Introduction
The epithelial-to-mesenchymal transition (EMT) is a
process in which cells lose epithelial properties and acquire the
properties of mesenchymal cells. This is a normal developmental
process in the early embryo, but is also a feature of tumor cells
(1,2).
EMT is crucial in the development of more invasive metastatic
tumors and the acquisition of resistance to anticancer therapies
(3–5).
Brachyury is a member of the T-box family of
transcription factors and is a highly conserved cellular protein.
It functions in the EMT process during cancer progression and is
involved in fetal mesoderm formation (6,7). High
expression of brachyury is associated with an increased likelihood
of recurrence and distant metastasis, invasion to the extracellular
matrix and development of resistance to chemotherapy (7–12). Thus,
brachyury has been implicated as a tool for predicting tumor
characteristics and prognosis. However, only a limited number of
studies have addressed the correlation between brachyury expression
and survival outcome in patients with breast cancer.
Triple-negative breast cancer (TNBC) features the
deleterious expression of receptors, including estrogen receptor
(ER), progesterone receptor (PR) and human epidermal growth factor
receptor 2 (HER2). Effective treatment of TNBC is difficult,
whereby recurrence and mortality rates are increased compared with
other subtypes of breast cancer (13–15). EMT
markers are highly expressed in TNBC cells, suggesting an
association between poor prognosis of TNBC and EMT (16,17).
Although lymphatic permeation is an important route
for breast cancer progression, to the best of our knowledge, only
two studies have addressed the involvement of brachyury in lymph
node metastasis (18,19). Data are limited concerning expression
of brachyury in recurrent tumors; however, these data may be
important in identifying patients for which the brachyury vaccine,
currently in clinical trials, is expected to be of therapeutic
benefit (20).
The present study investigated the expression of
brachyury in breast cancer tissues, including primary tumor,
axillary metastatic lymph nodes and recurrent tumor. The clinical
significance and value of brachyury as a biomarker to predict tumor
recurrence or survival in patients with breast cancer was then
explored. In addition, the association between expression of
brachyury and tumor characteristics was evaluated to understand its
biological behavior.
Materials and methods
Patients
The present retrospective study included consecutive
patients with breast cancer surgically resected at Kangbuk Samsung
Hospital (Seoul, Korea), between January 2005 and December 2011.
The 102 patients whose surgical samples were available consisted of
102 primary tumors, 21 metastatic axillary lymph nodes and 15
recurrent tumors. Primary invasive ductal carcinoma, not otherwise
specific tissue, metastatic cancer tissue from lymph nodes and
recurrent tumor tissue was obtained for tissue microarray analysis
(TMA). Specimens were fixed using 10% formalin solution at room
temperature for 24 h and embedded in paraffin using a standard
protocol. Tissue sections (3 µm thick) were stained using
hematoxylin (at room temperature for 90 sec) and eosin (at room
temperature for 40 sec) using a Dako Coverstainer fully automated
system (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA).
Hematoxylin and eosin (H&E) stained slides from all patients
were reviewed by two pathologists (Dr Sung-Im Do and Dr Seoung Wan
Chae, of the Department of Pathology, Kangbuk Samsung Hospital,
Seoul, Korea) in a blinded manner with an Olympus BX51 light
microscope, at ×200 magnification, to confirm histological data,
including tumor (T) and node (N) stage (based on the staging system
by the Union for International Cancer Control/American Joint
Committee on Cancer, 7th edition), (21) lymphatic invasion and other
characteristics. The most representative tumor area from each
H&E stained slide was selected and its location marked on the
particular paraffin block, the most representative tissue core was
obtained from each tumor specimen. TMA specimens were assembled
using a tissue-array instrument (Tissue-Tek Quick-Ray; Sakura
Finetek Europe B.V., Flemingweg, The Netherlands) consisting of
thin-walled stainless steel punches, and stylets for emptying and
transferring the needle contents. The assembly was held in an X-Y
position guide with a 1-mm increment between the individual
samples, a 4-mm punch depth stop device and semi-automatic
micrometers. The instrument was used to create holes in a recipient
block with defined array cores. The fit needle delivered the tissue
cores to the recipient block. Taking into account the limitations
of the representative areas of the tumor, duplicate 2 mm-diameter
tissue cores were used from each donor block. The tissue cores
taken from within the tumor represented >70% of the
material.
In addition, clinical data of the patients,
including age, sex, type of operation, radiotherapy, chemotherapy
and hormonal therapy were reviewed. No patients were treated for
breast cancer prior to surgery. The majority of the patients
(97/102) received adjuvant chemotherapy, usually
anthracycline-based with or without taxane regimen. Of the 97
patients, 27 (27.8%) were treated with an FEC regimen consisting of
500 mg/m2 5-Fluorouracil intravenously administered on
day 1 and 100 mg/m2 epirubicin intravenously
administered on day 1 and 500 mg/m2 cyclophosphamide
intravenously administered on day 1 every 3 weeks for 6 cycles, and
30 (30.9%) received an AC regimen consisting of 60 mg/m2
doxorubicin intravenously administered on day 1 and 600
mg/m2 cyclophophamide intravenously administered on day
1 every 3 weeks for 4 cycles, and 35 (36.1%) received a sequential
ACT regimen comprising 4 cycles of AC followed by 4 cycles of 100
mg/m2 docetaxel. In addition, all patients with ER- or
PR-positive tumors took tamoxifen (20 mg once a day) prior to
menopause, and aromatase inhibitor (anastrozol: 1 mg once a day or
letrozole: 2.5 mg once a day) following menopause for 5 years
unless recurrence occurred during follow-up. The patients with HER2
positive tumors who possessed >1 cm tumors or were pN1-3 also
received trastuzumab triweekly (6 mg/kg) for 1 year.
All patients underwent physical examination at
3-month intervals following surgery, breast ultrasonography at
6-month intervals, mammography and chest computed tomography, and
bone scan and breast magnetic resonance imaging at 1-year
intervals. The last follow-up date was December 31, 2016, for all
available patients. Disease-free survival (DFS) was defined as the
interval between the date of treatment for breast cancer and the
date of evidence of recurrence events, including the following:
Invasive recurrence in any sites or a novel invasive breast cancer
in the contralateral breast. Overall survival (OS) was defined as
the time until the time of last follow-up or of death from any
cause.
The study protocol was approved by the Institutional
Review Board of Kangbuk Samsung Hospital, Sungkyunkwan University
of Korea on August 30, 2016 (approval no. KBSMC
2016-10-022-001).
Immunohistochemical (IHC) scoring
Scoring for each IHC marker was performed using an
Olympus BX51 light microscope at ×200 magnification by an
experienced breast histopathologist who was blinded to the results
of other markers and patient identity. IHC analysis was performed
using Leica BOND MAX™ fully automated
immunohistochemistry system, according to the manufacture's
protocol (Leica Microsystems GmbH, Wetzlar, Germany). Briefly, 4 µm
thick sections were deparaffinized and pre-treated with the Epitope
Retrieval Solution 2 (EDTA buffer pH 8.8) at 98°C for 20 min. Once
the tissue washed three times with Bond TM Wash Solution 10X
concentrate (cat no. AR9590, Leica Microsystems, GmbH), peroxidase
blocking was performed for 10 min using the Bond Polymer Refine
Detection kit DS9800 (Leica Microsystems, GmbH) according to
manufacturer's protocol. Tissues were again washed three times with
Bond TM Wash Solution 10X concentrate (cat no. AR9590; Leica
Microsystems, GmbH) and then incubated with the primary antibodies
at room temperature for 60 min. Subsequently, tissues were
incubated with bond polymer (cat no. AR9352; Leica Microsystems,
GmbH) at room temperature for 10 min and developed using
3,3-diaminobenzidine at room temperature for 10 min. Primary
antibodies used are as follows: ER (cat no. RM-9101-F; 1:200
dilution; SP1 clone; Lab Vision Corporation, Fremont, CA, USA), PR
(cat no. M3569; 1:200 dilution; PgR636 clone; Dako; Agilent
Technologies, Inc.) and HER2 (cat no. RM-9103-R7-A; 1:200 dilution;
SP3 clone; Lab Vision Corporation) were used. For brachyury
staining, human tissues obtained were fixed in 10% formalin
solution at room temperature for 24 h, dehydrated through a graded
ethanol series (30, 50 and 100%), washed in xylene and processed
for embedding in paraffin wax, at room temperature and for 30 min.
Sections were incubated in a solution of 0.3%
H2O2 at room temperature for 15 min to
inhibit endogenous peroxidase activity. Antigen retrieval procedure
was performed using 10 mM Tris + 1 mM EDTA + 0.03% Tween-20
Solution at 98°C for 30 min in a presser cooker chamber.
Non-specific blocking was quenched by incubation with 4% bovine
serum albumin for 30 min. Sections were then incubated for 1 h at
room temperature with primary antibodies against brachyury (cat no
ab57480; Abcam, Cambridge, UK) diluted to 1:500. The detection
system EnVision+ for secondary horseradish peroxidase-conjugated
mouse antibodies (cat no. K4001; 1:2,000; Dako; Agilent
Technologies, Inc.) was applied according to the manufacturer's
protocol. The secondary antibodies were incubated at room
temperature for 8 min. Slides were stained with liquid
diaminobenzidine tetrahydrochloride, a high-sensitivity
substrate-chromogen system (cat no. K5007; Dako; Agilent
Technologies, Inc.). Counterstaining was performed with Meyer's
hematoxylin at room temperature for 1 min. A total of between three
and five randomly selected fields were evaluated for each slide.
For each field, the percentage of positive tumor cells was
calculated as follows: (Number of positive tumor cells/total number
of tumor cells) ×100. Nuclear staining was scored by examining for
brachyury in the nucleus. The relative staining intensity was
scored as weak (+) for pale brown intensity, moderate (++) for
intermediate brown intensity and strong (+++) for intense, dark
brown immunoprecipitate. Brachyury score was calculated using the
Allred scoring system of staining intensity (absent, 0; weak, 1;
moderate, 2; and strong, 3) added to another score of the
percentage of cells stained (none, 0; <1%, 1; 1–10%, 2; 11–33%,
3; 34–66%, 4; and 67–100%, 5) to yield a total score of 0 or 2–8,
using the Allred scoring system as previously described (22). The score was calculated as the
immunoactivity observed in the nucleus and cytoplasm.
Statistical analyses
All statistical analyses were performed using R
version 3.3.2 (23–25). All data were presented as the mean ±
standard deviation, or number and percentage. Associations among
variables were evaluated using Fisher's exact test or χ2
test. Continuous variables were compared using the Wilcoxon
rank-sum test. OS and DFS curves were determined using the
Kaplan-Meier method with differences assessed using the log-rank
test. Risk factors of DFS were analyzed by univariate analysis with
the log-rank test and multivariate analysis with Cox's proportional
hazard model. All tests were two-tailed and P<0.05 was
considered to indicate a statistically significant difference.
Results
Associations between brachyury protein
expression and clinicopathological factors in primary tumors
The present study consisted of 102 patients, of whom
all were female. Brachyury protein expression in primary,
metastatic and recurrent tumors was assessed using IHC staining.
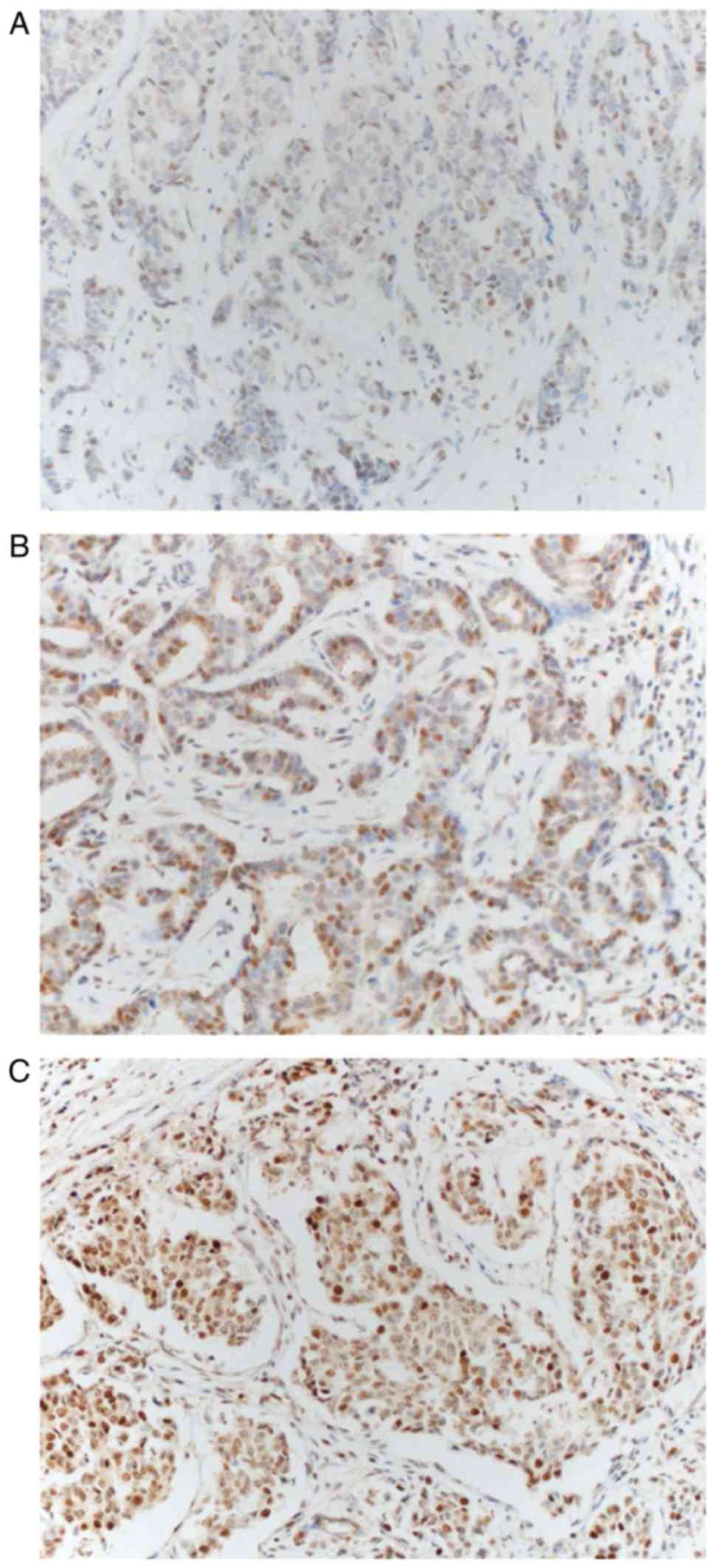
Brachyury protein expression occurred in the nucleus and cytoplasm
of tumor cells (Fig. 1). Brachyury
expression in the cytoplasm was observed in all tumor cells; the
expression intensity varied somewhat; however, the percentage of
cells stained typically exceeded 80%, so that the score difference
between the tumors was small (range, 5–8). Depending on the
presence or absence of brachyury expression in the nucleus, the
study population was divided into a brachyury negative group (n=40)
or a brachyury positive group (n=62) to compare the differences in
clinicopathological characteristics (Table I). Based on the median value of
brachyury expression in the cytoplasm 5–6 points were defined as
low brachyury and 7–8 as high brachyury. In the brachyury-positive
group, ER+ and PR+ tumors were more prevalent; however, neither
were significant. Microcalcification in tumors was significantly
associated with brachyury expression (P=0.025). The
brachyury-positive group possessed a high score, even in the
cytoplasm; however, without statistical significance (P=0.069). The
remaining clinicopathological factors did not demonstrate any
statistical differences.
 | Table I.Clinicopathological characteristics of
the patients according to brachyury expression in nucleus of
primary tumor. |
Table I.
Clinicopathological characteristics of
the patients according to brachyury expression in nucleus of
primary tumor.
| Parameter | Brachyury-negative
(n=40) | Brachyury-positive
(n=62) | P-value |
|---|
| Age,
yearsa |
|
| 0.397 |
| ≤45 | 12 (30.0) | 25 (40.3) |
|
|
>45 | 28 (70.0) | 37 (59.7) |
|
| pTa |
|
| 1.000 |
| 1 | 17 (42.5) | 27 (43.5) |
|
|
2–4 | 23 (57.5) | 35 (56.5) |
|
| pNa |
|
| 0.965 |
| 0 | 19 (47.5) | 31 (50.0) |
|
|
1–3 | 21 (52.5) | 31 (50.0) |
|
| Histological
gradea |
|
| 0.552 |
|
1–2 | 20 (50.0) | 36 (58.1) |
|
| 3 | 20 (50.0) | 26 (41.9) |
|
| Molecular
subtypea |
|
| 0.316 |
|
Non-TNBC | 19 (47.5) | 37 (59.7) |
|
|
TNBC | 21 (52.5) | 25 (40.3) |
|
| Lymphovascular
invasiona |
|
| 0.908 |
|
Absent | 20 (50.0) | 33 (53.2) |
|
|
Present | 20 (50.0) | 29 (46.8) |
|
|
Microcalcificationa |
|
| 0.025 |
|
Absent | 18 (45.0) | 43 (69.4) |
|
|
Present | 22 (55.0) | 19 (30.6) |
|
| Estrogen
receptora |
|
| 0.052 |
|
Negative | 28 (70.0) | 30 (48.4) |
|
|
Positive | 12 (30.0) | 32 (51.6) |
|
| Progesterone
receptora |
|
| 0.125 |
|
Negative | 30 (75.0) | 36 (58.1) |
|
|
Positive | 10 (25.0) | 26 (41.9) |
|
| HER2a |
|
| 0.494 |
|
Negative | 32 (80.0) | 54 (87.1) |
|
|
Positive | 8 (20.0) | 8 (12.9) |
|
| Type of
surgerya |
|
| 1.000 |
|
Conserving surgery | 5 (12.5) | 7 (11.3) |
|
| Total
mastectomy | 35 (87.5) | 55 (88.7) |
|
| Follow up periods,
monthsb | 79.2±35.2 | 82.2±42.8 | 0.712 |
|
Radiotherapya |
|
| 0.526 |
| No | 30 (75.0) | 51 (82.3) |
|
|
Yes | 10 (25.0) | 11 (17.7) |
|
|
Chemotherapya |
|
| 0.665 |
| No | 1 (2.5) | 4 (6.5) |
|
|
Yes | 39 (97.5) | 58 (93.5) |
|
| Cytoplasmic
brachyurya |
|
| 0.069 |
|
Low | 23 (57.5) | 23 (37.1) |
|
|
High | 17 (42.5) | 39 (62.9) |
|
Differences in nuclear brachyury
expression between primary and metastatic or recurrent tumors
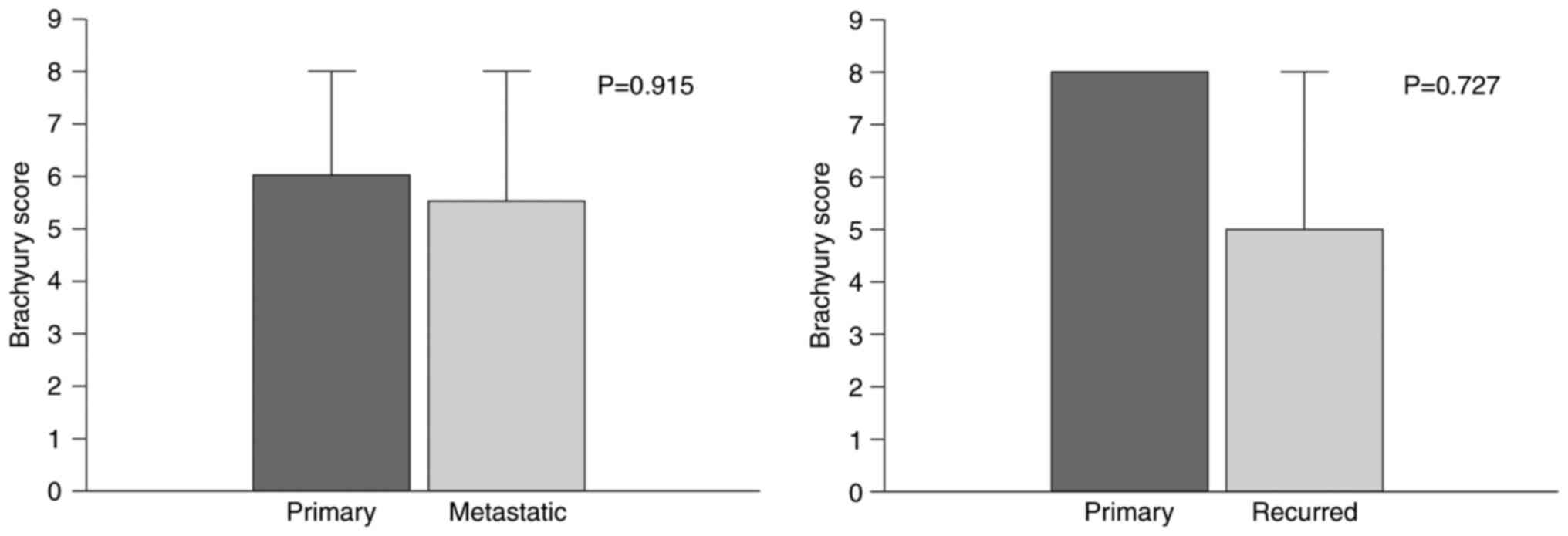
Brachyury staining of primary tumor samples was
performed in samples from 15 patients with recurrent tumors that
were available for immunostaining. The difference in scores was
compared between primary and recurrent tumors. Differences between
21 metastatic lymph nodes and primary tumors were similarly
compared. The comparisons did not identify any statistically
significant differences (primary vs. metastatic, P=0.915; primary
vs. recurred, P=0.727; Fig. 2).
Association between nuclear brachyury
expression in primary tumors and postoperative prognoses of
patients
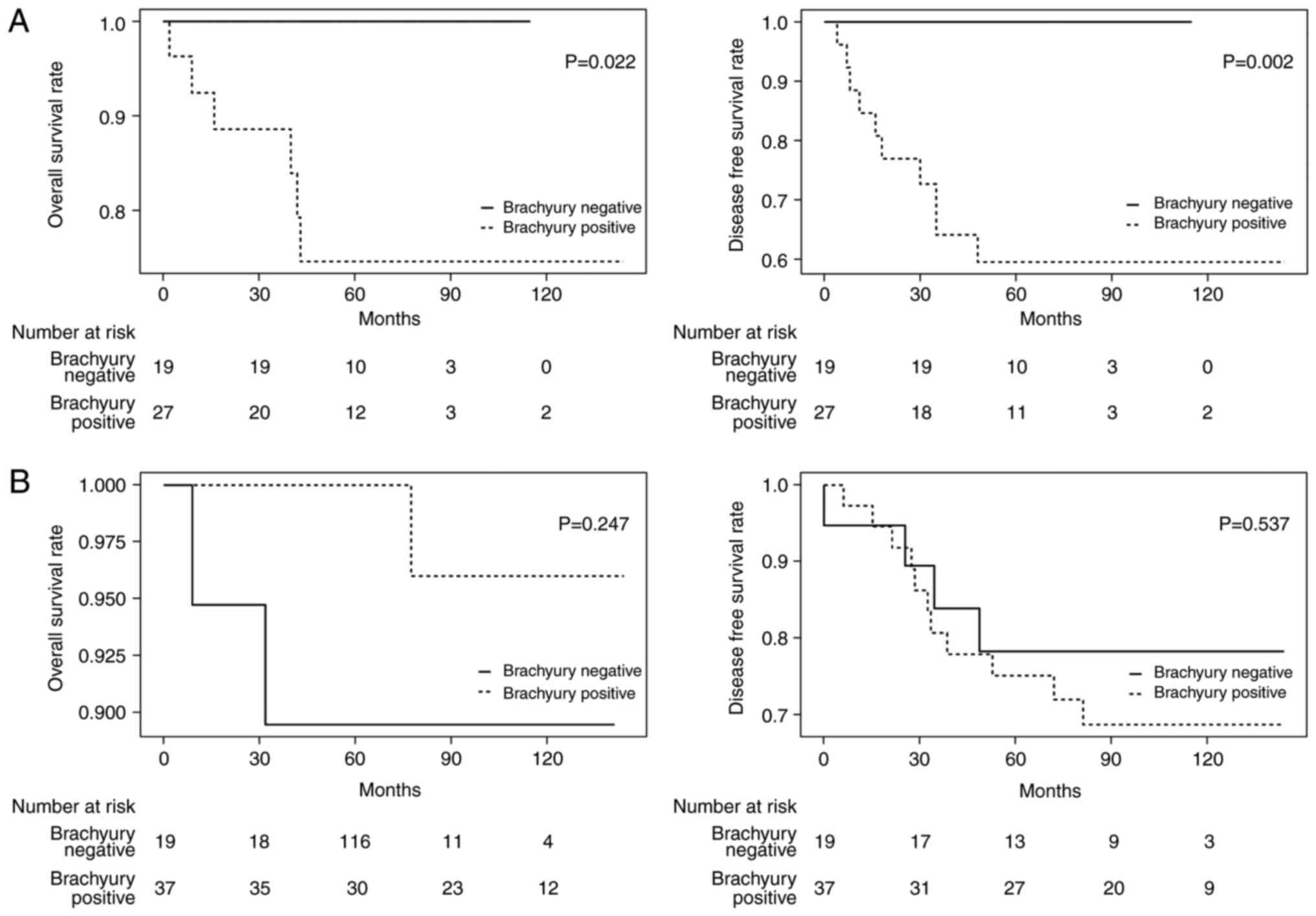
The effect of brachyury expression in primary tumors
on the prognosis of patients was investigated. Following a median
follow-up of 73.5 months (range, 2–145 months), 25 patients
experienced relapse and 9 patients succumbed. The 5-year DFS and OS
rates were 84.3 and 92.2%, respectively. OS and DFS appeared to
decrease in the brachyury negative group, compared with the
positive; however, no statistical significance was identified [OS
hazard ratio (HR), 1.4; 95% confidence interval (CI), 0.34–5.47;
P=0.656 and DFS HR, 2.2; 95% CI, 0.89–5.56; P=0.081; data not
shown]. Cox's proportional hazard regression models of DFS revealed
that the presence of lymph node metastasis, lymphovascular invasion
and HER2 positivity were associated with poor prognosis, whereas no
factor was significantly associated with an improved prognosis
(Table II). Multivariate analysis
that included variables with P<0.2 in univariate regression
analysis revealed brachyury expression in the nucleus of primary
tumor, HER2 and lymphovascular invasion as independent prognostic
factors for DFS (brachyury negative versus positive, HR 3.0,
P=0.024; HER2 negative vs. positive HR, 4.9; P=0.003 and
lymphovascular invasion absent vs. present HR, 3.5; P=0.020;
Table II).
 | Table II.Univariate and multivariate analyses
of disease free survival of patients with breast cancer. |
Table II.
Univariate and multivariate analyses
of disease free survival of patients with breast cancer.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
≤45 | ref |
|
|
|
|
>45 | 0.990
(0.440–2.200) | 0.973 |
|
|
| pT stage |
|
|
|
|
| 1 | ref |
|
|
|
|
2–4 | 2.170
(0.910–5.190) | 0.082 | 2.419
(0.882–6.632) | 0.086 |
| pN stage |
|
|
|
|
| 0 | ref |
|
|
|
|
1–3 | 2.420
(1.040–5.610) | 0.040 | 1.394
(0.498–3.902) | 0.527 |
| Histologic
grade |
|
|
|
|
|
1–2 | ref |
|
|
|
| 3 | 1.880
(0.850–4.140) | 0.119 | 1.345
(0.587–3.082) | 0.483 |
|
Microcalcification |
|
|
|
|
|
Absent | ref |
|
|
|
|
Present | 1.050
(0.470–2.330) | 0.909 |
|
|
| L/V invasion |
|
|
|
|
|
Absent | ref |
|
|
|
|
Present | 4.360
(1.740–10.920) | 0.002 | 3.481
(1.214–9.977) | 0.020 |
| Surgery type |
|
|
|
|
|
BCS | ref |
|
|
|
|
Mastectomy | 3.440
(0.470–25.440) | 0.226 |
|
|
| Estrogen
receptor |
|
|
|
|
|
Negative | ref |
|
|
|
|
Positive | 0.610
(0.270–1.390) | 0.239 |
|
|
| Progesterone
receptor |
|
|
|
|
|
Negative | ref |
|
|
|
|
Positive | 0.480
(0.190–1.220) | 0.123 | 0.787
(0.289–2.148) | 0.641 |
| HER2 |
|
|
|
|
|
Negative | ref |
|
|
|
|
Positive | 2.950
(1.270–6.840) | 0.012 | 4.889
(1.694–14.110) | 0.003 |
| Brachyury
(nucleus) |
|
|
|
|
|
Negative | ref |
|
|
|
|
Positive | 2.220
(0.890–5.560) | 0.089 | 3.004
(1.157–7.804) | 0.024 |
| Brachyury
(cytoplasm) |
|
|
|
|
|
Low | ref |
|
|
|
|
High | 0.880
(0.400–1.920) | 0.741 |
|
|
The hypothesis of the present study was that the
expression of brachyury is associated with poor outcome of patients
with TNBC based on a previous study (19). The prognoses of patients with TNBC
(n=46) and those with non-TNBC (n=56) were examined separately. In
the brachyury negative group in TNBC, no recurrence or mortalities
occurred during the follow-up period, and therefore a significantly
improved prognosis was identified in these patients compared with
the brachyury-positive group (OS, P=0.022; DFS, P=0.002; Fig. 3A). No statistical difference was
observed in those with non-TNBC (OS HR, 0.8; 95% CI, 0.02–2.94;
P=0.247 and DFS HR, 1.4; 95% CI, 0.46–4.49; P=0.537; Fig. 3B).
Association between nuclear brachyury
protein expression in metastatic/recurrent tumors and postoperative
prognoses of patients
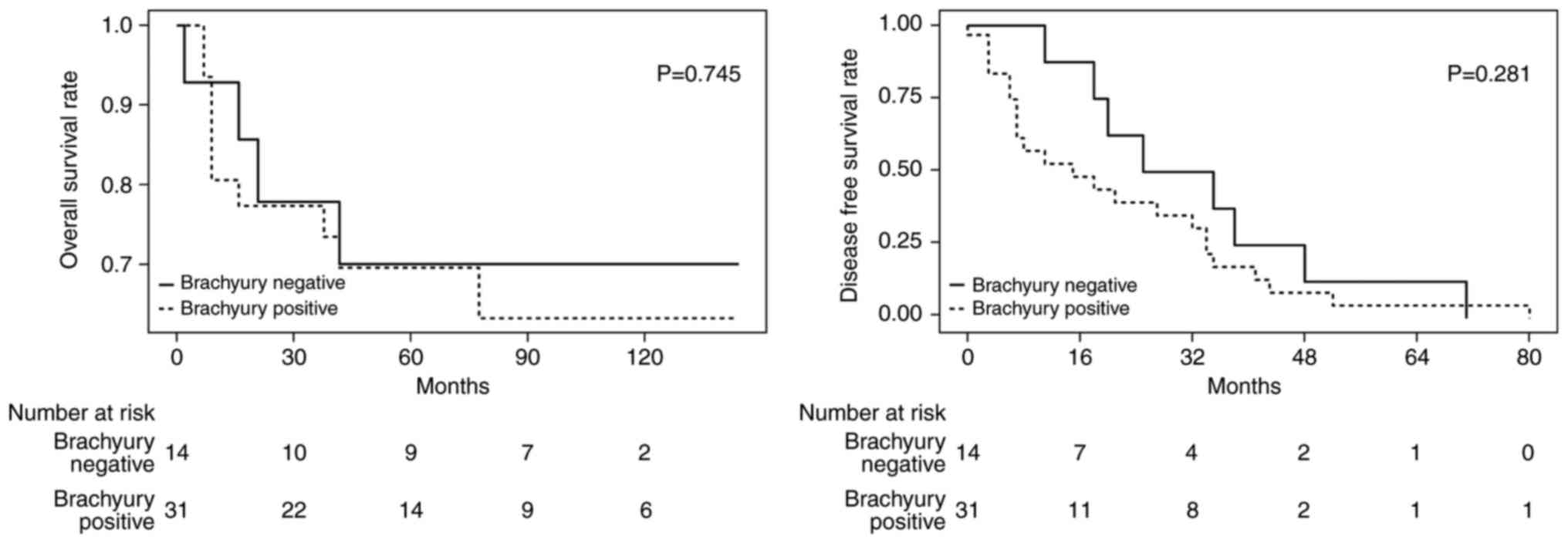
No significant differences in the degree of
brachyury expression in the primary tumor and metastatic/recurrent
tumor were identified in the present study. The way in which
brachyury expression in metastatic and recurrent tumors affected
survival was investigated. Among the patients with lymph node
metastasis observed at the first surgery, 23 samples were able to
be stained. Out of the patients with recurrence during follow-up,
22 samples were able to be stained and were grouped together with
lymph node metastasis samples (n=45). There were 14 (31%) brachyury
positive tumors and 31 (69%) brachyury negative tumors. The groups
demonstrated no significant difference in OS or DFS (OS HR, 1.2;
95% CI, 0.38–3.91; P=0.745 and DFS HR, 1.6; 95% CI, 0.69–3.54;
P=0.281; Fig. 4). It was intended
that the subgroups of patients with TNBC were to be analyzed;
however, the small number of samples did not allow for this.
Analysis of differences in survival
rates with cytoplasmic brachyury expression
Brachyury score in the cytoplasm ranged between 5
and 8 as expression of cytoplasmic brachyury was exhibited by
>80% of all stained cells, with the only differences observed
being in staining intensity. Therefore, the samples were divided
into low (5–6 points) brachyury expression and high (7–8 points)
expression groups to investigate the effect of brachyury expression
in the cytoplasm on patient prognosis. This analysis was also
divided into TNBC and non-TNBC according to molecular subtype,
since brachyury expression in the cytoplasm did not correlate with
prognosis in the previous Cox's proportional hazard regression
models. No statistical significance was evident between the high
and low group, regardless of the molecular subtype (TNBC OS,
P=0.996; DFS, P=0.228 and non-TNBC OS, P=0.12; DFS, P=0.533).
Discussion
Brachyury has been associated with cancer cell
invasion, metastatic progression and chemoresistance (7–12). Thus,
the present study investigated the effects of brachyury expression
on the prognosis of patients with breast cancer, whilst also
exploring the expression of brachyury in recurrent and metastatic
tumors, addressed in only two prior studies (19,20), to
the best of our knowledge. The present study is, to the best of our
knowledge, the first to demonstrate the association between
brachyury expression in recurrent and metastatic tumors, and
prognosis.
Brachyury has been increasingly studied as a
targeting protein of interest in immune therapy (19,20).
Previous studies have demonstrated that brachyury expression is
prognostic only in TNBC (18). In the
present study, all recurrences or mortalities in TNBC occurred in
the brachyury-positive group, while no recurrence or mortalities
occurred during the follow-up period in the brachyury-negative
group. These results contribute toward increased anticipation of
the results from ongoing clinical trials of a brachyury vaccine as
a potential treatment for TNBC. However, why brachyury is more
relevant to tumor prognosis in TNBC is not clear, and should be
addressed in further studies of the association of brachyury with
other factors and associated signaling pathways. This may allow for
the selection of the most appropriate patient group for brachyury
targeted therapy and to investigate the synergistic effects of the
combination therapy with other drugs.
Presently, brachyury expression was not
statistically associated with molecular subtype, including PR and
HER2. ER positivity had a marginally statistically significant
association (P=0.052), in contrast with previous studies (18,19). The
association of these biomarkers remains a subject of debate, with
dichotomous results from different studies. The relevance of these
biomarkers requires further investigation.
A previous study examined stained tissue for the
presence of brachyury in the cytoplasm of breast tumor cells and
brachyury expression in tumors was calculated as the sum of the
brachyury expression in the nucleus and cytoplasm (19). In the present study, unlike previous
studies, each cytoplasm and nucleus were separately analyzed for
prognosis. Expression in the nucleus was significantly associated
with a poor prognosis, whereas expression in cytoplasm was not
significantly associated with prognosis. The reasons for these
outcomes may be hypothesized using other studies about breast
cancer. In a study of the androgen receptor (AR), AR without ligand
which attached to heat shock proteins is present mainly in the
cytoplasm. In the presence of ligand, the ligand-binding domain is
unbound from heat shock protein and becomes active in translocating
into the nucleus (26). A similar
phenomenon may be envisaged for brachyury. In support of this
hypothesis, in the present study, brachyury expression in the
cytoplasm was observed in all stained samples, compared with
expression in the nucleus being observed in only 60.8% of the
samples. The effect of expression in the cytoplasm and nucleus on
prognosis was different. Thus, as demonstrated with the AR, any
receptor that responds to brachyury antibody may serve a function
in chemoresistance, tumor invasiveness and metastasis if it is
activated and translocated into the nucleus.
The exact mechanism by which microcalcification
forms in breast cancer is not understood. Previous studies have
revealed that microcalcification appears in tumor cells in a manner
similar to that occurring in physiological phenomena (27,28). Cells
involved in bone development, such as osteoblasts and hypertrophic
chondrocytes, are mineralization-competent cells with a mesenchymal
origin (29,30). Thus, microcalcification may form by
mesenchymal cells that are translocated while the EMT process takes
place in tumor cells (31). In the
present study, microcalcification was significantly more frequent
in tumors expressing the EMT driver, brachyury. This result may
assist in validating the hypothesis of previous studies.
Understand is limited about the expression of
brachyury in metastatic lymph nodes. a previous study compared the
level of brachyury expression in primary and metastatic cancer in
115 patients with lung cancer and identified increased expression
in metastatic lymph nodes compared with primary cancer (22). In addition, brachyury expression in
metastatic lymph nodes suggested a poor prognosis as in primary
tumors. The authors suggested two explanations for the abundant
expression of brachyury in metastatic lymph nodes. First,
expression of brachyury in primary tumors enhances the metastatic
and invasive ability, which in turn metastasizes into lymph nodes.
Second, expression of brachyury in metastatic lymph node is
increased in one of the preparations to form the so-called
pre-metastatic niche. In addition, mesenchymal markers, including
snail family transcriptional repressor 2 and interleukin-8 were
increased in these metastatic lymph nodes, whereas the epithelial
marker E-cadherin was decreased. In another study of 42 patients
with breast cancer, increased EMT-associated genes (twist family
BHLH transcription factor 1 and snail family transcriptional
repressor 1 and 2) in metastatic lymph nodes were associated with
poor prognosis (32).
To the best of our knowledge, the present study is
the first to compare the expression of brachyury in primary breast
carcinoma and metastatic lymph nodes in patients with breast
cancer, and the first study to compare the effects of brachyury
expression in metastatic lymph nodes on prognosis. However, unlike
one previous study of lung cancer (22), the expression of brachyury in
metastatic lymph nodes did not differ from the expression in
primary tumor and did not demonstrate any association with
prognosis. It may be that the metastatic mechanism of breast cancer
is different from other types of cancer, or it may be an error
caused by a small number of samples. Further studies will assist to
clarify this argument.
In conclusion, the present study demonstrated that
expression of brachyury in the nucleus of primary tumor possesses
value as an independent marker that predicts poor prognosis. This
was particularly evident in TNBC, where no recurrence or
mortalities occurred in the brachyury negative group during the
follow-up period. In other words, all recurrences or mortalities
only occurred in the brachyury positive group. The expression of
brachyury in the metastatic or recurrent site was not different
from that of the primary tumor, and there was no prognostic value.
Current clinical trials of brachyury targeting vaccines are likely
to be of use in patients with TNBC, although this potential should
be demonstrated by a well-designed randomized prospective
trial.
Acknowledgements
The present study was supported by the Medical
Research Funds from Kangbuk Samsung Hospital.
Glossary
Abbreviations
Abbreviations:
|
AR
|
androgen receptor
|
|
CI
|
confidence interval
|
|
DFS
|
disease-free survival
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ER
|
estrogen receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
HR
|
hazard ratio
|
|
HRP
|
horseradish peroxidase
|
|
H&E
|
hematoxylin and eosin
|
|
IHC
|
immunohistochemical
|
|
OS
|
overall survival
|
|
PR
|
progesterone receptor
|
|
TMA
|
tissue microarray analysis
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Larocca C, Cohen JR, Fernando RI, Huang B,
Hamilton DH and Palena C: An autocrine loop between TGF-β1 and the
transcription factor brachyury controls the transition of human
carcinoma cells into a mesenchymal phenotype. Mol Cancer Ther.
12:1805–1815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernando RI, Litzinger M, Trono P,
Hamilton DH, Schlom J and Palena C: The T-box transcription factor
Brachyury promotes epithelial-mesenchymal transition in human tumor
cells. J Clin Invest. 120:533–544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rowley M, Grothey E and Couch FJ: The role
of Tbx2 and Tbx3 in mammary development and tumorigenesis. J
Mammary Gland Biol Neoplasia. 9:109–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sinclair CS, Adem C, Naderi A, Soderberg
CL, Johnson M, Wu K, Wadum L, Couch VL, Sellers TA, Schaid D, et
al: TBX2 is preferentially amplified in BRCA1- and BRCA2-related
breast tumors. Cancer Res. 62:3587–3591. 2002.PubMed/NCBI
|
|
10
|
Wang B, Lindley LE, Fernandez-Vega V,
Rieger ME, Sims AH and Briegel KJ: The T box transcription factor
TBX2 promotes epithelial-mesenchymal transition and invasion of
normal and malignant breast epithelial cells. PLoS One.
7:e413552012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodriguez M, Aladowicz E, Lanfrancone L
and Goding CR: Tbx3 represses E-cadherin expression and enhances
melanoma invasiveness. Cancer Res. 68:7872–7881. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang B, Cohen JR, Fernando RI, Hamilton
DH, Litzinger MT, Hodge JW and Palena C: The embryonic
transcription factor Brachyury blocks cell cycle progression and
mediates tumor resistance to conventional antitumor therapies. Cell
Death Dis. 4:e6822013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carey LA, Perou CM, Livasy CA, Dressler
LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S,
et al: Race, breast cancer subtypes, and survival in the Carolina
Breast Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haffty BG, Yang Q, Reiss M, Kearney T,
Higgins SA, Weidhaas J, Harris L, Hait W and Toppmeyer D:
Locoregional relapse and distant metastasis in conservatively
managed triple negative early-stage breast cancer. J Clin Oncol.
24:5652–5657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong H, Ryu YJ, An J, Lee Y and Kim A:
Epithelial-mesenchymal transition in breast cancer correlates with
high histological grade and triple-negative phenotype.
Histopathology. 60:E87–E95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sarrió D, Rodriguez-Pinilla SM, Hardisson
D, Cano A, Moreno-Bueno G and Palacios J: Epithelial-mesenchymal
transition in breast cancer relates to the basal-like phenotype.
Cancer Res. 68:989–997. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palena C, Roselli M, Litzinger MT, Ferroni
P, Costarelli L, Spila A, Cavaliere F, Huang B, Fernando RI,
Hamilton DH, et al: Overexpression of the EMT driver brachyury in
breast carcinomas: Association with poor prognosis. J Natl Cancer
Inst. 106:dju0542014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hamilton DH, Roselli M, Ferroni P,
Costarelli L, Cavaliere F, Taffuri M, Palena C and Guadagni F:
Brachyury, a vaccine target, is overexpressed in triple-negative
breast cancer. Endocr Relat Cancer. 23:783–796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heery CR, Singh BH, Rauckhorst M, Marte
JL, Donahue RN, Grenga I, Rodell TC, Dahut W, Arlen PM, Madan RA,
et al: Phase I Trial of a Yeast-Based Therapeutic Cancer Vaccine
(GI-6301) targeting the transcription factor brachyury. Cancer
Immunol Res. 3:1248–1256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edge SB and Compton CC: American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimamatsu S, Okamoto T, Haro A, Kitahara
H, Kohno M, Morodomi Y, Tagawa T, Okano S, Oda Y and Maehara Y:
Prognostic significance of expression of the epithelial-mesenchymal
transition-related factor brachyury in intrathoracic lymphatic
spread of non-small cell lung cancer. Ann Surg Oncol. 23 Suppl
5:S1012–S1020. 2016. View Article : Google Scholar
|
|
23
|
R Development Core Team, . R: A Language
and Environment for Statistical Computing. The R Foundation for
Statistical Computing; Vienna: 2011, http://www.r-project.org/
|
|
24
|
Therneau TM: Survival: Survival Analysis.
R package version 2.38. 2015, https://CRAN.R-project.org/package=survival
|
|
25
|
Therneau TM and Grambsch PM: Modeling
survival data: Extending the Cox model. Springer; New York, NY:
2010
|
|
26
|
Kono M, Fujii T, Lim B, Karuturi MS,
Tripathy D and Ueno NT: Androgen receptor function and androgen
receptor-targeted therapies in breast cancer: A Review. JAMA Oncol.
3:1266–1273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kirsch T: Determinants of pathological
mineralization. Curr Opin Rheumatol. 18:174–180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cox RF, Jenkinson A, Pohl K, O'Brien FJ
and Morgan MP: Osteomimicry of mammary adenocarcinoma cells in
vitro; increased expression of bone matrix proteins and
proliferation within a 3D collagen environment. PLoS One.
7:e416792012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Soltanoff CS, Yang S, Chen W and Li YP:
Signaling networks that control the lineage commitment and
differentiation of bone cells. Crit Rev Eukaryot Gene Expr.
19:1–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anderson HC: Vesicles associated with
calcification in the matrix of epiphyseal cartilage. J Cell Biol.
41:59–72. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scimeca M, Giannini E, Antonacci C,
Pistolese CA, Spagnoli LG and Bonanno E: Microcalcifications in
breast cancer: An active phenomenon mediated by epithelial cells
with mesenchymal characteristics. BMC Cancer. 14:2862014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Markiewicz A, Ahrends T,
Wełnicka-Jaśkiewicz M, Seroczyńska B, Skokowski J, Jaśkiewicz J,
Szade J, Biernat W and Zaczek AJ: Expression of epithelial to
mesenchymal transition-related markers in lymph node metastases as
a surrogate for primary tumor metastatic potential in breast
cancer. J Transl Med. 10:2262012. View Article : Google Scholar : PubMed/NCBI
|