Introduction
Gastric cancer is one of the most common types of
malignant tumor in clinical practice and is a leading cause for
mortality (1). Gastric cancer causes
the second highest number of mortalities of all types of malignant
tumor (2). The majority of tumor
types, particularly solid tumors, are associated with infection and
chronic inflammation (3). The
occurrence and development of gastric cancer are influenced by
interactions with cells in the tumor microenvironment, and it is
particularly associated with chronic inflammation (4). In gastric tumors there is a combination
of gastric cancer cells and a range of types of immune cell, which
may either inherently exist in the tumor or migrate from other
tissues (4,5). Immune cells in the tumor
microenvironment may alter the phenotype of gastric cancer cells
and regulate the inflammatory response through a number of
signaling pathways, thereby promoting the development of gastric
cancer (6).
Myeloid cells are the principal type of
immunosuppressive cell in the tumor microenvironment (7). Mast cells are a type of terminally
differentiated myeloid cell and serve functions in type I allergic
reactions, innate and acquired immunity by identifying and
eliminating pathogens, releasing bio-active factors, and preventing
invasion by pathogens (8). Previous
studies have identified that the extent of mast cell infiltration
was enhanced in a number of types of tumor tissue, including breast
cancer and lymphoma, suggesting that mast cells serve a function in
the development of tumors (9,10). However, a limited number of studies
have been conducted on mast cells in gastric cancer. The effects of
mast cells on the occurrence and development of gastric cancer, and
the mechanism of inflammatory regulation, remain unknown.
Chemokines are small, soluble proteins that induce
the directional chemotaxis of cells by forming a concentration
gradient (11). C-C motif chemokine
ligand 2 (CCL-2) is a member of the CC chemokine subfamily that may
be secreted by monocytes, epithelial cells, astrocytes or tumor
cells, and is a key molecule for the regulation of inflammation
(12). C-C chemokine receptor 2
(CCR2) is a member of the G protein-coupled receptors superfamily
and binds CCL-2 with high affinity. Previous studies indicated that
CCL-2 or CCR2 are highly expressed in a number of types of tumor
tissue (13), and that a CCL-2
recombinant may induce the malignant transformation of breast
epithelial cells (14). In addition,
it was identified that the increased expression of CCL-2 in gastric
cancer tissue and peripheral blood was associated with disease
progression (15); however, whether
CCL-2 and CCR2 are involved in the development of gastric cancer,
and the underlying molecular mechanism of this interaction, remains
unknown.
Stem cell factors (SCFs), also known as mast cell
growth factors, may regulate the growth of a number of types of
cell, induce the proliferation and survival of and mobilize
stem/progenitor cells, and regulate the development of mast cells
(16,17). Mast/stem cell growth factor receptor
(known as c-Kit or CD117) is a transmembrane protein with tyrosine
kinase activity that acts as a receptor for SCFs; the activation of
c-Kit is associated with the proliferation and differentiation of
cells (18). It has been demonstrated
that a mutation of the c-Kit gene may cause the deregulation of the
proliferation and apoptosis of cells, promoting the development of
malignant tumors (19). c-Kit has
been identified as highly expressed in lung cancer,
gastrointestinal stromal tumors, ovarian cancer and other types of
malignant tumor (19). However, the
association between the activation of the SCF/c-kit signaling
pathway and the chemotaxis of mast cells, as induced by the
secretion of CCL-2, in gastric cancer tissue is yet to be
reported.
Materials and methods
Patient tissues
Cancer tissues and adjacent-normal tissue were
collected from 40 patients who underwent gastrectomy for gastric
cancer in the Affiliated Hospital of Qingdao University (Qingdao,
China) between January 2011 and December 2013. Of the 40 specimens,
26 were from male patients and 14 from female patients, with an age
range of 42–76 years, and a mean age of 56.25±11.37 years. All
patients provided written, informed consent. Fresh tissue specimens
were pathologically confirmed and diagnosed; differentiation and
stage were determined as previously described (20). Of the specimens, 7, 10 and 23 cases
were highly, moderately and poorly differentiated, respectively,
and 5, 9, 10, and 16 cases were classified as stage I, II, III and
IV, respectively.
Experimental materials
The human mast cell line HMC-1 was purchased from
Shanghai Meiyan Biological Technology Co., Ltd. (Shanghai, China).
HMC-1 cells were cultured in RPMI 1640 medium containing 10% fetal
bovine serum and 1% penicillin/streptomycin (all Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and incubated at 37°C
with 5% CO2 in air. The human gastric cancer cell line
BGC-823 was purchased from Shenzhen Baienwei Biological Technology
Co., Ltd, Shanghai, China. BGC-823 cells were maintained in
RPMI-1640 medium supplemented with 10% heated-inactivated calf
serum (Hangzhou Sijiqing Biological Engineering Materials Co.,
Ltd., Hangzhou, China) and 1% penicillin/streptomycin at 37°C with
5% CO2 in air.
The reagents for flow cytometry, included a
phycoerythrin (PE-) cyanine (Cy) 7-labeled anti-human cluster of
differentiation (CD) 45 antibody (560915, BD Biosciences, San Jose,
CA, USA), a peridinin chlorophyll protein complex (PerCP-)
Cy5.5-labeled anti-CD117 antibody (cat. no. 560557; BD
Biosciences), a fluorescein isothiocyanate (FITC)-labeled
anti-high-affinity immunoglobulin E receptor (FcεRIα) antibody
(cat. no. 610167; BD Biosciences), an allophycocyanin (APC)-labeled
anti-major histocompatibility complex class II-DR (HLA-DR) antibody
(cat. no. 559868; BD Biosciences).
RPMI 1640 and collagenase type IV were purchased
from Gibco; Thermo Fisher Scientific, Inc., and DNase type I was
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). An
immunohistochemical staining kit was purchased from Wuhan BOSHIDE
Biological Engineering Co., Ltd. (Wuhan, China) and the
EnVision™ G|2 System/AP kit was purchased from Dako;
Agilent Technologies, Inc. (Santa Clara, CA, USA).
The mouse anti-CCL-2 (cat. no. sc-1304) and
anti-CCR2 (cat. no. sc-6228) antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA) and an anti-GAPDH
(cat. no. sc-365062) antibody was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The rabbit anti-SCF (cat.
no. sc-9132), and anti-c-KIT (cat. no. sc-168) antibodies were
purchased from Santa Cruz Biotechnology, Inc., and anti-Akt (cat.
no. 9272) and anti-phosphorylated Akt (p-Akt; cat. no. 9271S) were
purchased from Cell Signaling Technology, Inc.
The reagents for reverse transcription-quantitative
polymerase chain reaction (RT-qPCR), including the RNA extraction
reagent, the RT kit and the SYBR kit, were purchased from Takara
Biological Co., Ltd. (Dalian, China). The primers for CCL-2, CCR2
and GAPDH, as described in Table I,
were purchased from Shanghai Yingjun Biotechnology Co. Ltd
(Shanghai, China).
 | Table I.Primer sequences of the target
genes. |
Table I.
Primer sequences of the target
genes.
| Gene | Forward primer | Reverse primer |
|---|
| CCL-2 |
GCTCATAGCAGCCACCTTCATTC |
CCGCCAAAATAACCGATGTGATAC |
| CCR2 |
CCAACTCCTGCCTCCGCTCTA |
TGCAGATTCTTGGGTTGTGGAG |
| GAPDH |
ACCACAGTCCATGCCATCAC |
TCCACCACCCTGTTGCTGTA |
Sample processing
The fresh specimens from patients with gastric
cancer, including the gastric cancer and normal adjacent tissues
(0–5 cm from the margin of the gastric cancer tissue), were
processed under aseptic conditions. Following 2–3 washes with 0.01
mol/l PBS, tissues were cut into small pieces and transferred into
tissue lysis buffer. The formula of the buffer was as follows:
Collagenase type IV (final concentration, 1 mg/ml), DNase type I
(final concentration, 100 µg/ml), CaCl2 (final
concentration, 2 mmol/l) and MgCl2 (final concentration,
2 mmol/l). The tissues were isolated using an automatic single cell
separator and incubated for 60 min at 37°C, with continuous
rotation. Subsequently, the samples were ground, passed through a
200-mesh steel net and centrifuged at 500 × g for 5 min at the room
temperature. Finally, the supernatant was discarded and the single
cell suspension was collected for staining and flow cytometry.
Flow cytometry staining
The single cell suspensions isolated from gastric
cancer and adjacent tissues were labeled using the PE-Cy7-labeled
anti-CD45 (1:600), FITC-labeled anti-FcεRIα (1:100),
PerCP-Cy5.5-labeled anti-CD117 (1:500), APC-labeled anti-HLA-DR
(1:300) antibodies at 4°C for 30 min. PBS containing 1% bovine
serum albumin (100 µl, Sigma-Aldrich; Merck KGaA) was used to wash
the samples. The mixtures were subsequently centrifuged at 500 × g
for 5 min at 4°C and fixed with 4% polyformaldehyde at 4°C for 30
min. Following a further wash in PBS, the precipitant was collected
by centrifuging at 500 × g for 5 min at 4°C, and 500 µl PBS was
added to re-suspend the cells. The mixture was incubated at 4°C in
lightproof conditions prior to flow cytometry to detect the
CD45+ and CD117+FcεRIα+ cell
subpopulations. FlowJo software version 7.6.2 (FlowJo LLC, Ashland,
OR, USA) was used for data processing.
Immunohistochemistry
Gastric cancer and adjacent tissue samples were
fixed in 10% formalin for 12 h at 4°C, embedded in paraffin and
sliced into 4-µm sections. The sections were dewaxed using xylene
twice (each for 10 min), rehydrated using an 80% ethanol series for
1 min and washed in distilled water. Antigen retrieval was
performed by immersing slides in ethylenediaminetetraacetic acid
for 15 min at 95°C, and the sections were allowed to cooled at room
temperature. Subsequently, sections were incubated with tryptase
(dilution, 1:100) and a primary antibody (anti-Akt, 1:1,000) for 30
min at room temperature and washed with PBS three times (each for 5
min). The IHC staining was developed with the EnVision™
G|2 System/AP kit, according to the manufacturer's protocol.
RT-qPCR detection
The gastric cancer tissue samples or BGC-823 cells
were washed with PBS and 1 ml TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was added. The tissue samples were ground.
Subsequently, total RNA was extracted using the phenol chloroform
extraction method, and the concentration and purity of tissue RNA
were determined using ultraviolet spectrophotometry. RNA was
reverse transcribed into cDNA using the RT kit according to the
manufacturer's protocol (Takara Biotechnology Co., Ltd., Dalian,
China). The expression of CCL-2, CCR2 and GAPDH mRNA was determined
using the SYBR qPCR kit with the primers listed in Table I. qPCR was performed using the Applied
Biosystems StepOne Real-Time PCR instrument (Thermo Fisher
Scientific, Inc.), and Cq values were automatically calculated with
the in-built software. The qPCR conditions were as follows:
Pre-denaturation at 95°C for 20 sec, then 95°C for 5 sec and 60°C
for 30 sec for 45 cycles. GAPDH was used as an internal reference
gene.
Western blot analysis
The gastric cancer and adjacent tissues were cut
into pieces, and the BGC-823 gastric cancer cells and tissue
samples were lysed with the aforementioned tissue lysis buffer
(Sample processing). The lysis solutions were centrifuged at 500 ×
g at 4°C for 15 min and the total protein in the supernatant was
extracted and cryopreserved at −80°C. The protein concentration was
determined using the bicinchoninic acid method. A total of 30 µg
protein per lane was separated using SDS-PAGE electrophoresis and
the products were transferred to a PVDF membrane with a 350 mA
current. The film was blocked with 5% skimmed milk powder for 1 h
and incubated with the following primary antibodies at 4°C
overnight: Anti-SCF (1:200), c-Kit (1:500), Akt (1:1,000), p-Akt
(1:1,000), CCL-2 (dilution, 1:1,000), CCR2 (1:1,000) and GAPDH
(1:2,000). The membrane was rinsed with Tris buffered saline with
Tween-20 (TBST) three times, for 10 min each time, and the
corresponding horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (cat. no. ab6721; dilution, 1:5,000, Abcam,
Cambridge, UK) was added. Following incubation at room temperature
for 2 h, the film was washed with TBST (each time for 10 min), and
the target protein was developed using an enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc.), and the
relative density of the bands were analyzed using ImageLab software
version 4.1 (Bio-Rad Laboratories Inc., Hercules, MA, USA). Using
GAPDH as an internal reference, the relative expression of CCL-2
and CCR2 proteins were calculated.
Transwell migration and invasion
assay
Prior to the migration assay, 600 µl blank control
(PBS) and HMC-1 mast cells (5×104 cells/ml) were added
to the lower chambers of 24-well plates, with triplicates for each
well. BGC-823 cell suspensions (200 µl, density, 2×104
cells/ml) were seeded in the upper chamber of Transwell inserts and
incubated for 24 h. The plate was washed with distilled water,
allowed to dry, fixed with 70% paraformaldehyde for 15 min and
stained with 0.05% crystal violet (Bioteke Corporation, Beijing,
China) for 30 min. Each plate was viewed with an optical microscope
at ×100 magnification in five fields of view (upper, lower, left,
right and center) and the numbers of migrating cells were counted.
The assay was repeated 3 times.
The invasion assay was similar to the migration
assay, except 12 h prior to the addition of cells, RPMI 1640 was
mixed with Matrigel, at a ratio of 3:1 at 4°C, and 40 µl of the
mixture was added into the upper Transwell chambers. The density of
the inoculated gastric cancer cells was 4×104 cells/ml
for the invasion assay.
Cell Counting kit (CCK)-8
proliferation assay
BGC-823 cells in the logarithmic growth stage were
inoculated in 96-well plates at a density of 5×103
cells/well and incubated at 37°C with 5% CO2. Once the
cells had adhered, the old culture medium was discarded and
replaced with fresh RPMI 1640. HMC-1 mast cells were added to a
concentration of 105 cells/ml. The negative control group, without
HMC-1 cells, was also seeded. A total of 300 nM Wortmannin (LC
Laboratory, Woburn, MA, USA) was added into 96-well plates 1 h
before the CCK-8 assay. A CCK-8 assay was performed at 12 and 24 h
post-incubation; 10 µl CCK-8 solution was added into each well and
incubated for 2 h. The absorbance value was measured at 490 nm
using a microplate reader. The assay was repeated three times, in
triplicate.
Acridine orange (AO)/ethidium bromide
(EB) double fluorescent staining apoptosis
Following co-cultivation, BGC-823 cells were seeded
at a density of 5×104 cells/well and HMC-1 cells were
seeded at a density of 2×104 cells/well in 24-well
plates for 24 h, and apoptosis was induced by adding 100 µM
H2O2 for 12 h. Subsequent to washing in PBS,
the cells were fixed with 4% paraformaldehyde at room temperature
for 10 min. PBS was added and agitated two times (each for 3 min).
AO solution was mixed with EB solution at a ratio of 1:1. AO/EB
mixture (10 µl) was removed and combined with 90 µl Buffer A
(Abcam). The 100 µl mixture was added into each well, incubated for
5 min at room temperature, washed with PBS twice for 3 min, and
allowed to dry in light proof conditions. Anti-fluorescence
quenching mounting media was added prior to observing changes to
cell morphology and nuclear structures using an Olympus IX50
inverted fluorescence microscope (Olympus Corporation, Tokyo,
Japan) at ×400 magnification. Normal cells exhibited uniform green
fluorescence, whereas chromatin condensation and nuclear
dissolution were visible in the apoptotic cells, and compact,
hyperchromatic yellow green particles or buds in different sizes
could be observed. This assay was repeated 3 times.
Statistical analysis
The data were analyzed using SPSS software (version
19.0; IBM Corp., Armonk, NY, USA) and were presented as the mean ±
standard deviation. Comparisons between two groups were performed
using a t-test and comparisons between three groups were examined
by one-way analysis of variance followed by a Dunnett's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Mast cells are upregulated in tumor
tissue compared with adjacent tissues
Single cell suspensions were prepared from the
surgical tissue specimens of 40 cases of patients with gastric
cancer. The infiltration ratio of mast cells in the gastric cancer
tissues and para-cancerous tissues was analyzed by calculating the
ratio of CD117+FcεRIα+ mast cells to
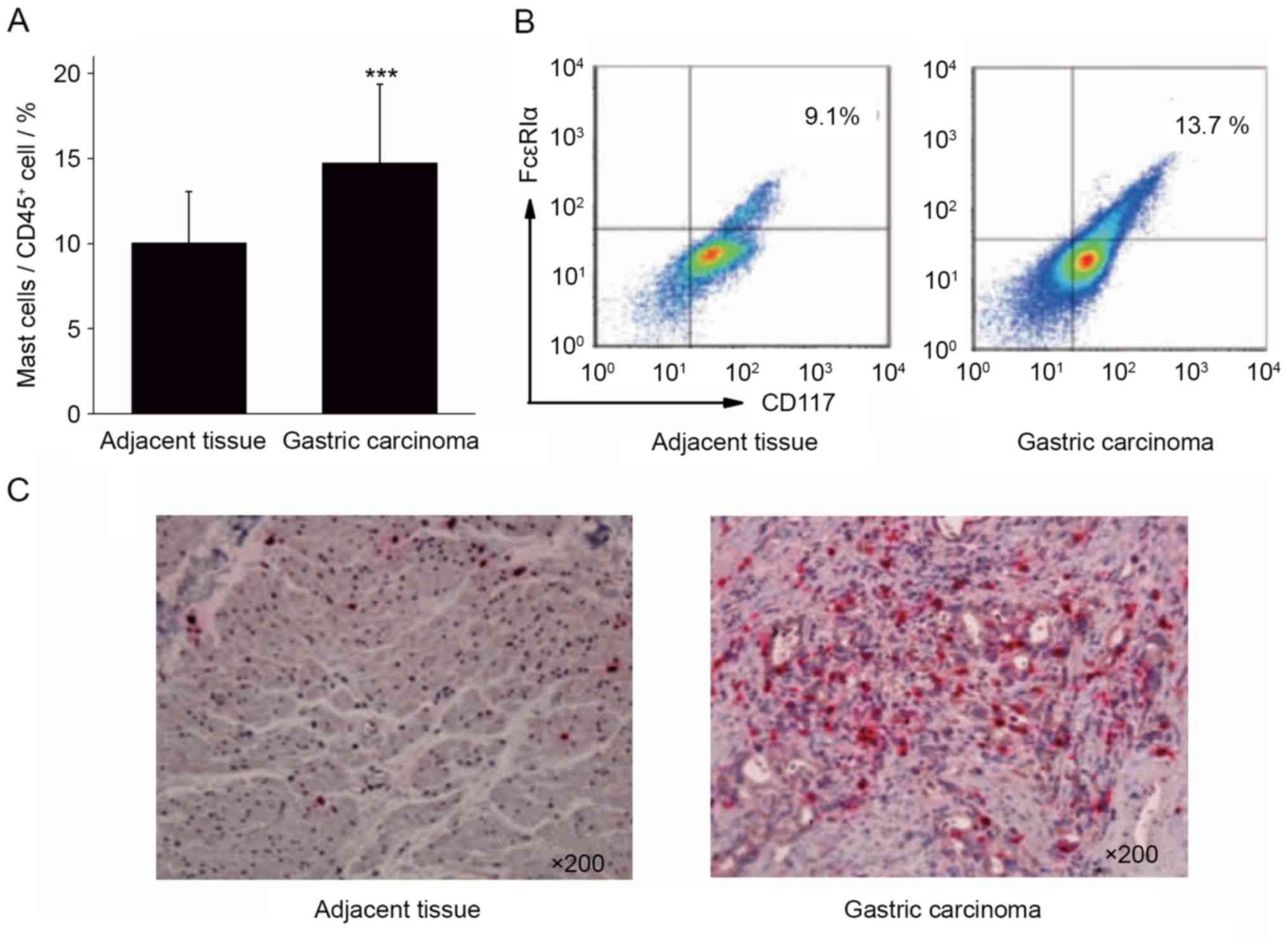
CD45+ cells. The results of flow cytometry demonstrated
that the proportion of mast cells in gastric cancer tissue
(14.73±4.62%) was significantly increased compared with the
adjacent tissues (10.03±3.02%; Fig. 1A
and B; P<0.001).
Immunohistochemical staining was performed to stain
mast cells red with tryptase in gastric cancer and adjacent
tissues. The results of immunohistochemistry revealed that the
number of mast cells in gastric cancer tissue was increased,
compared with that in the adjacent cancer tissue (Fig. 1C). The results of flow cytometry and
immunohistochemistry indicated that gastric cancer tissue was
enriched in mast cells, which may serve a function in the
development of gastric cancer.
CCL-2 and CCR2 are upregulated in
tumor tissue compared with adjacent tissues
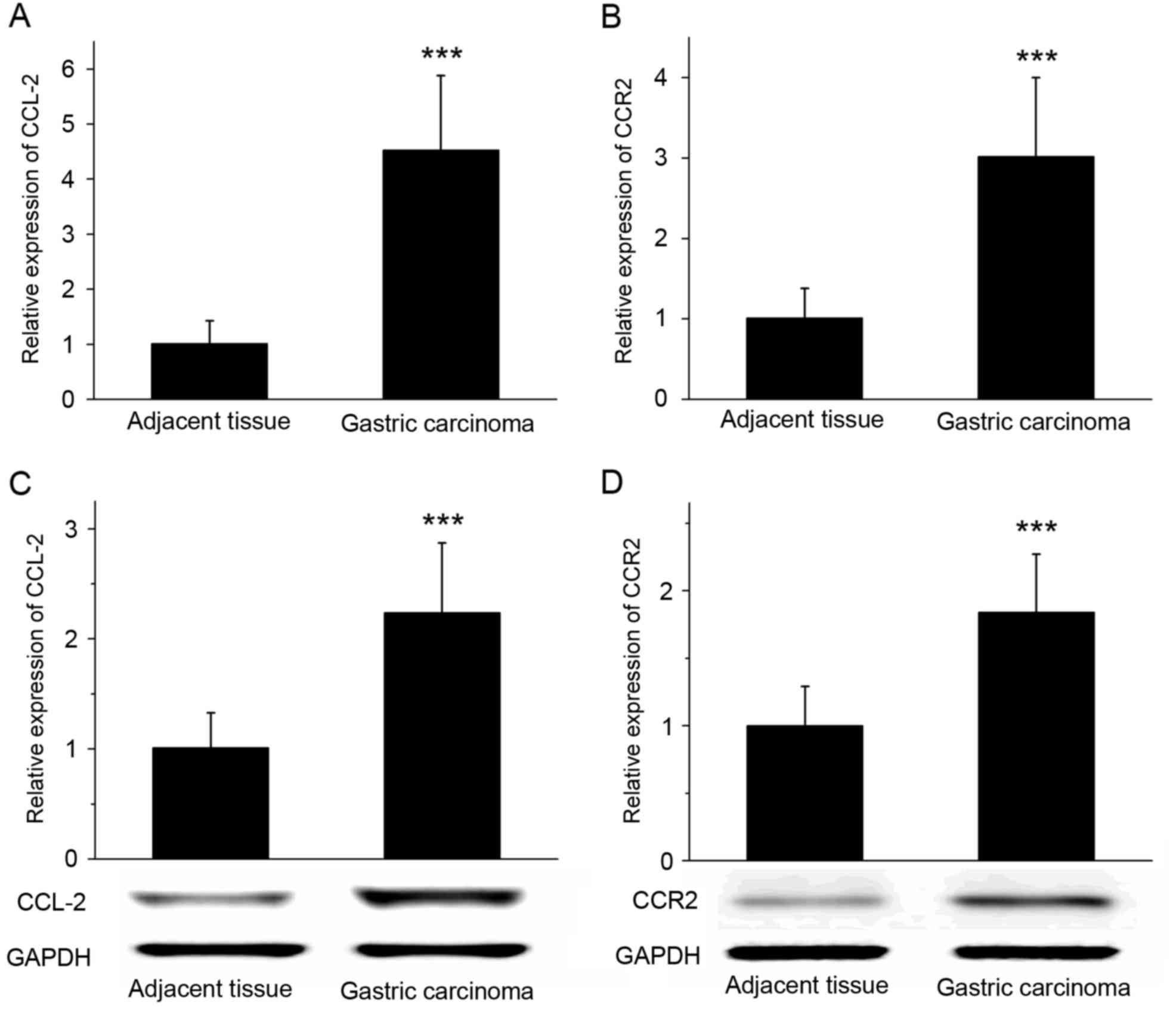
RT-qPCR revealed that the expression of CCL-2 and
CCR2 mRNA was significantly increased in gastric cancer tissues
compared with that in the adjacent tissues (P<0.001; Fig. 2A and B). The results of western blot
analysis demonstrated that the expression of CCL-2 and CCR2 protein
was significantly increased in gastric cancer tissues compared with
adjacent tissues (Fig. 2C and D;
P<0.001). RT-qPCR and western blot analysis suggested that the
expression levels of chemokine CCL-2 and its receptor CCR2 in
gastric cancer tissues were increased compared with the adjacent
tissues.
Mast cells significantly enhance the
migratory and invasive abilities of gastric cancer cells
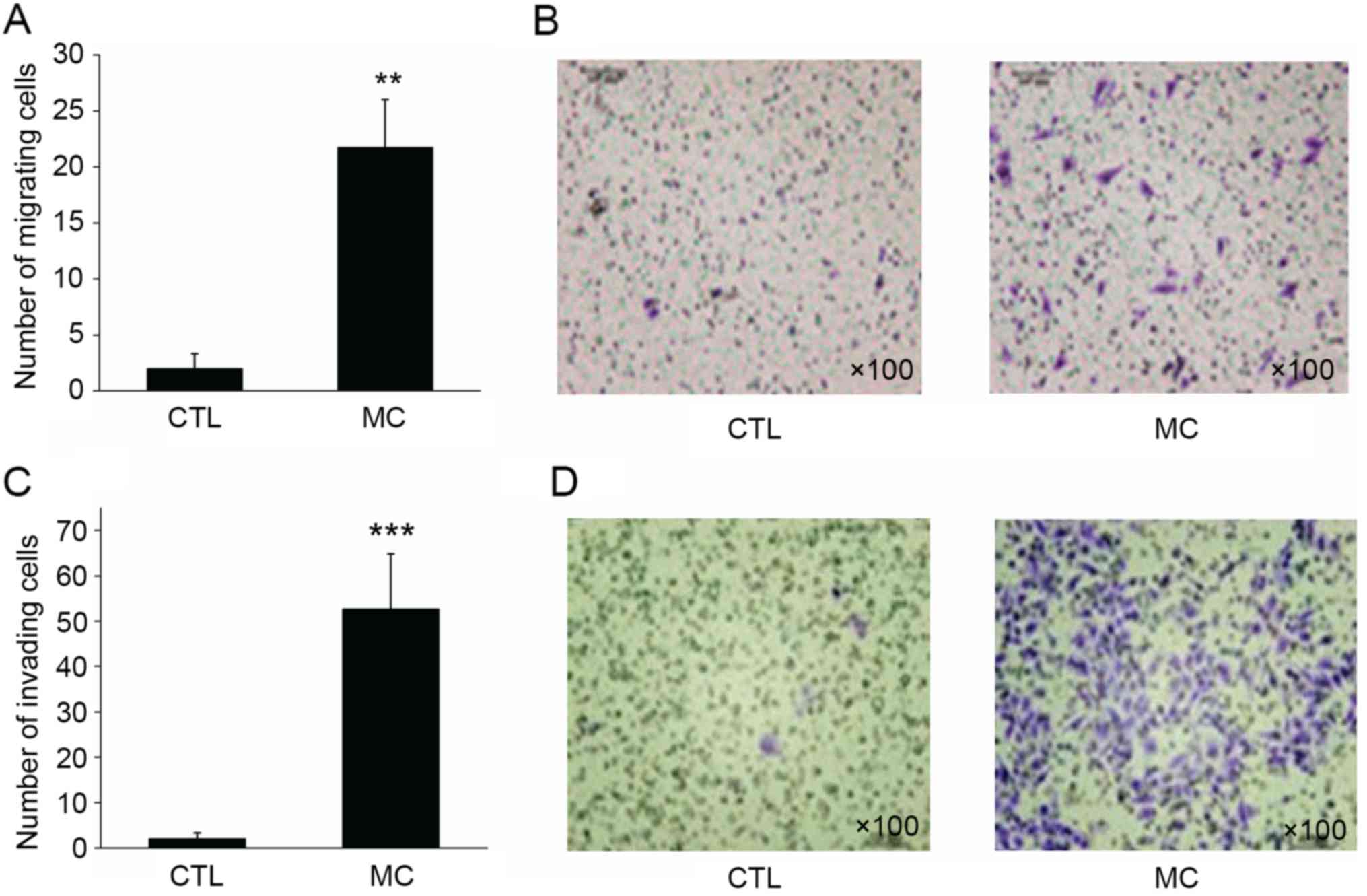
In the migration assay, BGC-823 gastric cancer cells
in the control group demonstrated a limited capacity for migration,
with a mean of 2.15±1.36 cells per well migrating to the lower
surface of the membrane. However, when HMC-1 mast cells were added
to the lower chamber, the migratory ability of BGC-823 cells was
enhanced, with a mean of 21.68±4.29 cells per well migrating to the
lower surface (Fig. 3A and B;
P<0.01).
Similar to the migration assay result, 2.01±1.30
BGC-823 cells per well invaded to the lower surface of the
membrane. However, when HMC-1 mast cells were added to the lower
chamber, the invasive ability of gastric cancer cells was
significantly enhanced, with 52.73±12.08 cells invading to the
lower surface of the membrane (Fig. 3C
and D; P<0.001). Transwell migration and invasion assays
revealed that mast cells may significantly enhance the migratory
and invasive abilities of gastric cancer cells.
Co-culturing with mast cells promotes
the proliferation and inhibits the apoptosis of gastric cancer
cells
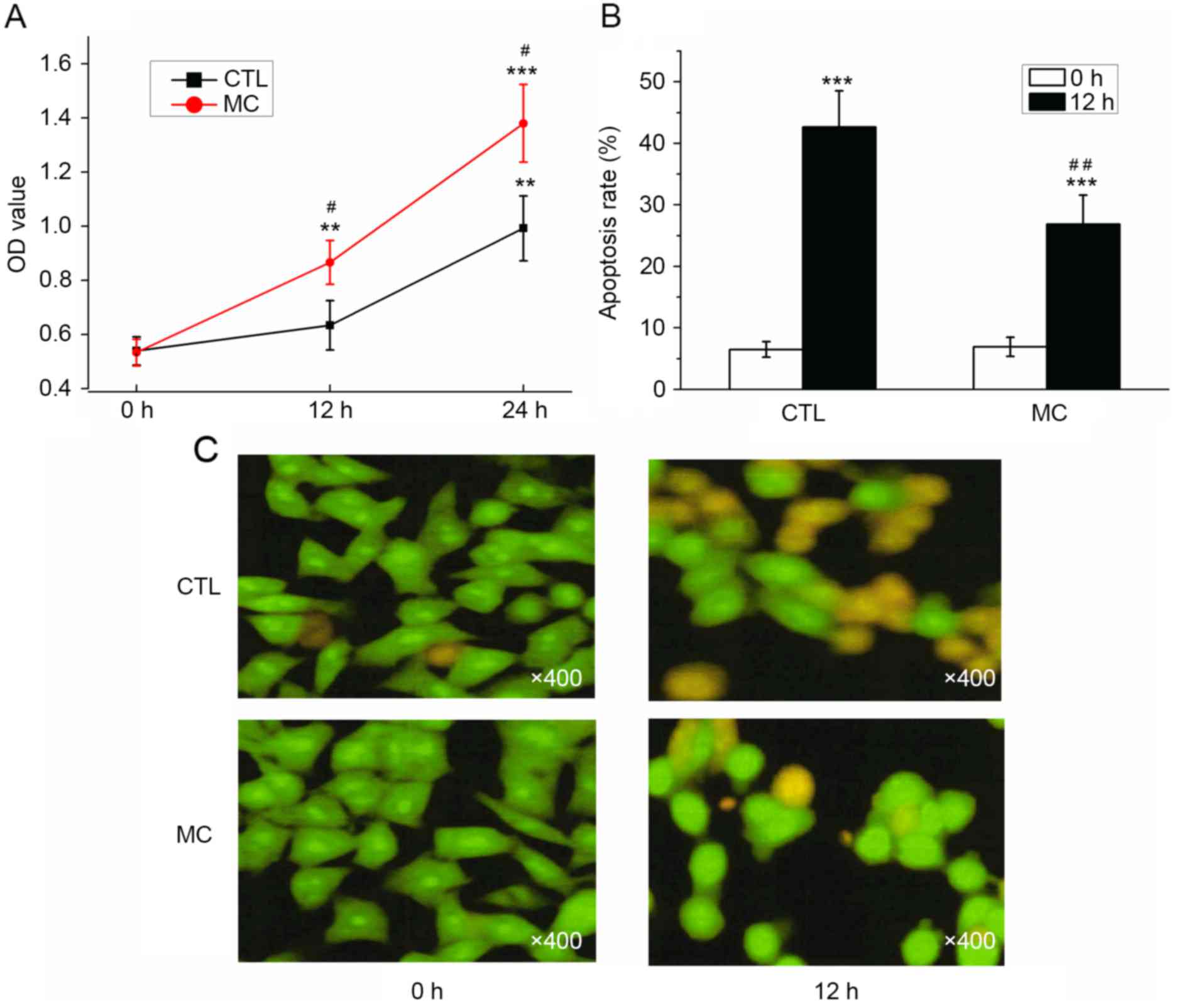
As determined with a CCK-8 assay, the OD values for
BGC-823 cells with or without HMC-1 cells increased; however, the
OD value determined in the co-culture group was significantly
increased compared with the control group at 12 and 24 h (Fig. 4A; P<0.05). Prior to the induction
of apoptosis by H2O2, the apoptosis rates
were 6.50±1.25 (co-culture group) and 6.93±1.55% (control group),
which was not significantly different. Following co-culture with
HMC-1 cells for 24 h, the apoptosis rate of gastric cancer cells
was significantly decreased (26.87±4.68%) compared with the control
group (42.63±5.89%; Fig. 4B;
P<0.01). Prior to H2O2 treatment, the
majority of cells demonstrated a uniform green fluorescence with a
limited amount of yellow fluorescence. Following
H2O2 treatment for 12 h, the number of cells
with incomplete nuclear membranes increased, dense patches could be
observed in the membrane, and orange or yellow apoptotic bodies
were observed (Fig. 4C). Following
the co-culture with mast cells, a reduced number of pale yellow
gastric cancer cells and apoptotic bodies were observed (Fig. 4C). The results of AO/EB double
fluorescence staining demonstrated that co-culturing with mast
cells may promote the proliferation, and inhibit the apoptosis, of
gastric cancer cells.
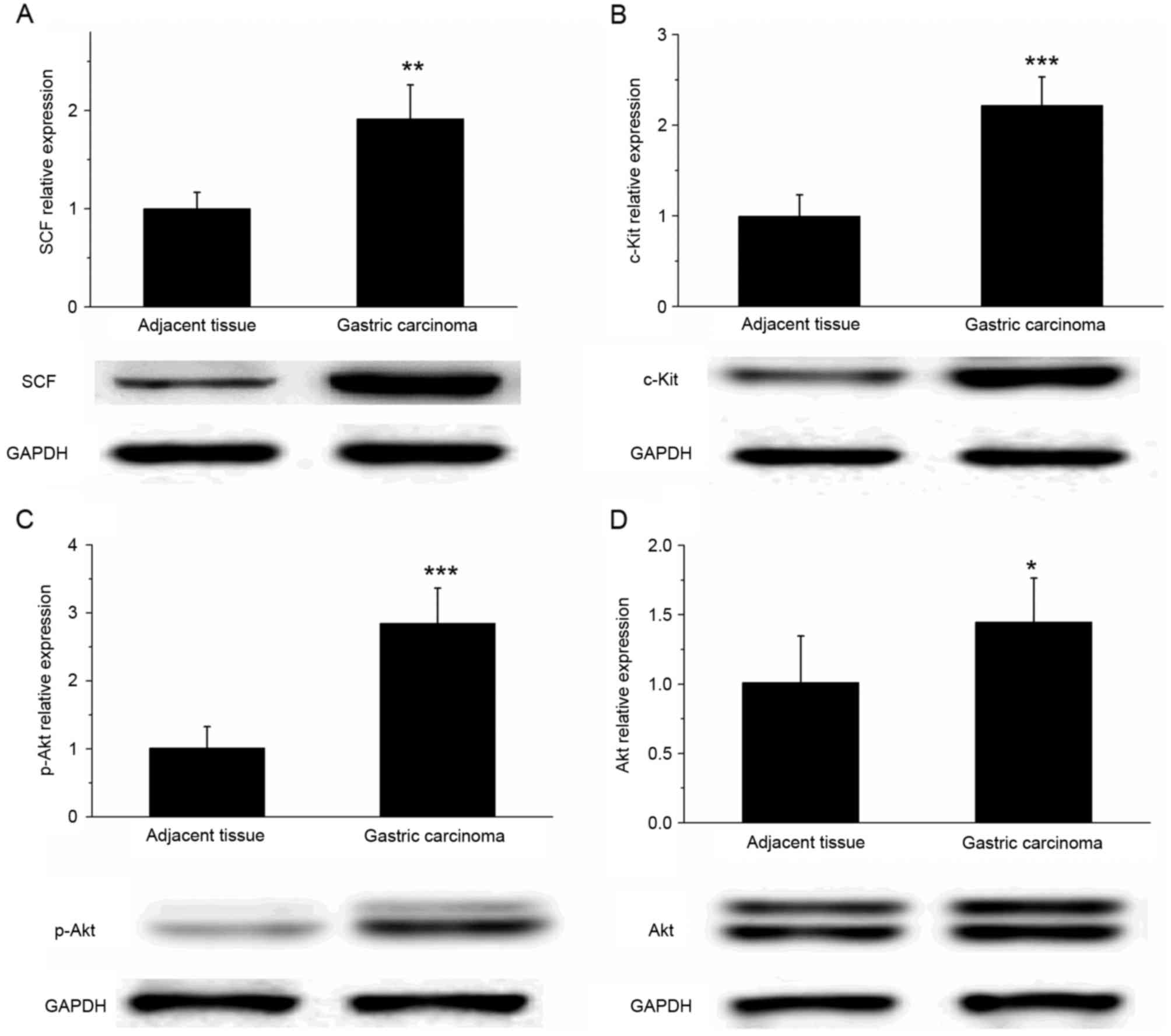
SCF and c-Kit are upregulated in
gastric cancer tissue
The results of western blot analysis revealed that
the expression of SCF and c-Kit protein was significantly increased
in gastric cancer tissues, compared with that in the adjacent
tissues (Fig. 5A and B; P<0.01).
In addition, the expression of p-Akt in gastric cancer tissue was
significantly increased compared with that in the adjacent tissues
(P<0.001); however, the difference in the Akt expression level
of the two types of tissue was relatively small (Fig. 5C and D; P<0.05). These results
suggested that the SCF/c-Kit signaling pathway may activate the
phosphoinositide 3-kinase (PI3K)-Akt signaling pathway to
participate in the development and metastasis of gastric
cancer.
Pre-treatment with wortmannin inhibits
the expression of CCL-2 and reduces the migratory abilities of
gastric cancer cells
RT-qPCR and western blot analysis revealed that
CCL-2 expression was increased in BGC-823 cells co-cultured with
HMC-1 cells; however, if cells were pre-treated with the specific
PI3K inhibitor wortmannin, the mRNA and protein expression of CCL-2
was significantly inhibited (Fig. 6A and
B; P<0.05).
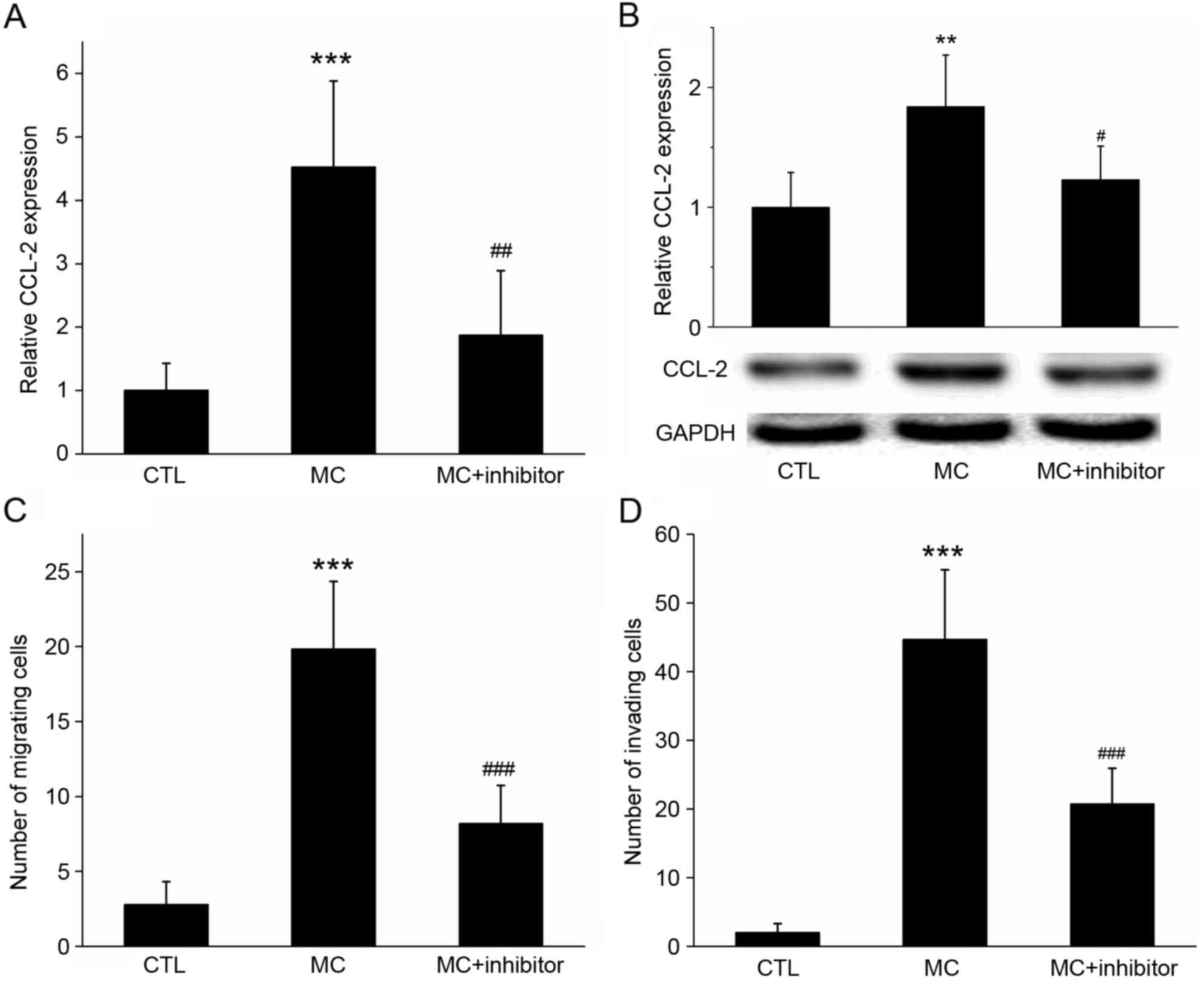 | Figure 6.Effect of the inhibition of PI3K
activation on co-cultured cell CCL-2 expression, invasion and
migration. (A) Reverse transcription-quantitative polymerase chain
reaction and (B) western blot analysis determined that, following
co-culture with MC, the relative expression of CCL-2 mRNA and
protein in gastric cancer cells was increased, compared with the
control. However, if cells were pre-treated with the specific PI3K
inhibitor wortmannin, the increase in the gene and protein
expression levels of CCL-2 was significantly inhibited. GADPH was
used as the loading control. (C) Migration and (D) invasion assays
demonstrating that the migration of gastric cancer cells increased
with MC co-culture, but decreased following the addition of the
PI3K inhibitor wortmannin. ***P<0.001 MC vs. CTL;
#P<0.05, ##P<0.01 and
###P<0.001 MC vs. MC+ inhibitor. PI3K,
phosphoinositide 3-kinase; CCL-2, C-C motif chemokine ligand 2;
CCR2, C-C motif chemokine receptor 2; MC, mast cell co-culture
group; CTL, control group. |
In the migration assay, when HMC-1 cells were added
to the lower chamber, the number of BGC-823 cells on the lower
surface of the membrane was 19.84±4.52; however, the number
decreased to 8.22±2.49 (P<0.01) following wortmannin treatment
(Fig. 6C). In the invasion assay,
when HMC-1 cells were added to the lower chamber, the number of
BGC-823 cells on the lower surface of the membrane was 44.73±10.09;
however, this decreased to 20.73±5.21 (P<0.001) following
wortmannin treatment (Fig. 6D). These
results suggested that the PI3K inhibitor may have inhibited the
expression of CCL-2 in gastric cancer cells, and the inhibitor may
inhibit the migration and invasion of gastric cancer cells promoted
by mast cells.
Discussion
Gastric cancer is one of the most common types of
cancer and the leading causes of cancer-associated mortalities in
china (21). Despite a sustained
period of significant progress in basic and clinical cancer
research, the current status of the diagnosis and treatment of
gastric cancer is poor; the majority of patients with gastric
cancer are diagnosed at the intermediate-to-advanced stage, and the
5-year survival rate is low (21,22).
Despite resective surgery, recurrence and metastasis occur in
>50% of the patients (2).
Therefore, it is necessary to explore the underlying molecular
mechanisms for gastric cancer metastasis, and identify novel
markers and therapeutic targets, in order to determine an improved
theoretical basis for the diagnosis and treatment of gastric
cancer. The underlying molecular mechanism of gastric cancer is
complex; the disease course and risk factors are associated with
inflammation (3). The progression of
gastric cancer is regulated by the interactions of a number of
types of cell in the tumor microenvironment, and the
microenvironment of gastric cancer may alter the phenotype of
immunoinflammatory cells (6). It is
now evident that inflammatory cells have powerful effects on tumor
development, and gastric cancer may be included (6).
The infiltration of mast cells was previously
observed to increase in lymphoma, breast cancer and other types of
tumor compared with non-tumor tissue (9,10). The
analysis of tissue samples from 30 patients with gastric cancer
demonstrated that that the number of mast cells exhibited a
positive association with the vascular density, and a negative
association with the patient prognosis (23). The present study provides evidence
that mast cells serve a function in gastric cancer. The
infiltration ratio of mast cells in 40 gastric cancer tissues and
adjacent cancer tissues were analyzed. The results demonstrated
that the proportion of mast cells in gastric cancer tissues were
significantly increased, compared with that in the paracancerous
tissues, which was validated using immunohistochemical staining
with tryptase. In addition, the Transwell migration and invasion
assay results revealed that co-culture with mast cells
significantly enhanced the migratory and invasive abilities of
gastric cancer cells. Furthermore, the results of the CCK-8 assay
suggested that co-culture with mast cells may promote the
proliferation of gastric cancer cells, whereas the double
fluorescence staining result suggested that co-culture inhibited
the H2O2-induced apoptosis of gastric cancer
cells.
It was identified in previous studies that the role
of mast cells in different tumor types varies; mast cell
infiltration has been associated with both tumor promotion and
suppression. For example, in primary cutaneous lymphoma, it was
demonstrated that mast cells promoted the growth of tumor cells
(24). In colorectal cancer, however,
the infiltration of the tumor by mast cells was negatively
associated with lymph node or distant metastasis (25). Previous studies have suggested that
interstitial mast cells in breast cancer were associated with an
improved prognosis (26,27). Therefore, the effect of mast cells may
be associated with the specific type and stage of the tumor.
A previous study indicated that chemokines and their
receptors are involved in tumor cell biology, including the
regulation of cell proliferation, apoptosis, metastasis,
angiogenesis and the immune response to tumors (13). CCL-2 is a key molecule in the
regulation of inflammation that is secreted by monocytes,
epithelial cells or certain types of tumor cell (12). CCR2, as a member of the chemokine
receptor family, is associated with cell migration and other
functions in tumors (28). Studies
have indicated that CCL-2 or CCR2 are highly expressed in a number
of types of tumor and serve functions in their development
(13,15). The results of RT-qPCR and western blot
analysis in the present study validated that the expression of
CCL-2 and CCR2 mRNA and proteins in gastric cancer tissues were
significantly increased compared with adjacent tissues, consistent
with a previous study (29). At
present, the proposed molecular mechanisms for the promotion of
tumor development by CCL-2 expression are: i) The recruitment of
macrophages and influence of their phenotype, quantity and
function; ii) the promotion of tumor angiogenesis; iii) effects on
the regulation of the immune response; and iv) acting directly on
tumor cells, e.g. stimulating tumor cells to promote stem cell
characteristics and spheroiding phenotype (30,31). CCL-2
may regulate the association between normal cells and tumor cells
in the microenvironment, and promote the metastasis of prostate
cancer (32). It was previously
identified that the expression of CCL-2 in the tissue and serum of
patients with oral squamous cell carcinoma was upregulated, and the
tumor size of mice was significantly decreased when CCL-2 was
inhibited (33). Rokavec et al
(14) demonstrated that CCL-2 may
directly promote the transformation of mammary epithelial cells.
The results of the present study suggested that when gastric cancer
cells were co-cultured with mast cells, the expression of CCL-2
mRNA and protein increased; however, when the gastric cancer cells
were pre-treated with a PI3K inhibitor, wortmannin, the elevation
of CCL-2 was inhibited, in addition to the effects of mast cells on
gastric cancer cell proliferation, invasion and migration. This
result indicated that CCL-2 may promote the migration and invasion
of gastric cancer cells.
SCF is produced by stromal cells in the bone marrow
microenvironment and may regulate mast cell growth through
coordination with other types of cytokine, induction of the
proliferation of stem/progenitor cells and the inhibition of
apoptosis, and thus affect the development of mast cells (16). c-Kit is a transmembrane protein with
tyrosine kinase activity; its ligand is SCF, which is expressed by
a number of types of cell, including hematopoietic stem cells, mast
cells, melanocytes and Cajal cells from the gastrointestinal tract
(19). It was demonstrated that c-Kit
is highly expressed in a number of types of malignant tumor,
including mast cell leukemia, neuroblastoma, malignant melanoma,
endometrial cancer, ovarian cancer and small cell lung cancer
(19). Furthermore, c-Kit is used as
a marker for the diagnosis of the gastrointestinal stromal tumors,
and a target for therapy (19). The
results of the present study indicated that the expression levels
of SCF and c-Kit proteins in gastric cancer tissues were
significantly increased compared the adjacent tissues. In addition,
it was identified that the expression of p-Akt in gastric cancer
tissues was significantly increased compared with the adjacent
tissues. Based on the data of the present study, we hypothesize
that the SCF/c-Kit signaling pathway may activate the PI3K-Akt
signaling pathway in gastric cancer, and participate in the
development and metastasis of gastric cancer. To test this, the
specific PI3K inhibitor wortmannin was used; when treated with
wortmannin, the extent of the promotion of gastric cancer cell
migration and invasion of by mast cells was significantly
inhibited. Therefore, activation of the SCF/c-Kit signaling pathway
may activate PI3K-Akt and enhance the effect of mast cells by
upregulating CCL-2 secretion in gastric cancer cells.
In the present study, the underlying molecular
mechanisms of mast cells in gastric cancer were investigated. It
was identified with flow cytometry and immunohistochemistry that
the infiltration ratio of mast cells in gastric cancer tissues was
significantly increased compared with the adjacent cancer tissues.
Additionally, the expression level of CCL-2 and CCR2 mRNA and
protein was increased significantly in gastric cancer tissues
compared with adjacent tissues. Furthermore, in vitro
cellular experimental results demonstrated that mast cells may
promote the proliferation, migration and invasion of gastric cancer
cells, and inhibit apoptosis. We therefore hypothesize that
activation of the SCF/c-Kit signaling pathway may promote the
expression of CCL-2 by activating PI3K-Akt to promote the
occurrence and development of gastric cancer.
In conclusion, the results of the present study
provide new insight on the mechanism of gastric cancer development,
and may identify potential novel targets for clinical
treatment.
References
|
1
|
Rugge M, Fassan M and Graham DY:
Epidemiology of gastric cancer. Gastric Cancer. 1–34.
2015.PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trinchieri G: Cancer and inflammation: An
old intuition with rapidly evolving new concepts. Annu Rev Immunol.
30:677–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung HW and Lim JB: Role of the tumor
microenvironment in the pathogenesis of gastric carcinoma. World J
Gastroenterol. 20:1667–1680. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mroczko B, Łukaszewicz-Zajac M,
Guzińska-Ustymowicz K, Gryko M, Czyzewska J, Kemona A, Kedra B and
Szmitkowski M: Expression of matrix metalloproteinase-9 in the
neoplastic and interstitial inflammatory infiltrate cells in
gastric cancer. Folia Histochem Cytobiol. 47:491–496.
2009.PubMed/NCBI
|
|
6
|
Dalgleishl AG and O'Byrne K: Inflammation
and cancer. Nature. 420:1–3849. 2014.
|
|
7
|
Rivera LB, Meyronet D, Hervieu V,
Frederick MJ, Bergsland E and Bergers G: Intratumoral myeloid cells
regulate responsiveness and resistance to antiangiogenic therapy.
Cell Rep. 11:577–591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Theoharides TC, Alysandratos K-D,
Angelidou A, Delivanis D-A, Sismanopoulos N, Zhang B, Asadi S,
Vasiadi M, Weng Z, Miniati A and Kalogeromitros D: Mast cells and
inflammation. Biochim Biophys Acta. 1822:21–33. 2013. View Article : Google Scholar
|
|
9
|
Raica M, Cimpean AM, Ceausu R, Ribatti D
and Gaje P: Interplay between mast cells and lymphatic vessels in
different molecular types of breast cancer. Anticancer Res.
33:957–963. 2013.PubMed/NCBI
|
|
10
|
Rabenhorst A, Schlaak M, Heukamp LC,
Förster A, Theurich S, von Bergwelt-Baildon M, Büttner R, Kurschat
P, Mauch C, Roers A and Hartmann K: Mast cells play a
protumorigenic role in primary cutaneous lymphoma. Blood.
120:2042–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Griffith JW, Sokol CL and Luster AD:
Chemokines and chemokine receptors: Positioning cells for host
defense and immunity. Annu Rev Immunol. 32:659–702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsaur I, Noack A, Makarevic J, Oppermann
E, Waaga-Gasser AM, Gasser M, Borgmann H, Huesch T, Gust KM, Reiter
M, et al: CCL2 chemokine as a potential biomarker for prostate
cancer: A pilot study. Cancer Res Treat. 47:306–312. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rokavec M, Wu W and Luo JL: IL6-mediated
suppression of miR-200c directs constitutive activation of
inflammatory signaling circuit driving transformation and
tumorigenesis. Mol Cell. 45:777–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Liu X and Wang Y: Predictive value
of preoperative serum CCL2, CCL18 and VEGF for the patients with
gastric cancer. BMC Clin Pathol. 13:152013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ray P, Krishnamoorthy N and Ray A:
Emerging functions of c-kit and its ligand stem cell factor in
dendritic cells: Regulators of T cell differentiation. Cell Cycle.
7:2826–2832. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amagai Y, Tanaka A, Matsuda A, Jung K,
Ohmori K and Matsuda H: Stem cell factor contributes to
tumorigenesis of mast cells via an autocrine/paracrine mechanism. J
Leukoc Biol. 93:245–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suphanantachat S, Iwata T, Ishihara J,
Yamato M, Okano T and Izumi Y: A role for c-Kit in the maintenance
of undifferentiated human mesenchymal stromal cells. Biomaterials.
35:3618–3626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashman LK and Griffith R: Therapeutic
targeting of c-KIT in cancer. Expert Opin Investig Drugs.
22:103–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huscher CG, Mingoli A, Sgarzini G,
Sansonetti A, Di Paola M, Recher A and Ponzano C: Laparoscopic
versus open subtotal gastrectomy for distal gastric cancer:
Five-year results of a randomized prospective trial. Ann Surg.
241:232–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H
and Zou X: Report of cancer incidence and mortality in China, 2010.
Ann Transl Med. 2:612014.PubMed/NCBI
|
|
22
|
Ferro A, Peleteiro B, Malvezzi M, Bosetti
C, Bertuccio P, Levi F, Negri E, La Vecchia C and Lunet N:
Worldwide trends in gastric cancer mortality (1980–2011), with
predictions to 2015, and incidence by subtype. Eur J Cancer.
50:1330–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ribatti D, Guidolin D, Marzullo A, Nico B,
Annese T, Benagiano V and Crivellato E: Mast cells and angiogenesis
in gastric carcinoma. Int J Exp Pathol. 91:350–356. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rabenhorst A, Schlaak M, Heukamp LC,
Förster A, Theurich S, von Bergwelt-Baildon M, Büttner R, Kurschat
P, Mauch C, Roers A and Hartmann K: Mast cells play a
protumorigenic role in primary cutaneous lymphoma. Blood.
120:2042–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan SY, Fan Y, Luo HS, Shen ZX, Guo Y and
Zhao LJ: Prognostic significance of cell infiltrations of
immunosurveillance in colorectal cancer. World J Gastroenterol.
11:1210–1214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rajput AB, Turbin DA, Cheang MC, Voduc DK,
Leung S, Gelmon KA, Gilks CB and Huntsman DG: Stromal mast cells in
invasive breast cancer are a marker of favourable prognosis: A
study of 4,444 cases. Breast Cancer Res Treat. 107:249–257. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amini RM, Aaltonen K, Nevanlinna H,
Carvalho R, Salonen L, Heikkilä P and Blomqvist C: Mast cells and
eosinophils in invasive breast carcinoma. BMC Cancer. 7:1652007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang Y, Chen H, Wang J, Bunjhoo H, Xiong
W, Xu Y and Zhao J: Relationship between CCR2-V64I polymorphism and
cancer risk: A meta-analysis. Gene. 524:54–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tao LL, Shi SJ, Chen LB and Huang GC:
Expression of monocyte chemotactic protein-1/CCL2 in gastric cancer
and its relationship with tumor hypoxia. World J Gastroenterol.
20:4421–4427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast tumor metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Low-Marchelli JM, Ardi VC, Vizcarra EA,
van Rooijen N, Quigley JP and Yang J: Twist1 induces CCL2 and
recruits macrophages to promote angiogenesis. Cancer Res.
73:662–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Patel L and Pienta KJ: CC
chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis
and metastasis. Cytokine Growth Factor Rev. 21:41–48. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Xu Q, Wu Y, Li J, Tang D, Han L and
Fan Q: A CCL2/ROS autoregulation loop is critical for cancer
associated fibroblasts-enhanced tumor growth of oral squamous cell
carcinoma. Carcinogenesis. 35:1362–1370. 2014. View Article : Google Scholar : PubMed/NCBI
|