 12 h. Different concentrations
(0–32 µg/ml) of TT-1 were added to the cells, and the cells were
further incubated for 24, 48 and 72 h at 37°C. Following
incubation, the cells were incubated with MTT solution (5 µg/ml)
for 4 h at 37°C followed by the addition of 150 µl DMSO per well
and then shaken for 5 min prior to measuring the absorbance at 490
nm.
Cell apoptosis assay
Cell apoptosis assays (22) were conducted by double staining with
AV and propidium iodide (PI) kit (ebioscience; Thermo Fisher
Scientific, Inc.) to investigate whether TT-1 is able to induce
apoptosis in TT cells. TT cells (5×105 cells/well) were
placed in 6-well plastic plates 24 h prior to the TT-1 treatments.
Following the replacement of medium supernatant, various
concentrations of TT-1 (0–8 µg/ml) diluted in PBS were added.
Following incubation at 37°C with 5% CO2 for 48 h, the
cells were harvested. The AV/PI assays were performed following the
manufacturer's instructions. Subsequently, the cell samples were
analyzed by flow cytometry (BD Biosciences, San Jose, CA, USA) and
the data was analyzed by the flowjo 9 software (FlowJo LLC,
Ashalnd, OR, USA).
Western blot analysis for Bax and
Bcl-2 protein expression
The effects of TT-1 on Bax and Bcl-2 expression of
TT cells were examined by western blotting as previously described
(23). TT cells treated with or
without TT-1 (0–8 µg/ml) for 48 h were homogenized in lysis buffer
and centrifuged at 10,000 × g for 20 min at 4°C. Then, the
supernatants were analyzed for protein content by BCA assay. Equal
amounts of protein sample (50 µg) were loaded and separated by 12%
SDS-PAGE and electrically transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). Afterward, the
membranes were blocked in TBST supplemented with 5% bovine serum
albumin for 2 h at 37°C followed by incubation at 4°C overnight in
primary anti-Bax and anti-Bcl-2 antibodies, and finally incubation
at 37°C for 1 h with secondary antibodies [goat anti-mouse IgG
(H&L) (HRP), cat. no. KC-MM-035; goat anti-rabbit IgG (H&L)
(HRP) or cat. no. KC-RB-035; both diluted to 1:5,000; both Zhejiang
Kangchen Biotech Co., Ltd., Wuhan, China]. Following washing with
TBST three times for 10 min, the membranes were exposed by a
chemiluminescence (ECL) detection kit. Bio-Imaging Image Lab 6.0
System software (Image Lab, Hercules, CA, USA) was used to detect
the blot. Meanwhile, all blots were stripped and reprobed using a
monoclonal anti-β-actin antibody (Santa Cruz Biotechnology, Inc.;
grant no. sc-47778; dilution 1:1,000) to determine whether the
proteins were equally loaded.
Determining the activity of caspase-3
and −9
The effects of TT-1 on the activity of caspase-3 and
−9 in TT cells were determined using a fluorometric assay kit
(Calbiochem; EMD Millipore) according to the manufacturer's
protocol as previously described (24). Briefly, TT cells were treated with
TT-1 (0–8 µg/ml) for 48 h and harvested prior to the preparation of
cell lysates. Then, the reaction buffer and the corresponding
fluorogenic peptide substrate, Ac-DEVD-AMC (caspase-3) and
Ac-LEHD-AMC (caspase-9), were added to the cell lysates and
incubated for 2 h at 37°C in the dark. The activity of caspase-3
and −9 in TT-1-treated TT cells were investigated using a
microplate reader at 390 nm (excitation) and 500 nm (emission).
Quantitative reverse transcription
polymerase chain reaction (RT-qPCR)
The effects of TT-1 on the Bax, Bcl-2, caspase-3 and
caspase-9 RNA expression in TT cells were examined by RT-qPCR as
previously described (25,26). Total RNA was extracted from TT cells
with or without TT-1 treatment (0–8 µg/ml for 48 h) by using Trizol
reagent and then purified with an RNeasy Mini kit (Qiagen GmbH,
Hilden, Germany). Afterward, qPCR was conducted with an ABI PRISM
7300 sequence detection system (Applied Biosystems; Thermo Fisher
Scientific Inc.), and 3 wells were used for each reaction. The
relative mRNA expression was calculated using the comparative Cq
(2−ΔΔCq) method (27). The
primers were as follows: Caspase-3 forward, 5′-AGGAAGGTGGCAACG-3′
and reverse, 5′-CGCCAAATCTTGCTAAT-3′; caspase-9 forward,
5′-GGCTGTCTACGGCACAGATGGA-3′ and reverse,
5′-CTGGCTCGGGGTTACTGCCAG-3′; Bax forward,
5′-GGCCCACCAGCTCTGAGCAGA-3′ and reverse,
5′-GCCACGTGGGCGGTCCCAAAGT-3′; Bcl-2 forward,
5′-GTGGAGGAGCTCTTCAGGGA-3′ and reverse,
5′-AGGCACCCAGGGTGAGCAA-3′.
TT-xenograft mouse model and TT-1
administration in vivo
Ethical approval for the present study was obtained
from the Institutional Animal Care and Use Committee at Jilin
University (Jilin, China). A total of 40 4-week-old nude mice
(male; weight, 16–18 g) were purchased from the Jilin University
Bethune School of Medicine and housed in a rodent facility at 22°C
with a 12 h light-dark cycle. The mice were provided with
continuous standard rodent chow and water. TT cells
(1×107) were collected in the logarithmic phase of
growth, diluted with normal saline and then inoculated
intradermally into the hind flank. The tumors were inoculated for
12 days. The nude mice were randomly (n=10 per group) divided into
four groups, a model control group administered with normal saline
and three TT-1-treated groups, which were administered at 0.04, 0.2
or 1 mg/kg body weight with intra-tumor injection three times a
week. At the indicated time points, the mice from all the groups
were sacrificed by overdose of anesthetics 24 h following final
administration. Tumor weights of the mice were measured.
Additionally, the tumor volume of each mouse was measured every
three days during the treatment. The antitumor activities were
expressed as inhibitory rate (%) and calculated as [(A-B)/A] ×100%,
where A and B were the mean tumor weight of the model and TT-1
groups, respectively. The tumor volumes (TV) were calculated using
the following formula: TV=1/2xaxb2, where a and b are
the long and short diameters of the tumors in each mouse,
respectively.
Statistical analysis
All experiments conducted in the present study were
performed in triplicate. The data are presented as the mean ±
standard deviation. Statistical analysis was performed by Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
TT-1 selectively inhibits the
viability of TT cancer cells but not normal human thyroid
cells
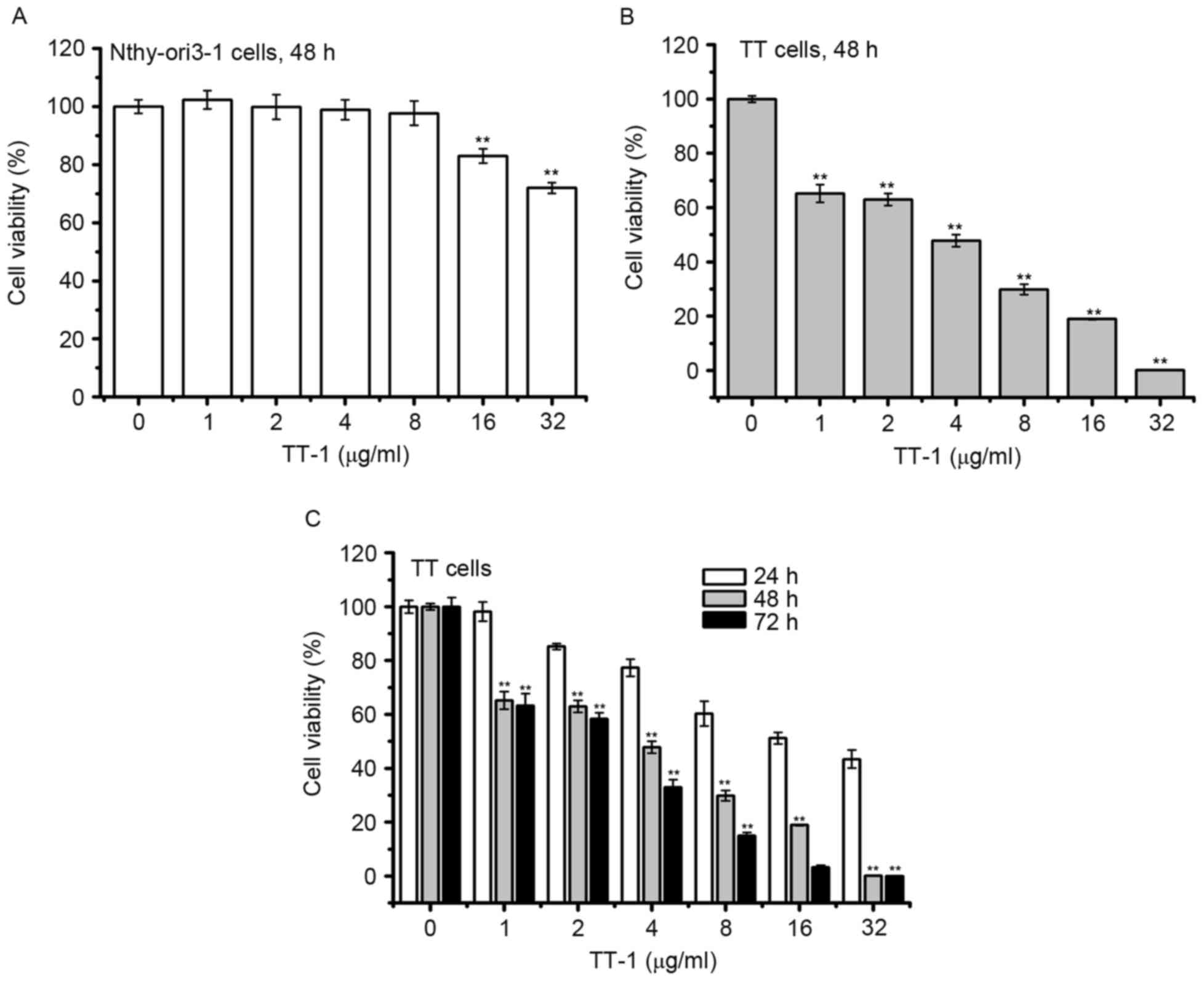
To determine the cytotoxic activity of TT-1, MTT
assay was conducted. As shown in Fig.
1A, the viabilities of normal human thyroid follicular
epithelial cells Nthy-ori3-1 did not decrease in response to TT-1
treatment at concentrations up to 8 µg/ml for 48 h. Furthermore,
treatment with TT-1 significantly inhibited the proliferation of TT
cells in a dose and time-dependent manner (Fig. 1B and C). The 50% inhibitory
concentrations (IC50) of TT-1 on TT cells were
18.23±2.81, 3.87±0.34, 2.76±0.32 µg/ml at 24, 48 and 72 h,
respectively. Specifically, at 4 µg/ml TT-1, the viability of TT
cells at 48 h decreased to 47.8%, which was much lower compared
with the viability of Nthy-ori3-1 cells. The results showed that
TT-1 exhibited high cytotoxicity on TT cancer cells and low
cytotoxicity to normal human thyroid cells.
TT-1-induces apoptosis of TT cells in
vitro
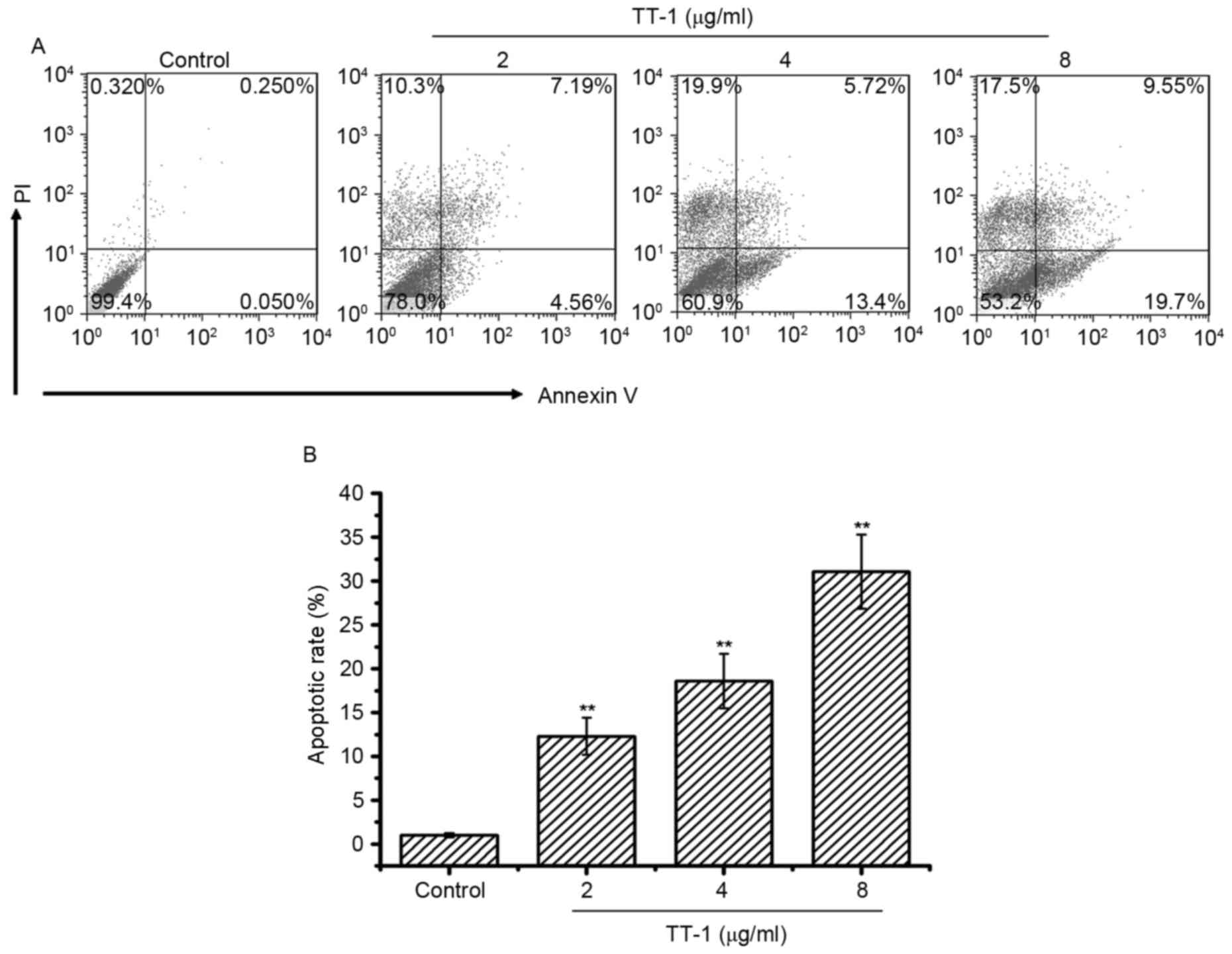
To investigate whether apoptosis was involved in
TT-1-induced anti-TT activity, an AV/PI assay was conducted. The
cells double-labeled with AV and PI, which discriminated between
unaffected and apoptotic cells. AV-positive cells indicated the
loss of membrane polarity, which leads to the complete loss of
membrane integrity and subsequently, to apoptosis and PI
infiltration. As shown in Fig. 2,
treatment with TT-1 induced apoptosis of TT cells in a
dose-dependent manner. Specifically, the average apoptotic cell
accumulations reached 12.31, 18.62 and 31.07% for 2, 4 and 8 µg/ml
TT-1 concentrations, respectively (Fig.
2B). Apoptosis may be one of the mechanisms by which TT-1 is
able to prevent proliferation.
TT-1 upregulates Bax and downregulates
Bcl-2 expression in TT cells
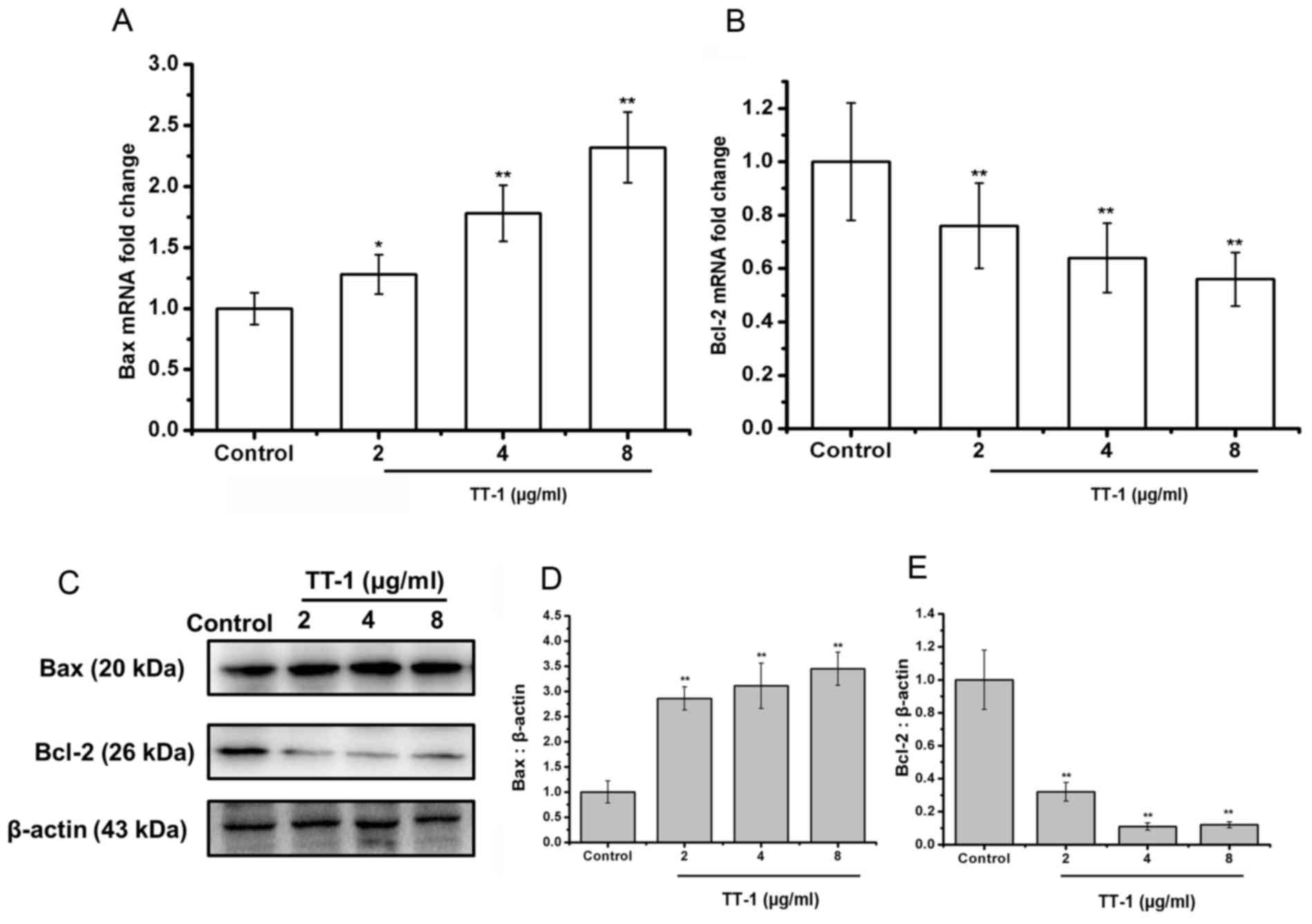
The Bcl-2 family of proteins is known to have
critical roles in regulating apoptosis (28). Therefore, the authors of the present
study examined the expression of Bax, the pro-apoptotic protein,
and Bcl-2, the anti-apoptotic protein, on the treatment of TT cells
with TT-1 at the level of transcription and translation. As shown
in Fig. 3A, the levels of Bax mRNA in
TT cells was upregulated by TT-1 treatment in a dose-dependent
manner.
Additionally, treatment with TT-1 decreased the
levels of Bcl-2 mRNA in TT cells in a dose-dependent manner
(Fig. 3B). Specifically, at 8 µg/ml
TT-1, the mRNA expression of Bax and Bcl-2 were 2.23-fold higher
and 0.56-fold lower, respectively compared with the control group.
These changes were confirmed at the protein level using a western
blot analysis (Fig. 3C-E).
TT-1 treatment activates the caspase-3
and −9 in TT cells
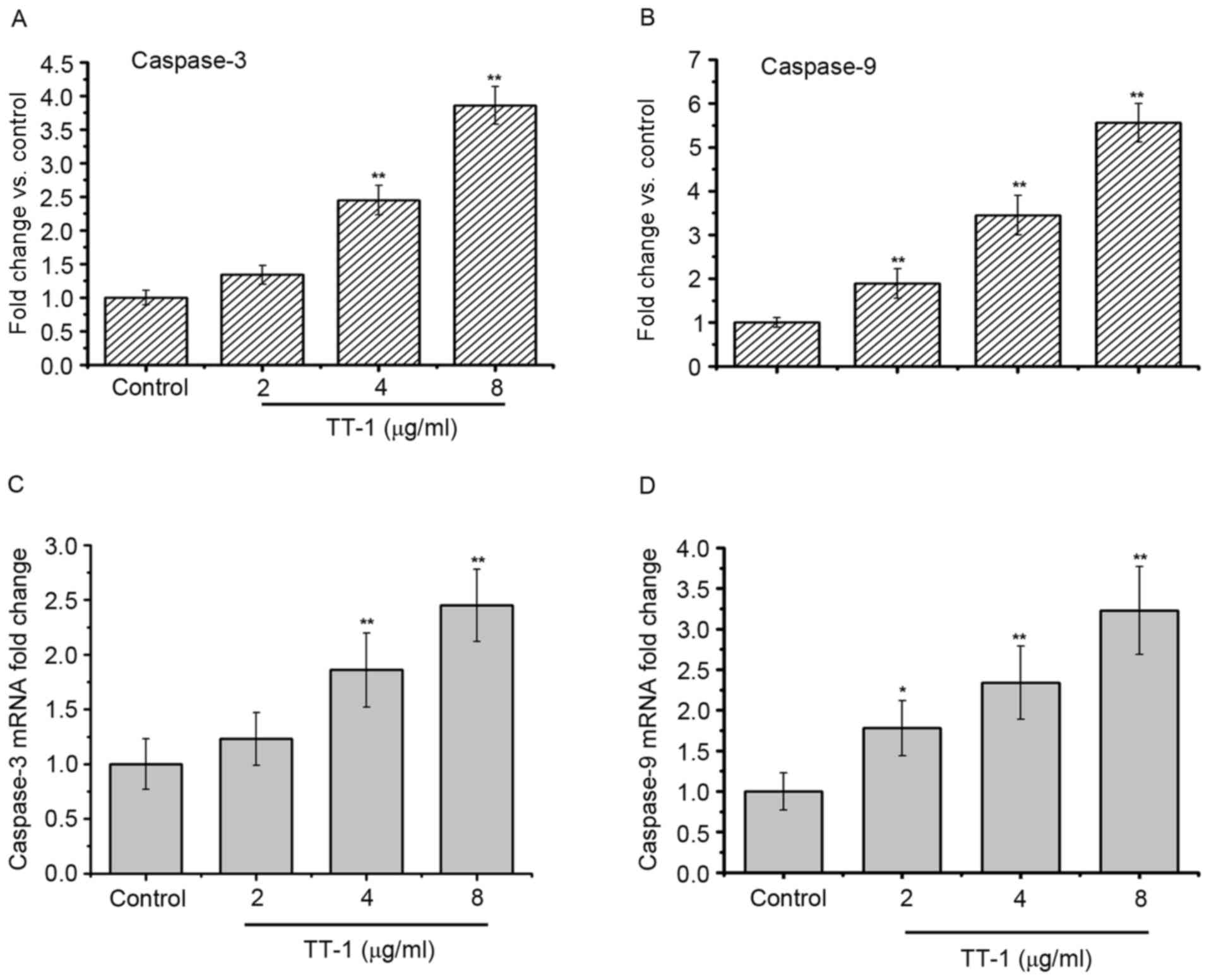
As apoptosis was observed in TT-1-treated TT cells,
the present study measured the activity of different caspases as
key factors of apoptosis using a fluorometric assay (Fig. 4). The results showed that caspase-3
and −9 were activated in the TT-1-treated TT cells in
dose-dependent manners from 2–8 µg/ml (Fig. 4A and C). Furthermore, similar results
were obtained in the RT-qPCR assays. The levels of caspase-3 and −9
RNA in the TT cells increased following exposure to TT-1 (Fig. 4B and D).
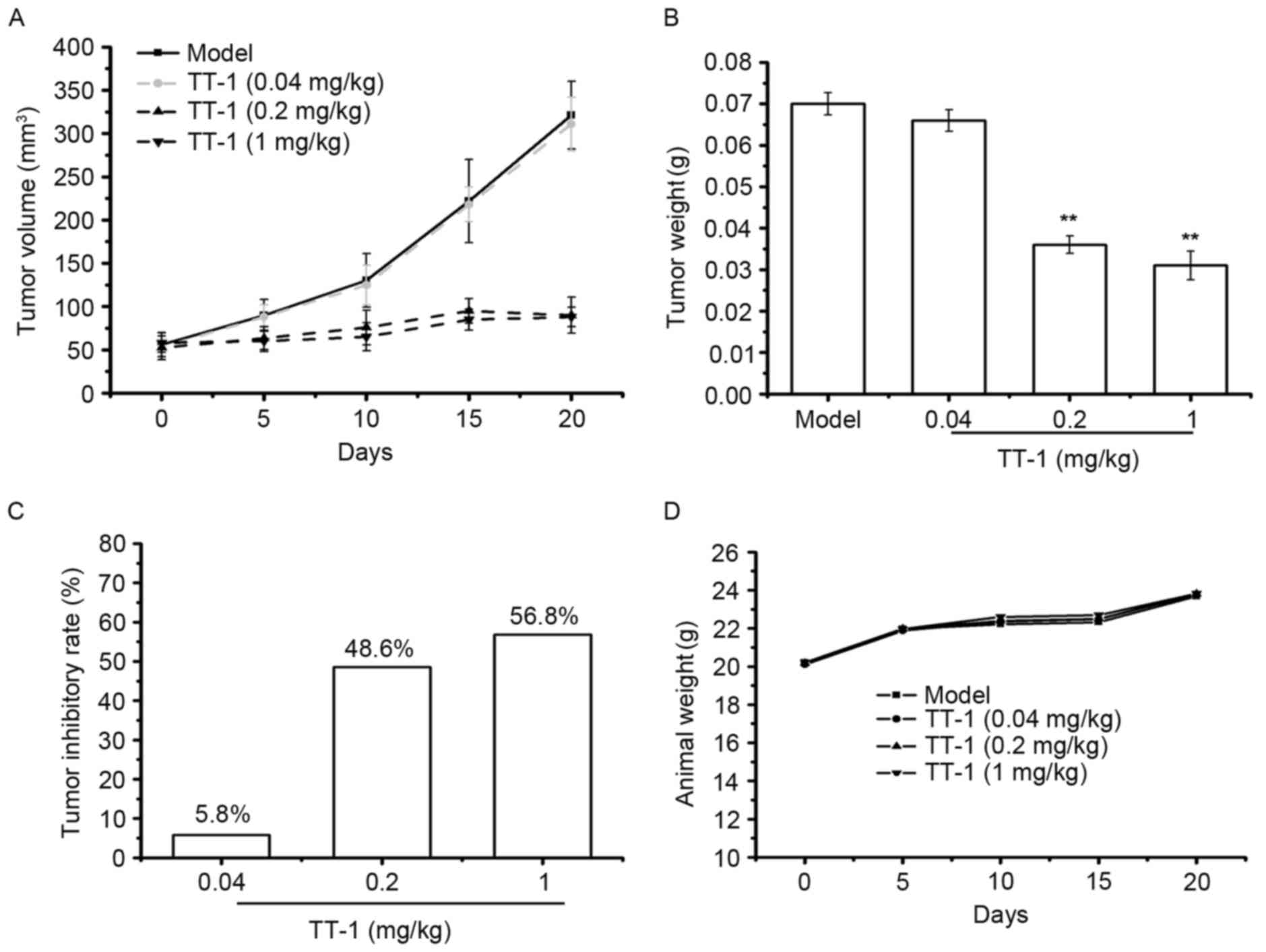
TT-1 treatment suppresses the tumor
growth in TT-bearing mice
To investigate whether TT-1 is able to inhibit tumor
growth in vivo, a TT xenograft model was established.
Following TT-1 treatment, the volume and weight of the tumors were
measured. As indicated in Fig. 5A-C,
TT-1 was able to suppress the tumor growth in nude mice in a dose
dependent manner. Compared with the model group, the tumor
inhibitory rates of the TT-1-treated groups were 30.00, 34.28 and
55.71% at treatment concentrations 0.04, 0.2 and 1 mg/kg,
respectively. The weight of the animals in the TT-1-treated groups
to those of the model control group (Fig.
5D), indicating that there were no significant changes in body
weight during TT-1 treatment. Therefore, cancer cell growth was
significantly suppressed in mice treated with TT-1 without a
significant loss in body weight.
Discussion
Thyroid cancer is the most frequent neoplasm of the
endocrine system (2). The prognosis
of thyroid cancer is excellent at the initial stages of disease.
However, for advanced or metastatic diseases, limited therapeutic
options are available (29).
Additionally, conventional chemotherapy and radiotherapy often have
severe side effects on healthy cells and tissues (30). As a result, the most promising drugs
are those with low cytotoxicity, target selectivity and
availability for chronic treatment.
Antimicrobial peptides have recently attracted
significant attention as novel anticancer agents due to their novel
mechanisms, decreased likelihood of drug resistance, and low
intrinsic cytotoxicity (31).
Melittin, which consists of 26 amino acid residues, is a cationic,
hemolytic peptide isolated from honeybee venom. Previous studies
have demonstrated that melittin has antibacterial, anti-arthritic
and anti-inflammatory activities in various cell lines (6). Additionally, melittin has been shown to
be a promising anticancer drug. A number of types of cancer cells,
including renal, lung, breast, and bladder cells, have been
reported to be selectively killed by melittin in vitro
(14). In the present study, the
authors designed a novel peptide, TT-1, based on the amphipathic
structure of melittin. The peptide sequence of TT-1 was
KIKAVLKVLTT, consisting of only 11 amino acids, which is much
shorter than the peptide sequence length of melittin. The
antimicrobial peptide database indicated that total hydrophobic
ratio of TT-1 was 54% and the net charge was 5. These parameters
indicated that TT-1 would be effective in treating cancer cells.
Therefore, the relative activity and mechanism of TT-1 were further
investigated.
It has been reported that melittin exhibits
cytotoxic activity toward tumors and normal cells (32). In the present study, an in
vitro study of the cytotoxic effect of TT-1 revealed that TT-1
was able to inhibit the proliferation of TT cells in a dose and
time-dependent manner but had no significant growth inhibitory
effects on normal thyroid follicular epithelial Nthy-ori3-1 cells.
Therefore, TT-1 displayed selective anticancer activity.
Apoptosis, a very tightly programmed cell death with
distinct biochemical and genetic pathways (33), is generally identified via specific
morphological cellular characteristics, including cell shrinkage,
nuclear or cytoplasmic fragmentation and chromatin condensation
(34). A class of cysteine proteases,
including caspase-3, −8 and −9, is commonly involved in the
apoptotic pathways (35). On the
other hand, the Bcl-2 family also has an important role in the
regulation of apoptosis (36).
Defects in apoptotic mechanisms have important roles in tumor
pathogenesis, which allows neoplastic cells to survive over
intended lifespans, subverts the need for exogenous survival
factors and provides protection from oxidative stress and hypoxia
as the tumor mass expands (32).
Therefore, the ability to induce apoptosis is necessary for
effective anticancer therapies (37).
In the present study, double staining of cells with AV/PI revealed
that TT-1-induced apoptosis may be one of the mechanisms by which
TT-1 prevents the growth of TT cells in vitro.
Bcl-2 and Bax, members of the Bcl-2 family of
proteins, are important components of ischemia-reperfusion
injury-induced apoptosis (28). These
two proteins can form either homodimers or heterodimers, which
depends on the levels of each component that is present. Bax forms
a heterodimer with Bcl-2 and functions as an apoptotic activator by
increasing the opening of the mitochondrial voltage-dependent anion
channel, which leads to the loss in membrane potential (38). Therefore, high expression of Bcl-2 is
able to inhibit apoptosis, while high expressions of Bax can
stimulate apoptosis. A change in the expression ratio of these two
factors determines whether apoptosis occurs (39). In the present study, the results shown
in Fig. 3 suggest that the apoptotic
mechanism of TT-1 in TT cells include the downregulation of Bcl-2
expression and the upregulation of Bax expression at the level of
transcription and translation.
Additionally, caspase family members have major
roles in cell apoptosis (40). The
caspase cleavage cascade begins with initiator caspase being
activated by intrinsic or extrinsic pathways. In the present study,
the authors examined two typical caspase family members, caspase-3
and −9. Caspase-3 is an effector caspase that mediates the cleavage
of many proteins, while caspase-9 is the key initiator caspase in
the intrinsic pathway that induces cell death and the activation of
which occurs at the mitochondrial membrane (41). The present study showed that caspase-3
and −9 mRNA levels were significantly increased in the TT-1-treated
group compared with the untreated controls and indicated that TT-1
may induce the apoptosis of TT cells, which may be partly due to
the activation of caspase-3 and −9 (Fig.
4).
From these results, TT-1 exhibited a marked
inhibitory effect on cell viability on TT cells in vitro,
and it was also demonstrated that TT-1 exhibited anti-tumor
activity on TT cells in vivo (Fig.
5). Compared with the control, TT-1 was able to suppress the
proliferation of TT cells tumor-bearing mice in a dose dependent
manner, with an observed 55.71% inhibition at 1 mg/kg TT-1. This
finding was further confirmed by results of TT tumor growth in
vivo, and TT-1 treatment had no effect on the weight of mice,
indicating that TT-1 may be a potential high efficiency and low
toxicity anticancer agent.
In summary, TT-1 inhibited the proliferation of
human TT cells in vitro and in vivo through the
upregulation of Bax, the downregulation of Bcl-2 and the activation
of caspase-3 and −9. These results further suggested that TT-1 may
be a potential candidate for the treatment of thyroid cancer.
Acknowledgements
This study was financially supported by a grant
fromJilin Provincial Finance Department (grant no. SCZSY201504) and
the Outstanding Young Talent Foundation Project of Science and
Technology Department in Jilin Province (grant no. 20170520018JH),
China.
References
|
1
|
Yan JX, Wang KR, Chen R, Song JJ, Zhang
BZ, Dang W, Zhang W and Wang R: Membrane active antitumor activity
of NK-18, a mammalian NK-lysin-derived cationic antimicrobial
peptide. Biochimie. 94:184–191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
6:69–90. 2011. View Article : Google Scholar
|
|
4
|
Matsuno A, Murakami M, Hoya K, Yamada SM,
Miyamoto S, Yamada S, Son JH, Nishido H, Ide F, Nagashima H, Sugaya
M, et al: Clinicopathological and molecular histochemical review of
skull base metastasis from differentiated thyroid carcinoma. Acta
Histochem Cytochem. 46:129–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xing M, Haugen BR and Schlumberger M:
Progress in molecular-based management of differentiated thyroid
cancer. Lancet. 381:1058–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown CK and Kirkwood JM: Medical
management of melanoma. Surg Clin North Am. 83:283–322, viii. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lienkamp K and Tew GN: Synthetic mimics of
antimicrobial peptides-aversatile ring-opening metathesis
polymerization based platform for the synthesis of selective
antibacterial and cell-penetrating polymers. Chemistry.
15:11784–11800. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen C, Hu J, Zhang S, Zhou P, Zhao X, Xu
H, Zhao X, Yaseen M and Lu JR: Molecular mechanisms of
antibacterial and antitumor actions of designed surfactant-like
peptides. Biomatials. 33:592–603. 2012.
|
|
9
|
Brogden KA: Antimicrobial peptides: Pore
formers or metabolic inhibitors in bacteria? Nav Rev Microbiol.
3:238–250. 2005. View Article : Google Scholar
|
|
10
|
Mader JS and Hoskin DW: Cationic
antimicrobial peptides as novel cytotoxic agents for cancer
treatment. Expert Opin Investig Grugs. 15:933–946. 2006. View Article : Google Scholar
|
|
11
|
Takahashi T, Nomura F, Yokoyama Y,
Tanaka-Takiguchi Y, Homma M and Takiguchi K: Multiple membrane
interactions and versatile vesicle deformations elicited by
melittin. Toxins. 5:637–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han SM, Kim JM, Park KK, Chang YC and Pak
SC: Neuroprotective effects of melittin on hydrogen
peroxide-induced apoptotic cell death in neuroblastoma SH-SY5Y
cells. BMC Complement Altern Med. 14:2862014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sommer A, Fries A, Cornelsen I, Speck N,
Koch-Nolte F, Gimpl G, Andrä J, Bhakdi S and Reiss K: Melittin
modulates keratinocyte function through P2 Receptor-dependent ADAM
Activation. J Biol Chem. 287:23678–23689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Son DJ, Lee JW, Lee YH, Song HS, Lee CK
and Hong JT: Therapeutic application of anti-arthritis,
pain-releasing and anticancer effects of bee venom and its
constituent compounds. Pharmacol Ther. 115:246–270. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oršolić N: Bee venom in cancer therapy.
Cancer Metastasis Rev. 31:173–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raghuraman H and Chattopadhyay A:
Melittin: A membrane-active peptide with diverse functions. Biosci
Rep. 27:189–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li SA, Lee WH and Zhang Y: Efficacy of
OH-CATH30 and its analogs against drug-resistant bacteria in vitro
and in mouse models. Antimicrob Agents Chemother. 56:3309–33017.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma Q, Jiao W, Lv Y, Dong N, Zhu X and Shan
A: Structure-function relationship of Val/Arg-rich peptides:
Effects of net charge and pro on activity. Chem Biol Drug Des.
84:348–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ozgur B and Sayar M: Role of
hydrophobic/aromatic residues on the stability of double-wall
β-sheet structures formed by a triblock peptide. J Phys Chem B.
121:4115–4128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Starenki D and Park JI:
Mitochondria-targeted nitroxide, mito-CP, suppresses medullary
thyroid carcinoma cell survival in vitro and in vivo. J Clin
Endocrinol Metab. 98:1529–15240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Ke M, Tian Y, Wang J, Li B, Wang
Y, Dou J and Zhou C: BF-30 selectively inhibits melanoma cell
proliferation via cytoplasmic membrane permeabilization and
DNA-binding in vitro and in B16F10-bearing mice. Eur J Pharmaco.
707:1–10. 2013. View Article : Google Scholar
|
|
22
|
Paredes-Gamero EJ, Martins MN, Cappabianco
FA, Ide JS and Miranda A: Characterization of dual effects induced
by antimicrobial peptides: Regulated cell death or membrane
disruption. Biochim Biophys Acta. 1820:1062–91072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Massaoka MH, Matsuo AL, Figueiredo CR,
Farias CF, Girola N, Arruda DC, Scutti JA, Romoff P, Favero OA,
Ferreira MJ, et al: Jacaranone induces apoptosis in melanoma cells
via ROS-mediated down regulation of Akt and p38 MAPK activation and
displays antitumor activity in vivo. PLoS One. 7:e386982012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu C, Geng X, Wan S, Hou H, Yu F, Jia B
and Wang L: Cecropin-P17, an analog of Cecropin B, inhibits human
hepatocellular carcinoma cell HepG-2 proliferation via regulation
of ROS, Caspase, Bax, and Bcl-2. J Pept Sci. 21:661–668. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu J, Yao YY, Dai QM, Ma GS, Zhang SF, Cao
L, Ren LQ and Liu NF: Erythropoietin attenuates cardiac dysfunction
by increasing myocardial angiogenesis and inhibiting
interstitialfibrosis in diabetic rats. Cardiovasc Diabetol.
11:1052012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tano T, Okamoto M, Kan S, Nakashiro K,
Shimodaira S, Koido S, Homma S, Sato M, Fujita T, Kawakami Y and
Hamakawa H: Prognostic Impact of Expression of Bcl-2 and Bax Genes
in Circulating Immune Cells Derived from Patients with Head and
Neck Carcinoma. Neoplasia. 15:305–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ng CS, Wan S and Yim AP: Pulmonary
ischemia-reperfusion injury: Role of apoptosis. Eur Respir J.
25:356–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stjepanovic N and Capdevila J: Multikinase
inhibitors in the treatment of thyroid cancer: Specific role of
lenvatinib. Biologics. 8:129–139. 2014.PubMed/NCBI
|
|
30
|
Leung HW, Yang WH, Lai MY, Lin CJ and Lee
HZ: Inhibition of 12-lipoxygenase during baicalein-induced human
lung non-small carcinoma H460 cell apoptosis. Food Chem Toxicol.
45:403–411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schweizer F: Cationic amphiphilic peptides
with cancer selective toxicity. Eur J Pharmacol. 625:190–194. 2007.
View Article : Google Scholar
|
|
32
|
Sun D, Sun M, Zhu W, Wang Z, Li Y and Ma
J: The anti-cancer potency and mechanism of a novel tumor-activated
fused toxin, DLM. Toxins (Basel). 7:423–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lockshin RA and Williams CM: Programmed
cell death-I. Cytology of degeneration in the intersegmental
muscles of the Pernyi silkmoth. J Insect Physiol. 11:123–133. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bottone MG, Santin G, Aredia F, Bernocchi
G, Pellicciari C and Scovassi AI: Morphological features of
organelles during apoptosis: An overview. Cells. 2:294–305. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nuñez G, Benedict MA, Hu Y and Inohara N:
Caspases: The proteases of the apoptotic pathway. Oncogene.
17:3237–3245. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji ES, Kim YM, Shin MS, Kim CJ, Lee KS,
Kim K, Ha J and Chung YR: Treadmill exercise enhances spatial
learning ability through suppressing hippocampal apoptosis in
Huntington's disease rats. J Exerc Rehabil. 11:133–139. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tseng TH, Shen CH, Huang WS, Chen CN,
Liang WH, Lin TH and Kuo HC: Activation of
neutral-sphingomyelinase, MAPKs, and p75 NTR-mediating caffeic acid
phenethyl ester-induced apoptosis in C6 glioma cells. J Biomed Sci.
21:612014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vadde R, Radhakrishnan S, Reddivari L and
Vanamala JK: Triphala extract suppresses proliferation and induces
apoptosis in human colon cancer stem cells via suppressing
c-Myc/Cyclin D1 and Elevation of Bax/Bcl-2 Ratio. Biomed Res Int.
2015:6492632015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang C, Guo Z, Liu H, Shi Y and Ge S:
Influence of levosimendan postconditioning on apoptosis of rat lung
cells in a model of ischemia-reperfusion injury. PLoS One.
10:e01149632015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Floyd DH, Zhang Y, Dey BK, Kefas B, Breit
H, Marks K, Dutta A, Herold-Mende C, Synowitz M, Glass R, et al:
Novel anti-apoptotic microRNAs 582-5p and 363 promote human
glioblastoma stem cell survival via direct inhibition of caspase 3,
caspase 9 and Bim. PLoS One. 9:e962392014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wyllie AH: ‘Where, O death, is thy sting?’
A brief review of apoptosis biology. Mol Neurobiol. 42:4–9. 2010.
View Article : Google Scholar : PubMed/NCBI
|