Introduction
Hepatocellular carcinoma (HCC) is a common
malignancy globally, and its mortality rate ranked fourth in 2015
(1,2).
In total, ~60% of patients with HCC are diagnosed with unresectable
HCC, which represents an incurable disease (3,4). Barcelona
Clinic Liver Cancer (BCLC) guidelines recommend patients with
intermediate-stage HCC to receive trans-arterial chemoembolization
(TACE) as a standard treatment. Two randomized controlled clinical
trials demonstrated the benefits of TACE treatment for patients
with HCC (5,6). According to BCLC guidelines, patients
with HCC who present an asymptomatic HCC, lack of portal vein
thrombosis or extra-hepatic spread, compensated cirrhosis, and
patients classified with Child-Pugh scores <8 and a performance
status (PS) of 0, are advised to receive TACE treatment (4). In total, ~20% of patients with HCC are
at an intermediate stage. These patients form a heterogeneous
group, owing to discrepancies in tumor burden, serum biomarker,
liver function, performance status, etiology, etc. However, BCLC
guidelines do not consider all these parameters. The reported
survival rates of patients with intermediate-stage HCC varies
between 11 and 45 months (7).
Staging systems, including model to estimate
survival rates for hepatocellular carcinoma (MESH), hepatoma
arterial embolization prognostic score (HAP), modified HAP (mHAP),
performance status combined Japan Integrated Staging system (PSJIS)
and tumor-node-metastasis (TNM), have been developed to determine
the optimal treatment for the patients. However, it remains unknown
which system is optimal for the prediction of patient survival
rates.
In the present study, the performance of five
staging systems, i.e., MESH, HAP, mHAP, PSJIS and TNM, was compared
in predicting 3-month survival, 6-month survival, 1-year survival
and OS survival rates of patients with HBV-associated HCC
undergoing TACE. The aim of the present study was to determine the
optimal staging system for patients with HCC.
Patients and methods
Patients
A total of 220 sequential patients with HCC treated
with TACE were retrospectively reviewed at the Third Affiliated
Hospital of Sun Yat-Sen University (Guangzhou, China) between July
2009 and June 2012. Patient characteristics are provided in
Table I. The diagnosis of HCC was
confirmed using pathology or magnetic resonance imaging
(MRI)/computed tomography (CT) according to the American
Association for the Study of Liver Diseases guidelines (3). All participants studied were patients
with HBV-associated HCC and received TACE following
multidisciplinary team discussion. Patients with advanced HCC
classified as Child-Pugh grade A or B and with a performance status
(PS) of 0 to 2, platelet count ≥30×109 cells/l and
hemoglobin level ≥60 g/l, were eligible for enrollment. However,
patients were excluded if a second type of cancer and/or
intractable comorbid medical illness existed. Patients classified
as Child-Pugh grade C were also excluded.
 | Table I.Baseline demographic and clinical
characteristics of patients. |
Table I.
Baseline demographic and clinical
characteristics of patients.
| Characteristic | Patients |
|---|
| Total patients, n
(%) | 220 (100) |
| Sex, n (%) |
|
| Male | 200 (90.90) |
|
Female | 20 (9.10) |
| Median age, years
(range) | 52.5 (11–84) |
| Etiology,
n (%) |
|
|
HBsAg | 220 (100) |
| Tumor size, n
(%) |
|
| <2
cm | 20 (9.1) |
| 2-5
cm | 62 (28.2) |
| >5
cm | 138 (62.7) |
| Ascites,
n (%) | 55 (25.0) |
| Portal
vein invasion (segmental), n (%) | 102 (46.4) |
|
Extrahepatic spread | 21 (9.5) |
| White blood cell
count, ×109 cells/l | 5.90 (1.54–20.6) |
| (range) |
| α-fetoprotein, ng/ml
(range) | 503.47 (1–1210) |
| Albumin, g/l
(range) | 38.06
(22.0–53.3) |
| Creatinine, µmol/l
(range) | 73.67
(41.0–160.1) |
| Alkaline phosphatase,
U/l (range) | 139.90 (44–1048) |
| Platelets,
×109 cells/l (range) | 161.31 (31–520) |
| Hemoglobin, g/l
(range) | 130.72 (60–190) |
| Fibrinogen, g/l
(range) | 3.48 (1.26–9.39) |
| Total bilirubin,
µmol/l (range) | 21.28
(4.7–109.8) |
| AST, IU/l
(range) | 82.28 (12–931) |
|
γ-glutamyltransferase, U/l (range) | 166.50 (17–1136) |
| Blood urea nitrogen,
mmol/l (range) | 5.24
(2.08–12.85) |
| PT, sec (range) | 14.18
(11.2–24.3) |
| INR (range) | 1.11 (0.84–2.21) |
| 90-day survival
rate, n (%) | 194 (88.18) |
| 6-month survival
rate, n (%) | 166 (75.45) |
| 1-year survival
rate, n (%) | 132 (60.00) |
| 2-year survival
rate, n (%) | 108 (49.09) |
| 5-year survival
rate, n (%) | 18 (8.18) |
| TNM 7th edition, n
(%) |
|
| I | 14 (6.4) |
| II | 52 (23.6) |
|
III | 38 (17.3) |
| IV | 116 (52.7) |
| Child-Pugh class, n
(%) |
|
| A | 153 (69.5) |
| B | 67 (30.5) |
| BCLC, n (%) |
|
| A | 6 (2.7) |
| B | 12 (5.5) |
| C | 202 (91.8) |
| MESH, n (%) |
|
| 0 | 5 (2.3) |
| 1 | 27 (12.3) |
| 2 | 43 (19.5) |
| 3 | 60 (27.3) |
| 4 | 53 (24.1) |
| 5 | 31 (14.1) |
| 6 | 1 (0.5) |
| HAP, n (%) |
|
| A | 26 (11.8) |
| B | 59 (26.8) |
| C | 76 (34.5) |
| D | 59 (26.8) |
| mHAP, n (%) |
|
| A | 50 (22.7) |
| B | 74 (33.6) |
| C | 74 (33.6) |
| D | 22 (10.0) |
| JIS, n (%) |
|
| 0 | 6 (2.7) |
| 1 | 52 (23.6) |
| 2 | 81 (36.8) |
| 3 | 56 (25.5) |
| 4 | 22 (10.0) |
| 5 | 3 (1.4) |
| PSJIS, n (%) |
|
| 0 | 1 (0.5) |
| 1 | 12 (5.5) |
| 2 | 44 (20.0) |
| 3 | 76 (34.5) |
| 4 | 46 (20.9) |
| 5 | 27 (12.3) |
| 6 | 12 (5.5) |
| 7 | 2 (0.9) |
Data collection
The Institutional Review Board of the Third
Affiliated Hospital of Sun Yat-Sen University reviewed and approved
the present study. Prior to enrollment, all participants provided
written informed consent for data sharing.
A range of demographic data were collected including
risk factors, blood results, imaging and therapy data. Collected
data also included sex, age, date of diagnosis, date of mortality
or last follow-up date. The clinical records of the patients were
retrospectively assessed. Tumor characteristics, including tumor
size and extension, vascular invasion and lymph node metastases,
were assessed using CT or MRI. Routine blood tests, liver function
and coagulation tests were also conducted.
Staging
Baseline data were collected to classify patients
according to MESH, HAP, mHAP, PSJIS and TNM systems. All eligible
patients were classified by MESH, HAP, mHAP, PSJIS, TNM and BCLC in
the first diagnosis, and 91.8% of patients with HCC were classified
according to BCLC-C. Patients with a PS of 2 were also the
classified according to BCLC-C at first diagnosis. A baseline
evaluation that included laboratory studies, imaging studies (CT or
MRI) and clinical examination was performed. Data were collected at
the time the patients were diagnosed with advanced HBV-associated
HCC. Survival times were defined as the time from first TACE
treatment until mortality or last follow-up. Patients who lacked
the required data or who were lost to follow-up within 3 months of
diagnosis were excluded from the present study.
Statistical analysis
The primary endpoints of the present study were
3-month survival and OS. The secondary endpoints of the study were
6-month and 1-year survival. Kaplan-Meier estimator survival curves
and log rank tests were used to evaluate the OS rate. Likelihood
ratio tests (LRTs) were used to compare different staging systems.
The degree of freedom was set at 1, so that different prognostic
systems with different numbers of stages could be compared. Bias
correction of Akaike information criterion (AIC) was applied. Lower
AIC and higher likelihood ratio values indicate improved prognosis
capacity of a staging system. Statistically significant prognostic
variables in univariate analyses were identified by multivariate
analysis using Cox's proportional hazards model. Receiver operating
characteristic (ROC) curve analysis was performed for each staging
system's predictive value for predicting 3-month, 6-month and
1-year mortality. Higher area under curve (AUC) values of the ROC
curves indicate better predictive ability. Data were analyzed using
SPSS (version 22.0; IBM Corp., Armonk, NY, USA) and SAS (version
9.0; SAS Institute, Inc., Cary, NC, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient characteristics
A total of 220 patients were classified using the
MESH, HAP, mHAP, TNM and PSJIS systems. The baseline
characteristics of all patients studied are presented in Table I.
Staging system comparison
Analysis of the prognostic performance of the
staging systems to predict 3-month, 6-month and 1-year survival
rates was performed. The AUC values for predicting 3-month survival
rates for MESH, HAP, mHAP, PSJIS and TNM systems were 0.858, 0.728,
0.690, 0.688 and 0.699, respectively. Additionally, the AUC values
of MESH, HAP, mHAP, PSJIS and TNM for predicting 6-month survival
rates were 0.822, 0.747, 0.720, 0.722 and 0.715, whereas the
respective values for predicting 1-year survival rates were 0.725,
0.664, 0.672, 0.645 and 0.654.
Pairwise comparison of the AUC to predict 3-month,
6-month and 1-year survival rates revealed that the MESH staging
system performed optimally in predicting 3- and 6-month survival
rates (Tables II and III)(Figs 1
and 2). A statistical trend was only
observed when MESH was compared with mHAP in predicting 1-year
survival rate (P=0.0797; Table IV;
Fig. 3). MESH exhibited an improved
performance compared with HAP, PSJIS and TNM in predicting 1-year
survival rate (P<0.05), while mHAP performed equally well
compared with PSJIS and TNM in predicting 1-year survival rate
(P>0.05; Table IV).
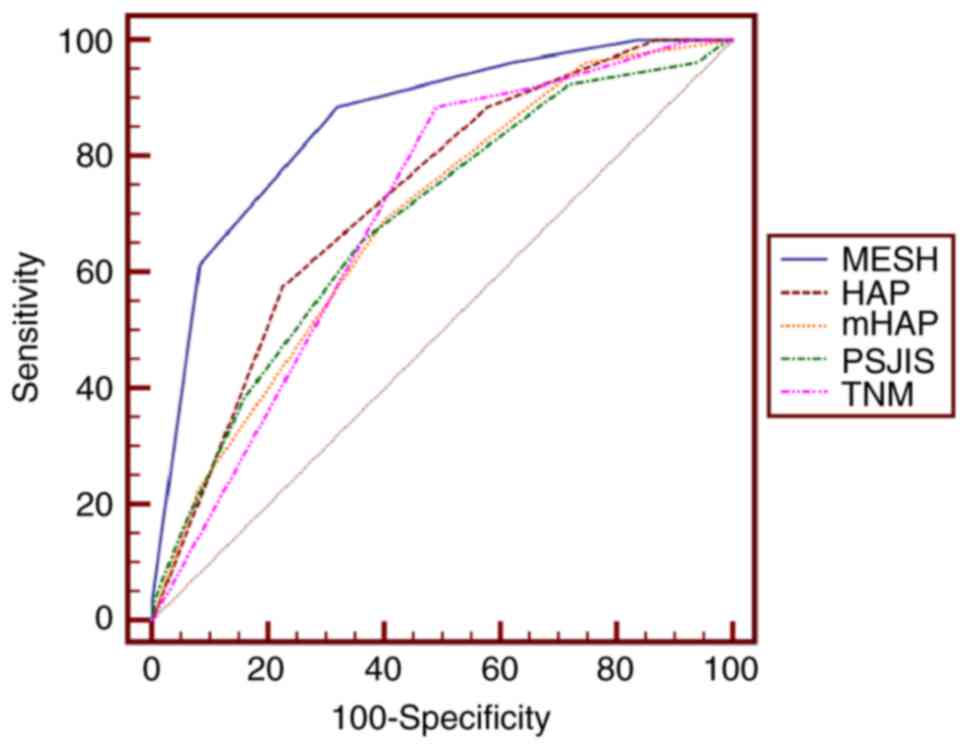 | Figure 1.Receiver operating characteristic
curves of MESH, HAP, mHAP, TNM and PSJIS for predicting 3-month
survival. The optimal threshold value of MESH was 3 and the area
under the curve of MESH was 0.858 (95% confidence interval,
0.805–0.901; P<0.001). P<0.001 for MESH vs. HAP, mHAP, PSJIS
or TNM. MESH, model to estimate survival for hepatocellular
carcinoma; HAP, hepatoma arterial embolization prognostic score;
mHAP, modified HAP; PSJIS, performance status combined Japan
Integrated Staging system; TNM, tumor-node-metastasis. |
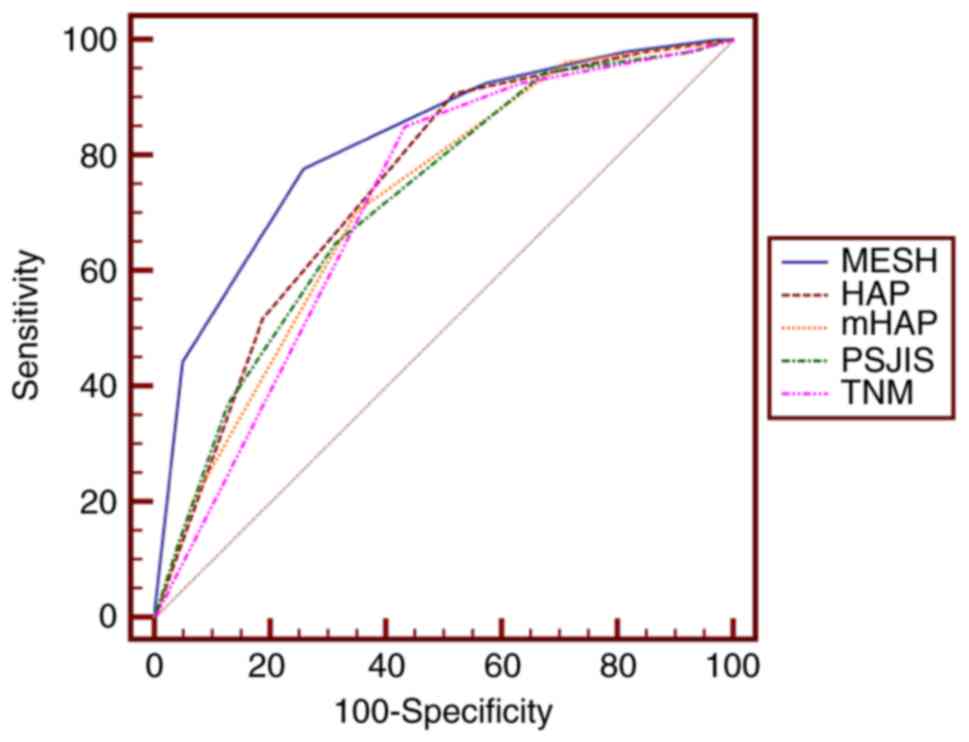 | Figure 2.Receiver operating characteristic
curves of MESH, HAP, mHAP, TNM and PSJIS for predicting 6-month
survival. The optimal threshold value of MESH was 3 and the area
under the curve of MESH was 0.822 (95% confidence interval,
0.765–0.870; P<0.001). P<0.01 for MESH vs. HAP, mHAP, PSJIS
or TNM. MESH, model to estimate survival for hepatocellular
carcinoma; HAP, hepatoma arterial embolization prognostic score;
mHAP, modified HAP; PSJIS, performance status combined Japan
Integrated Staging system; TNM, tumor-node-metastasis. |
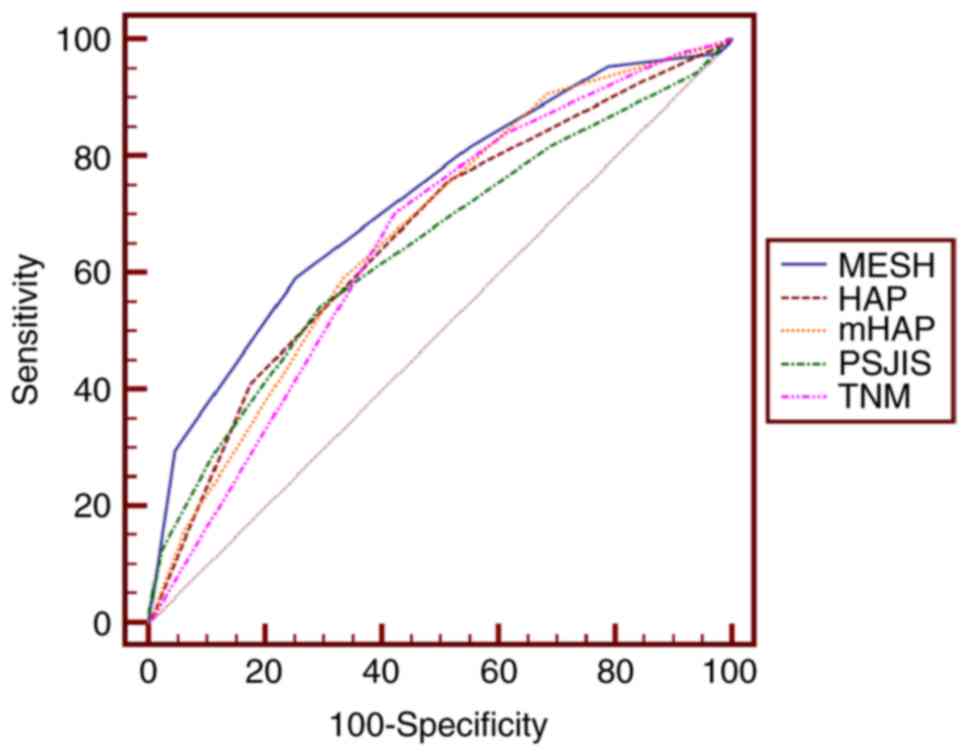 | Figure 3.Receiver operating characteristic
curves of MESH, HAP, mHAP, TNM and PSJIS when predicting 1-year
survival. The optimal threshold value of MESH was 3 and the area
under the curve of MESH was 0.725 (95% confidence interval,
0.661–0.783; P<0.01). P<0.05 for MESH vs. HAP, PSJIS or TNM.
MESH, model to estimate survival for hepatocellular carcinoma; HAP,
hepatoma arterial embolization prognostic score; mHAP, modified
HAP; PSJIS, performance status combined Japan Integrated Staging
system; TNM, tumor-node-metastasis. |
 | Table II.Pairwise comparison of receiver
operating characteristic curves predicting 3-month survival
rates. |
Table II.
Pairwise comparison of receiver
operating characteristic curves predicting 3-month survival
rates.
| System 1 | System 2 | Difference | P-value |
|---|
| MESH | HAP | 0.130 | 0.0008 |
| MESH | mHAP | 0.168 | 0.0001 |
| MESH | PSJIS | 0.170 | 0.0002 |
| MESH | TNM | 0.159 | <0.0001 |
| HAP | mHAP | 0.0373 | 0.1839 |
| HAP | PSJIS | 0.0395 | 0.4660 |
| HAP | TNM | 0.0283 | 0.5530 |
| mHAP | PSJIS | 0.00218 | 0.9706 |
| mHAP | TNM | 0.00902 | 0.8639 |
| PSJIS | TNM | 0.0112 | 0.8479 |
 | Table III.Pairwise comparison of receiver
operating characteristic curves predicting 6-month survival
rates. |
Table III.
Pairwise comparison of receiver
operating characteristic curves predicting 6-month survival
rates.
| System 1 | System 2 | Difference | P-value |
|---|
| MESH | HAP | 0.0752 | 0.0076 |
| MESH | mHAP | 0.102 | 0.0016 |
| MESH | PSJIS | 0.0999 | 0.0032 |
| MESH | TNM | 0.107 | 0.0002 |
| HAP | mHAP | 0.0266 | 0.2054 |
| HAP | PSJIS | 0.0247 | 0.5311 |
| HAP | TNM | 0.0320 | 0.3498 |
| mHAP | PSJIS | 0.00184 | 0.9639 |
| mHAP | TNM | 0.00541 | 0.8813 |
| PSJIS | TNM | 0.00725 | 0.8446 |
 | Table IV.Pairwise comparison of receiver
operating characteristic curves predicting 1-year survival
rates. |
Table IV.
Pairwise comparison of receiver
operating characteristic curves predicting 1-year survival
rates.
| System 1 | System 2 | Difference | P-value |
|---|
| MESH | HAP | 0.0610 | 0.0318 |
| MESH | mHAP | 0.0525 | 0.0797 |
| MESH | PSJIS | 0.0796 | 0.00116 |
| MESH | TNM | 0.0704 | 0.0189 |
| HAP | mHAP | 0.00848 | 0.6508 |
| HAP | PSJIS | 0.0186 | 0.6167 |
| HAP | TNM | 0.00943 | 0.7866 |
| mHAP | PSJIS | 0.0271 | 0.4793 |
| mHAP | TNM | 0.0179 | 0.6189 |
| PSJIS | TNM | 0.00917 | 0.7800 |
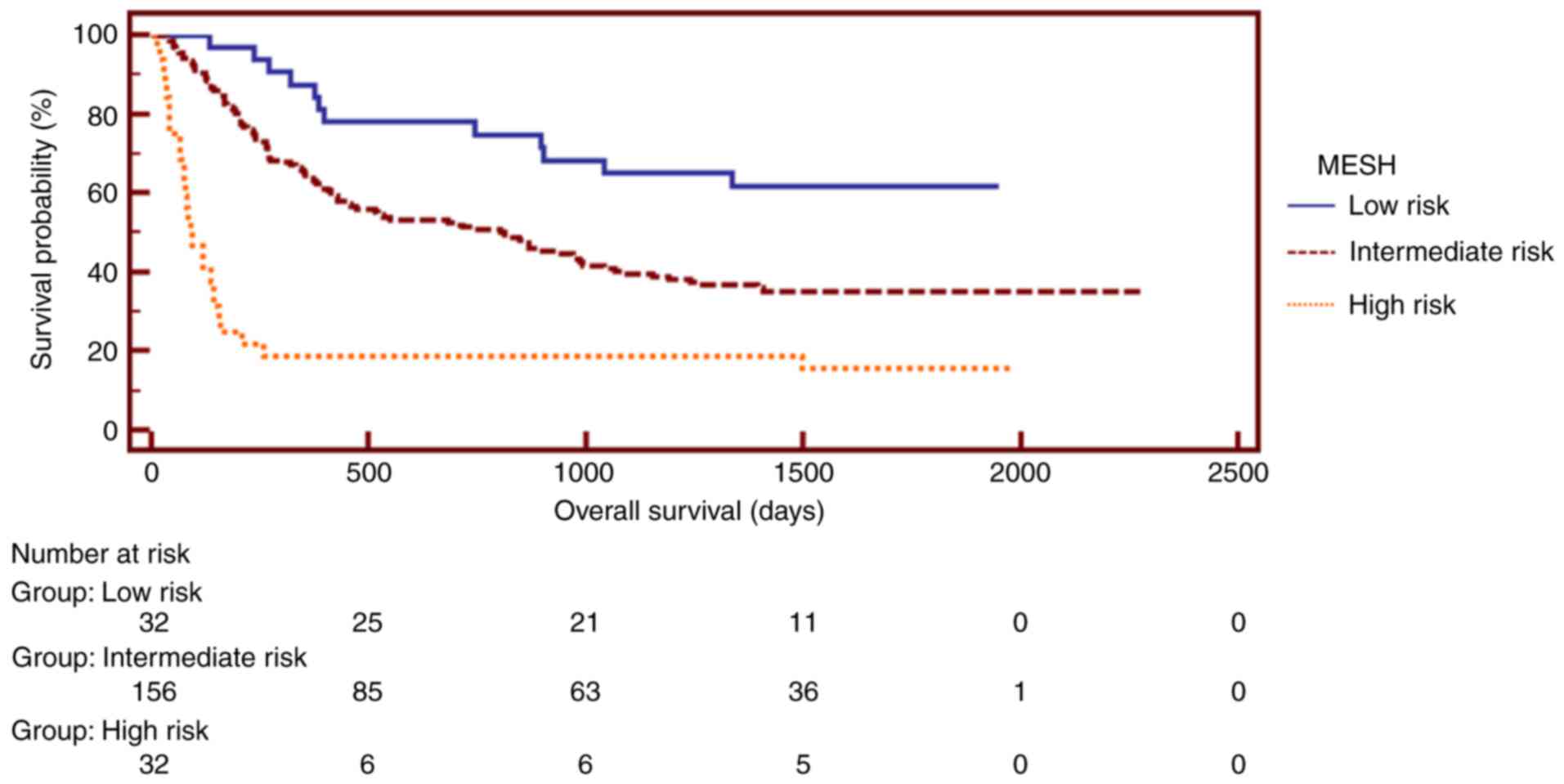
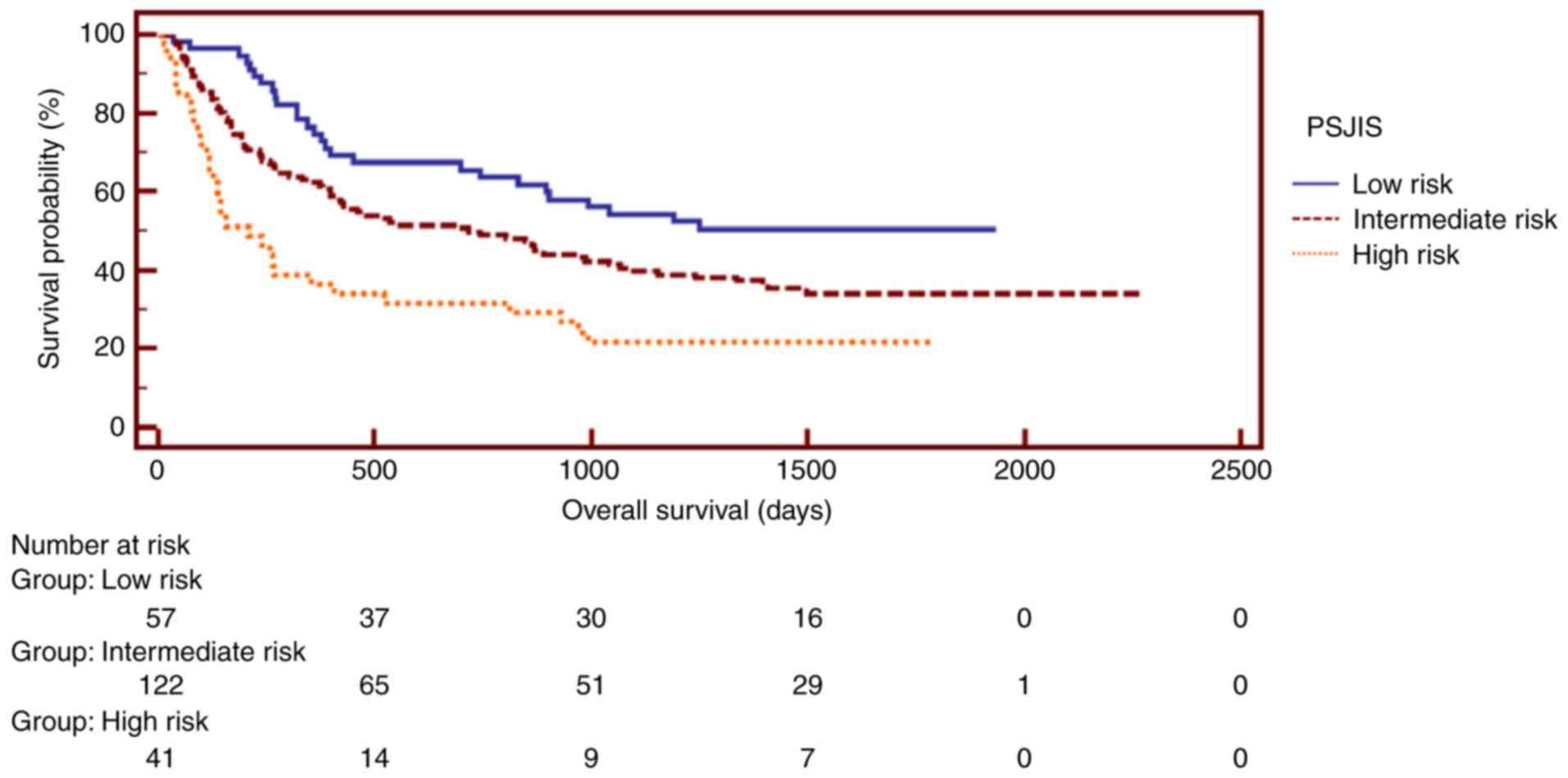
Additionally, the staging systems, including MESH,
HAP, mHAP, TNM and PSJIS were analyzed separately, using
Kaplan-Meier estimator curves (Figs.
4–8, respectively). For the
analysis of the MESH system, patients were assigned to risk groups
(MESH score 0–1, low risk; score 2–4, intermediate risk; score 5–6,
high risk). The median survival was 3 months for the high-risk
group, 27 months for the intermediate-risk group and 41 months for
the low-risk group, indicating that high-risk patients had a poor
survival rate (8). For the analysis
of the PSJIS system, patients with a score of 0–2 represented a
low-risk group, whereas patients in the high-risk group had a score
of 5–7 (9). The Kaplan-Meier
estimator curves exhibited different prognostic strata for MESH,
HAP, mHAP, PSJIS and TNM, which was statistically different
(log-rank P<0.05 in all cases). Subsequently, Kaplan-Meier
estimator analysis of survival rate revealed that the MESH staging
system exhibited an excellent stratified prognostic capacity.
Following comparison of the LRT χ2 and
AIC values of the five staging systems, MESH demonstrated the
highest χ2 and lowest AIC value, thus suggesting an
improved predictive performance compared with that of the HAP,
mHAP, PSJIS and TNM systems (Table
V).
 | Table V.Homogeneity LRT χ2 test
and AIC of different staging systems. |
Table V.
Homogeneity LRT χ2 test
and AIC of different staging systems.
| Staging system | Homogeneity LRT
χ2 test | AIC | P-value |
|---|
| MESH | 31 | 1339 | <0.01 |
| PSJIS | 19 | 1354 | <0.01 |
| TNM | 16 | 1354 | <0.01 |
| mHAP | 14 | 1358 | <0.01 |
| HAP | 11 | 1361 | <0.01 |
Prognostic factors of survival in
patients with HCC
Independent prognostic factors including tumor size,
portal vein invasion (segmental), antiviral therapy and bilirubin
for OS were revealed by univariate and multivariate analyses
(Table VI).
 | Table VI.Univariate and multivariate analysis
of prognostic factors for overall survival in 220 patients with
hepatocellular carcinoma undergoing trans-arterial
chemoembolization. |
Table VI.
Univariate and multivariate analysis
of prognostic factors for overall survival in 220 patients with
hepatocellular carcinoma undergoing trans-arterial
chemoembolization.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex
(male/female) | 0.77 | 0.41 to 1.46 | 0.43 |
|
|
|
| Age, years
(>53/≤53) | 0.95 | 0.68 to 1.32 | 0.75 |
|
|
|
| Tumor size (>50%
of liver/≤50% of liver) | 2.07 | 1.48 to 2.90 | <0.01 | 1.64 | 1.11 to 2.42 | 0.01 |
| Node status
(N0/N1) | 1.99 | 1.27 to 3.11 | 0.01 | 1.24 | 0.75 to 2.04 | 0.40 |
| Extrahepatic spread
(yes/no) | 1.87 | 1.11 to 3.14 | 0.02 | 1.20 | 0.67 to 2.14 | 0.54 |
| Portal vein
invasion (segmental; yes/no) | 2.12 | 1.52 to 2.97 | <0.01 | 1.64 | 1.13 to 2.37 | 0.01 |
| AFP (>400/≤400
ng/ml) | 1.41 | 1.01 to 1.97 | 0.04 | 1.03 | 0.73 to 1.48 | 0.85 |
| Child-Pugh grade
(A/B) | 1.32 | 0.93 to 1.88 | 0.12 |
|
|
|
| Antiviral therapy
(yes/no) | 0.64 | 0.45 to 0.89 | <0.01 | 0.70 | 0.49 to 1.01 | 0.05 |
| AST (>40/≤40
U/l) | 0.58 | 0.39 to 0.86 | <0.01 | 0.75 | 0.50 to 1.13 | 0.18 |
| Bilirubin
(>51.3/≤51.3 µmol/l) | 2.22 | 1.09 to 4.54 | 0.03 | 2.54 | 1.21 to 5.36 | 0.01 |
Discussion
Many patients with HCC are diagnosed at an advanced
stage and TACE is a major therapeutic approach. BCLC guidelines
recommend patients with intermediate-stage HCC to receive TACE
treatment for first-line therapy. Evidence comes from two
randomized controlled clinical trials (5,6). Llovet
et al (5) conducted a
randomized controlled trial and analyzed 112 patients. It was
identified that patients with HCC undergoing chemoembolization
exhibited a longer survival time compared with patients undergoing
conservative treatment. Chemoembolization decreased mortality by
53%. It was concluded that under careful selection, patients with
unresectable HCC received a survival benefit from chemoembolization
(5). Another trial was conducted by
Lo et al (6), in which 80
patients with HCC were examined. The survival rate of the
chemoembolization group was significantly increased compared with
that of the control group. Chemoembolization decreased mortality by
51%. This study verified the previous results that TACE treatment
significantly prolonged the survival of Asian patients with HCC at
an unresectable stage.
However, it is difficult to predict which group of
patients would benefit most from TACE treatment. Precise prognosis
for patients with HCC under TACE treatment is needed. First, many
patients may not respond to TACE although they fulfill the
eligibility criteria. Furthermore, patients receiving their first
course of TACE may develop liver failure and become unsuitable to
receive the second embolization (10). Lastly, the development of TACE
techniques has broadened the use of TACE beyond the initial
eligibility criteria, which widens the heterogeneity of the
treatment group survival.
Several staging systems, including MESH, HAP, mHAP
and PSJIS, have been developed for more precise prognosis for
patients with HCC undergoing TACE. Liu et al (8) proposed the MESH staging system. This
model was derived from the analysis of 3,182 patients with HCC from
Taiwan (8), where multiple factors,
including vascular invasion or metastasis, tumor size, serum
α-fetoprotein (AFP) and alkaline phosphatase (ALP) levels, were
employed. MESH scores range between 0 and 6. The authors identified
that MESH improved prognostic accuracy and refined treatment
strategies for patients with HCC when compared with other staging
systems including BCLC, Taipei Integrated Scoring (TIS), Cancer of
the Liver Italian Program (CLIP) and Hong Kong Liver Cancer (HKLC)
(8). The second system, known as HAP,
was established by Kadalayil et al (11) following examination of 281 patients
with HCC (114 in the training set; 167 in the validation set)
undergoing TACE/trans-arterial embolization (TAE). The authors
employed independent prognostic factors analyzed using Cox's
regression (11). Those parameters
included albumin levels, tumor size, AFP levels and bilirubin
levels (albumin, <36 g/dl; maximum tumor diameter, >7 cm;
AFP, >400 ng/ml; and bilirubin, >17 µmol/l). Patients were
categorized into HAP groups A-D. The median survival rates for HAP
A, B, C and D groups were 27.6, 18.5, 9.0 and 3.6 months,
respectively. Patients in the HAP C and D groups were recommended
not to receive TACE owing to the poor survival rate. The HAP
scoring system was further validated by Pinato et al
(12) who examined 923 patients with
HCC from Asia and Europe. The authors proposed a modified version
of the HAP score (mHAP), based on the tumor size, albumin levels
and AFP levels, but not bilirubin levels. This mHAP system was
identified to offer an improved prediction of overall survival (OS)
rate compared with HAP (12). Another
system, the Japan Integrated Score (JIS), was established based on
analysis of 722 Japanese patients with HCC (13). In addition to this system, Nishikawa
et al (9) proposed the PSJIS
system, which is a combination of PS with JIS and derived from
1,170 patients with HCC and with liver cirrhosis. PSJIS was
identified to be an improvement over the original JIS system and
the BCLC, TNM and CLIP scoring systems in predicting 1-, 3- and
5-year survival rates in patients with transcatheter arterial
therapies (9).
HAP and mHAP staging systems were established based
on the prognostic analysis of TACE/TAE-treated patients with HCC
(11,12). Patients with poor prognosis may not
benefit from TACE. In the HAP staging system, patients were
classified into HAP groups A-D. Median survival rates of patients
classified as HAP A, B, C and D was 27.6, 18.5, 9.0 and 3.6 months,
respectively (11). Patients in the
HAP C and D group were advised not to receive TACE because of poor
survival. The MESH score includes six common clinical variables
including Child-Pugh score, vascular invasion or metastasis
presence, tumor number and tumor size, PS, AFP and ALP. The MESH
score considers tumor burden, serum biomarker, liver function and
PS. It was demonstrated that for BCLC stage B-D patients with HCC,
patients may be classified into different prognostic groups based
on MESH score (8). The MESH score
provided an improvement over TIS, HKLC (14) and CLIP (15).
To the best of our knowledge, MESH has not been
studied in geographical areas other than Taiwan, therefore the
present study is the first to compare MESH and other staging
systems, including HAP, mHAP, PJIS and TNM in patients with
HBV-associated HCC under TACE therapy. According to the results of
the present study, the MESH score exhibited the highest AUC value
when predicting 3-month, 6-month and 1-year survival rates. Life
expectancy >3 months is a common inclusion criterion of TACE
clinical trials. Routinely, TACE is repeated every 2–3 months
(5). As for OS, MESH exhibited the
highest χ2 value and the lowest AIC value, suggesting
that MESH exhibited the optimum performance in terms of
discriminatory ability, homogeneity and monotonicity. The MESH
score is user-friendly and precise. The median survival of patients
with HCC with a MESH score of 5–6 in the present study was 3
months. They would not benefit from TACE due to their poor survival
rates.
Sub-classification of the intermediate stage of BCLC
was proposed by Bolondi (16).
Patients with intermediate-stage HCC were classified into four
sub-classes (B1-B4) based on Child-Pugh score, tumor burden
(assessed by the Milan criteria), PS and portal vein thrombosis. In
the present study, the majority of patients with HCC were
classified as BCLC-C, therefore Bolondi's sub-classification was
not evaluated (16).
Antiviral therapy was identified as an independent
prognostic factor by multivariate analyses. In the present study,
all patients with HCC were associated with HBV. In total ~50% of
the patients received antiviral therapy. HBV reactivation and
hepatic decompensation are major risks in patients with
HBV-associated HCC undergoing TACE. Previous studies have also
confirmed that patients with HCC and with HBV should be considered
for antiviral therapy for preventing hepatic decompensation and HCC
development (17–19).
The present study has certain limitations. First,
the patients included were restricted to a single center and the
number of patients was limited. Additionally, the patients had
HBV-associated advanced HCC. Whether the results of the present
study are applicable to patients with HCC not associated with HBV
is uncertain. Additional etiologies of HCC, including HCV and
alcohol, require further study. Therefore, standard investigations
and large-scale prospective studies are required to validate the
results of the present study.
In conclusion, MESH score was identified as the most
accurate score system for predicting 3-month survival, 6-month
survival, 1-year survival and OS rates among the five systems
analyzed in the patients with HCC who received TACE treatment in
the present study.
Acknowledgements
The present study was supported by National Natural
Science Foundation (grant nos. 31600710 and 81372374), Natural
Science Foundation of Guangdong (grant nos. 2014A030313146 and
2016A030313302) and Project on the Integration of Industry,
Education and Research of Guangdong Province (grant no.
2012B091100460). The authors thank Mr. Teddy Huang (Bruker Nano
Surfaces, Goleta, CA, USA) for his help in language editing.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
GBD 2015 mortality and causes of death
collaborators, . Global, regional, and national life expectancy,
all-cause mortality, and cause-specific mortality for 249 causes of
death, 1980–2015: A systematic analysis for the global burden of
disease study 2015. Lancet. 388:1459–1544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J and Sherman M; Practice Guidelines
Committee, ; American Association for the Study of Liver Diseases,
: Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
European Association For Study Of Liver, ;
European Organization For Research And Treatment Of Cancer, .
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. Eur J Cancer. 48:599–641. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al:
Arterial embolization or chemoembolization versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: A
randomized controlled trial. Lancet. 359:1734–1739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lo CM, Ngan H, Tso WK, Liu CL, Lam CM,
Poon RT, Fan ST and Wong J: Randomized controlled trial of
transarterial lipiodol chemoembolization for unresectable
hepatocellular carcinoma. Hepatology. 35:1164–1171. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burrel M, Reig M, Forner A, Barrufet M, de
Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI and Bruix J:
Survival of patients with hepatocellular carcinoma treated by
transarterial chemoembolization (TACE) using drug eluting beads.
Implications for clinical practice and trial design. J Hepatol.
56:1330–1335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH,
Su CW, Lee FY, Lin HC and Huo TI: Proposal and validation of a new
model to estimate survival for hepatocellular carcinoma patients.
Eur J Cancer. 63:25–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishikawa H, Kita R, Kimura T, Endo M,
Ohara Y, Sakamoto A, Saito S, Nishijima N, Nasu A, Komekado H and
Osaki Y: Proposal of the performance status combined Japan
integrated staging system in hepatocellular carcinoma complicated
with cirrhosis. Int J Oncol. 46:2371–2379. 2015.PubMed/NCBI
|
|
10
|
Raoul JL, Gilabert M and Piana G: How to
define transarterial chemoembolization failure or refractoriness: A
European perspective. Liver Cancer. 3:119–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kadalayil L, Benini R, Pallan L, O'Beirne
J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK, et
al: A simple prognostic scoring system for patients receiving
transarterial embolization for hepatocellular cancer. Ann Oncol.
24:2565–2570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pinato DJ, Arizumi T, Allara E, Jang JW,
Smirne C, Kim YW, Kudo M, Pirisi M and Sharma R: Validation of the
hepatoma arterial embolization prognostic score in european and
asian populations and proposed modification. Clin Gastroenterol
Hepatol. 13:1204–1208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kubo S, Tanaka H, Shuto T, Takemura S,
Yamamoto T, Uenishi T, Tanaka S, Hai S, Yamamoto S, Ichikawa T, et
al: Prognostic effects of causative virus in hepatocellular
carcinoma according to the Japan integrated staging (JIS) score. J
Gastroenterol. 40:972–979. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yau T, Tang VY, Yao TJ, Fan ST, Lo CM and
Poon RT: Development of Hong Kong liver cancer staging system with
treatment stratification for patients with hepatocellular
carcinoma. Gastroenterology. 146:1691–1700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsu CY, Huang YH, Hsia CY, Su CW, Lin HC,
Loong CC, Chiou YY, Chiang JH, Lee PC, Huo TI and Lee SD: A new
prognostic model for hepatocellular carcinoma based on total tumor
volume: The taipei integrated scoring system. J Hepatol.
53:108–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bolondi L, Burroughs A, Dufour JF, Galle
PR, Mazzaferro V, Piscaglia F, Raoul JL and Sangro B: Heterogeneity
of patients with intermediate (BCLC B) hepatocellular carcinoma:
Proposal for a subclassification to facilitate treatment decisions.
Semin Liver Dis. 32:348–359. 2012.PubMed/NCBI
|
|
17
|
Li X, Zhong X, Chen ZH, Xing YF, Wu DH,
Chen J, Ma XK, Lin Q, Wen JY, Wei L, et al: Hepatitis B virus DNA
negativity acts as a favorable prognostic factor in hepatocellular
carcinoma patients. Asian Pac J Cancer Prev. 15:9635–9641. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roderburg C, Tacke F and Trautwein C:
Antiviral therapy in patients with viral hepatitis and
hepatocellular carcinoma: Indications and prognosis. Visc Med.
32:121–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu LH, Li N, Shi J, Guo WX, Wu MC and
Cheng SQ: Does anti-HBV therapy benefit the prognosis of
HBV-related hepatocellular carcinoma following hepatectomy? Ann
Surg Oncol. 21:1010–1015. 2014. View Article : Google Scholar : PubMed/NCBI
|