Introduction
The number of patients with newly diagnosed ovarian
cancer has gradually decreased, however, the 5-year survival rate
of this disease is still 40%, which remains a lethal gynecological
malignancy (1). In ~60% of patients
with ovarian cancer, the tumor has spread beyond the ovaries at
diagnosis, and these patients require surgery combined with
chemotherapy (2). Platinum-taxane
combination chemotherapy is the current gold standard treatment for
ovarian cancer. However, irrespective of the initial tumor
response, long-term survival is poor, particularly in patients with
advanced disease at the point of diagnosis. Relapse and
non-response to initial chemotherapy are thought to be associated
with tumor drug resistance (3).
Recently, the resistance of ovarian cancer to chemotherapy agents
has been studied extensively and multiple targets for novel
treatments have been proposed (4).
Chemotherapy treatment is often aimed at inducing tumor cell
apoptosis, and research has been focused towards either the
activation of apoptosis or lowering the apoptotic threshold via the
administration of cytotoxic drugs (5).
Evasion of apoptosis is a hallmark of cancer,
leading to the failure of chemotherapy treatment and subsequent
tumor progression (6). Autophagy, an
alternative caspase-independent cell death program, .is a highly
conserved process of degradation, by which cytoplasmic components
are sequestered in lysosomal vesicles and recycled to provide
energy (7,8). In addition, autophagy is induced by
various stresses, including chemotherapy (9,10). The
autophagy-associated genes which have currently been identified
include ~30 yeast genes and 16 human homologues, among which beclin
1 (BECN1), 1A/1B-light chain 3 (LC3), and high mobility group box-1
protein (HMGB-1) are important for mammalian autophagy (11–14). The
involvement of autophagy in chemoresistance in the progression of
ovarian cancer and the underlying molecular mechanisms involved
remain unclear. To the best of our knowledge, the
clinicopathological significance of the expression of key
autophagy-associated molecules in ovarian cancer, including BECN1,
LC3, and HMGB-1, has not yet been established. Therefore, the aim
of the present study was to clarify the relevance of the expression
of these autophagy-associated proteins to the prognosis of ovarian
cancer.
Materials and methods
According to previous studies, the positive rates of
Beclin 1 were significantly higher in ovarian cancer than in benign
ovarian tumor or normal ovarian tissue (15). Ovarian tissue samples were submerged
in 10% formalin for 24 to 48 h at room temperature.
Paraffin-embedded tissue samples from 141 ovarian cancers were
analyzed, including samples of 34 serous carcinomas, 20 mucinous
carcinomas, 60 clear cell carcinomas and 27 endometrioid
carcinomas. Tumor tissues from female patients were obtained from
the Department of Obstetrics and Gynecology at Shimane University
Hospital (Izumo, Japan), and the Department of Obstetrics and
Gynecology at Seirei Hamamatsu General Hospital (Hamamatsu, Japan),
all of whom underwent surgery at this hospital between January 1998
and December 2008. All patients with ovarian cancer were aged from
46 to 76 years (median, 61 years). Diagnosis was based on the
conventional examination of hematoxylin-eosin stained sections.
Tumors were classified according to the World Health Organization
classification and staging was performed according to the
International Federation of Gynecology and Obstetrics (FIGO)
classification (16). All patients
underwent primary cytoreductive surgery and received 6–12 cycles of
adjuvant platinum-taxane combination chemotherapy [carboplatin
(CBDCA, AUC 5) with paclitaxel; 175 mg/m2 or docetaxel;
70 mg/m2]. The Institutional Review Board of Shimane
University Hospital and Seirei Hamamatsu General Hospital approved
the examination of tumor tissues and the research was conducted
according to the Declaration of Helsinki. Written informed consent
was obtained from all of the subjects. Tumor cores (3 mm in
diameter) from the paraffin-embedded blocks were organized on
tissue microarrays (TMAs). Tumor cores were selected by a
gynecologic oncologist (K.N, Shimane University, Shimane, Japan)
and a pathology technician (K.I, Shimane University), and criteria
were based on a review of the hematoxylin-eosin stained slides.
Immunohistochemistry (IHC)
The expression of BECN1, LC3, and HMGB-1 proteins
were assessed by IHC staining performed on 4 µm tissue sections
from core tumor biopsies (3 mm in diameter). TMA sections were
incubated in sodium citrate buffer (pH 7.0) for 30 min at 97° to
perform antigen retrieval. Briefly, tissue sections were dewaxed in
xylene for 10 min at 20°C, rehydrated in 70% graded ethanol and
100% ethanol, washed in PBS (pH 7.25) for 5 min and quenched in
peroxidase-blocking reagent for 5 min at 20°C to remove endogenous
peroxidase activity. TMA sections were incubated overnight at 4°C
with mouse monoclonal primary antibodies against BECN1 (Cat. no.
NB110-55556; dilution, 1:100; Novus Biologicals, LLC Littleton, CO,
USA), LC3 (Cat. no. NB100-2220; dilution, 1:400; Novus Biologicals,
LLC), and HMGB-1 (Cat. no. 6893; dilution, 1:400; Cell Signaling
Technology, Inc., Danvers, MA, USA). The following day, slides were
washed three times with PBS prior to the detection of antigens
using the two-step DAKO EnVision+ Peroxidase System (DAKO; Agilent
Technologies, Inc., Santa Clara, CA, USA), according to the
manufacturer's protocol at room temperature for 30 min. After
rinsing with PBS, the sections were incubated for 5 min in 0.05%
diaminobenzidine in PBS with 0.03% H2O2. The
slides were counterstained with hematoxylin, dehydrated and
mounted. The serial sections were routinely incubated with
irrelevant mouse IgG at 4°C for 8 h as a negative control (Cat. no.
NC494H; dilution 1:100; Biocare Medical, LLC, Paheco, CA, USA). A
previously identified ovarian carcinoma sample was included as the
positive control (17), and isotype
control immunoglobulin G represents the negative control.
Counterstaining was performed using hematoxylin and eosin for 3 min
at room temperature. Slides were examined under a light microscope
at ×100 magnification in 3 fields of interest for each section, by
two independent researchers blinded to the clinicopathological
factors. The distribution of immunoreactivity for BECN1, LC3, or
HMGB-1 was usually homogenous within a tumor and immunoreactivity
was scored as follows: 0 (undetectable), 1+ (weakly positive), 2+
(moderately positive), and 3+ (strongly positive). There were no
significant differences of immunoreactivity scores between the two
researchers. If discrepancies occurred, a third investigator was
assigned to score the tumor. The score to be assigned was
determined by a majority vote. For analyses of clinicopathological
factors and survival, patients with no expression were assigned to
a negative expression group, while patients with weak, moderate, or
strong expression were assigned to a positive expression group.
Statistical analysis
Progression-free survival and overall survival were
calculated from the date of diagnosis to the date of first relapse
or final follow up. Survival data were plotted on Kaplan-Meier
curves and were compared using the log-rank test. Data were
excluded when patients did not continue to follow-up. The
chi-square test or Fisher's exact test were used for the comparison
of categorical data. All analyses were conducted with the StatView
statistical package, version 5.0 (Abacus Concepts, Piscataway, NJ,
USA). Reported P-values were two-tailed and P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification of BECN1, LC3, and
HMGB-1 expression in ovarian tumors
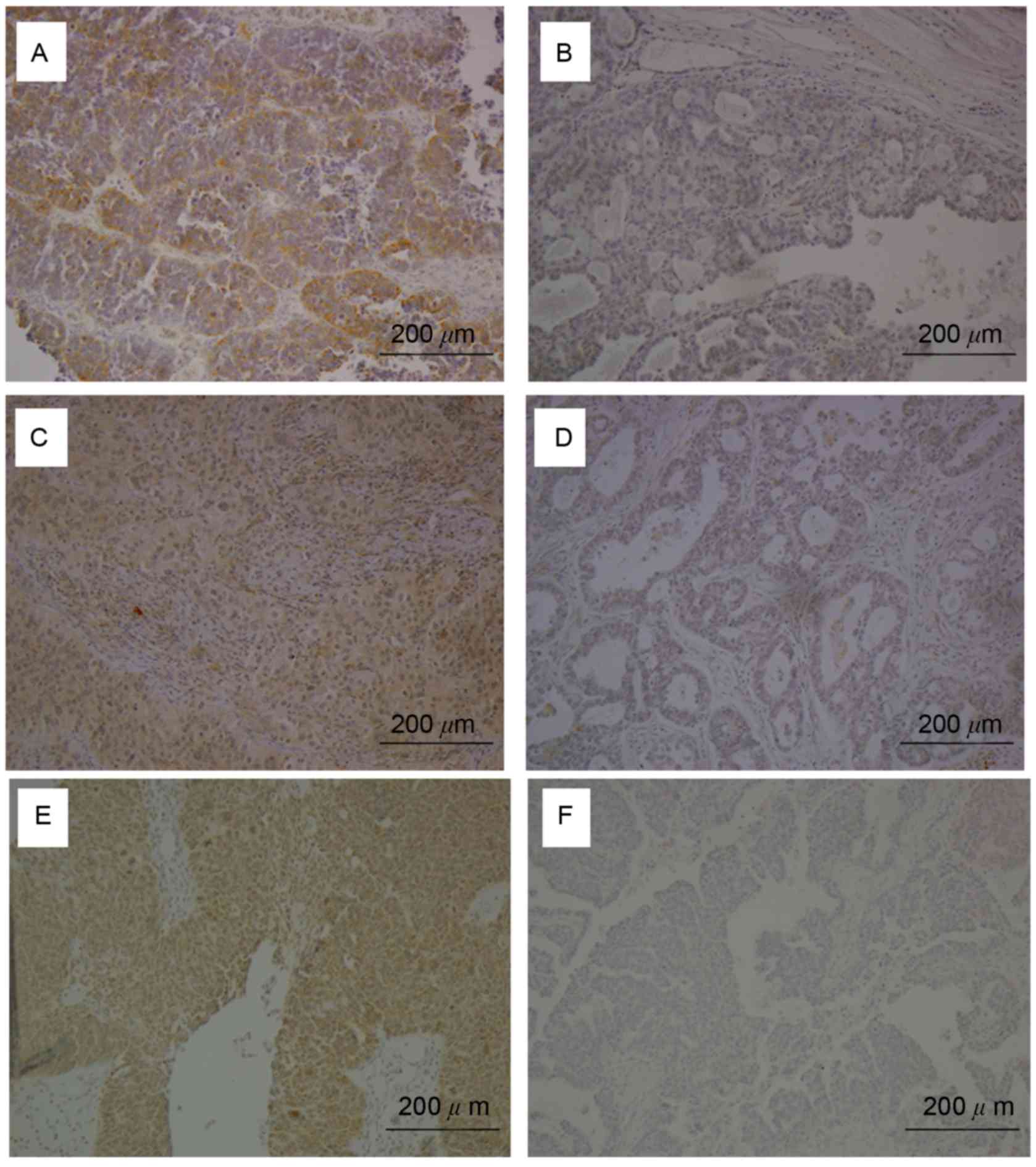
BECN1, LC3, and HMGB-1 expression was examined by
IHC (Fig. 1). Moderate to strong
cytoplasmic expression of BECN1, LC3, and HMGB-1 was detected in
ovarian carcinoma cells of at least 50% of patients (Fig. 1A, C, E, respectively), but was absent
in other ovarian tumor sections (Fig. 1B,
D, F). BECN1, LC3, and HMGB-1 were positively expressed in
58.2% (82/141), 75.2% (106/141), and 53.2% (75/141) of ovarian
tumor tissues, respectively.
Associations between the expression of
autophagy-associated proteins and clinicopathological factors
BECN1 expression was significantly associated with
the expression of LC3 (P<0.0001) and HMGB-1 (P<0.0001).
However, there were no significant associations observed between
BECN1, LC3, or HMGB-1 expression and any clinicopathological
factors examined (Table I).
 | Table I.Associations between BECN1 expression
and clinicopathological factors in patients with ovarian
cancer. |
Table I.
Associations between BECN1 expression
and clinicopathological factors in patients with ovarian
cancer.
|
|
| BECN1 expression |
|
|---|
|
|
|
|
|
|---|
| Factors | Patients | Negative | Positive | P-valuea |
|---|
| FIGO stage |
|
|
|
|
| III,
IV | 61 | 31 | 30 | 0.0592 |
| I,
II | 80 | 28 | 52 |
|
| Grade |
|
|
|
|
| G2,
G3 | 124 | 53 | 71 | 0.3798 |
| G1 | 16 | 5 | 11 |
|
| Histology |
|
|
|
|
|
Serous | 34 | 19 | 15 | 0.0568 |
|
Others | 107 | 40 | 67 |
|
| Age, years |
|
|
|
|
| ≥60 | 59 | 27 | 32 | 0.4236 |
|
<60 | 82 | 32 | 50 |
|
| Residual tumor,
cm |
|
|
|
|
| ≥1 | 55 | 25 | 30 | 0.487 |
|
<1 | 86 | 34 | 52 |
|
| LC3 expression |
|
|
|
|
|
Negative | 35 | 25 | 10 |
<0.0001a |
|
Positive | 106 | 34 | 72 |
|
| HMGB-1
expression |
|
|
|
|
|
Negative | 66 | 41 | 25 |
<0.0001a |
|
Positive | 75 | 18 | 57 |
|
Associations of BECN1, LC3, and HMGB-1
with progression-free survival and overall survival in patients
with ovarian cancer receiving platinum-taxane chemotherapy
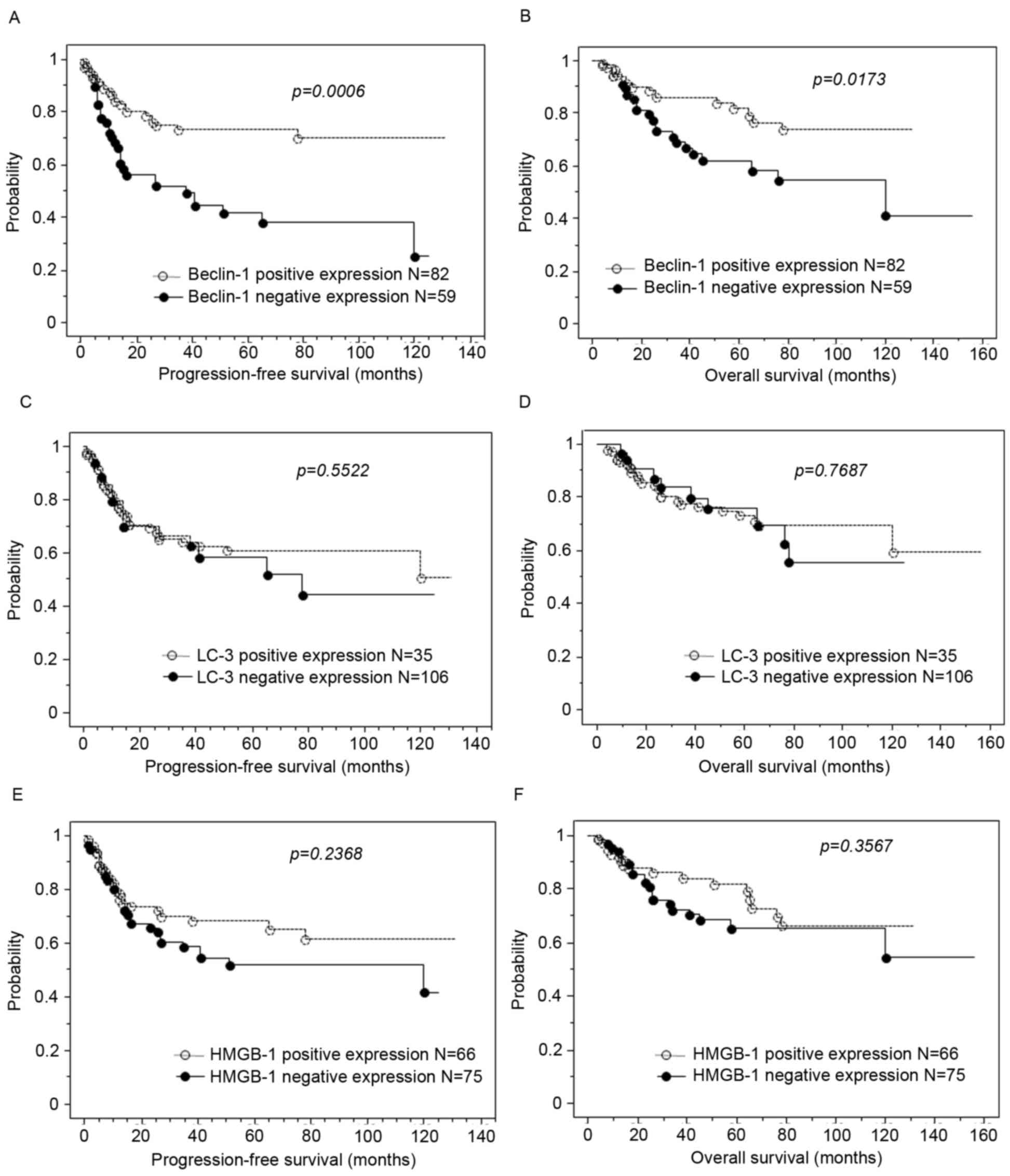
Kaplan-Meier curves present estimates of progression
free and overall survival in patients with ovarian carcinoma
(Fig. 1). Loss of BECN1 expression
was significantly associated with shorter progression-free survival
and shorter overall survival (P=0.001 and 0.017, respectively;
Fig. 2A and B). However, loss of LC3
or HMGB-1 expression was not associated with progression-free
survival or overall survival (Fig.
2C-F). According to univariate analysis, FIGO stage III–IV
(P<0.0001; log-rank test), high tumor grade 3 (P=0.033; log-rank
test), postoperative residual tumor ≥1 cm (P<0.0001; log-rank
test), and loss of BECN1 expression (P=0.0006; log-rank test) were
significantly associated with shorter progression-free survival
(Table II). Multivariate analysis
revealed that postoperative residual tumor ≥1 cm (P=0.002) and loss
of BECN1 expression (P=0.046) were independently associated with
shorter progression-free survival (Table
II). In addition, FIGO stage III–IV (P<0.0001; log-rank
test), postoperative residual tumor ≥1 cm (P<0.0001; log-rank
test), and loss of BECN1 expression (P=0.017; log-rank test) were
revealed to be associated with overall survival by univariate
analysis (Table III). Multivariate
analysis confirmed that postoperative residual tumor ≥1 cm
(P=0.001) and loss of BECN1 expression (P=0.036) were independently
associated with shorter overall survival (Table III).
 | Table II.Univariate and multivariate analyses
of progression-free prognostic factors in patients with ovarian
cancer. |
Table II.
Univariate and multivariate analyses
of progression-free prognostic factors in patients with ovarian
cancer.
|
|
| Univariate
analysis | Multivariate |
|---|
|
|
|
|
|
|---|
| Factors | Patients | Hazard ratio | 95% CI |
P-valuea | Hazard ratio | 95% CI |
P-valuea |
|---|
| FIGO stage |
|
|
|
|
|
|
|
| III,
IV | 61 | 3.8 | 2.1–6.8 |
<0.0001a | 1.6 | 0.7–3.9 | 1.594 |
| I,
II | 80 |
|
|
|
|
|
|
| Grade |
|
|
|
|
|
|
|
| G2,
G3 | 124 | 8.7 | 1.2–62.8 | 0.0325a | 2.2 | 0.3–16.4 | 0.4391 |
| G1 | 16 |
|
|
|
|
|
|
| Histology |
|
|
|
|
|
|
|
|
Serous | 34 | 1.5 | 0.8–2.7 | 0.1658 | NA | NA | NA |
|
Others | 107 |
|
|
|
|
|
|
| Age, years |
|
|
|
|
|
|
|
|
≥60 | 59 | 1.7 | 1.0–2.9 | 0.0576 | NA | NA | NA |
|
<60 | 82 |
|
|
|
|
|
|
| Residual tumor,
cm |
|
|
|
|
|
|
|
| ≥1 | 55 | 11.7 | 4.4–30.8 |
<0.0001a | 3.9 | 1.6–9.2 | 0.0024a |
|
<1 | 86 |
|
|
|
|
|
|
| BECN1
expression |
|
|
|
|
|
|
|
|
Negative | 59 | 2.6 | 1.5–4.5 | 0.0006a | 1.9 | 1.0–3.6 | 0.0462a |
|
Positive | 82 |
|
|
|
|
|
|
| LC3 expression |
|
|
|
|
|
|
|
|
Negative | 35 | 1.2 | 0.7–2.2 | 0.5522 | NA | NA | NA |
|
Positive | 106 |
|
|
|
|
|
|
| HMGB-1
expression |
|
|
|
|
|
|
|
|
Negative | 66 | 1.4 | 0.8–2.4 | 0.2368 | NA | NA | NA |
|
Positive | 75 |
|
|
|
|
|
|
 | Table III.Univariate and multivariate analysis
of overall prognostic factors in patients with ovarian cancer. |
Table III.
Univariate and multivariate analysis
of overall prognostic factors in patients with ovarian cancer.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factors | Patients | Hazard ratio | 95% CI |
P-valuea | Hazard ratio | 95% CI |
P-valuea |
|---|
| FIGO stage |
|
|
|
|
|
|
|
| III,
IV | 61 | 3.8 | 2.1–6.8 | 0.0001a | 2 | 0.8–5.1 | 0.122 |
| I,
II | 80 |
|
|
|
|
|
|
| Grade |
|
|
|
|
|
|
|
| G2,
G3 | 124 | 5.3 | 0.7–38.5 | 0.1011 | NA | NA | NA |
| G1 | 16 |
|
|
|
|
|
|
| Histology |
|
|
|
|
|
|
|
|
Serous | 34 | 1.5 | 0.8–2.7 | 0.1658 | NA | NA | NA |
|
Others | 107 |
|
|
|
|
|
|
| Age, years |
|
|
|
|
|
|
|
|
≥60 | 59 | 1.7 | 1.0–2.9 | 0.0576 | NA | NA | NA |
|
<60 | 82 |
|
|
|
|
|
|
| Residual tumor,
cm |
|
|
|
|
|
|
|
| ≥1 | 55 | 11.7 | 4.4–30.8 |
<0.0001a | 4.4 | 1.8–11.0 | 0.0013a |
|
<1 | 86 |
|
|
|
|
|
|
| BECN1
expression |
|
|
|
|
|
|
|
|
Negative | 59 | 2.2 | 1.1–4.2 | 0.0173 | 2 | 1.0–3.8 | 0.0364a |
|
Positive | 82 |
|
|
|
|
|
|
| LC-3
expression |
|
|
|
|
|
|
|
|
Negative | 35 | 1.1 | 0.7–2.3 | 0.7687 | NA | NA | NA |
|
Positive | 106 |
|
|
|
|
|
|
| HMGB-1
expression |
|
|
|
|
|
|
|
|
Negative | 66 | 1.4 | 0.7–2.6 | 0.3567 | NA | NA | NA |
|
Positive | 75 |
|
|
|
|
|
|
Discussion
The present study demonstrated that the loss of
BECN1 expression was an independent predictor of shorter
progression-free survival and shorter overall survival in patients
with ovarian carcinoma receiving platinum-taxane combination
chemotherapy. Currently, there are limited available biomarkers
that accurately predict early recurrence of ovarian carcinoma
(18,19). Loss of BECN1 expression may
potentially be used alone or in combination with other biomarkers
to identify patients with ovarian carcinoma, who demonstrate an
increased susceptibility to early disease recurrence. This is
clinically relevant given that ~60% of patients with advanced
ovarian carcinoma who demonstrate a complete response to primary
therapy, will develop recurrence (20). Potentially, the assessment of BECN1
may have an impact on disease management. Patients with recurrent
ovarian carcinoma gain the greatest benefit from secondary
cytoreductive therapy if the recurrent tumors remain small and
localized (20–24). Therefore, an increased frequency of
follow-up in patients with loss of BECN1, to detect recurrence
earlier, may allow patients to quickly receive further beneficial
cytoreductive surgery or second-line chemotherapy.
Multiple previous studies have demonstrated
comparable data in line with the present study, in that the loss of
BECN1 was reported to be associated with an unfavorable prognosis
of several solid cancers, including ovarian carcinoma (15,25–27). In
contrast, other studies demonstrated that the loss of BECN1 was
significantly correlated with improved survival in patients with
endometrial cancer, renal cell cancer, and colorectal cancer
(28–30). These differences may be due to a lack
of consistency between studies and/or variations in methodologies,
or may be disease-specific. The variations in methodologies between
studies, including the specific antibody and dilution used, and/or
the method for assessing immunohistochemical staining, may lead to
significant variability in results. Furthermore, several studies,
the present one included, had relatively small sample sizes, and
larger prospective studies are required to definitively determine
the association of BECN1 expression with the prognosis of ovarian
cancer. Finally, discrepancies between studies may be associated
with tissue-specific differences of oncogenic pathways, and
therefore tissue-specific differences in the loss of BECN1
expression require further investigation.
The present study demonstrated a strong association
between a poor prognosis and loss of BECN1 expression in patients
with ovarian carcinoma receiving platinum-taxane chemotherapy.
Although the expression of BECN1 was associated with LC3 and HMGB-1
expression, expression of the latter two proteins was not
associated with poor survival. Given that all three proteins are
involved in processes of autophagy, this was an unexpected
discovery. It is thus speculated that the association between the
loss of BECN1 and poor survival may be associated with a function
of BECN1 outside its involvement in autophagy. In a previous study,
Rohatgi et al (31) reported
that BECN1 regulates growth factor signaling, including the protein
kinase B (AKT) and extra-cellular signal-regulated kinase (ERK)
pathways. Therefore, reduction of BECN1 expression may cause
dysregulation of growth factor receptor signaling, leading to tumor
progression (31). This is indicative
of an autophagy-independent function for BECN1 in ovarian cancer.
Furthermore, our previous study revealed that BECN1 involvement in
autophagy was not associated with resistance of ovarian clear cell
carcinoma to platinum agents (17).
Thus, the results from the present study, and from previous studies
indicated that enhanced growth factor signaling due to the loss of
BECN1 may be a novel mechanism that explains treatment failure in
patients with loss of BECN1 expression.
The mechanisms underlying the association between
the loss of BECN1 expression and shorter survival in patients with
ovarian carcinoma are unknown. However, mortality in patients with
ovarian cancer is directly associated with disease recurrence
following chemotherapy. It is therefore hypothesized that the loss
of BECN1 expression confers chemoresistance and/or enhances cell
proliferation in recurrent tumors (17). The present study identified previously
unknown patterns of BECN1 expression in ovarian carcinoma, and
further investigation is warranted to elucidate the associations
between the loss of BECN1 expression and tumor sensitivity to other
chemotherapy drugs including paclitaxel, a key agent for ovarian
cancer.
To conclude, loss of BECN1 protein expression was
revealed to be an independent marker for poor progression-free
survival and poor overall survival in patients with ovarian
carcinoma receiving platinum-taxane chemotherapy.
Acknowledgements
The present study was supported by grants from the
Ministry of Education, Culture, Sports, Science and Technology in
Japan (grant no. 15K10717).
References
|
1
|
Allemani C, Weir HK, Carreira H, Harewood
R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A,
et al: Global surveillance cancer survival 1995–2009: Analysis of
individual data for 25,676,887 patients from 279 population-based
registries in 67 countries (CONCORD-2). Lancet. 385:977–1010. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Sosiety, . Cancer Facts
and Figures 2017. Atlanta, Ga: American Cancer Society; 2017
|
|
3
|
Pfisterer J and Ledermann JA: Management
of platinum-sensitive recurrent ovarian cancer. Semin Oncol. 33 2
Suppl 6:S12–S16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakayama K, Nakayama N and Miyazaki K:
Development of a novel ovarian cancer molecular target therapy
against cancer related transcriptional factor, NAC1. J Obstet
Gynaecol Res. 39:18–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agarwal R, Linch M and Kaye SB: Novel
therapeutic agents in ovarian cancer. Eur J Surg Oncol (EJSO).
32:875–886. 2006. View Article : Google Scholar
|
|
6
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimizu S, Kanaseki T, Mizushima N, Mizuta
T, Arakawa-Kobayashi S, Thompson CB and Tsujimoto Y: Role of Bcl-2
family proteins in a non-apoptotic programmed cell death dependent
on autophagy genes. Nat Cell Biol. 6:1221–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rashmi R, Pillai SG, Vijayalingam S,
Ryerse J and Chinnadurai G: BH3-only protein BIK induces
caspase-independent cell death with autophagic features in Bcl-2
null cells. Oncogene. 27:1366–1375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Furuya D, Tsuji N, Yagihashi A and
Watanabe N: Beclin 1 augmented cis-diamminedichloroplatinum induced
apoptosis via enhancing caspase-9 activity. Exp Cell Res.
307:26–40. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y, Liu JH, Jin L, Pan L, Sui YX, Yang
Y and Shi H: Beclin 1 influences cisplatin-induced apoptosis in
cervical cancer CaSki cells by mitochondrial dependent pathway. Int
J Gynecol Cancer. 22:1118–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J: Beclin 1 bridges autophagy,
apoptosis and differentiation. Autophagy. 4:947–948. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eskelinen EL and Saftig P: Autophagy: A
lysosomal degradation pathway with a central role in health and
disease. Biochim Biophys Acta. 1793:664–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mizushima N: Methods for monitoring
autophagy. Int J Biochem Cell Biol. 36:2491–2502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang D, Kang R, Cheh CW, Livesey KM, Liang
X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, et
al: HMGB1 release and redox regulates autophagy and apoptosis in
cancer cells. Oncogene. 29:5299–5310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai M, Hu Z, Liu J, Gao J, Liu C, Liu D,
Tan M, Zhang D and Lin B: Beclin 1 expressin in ovarian tissues and
its effect on ovarian cancer prognosis. Int Mol Sci. 15:5292–5303.
2014. View Article : Google Scholar
|
|
16
|
Odicino F, Pecorelli S, Zigliaani L and
Creasman WT: History of the FIGO cancer staging system. Int J
Gyneacol Obstet. 101:205–210. 2008. View Article : Google Scholar
|
|
17
|
Katagiri H, Nakayama K, Razia S, Nakamura
K, Sato E, Ishibashi T, Ishikawa M, Iida K, Ishikawa N, Otsuki Y,
et al: Loss of autophagy-related protein Beclin 1 may define poor
prognosis in ovarian clear cell carcinomas. Int J Oncol.
47:2037–2044. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katagiri A, Nakayama K, Rahman MT, Rahman
M, Katagiri H, Nakayama N, Ishikawa M, Ishibashi T, Iida K,
Kobayashi H, et al: Loss of ARID1A expression is related to shorter
progression-free survival and chemoresistance in ovarian clear cell
carcinoma. Mod Pathol. 25:1–288. 2012.PubMed/NCBI
|
|
19
|
Nakayama K, Rahman MT, Rahman M, Yeasmin
S, Ishikawa M, Katagiri A, Iida K, Nakayama N and Miyazaki K:
Biological role and prognostic significance of NAC1 in ovarian
cancer. Gynecol Oncol. 119:469–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Díaz-Montes TP and Bristow RE: Secondary
cytoreduction for patients with recurrent ovarian cancer. Curr
Oncol Rep. 7:451–458. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harter P and du Bois A: The role of
surgery in ovarian cancer with special emphasis on cytoreductive
surgery for recurrence. Curr Opin Oncol. 17:505–514. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gadducci A, Iacconi P, Cosio S, Fanucchi
A, Cristofani R and Riccardo Genazzani A: Complete salvage surgical
cytoreduction improves further survival of patients with late
recurrent ovarian cancer. Gynecol Oncol. 79:344–349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gadducci A, Iacconi P, Fanucchi A, Cosio
S, Teti G and Genazzani AR: Surgical cytoreduction during
second-look laparotomy in patients with advanced ovarian cancer.
Anticancer Res. 20:1959–1964. 2000.PubMed/NCBI
|
|
24
|
Zang RY, Li ZT, Tang J, Cheng X, Cai SM,
Zhang ZY and Teng NN: Secondary cytoreductive surgery for patients
with relapsed epithelial ovarian carcinoma: Who benefits? Cancer.
100:1152–1161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin HX, Qiu HJ, Zeng F, Rao HL, Yang GF,
Kung HF, Zhu XF, Zeng YX, Cai MY and Xie D: Decreased expression of
Beclin 1 correlates closely with Bcl-xL expression and poor
prognosis of ovarian carcinoma. PLoS One. 8:e605162013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou WH, Tang F, Xu J, Wu X, Yang SB, Feng
ZY, Ding YG, Wan XB, Guan Z, Li HG, et al: Low expression of Beclin
1, associated with high Bcl-xL, predicts a malignant phenotype and
poor prognosis of gastric cancer. Autophagy. 8:389–400. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiu DM, Wang GL, Chen L, Xu YY, He S, Cao
XL, Qin J, Zhou JM, Zhang YX and E Q: The expression of beclin-1,
an autophagic gene, in hepatocellular carcinoma associated with
clinical pathological and prognostic significance. BMC Cancer.
14:3272014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giatromanolaki A, Koukourakis MI,
Koutsopoulos A, Chloropoulou P, Liberis V and Sivridis E: High
Beclin 1 expression defines a poor prognosis in endometrial
adenocarcinomas. Gynecol Oncol. 123:147–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishikawa M, Miyake H, Liu B and Fujisawa
M: Expression pattern of autophagy-related markers in
non-metastatic clear cell renal cell carcinoma: Association with
disease recurrence following radical nephrectomy. J Cancer Res Clin
Oncol. 141:1585–1591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han Y, Xue XF, Shen HG, Guo XB, Wang X,
Yuan B, Guo XP, Kuang YT, Zhi QM and Zhao H: Prognostic
significance of Beclin-1 expression in colorectal cancer: A
meta-analysis. Asian Pac J Cancer Prev. 15:4583–4587. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rohatgi RA, Janusis J, Leonard D, Bellvé
KD, Fogarty KE, Baehrecke EH, Corvera S and Shaw LM: Beclin 1
regulates growth factor receptor signaling in breast cancer.
Oncogene. 34:5352–5362. 2015. View Article : Google Scholar : PubMed/NCBI
|