Introduction
Cholangiocarcinoma (CCA), a cancer of the bile duct,
is a major health problem in Northeastern Thailand and Southeast
Asia. It is associated with infestation by the liver fluke
Opisthorchis viverrini (1).
The incidence of CCA is high in East and Southeast Asia and its
incidence is also increasing in England, the USA and Australia
(2,3).
Diagnoses of CCA are usually made when the disease is advanced or
disseminated, meaning that the prognosis of patients is poor;
therefore, novel target biomarkers are required to enable early
diagnosis of CCA, as well as increase the therapeutic efficacy of
treatments for CCA.
Protein glycosylation is the most common
post-translational modification that occurs in human proteins
(4,5).
It is important in cell and tissue development, host-pathogen
interactions, inflammation and malignancy (6). Alterations in protein glycosylation have
been reported in various diseases, including different types of
cancer (7). Identifying altered
cancer-associated glycoproteins may facilitate the development of
potential biomarkers of cancer or novel targets for treatment.
A number of in vitro and in vivo
molecular studies investigating glycoproteins in CCA have been
performed. It has been demonstrated that the expression of
sialyl-LewisA in the tissues of patients with CCA is
associated with poor prognosis (8).
Furthermore, a study using monoclonal antibodies against serum
glycoprotein mucin 5AC revealed that levels of serum glycan epitope
(S121) are associated with patient prognosis and is specific to CCA
(9). This association was
investigated further in an animal model. It was demonstrated that
the glycan epitope (S121) was expressed in the cytoplasm and apical
surface of biliary cells during the early stages of tumor
development, and that this expression increased further with tumor
progression (10).
Immunohistochemical studies have revealed that
N-acetylglucosamine (GlcNAc) (11) and O-GlcNAc transferase are
overexpressed in CCA (12).
Furthermore, the results of ELISA performed on the serum of
patients with CCA revealed that the association between glycan
epitope CA-S27 and patient prognosis is specific to CCA and may
have immunodiagnostic value (13).
It has been demonstrated that the lectin
microarray-based sero-biomarker is able to detect O-linked
glycosylation in CCA (14).
Furthermore, using different CCA cell lines, it has been revealed
that different histological types of CCA exhibit differential
expression levels of O-glycans (15). In-depth characterization of the
glycans expressed in the serum of patients with CCA may facilitate
the identification of potential CCA biomarkers.
The present study assessed the structural glycomics
of N-glycans in the serum of patients with CCA compared with
healthy controls. Three candidate glycan markers were proposed and
it was hypothesized that these specific glycans may aid in the
development of diagnostic and/or therapeutic markers of CCA.
Patients and methods
Reagents
Sodium borohydride and sodium hydroxide were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Fetuin glycoprotein standard was obtained from Sigma-Aldrich; Merck
KGaA. D-galactose, D-mannose and N-acetyl-D-glucosamine were
obtained from EMD Millipore (Billerica, MA, USA).
Patients with CCA and healthy
controls
A total of 8 serum samples from patients with CCA
(mean age, 60.25±9.59; 3 females and 5 males) and 4 samples from
healthy controls (mean age, 41.75±16.88; 3 females and 1 male) were
obtained from participants recruited between January 2014 and May
2014 in the Liver Fluke and Cholangiocarcinoma Research Center,
Faculty of Medicine, Khon Kaen University (Khon Kaen, Thailand).
Patients were enrolled in the study if they had been diagnosed with
intrahepatic CCA and had no apparent chronic inflammatory diseases,
including diabetes mellitus or rheumatoid arthritis. The Ethics
Committee of Khon Kaen University reviewed and approved the study
protocol (registration number HE521209) and patients provided
informed consent for the use of their material in the present
study. Through peripheral venipuncture, a single blood sample was
drawn into a 10 ml BD Vacutainer sterile vacuum tube (BD
Biosciences, Franklin Lakes, NJ, USA) in the absence of
anticoagulant. Blood was immediately centrifuged at 1,000 × g for
10 min at room temperature. The serum supernatant was collected and
centrifuged at 2,500 × g for 10 min at room temperature. Following
liquidation, serum was maintained at −80°C until use.
Preparation of protein powder from the
serum of patients with CCA and healthy controls
Preparation of protein powder from the serum of
patients with CCA and healthy controls was performed following a
previously described protocol (16).
Briefly, 50 µl serum obtained from patients with CCA and healthy
controls were dissolved on ice in cold 50% methanol. The serum
mixture was then extracted in a 4:8:3 ratio of chloroform to
methanol to water for 2 h at room temperature. Extracts were
centrifuged at 2,500 × g for 15 min at room temperature. The
resulting pellets were then dried under nitrogen and stored at
−20°C until further use.
Preparation of glycopeptides and
release of N-glycans
The preparation of glycopeptides and release of
N-glycans was performed as previously described (16). Briefly, 1 mg protein powder from the
serum of patients with CCA and healthy controls was digested with
trypsin and chymotrypsin for 18 h at 37°C in 0.1 M Tris-HCl (pH
8.2) containing 1 mM CaCl2. Digestion products were
enriched and freed of contaminants using a 1 ml Sep-Pak C18
cartridge column (Waters Corporation, Milford, MA, USA), as
described by Aoki et al (17).
Glycopeptides were then digested with 2 µl peptide N-glycosidase F
(7.5 U/ml, New England BioLabs, Inc., Ipswich, MA, USA) in 50 µl 20
mM sodium phosphate buffer (pH 7.5) for 18 h at 37°C. Released
glycans were separated from peptides and enzymes by passing through
a 1 ml Sep-Pak C18 cartridge high-performance liquid chromatography
column.
Permethylation of glycans
Released glycan mixtures were permethylated as
described by Anumula and Taylor (18). Briefly, released glycan mixtures were
permethylated under water-free conditions using 500 µl DMSO, 10 µg
NaOH and 200 µl methyliodide (all Sigma-Aldrich; Merck KGaA) for 30
min at room temperature. Permethylated glycans were then extracted
with dichloromethane and dried under a stream of nitrogen.
Nanospray ionization-linear ion trap
mass spectrometry (NSI-MSn)
NSI-MSn was performed as described by
Aoki et al (17). Briefly,
permethylated glycans were dissolved in 1 mM NaOH in 50% methanol
and infused directly into a linear ion trap mass spectrometer (LTQ
Orbitrap Discovery; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) using a Thermo Fisher Scientific™ nanospray ion
source (Thermo Fisher Scientific, Inc.). MS analysis was performed
in a positive ion mode and MS/MS spectra (at 28% collision energy)
were obtained using the total ion mapping function of the Xcalibur
software (version 2; Thermo Fisher Scientific, Inc.). The
fragmentation derived from the MS/MS spectra was identified using
the nomenclature described by Domon and Costello (19).
Glycomic analysis of N-glycans in the
serum of patients with CCA and healthy controls
The expression of glycans from the serum of patients
with CCA and healthy controls were qualitatively and quantitatively
compared. The glycans from these sera were enzymatically released,
purified and analyzed in their permethylated forms using positive
ion NSI-MS/MS. Identification of the glycan structures was based on
the i) NSI-MS parent mass ion; ii) NSI-MS/MS fragmentation ion; and
iii) similarity to known glycan structures and known biosynthetic
limitations. The prevalence of each individual glycan (percentage
total profile) in each profile was quantified by comparing its
signal intensity to the sum of the signal intensities for all
identified glycans.
Statistical analysis
The respective prevalence of glycans (percentage
total profile) in the sera of patients with CCA vs. healthy
controls was reported as the mean ± standard deviation. The
difference in the expression between groups was analyzed using the
independent t-test. Cross-tabulations were analyzed using the
χ2 test to determine the association between
N-glycan expression and the clinicopathological features of
CCA. All analyses were performed using SPSS statistical software
(version 19.0; SPSS, Inc., Chicago, IL, USA) and P<0.05 was
considered to indicate a statistically significant difference.
Results
Structural characterization of
N-glycans in the serum from patients with CCA and healthy
controls
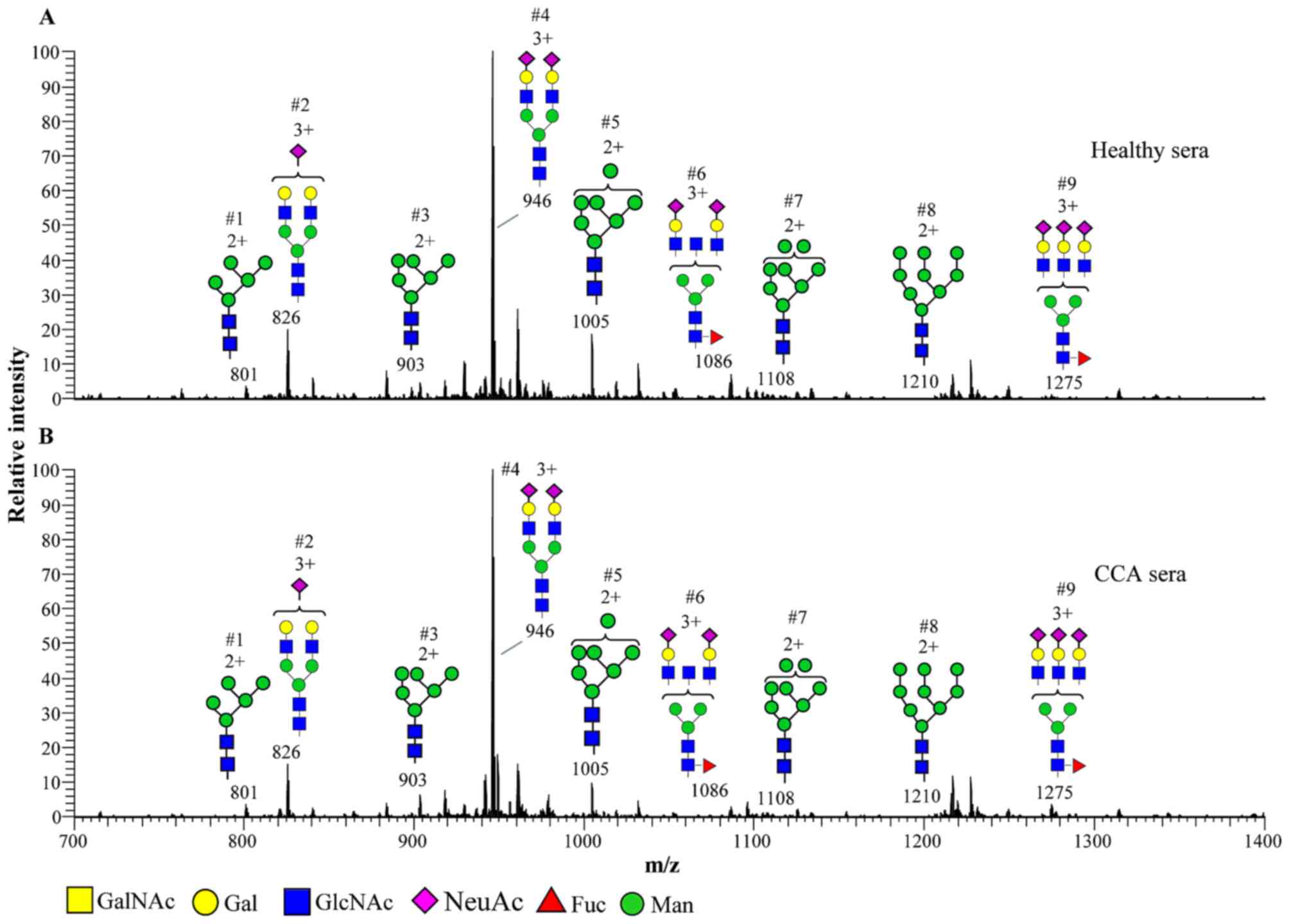
The N-glycan profiles in the serum of
patients with CCA and healthy controls are presented in Fig. 1. N-glycans were assigned as
high-mannose (M5–9N2; Structures 1, 3, 5, 7
and 8) or complex types
(NeuAc1–3Hex2–3HexNAc2–3 +
M3N2F0–1; Structures 2, 4, 6 and 9). High-mannose
N-glycans were detected from M5N2-M9N2. Complex
N-glycans were detected as bi-and tri-antennary structures
with the terminal galactose and sialic acid
(N-acetylneuraminic acid; NeuAc). A summary of
N-glycan structures in the sera of patients with CCA and
healthy controls, and their relative abundance is presented in
Table I. The representative
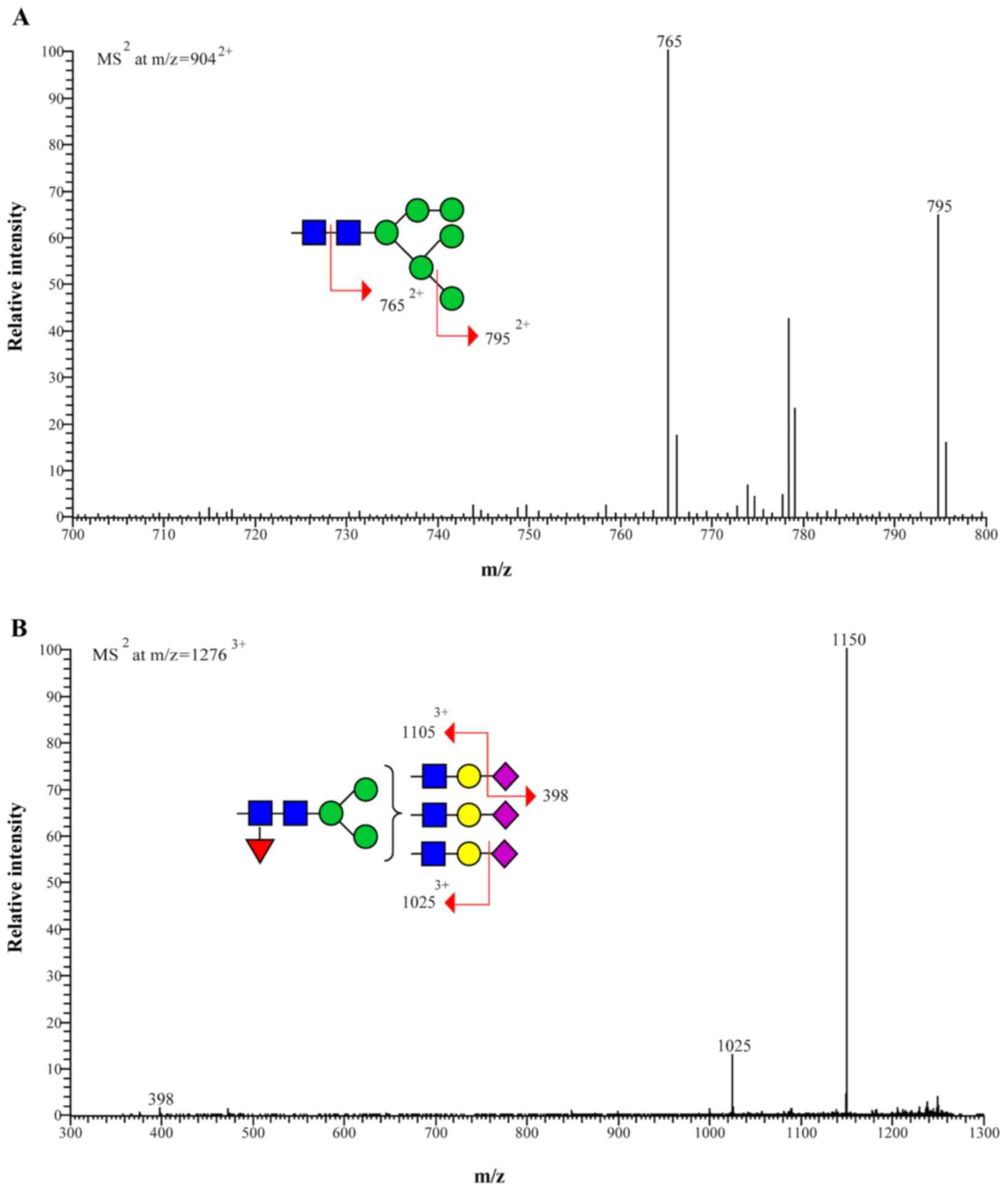
fragmentation of the high-mannose type and complex type
N-glycans are presented in Fig.
2.
 | Table I.Characteristics and prevalence of
N-linked glycans in the serum of patients with CCA compared
with healthy controls. |
Table I.
Characteristics and prevalence of
N-linked glycans in the serum of patients with CCA compared
with healthy controls.
| Structure | Group | Number | Relative abundance,
% | P-value |
|---|
| M5N2 | N | 4 | 2.45±0.24 | 0.247 |
|
| T | 8 | 2.19±0.39 |
|
| NeuAc1H2N2M3N2 | N | 4 | 14.21±2.18 | 0.248 |
|
| T | 8 | 12.58±2.17 |
|
| M6N2 | N | 4 | 3.13±0.56 | 0.044a |
|
| T | 8 | 3.91±0.55 |
|
| NeuAc2H2N2M3N2 | N | 4 | 61.17±2.55 | 0.162 |
|
| T | 8 | 64.68±4.23 |
|
| M7N2 | N | 4 | 12.12±2.54 | 0.106 |
|
| T | 8 | 9.27±2.66 |
|
|
NeuAc2H2N3M3N2F | N | 4 | 3.99±0.50 | 0.554 |
|
| T | 8 | 3.53±1.45 |
|
| M8N2 | N | 4 | 0.89±0.36 | 0.168 |
|
| T | 8 | 0.66±0.20 |
|
| M9N2 | N | 4 | 1.21±0.25 | 0.030a |
|
| T | 8 | 0.84±0.24 |
|
|
NeuAc3H3N3M3N2F | N | 4 | 0.80±0.30 | 0.002a |
|
| T | 8 | 2.36±0.68 |
|
Altered expression of N-glycan
structures in serum from patients with CCA compared with healthy
controls
N-glycans in the serum of patients with CCA and
healthy controls were qualitatively and quantitatively assessed
using positive ion NSI-MS/MS (Table
I). The detected N-glycans were high-mannose-and complex
types (bi-and tri-antennary structures) with the terminal galactose
and sialic acid (NeuAc). The expression of the high-mannose type
N-glycan, M6N2 (structure 3) and the complex tri-antennary
N-glycan containing a core fucose and terminal tri-sialic
acid, NeuAc3H3N3M3N2F (structure 9), were significantly increased
in the serum of patients with CCA compared with healthy controls
(P=0.044 and P=0.002, respectively). By contrast, the expression of
the high-mannose N-glycan M9N2 (structure 8) was
significantly decreased in patients with CCA compared with healthy
controls (P=0.030).
Serum N-glycan expression and
clinicopathological features of CCA
The association between the expression of the three
differentially expressed N-glycans in the serum of patients
with CCA and clinicopathological features of CCA were
quantitatively analyzed. High expression of M6N2 (structure 3) and
NeuAc3H3N3M3N2F (structure 9) were associated with an age <60
years (P=0.028 and P=0.005, respectively). However, there were no
significant associations between M9N2 (Structure 8) expression and
patient age, sex, histological type, tumor stage, vascular or
lymphatic invasion (Table II).
 | Table II.Association between N-linked
glycan expression and the clinicopathological features of patients
with CCA. |
Table II.
Association between N-linked
glycan expression and the clinicopathological features of patients
with CCA.
|
| M6N2
expression | M9N2
expression | NeuAc3H3N3M3N2F
expression |
|---|
|
|
|
|
|
|---|
| Variable | Low | High | P-value | Low | High | P-value | Low | High | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
|
|
<60 | 1 | 3 | 0.028a | 1 | 3 | 0.157 | 0 | 4 | 0.005a |
|
≥60 | 4 | 0 |
| 3 | 1 |
| 4 | 0 |
|
| Sex |
|
|
|
|
|
|
|
|
|
|
Male | 3 | 2 | 0.850 | 2 | 3 | 0.465 | 3 | 2 | 0.465 |
|
Female | 2 | 1 |
| 2 | 1 |
| 1 | 2 |
|
| Histological
type |
|
|
|
|
|
|
|
|
|
|
Papillary | 2 | 0 | 0.206 | 1 | 1 | 1.000 | 2 | 0 | 0.102 |
|
Non-papillary | 3 | 3 |
| 3 | 3 |
| 2 | 4 |
|
| Stage |
|
|
|
|
|
|
|
|
|
|
III | 2 | 1 | 0.850 | 2 | 1 | 0.465 | 2 | 1 | 0.465 |
| IV | 3 | 2 |
| 2 | 3 |
| 2 | 3 |
|
| Lymphatic
Invasion |
|
|
|
|
|
|
|
|
|
|
Present | 3 | 1 | 0.465 | 2 | 2 | 1.000 | 2 | 2 | 1.000 |
|
Absent | 2 | 2 |
| 2 | 2 |
| 2 | 2 |
|
| Vascular
Invasion |
|
|
|
|
|
|
|
|
|
|
Present | 2 | 0 | 0.206 | 2 | 0 | 0.102 | 2 | 0 | 0.102 |
|
Absent | 3 | 3 |
| 2 | 4 |
| 2 | 4 |
|
Discussion
Aberrant protein glycosylation has been reported in
various diseases, including cancer, and certain glycan structures
are well-known tumor markers. The present study demonstrated the
comparative structural glycomics of the N-glycans in the
serum from patients with CCA compared with healthy controls using
MS.
The expression of 3 N-glycans, including M6N2
(structure 3; P=0.044), M9N2 (structure 8, P=0.030) and
NeuAc3H3N3M3N2F (structure 9; P=0.002), differed significantly
between the 4 controls and 8 patients with CCA. The expression of
M6N2 and NeuAc3H3N3M3N2F were significantly increased in the serum
of patients with CCA, whereas M9N2 expression was significantly
decreased. The increased expression of high-mannose
N-glycans in patients with CCA is consistent with the
results of previous studies investigating N-glycan
expression in different types of cancer, including breast (20,21) and
colorectal cancer (22). The
increased expression of M6N2 high-mannose structures indicates an
incomplete maturation of the N-glycans in the glycosylation
process and an association with CCA tumor progression.
The significant increase of core fucosylated
tri-antennary N-glycans (NeuAc3H3N3M3N2F; structure 9) in
the serum of patients with CCA may be an example of the alteration
to the glycomic profile observed in different types of cancer,
including breast cancer (20),
colorectal cancer (22),
hepatocellular carcinoma (23) and
ovarian cancer (24). Furthermore,
core fucosylation has been identified as an important feature in
tumor progression and is associated with increased cancer
metastasis (25). Tri-antennary
N-glycans and core fucose structures have been associated
with cancer metastasis and serve as a useful tumor biomarker
(26); This suggests that these
N-glycans may be associated with tumor progression in
patients with CCA.
M9N2 was significantly decreased in CCA, indicating
an increase in the glycosylation process that decreases M9N2
expression to produce complex and hybrid oligosaccharides in CCA.
Decreased levels of high-mannose type of N-glycans have been
detected in ovarian (27) and gastric
cancer (28). Furthermore, the
alteration of high-mannose glycans, M6N2 and M9N2, may be due to a
more complex process that occurs during biosynthetic machinery,
involving CCA glycosylation.
Based on the glycan structural analysis, the changes
in glycan expression that occur during CCA may reflect specific
changes that occur in glycosyltransferase expression. The increase
of tri-antennary structures may be attributed to the altered
expression of glycosyltransferases, including
N-acetylglucosaminyltransferase (GnT)-III, -IV and-V. GnT-V is
markedly associated with cancer metastasis, whereas GnT-III is
associated with cancer suppression (26). The abundance of terminal sialic acid
(NeuAc) in CCA may be attributed to the dominant activity of
sialyltransferases, including ST3 β-galactoside
α-2,3-sialyltransferase 3 and ST6 β-galactoside
α-2,6-sialyltransferase, that represent the majority of
glycosyltransferases in CCA and may serve a pivotal role in cancer
progression (29).
The present study identified an association between
N-glycan expression and age in patients with CCA. The
increased expression of M6N2 and NeuAc3H3N3M3N2F is associated with
an age of <60 years old in patients with CCA. Age-related
changes in the expression of human serum N-glycans have been
reported in European (30) and
Chinese patients (31). CCA is rarely
diagnosed in patients <40 years old; changes in the expression
of N-glycans in the serum of patients with CCA may occur due
to tumorigenicity and aging.
In conclusion, the altered expression of
N-glycans in the serum of patients with CCA indicate that
they serve an important role in tumor growth and progression. M6N2
and NeuAc3H3N3M3N2F, which exhibit significantly increased
expression in the serum of patients with CCA, may therefore be
potentially promising biomarkers for CCA.
Acknowledgements
The present study was supported by the Suranaree
University of Technology (grant no. SUT6-606-58-12-04). The authors
wish to thank Mr. Bryan Roderick Hamman for assistance with the
English-language presentation of the present study.
References
|
1
|
Sripa B: Pathobiology of opisthorchiasis:
An update. Acta Trop. 88:209–220. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel T: Increasing incidence and
mortality of primary intrahepatic cholangiocarcinoma in the United
States. Hepatology. 33:1353–1357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaib YH, Davila JA, McGlynn K and
El-Serag HB: Rising incidence of intrahepatic cholangiocarcinoma in
the United States: A true increase? J Hepatol. 40:472–477. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Apweiler R, Hermjakob H and Sharon N: On
the frequency of protein glycosylation, as deduced from analysis of
the SWISS-PROT database. Biochim Biophys Acta. 1473:4–8. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong CH: Protein glycosylation: New
challenges and opportunities. J Org Chem. 70:4219–4225. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varki A and Gagneux P: Biological
Functions of GlycansEssentials of Glycobiology. 3rd. Varki A,
Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG,
Kinoshita T, Packer NH, Prestegard JH, Schnaar RL and Seeberger PH:
Cold Spring Harbor (NY): 2015, View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim EH and Misek DE: Glycoproteomics-based
identification of cancer biomarkers. Int J Proteomics.
2011:6019372011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Juntavee A, Sripa B, Pugkhem A, Khuntikeo
N and Wongkham S: Expression of sialyl Lewis(a) relates to poor
prognosis in cholangiocarcinoma. World J Gastroenterol. 11:249–254.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silsirivanit A, Araki N, Wongkham C,
Pairojkul C, Narimatsu Y, Kuwahara K, Narimatsu H, Wongkham S and
Sakaguchi N: A novel serum carbohydrate marker on mucin 5AC: Values
for diagnostic and prognostic indicators for cholangiocarcinoma.
Cancer. 117:3393–3403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sawanyawisuth K, Silsirivanit A, Kunlabut
K, Tantapotinan N, Vaeteewoottacharn K and Wongkham S: A novel
carbohydrate antigen expression during development of opisthorchis
viverrini-associated cholangiocarcinoma in golden hamster: A
potential marker for early diagnosis. Parasitol Int. 61:151–154.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Indramanee S, Silsirivanit A, Pairojkul C,
Wongkham C and Wongkham S: Aberrant glycosylation in
cholangiocarcinoma demonstrated by lectin-histochemistry. Asian Pac
J Cancer Prev. 13 Suppl:S119–S124. 2012.
|
|
12
|
Phoomak C, Silsirivanit A, Wongkham C,
Sripa B, Puapairoj A and Wongkham S: Overexpression of
O-GlcNAc-transferase associates with aggressiveness of mass-forming
cholangiocarcinoma. Asian Pac J Cancer Prev. 13 Suppl:S101–S105.
2012.
|
|
13
|
Silsirivanit A, Araki N, Wongkham C,
Vaeteewoottacharn K, Pairojkul C, Kuwahara K, Narimatsu Y, Sawaki
H, Narimatsu H, Okada S, et al: CA-S27: A novel Lewis a associated
carbohydrate epitope is diagnostic and prognostic for
cholangiocarcinoma. Cancer Sci. 104:1278–1284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuda A, Kuno A, Nakagawa T, Ikehara Y,
Irimura T, Yamamoto M, Nakanuma Y, Miyoshi E, Nakamori S, Nakanishi
H, et al: Lectin microarray-based sero-biomarker verification
targeting aberrant O-Linked glycosylation on mucin 1. Anal Chem.
87:7274–7281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Talabnin K, Talabnin C, Ishihara M, Azadi
P, Wongkham S and Sripa B: Differential expression of
O-glycoprotein glycans in cholangiocarcinoma cell lines. Asian Pac
J Cancer Prev. 17:691–695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Talabnin K, Aoki K, Saichua P, Wongkham S,
Kaewkes S, Boons GJ, Sripa B and Tiemeyer M: Stage-specific
expression and antigenicity of glycoprotein glycans isolated from
the human liver fluke, Opisthorchis viverrini. Int J Parasitol.
43:37–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aoki K, Perlman M, Lim JM, Cantu R, Wells
L and Tiemeyer M: Dynamic developmental elaboration of N-linked
glycan complexity in the Drosophila melanogaster embryo. J Biol
Chem. 282:9127–9142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anumula KR and Taylor PB: A comprehensive
procedure for preparation of partially methylated alditol acetates
from glycoprotein carbohydrates. Anal Biochem. 203:101–108. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Domon B and Costello CE: Structure
elucidation of glycosphingolipids and gangliosides using
high-performance tandem mass spectrometry. Biochemistry.
27:1534–1543. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Nie H, Zhang Y, Yao Y, Maitikabili
A, Qu Y, Shi S, Chen C and Li Y: Cell surface-specific N-glycan
profiling in breast cancer. PLoS One. 8:e727042013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Leoz ML, Young LJ, An HJ, Kronewitter
SR, Kim J, Miyamoto S, Borowsky AD, Chew HK and Lebrilla CB:
High-mannose glycans are elevated during breast cancer progression.
Mol Cell Proteomics. 10:M110.0027172011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sethi MK, Thaysen-Andersen M, Smith JT,
Baker MS, Packer NH, Hancock WS and Fanayan S: Comparative N-glycan
profiling of colorectal cancer cell lines reveals unique bisecting
GlcNAc and alpha-2,3-linked sialic acid determinants are associated
with membrane proteins of the more metastatic/aggressive cell
lines. J Proteome Res. 13:277–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakagawa T, Miyoshi E, Yakushijin T,
Hiramatsu N, Igura T, Hayashi N, Taniguchi N and Kondo A: Glycomic
analysis of alpha-fetoprotein L3 in hepatoma cell lines and
hepatocellular carcinoma patients. J Proteome Res. 7:2222–2233.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwedler C, Kaup M, Weiz S, Hoppe M,
Braicu EI, Sehouli J, Hoppe B, Tauber R, Berger M and Blanchard V:
Identification of 34 N-glycan isomers in human serum by capillary
electrophoresis coupled with laser-induced fluorescence allows
improving glycan biomarker discovery. Anal Bioanal Chem.
406:7185–7193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dennis JW, Laferté S, Waghorne C, Breitman
ML and Kerbel RS: Beta 1–6 branching of Asn-linked oligosaccharides
is directly associated with metastasis. Science. 236:582–585. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taniguchi N and Kizuka Y: Glycans and
cancer: Role of N-glycans in cancer biomarker, progression and
metastasis and therapeutics. Adv Cancer Res. 126:11–51. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kronewitter SR, De Leoz ML, Strum JS, An
HJ, Dimapasoc LM, Guerrero A, Miyamoto S, Lebrilla CB and
Leiserowitz GS: The glycolyzer: Automated glycan annotation
software for high performance mass spectrometry and its application
to ovarian cancer glycan biomarker discovery. Proteomics.
12:2523–2538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ozcan S, Barkauskas DA, Renee Ruhaak L,
Torres J, Cooke CL, An HJ, Hua S, Williams CC, Dimapasoc LM, Han
Kim J, et al: Serum glycan signatures of gastric cancer. Cancer
Prev Res (Phila). 7:226–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dall'Olio F, Malagolini N, Trinchera M and
Chiricolo M: Sialosignaling: Sialyltransferases as engines of
self-fueling loops in cancer progression. Biochim Biophys Acta.
1840:2752–2764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vanhooren V, Laroy W, Libert C and Chen C:
N-glycan profiling in the study of human aging. Biogerontology.
9:351–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding N, Nie H, Sun X, Sun W, Qu Y, Liu X,
Yao Y, Liang X, Chen CC and Li Y: Human serum N-glycan profiles are
age and sex dependent. Age Ageing. 40:568–575. 2011. View Article : Google Scholar : PubMed/NCBI
|