Introduction
Hepatocellular carcinoma (HCC) is the most common
type of liver cancer and among leading causes of cancer-related
death of humans (1–3). Rodent models of HCC have been proven
useful in revealing aspects of its multistep pathogenesis and
preclinical testing of anti-HCC treatments (1,2). Mice have
been shown to be particularly useful in that regard and a wide
variety of genetically engineered, xenograft and chemically induced
models are available for HCC research (1,2,4,5). Among
them, the chemically induced model that utilizes diethylnitrosamine
(DEN) for HCC initiation is widely used and well-characterized.
This model recapitulates aspects of liver injury and fibrosis and
hepatitis, which both are the basis of human HCC (1,2,4,6). For that,
and because it is comparable to its human counterpart in terms of
cancer-associated gene expression patterns and carcinogenetic
pathways, it is considered among the best-fit experimental models
of HCC (5).
Common molecular pathways of HCC pathogenesis
include phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target
of rapamycin (mTOR), c-MET, AMP-activated protein kinase (AMPK),
insulin growth factor 1 (IGF-1), H-Ras and vascular endothelial
growth factor (VEGF)-mediated angiogenesis (4,7). Recently,
the Wnt/β-catenin signaling pathway, mostly known for its
contributions in mucosal epithelia cancers, has been added to the
list of HCC pathways of carcinogenesis (8–13). In
humans, the percentage of HCCs showing activation of this pathway
have been reported to range from 20 to 90% (8–13).
Wnt proteins encode a large family of secreted
glycoproteins that act as extracellular cell signaling molecules.
Their binding to the transmembrane Frizzled (FZD) receptors
activates the Wnt/β-catenin pathway that eventually results in the
cytoplasmic accumulation and nuclear translocation of the β-catenin
protein (11–13). Intranuclear β-catenin binding to
T-cell factor 4 (Tcf4) consequently upregulates the expression of
many different cancer-related genes, including c-myc and
Cyclin-D1 (3,14).
Activating mutations of the β-catenin gene
(CTNNB1), loss-of-function mutations in APC and
Axin, as well as deregulation of other Wnt/β-catenin pathway
elements [ligands, such as Wnt-1; receptors and co-receptors, such
as Frizzled-1 (FZL-1), and inhibitors, such as DKK1] have all been
implicated in HCC (3,8–13).
Wnt/β-catenin as well as other molecular pathways of HCC
interrelate with important inflammation, proliferation and
apoptosis molecules, such as Forkhead box M1 (FOXM1), NF-κB, c-Jun
and B-cell lymphoma 2 (Bcl-2) with important roles in HCC evolution
and growth (15–19).
Although blocking of the Wnt/β-catenin pathway has
emerged as potential anti-HCC treatment, blocking of the Wnt
secreted ligands and especially Wnt-1 has not been adequately
tested (9,10). A small number of studies, however,
tested the depletion of Wnt-1 on HCC cell cultures and tumor
transplant mouse models grafted with HCC cells. These studies show
preliminary evidence of tumor suppressive effects (20–23).
In the light of this evidence, the present study
aimed to test the effects and the outcome of Wnt-1 blockade in the
DEN mouse model of chemically-induced spontaneous hepatocellular
carcinogenesis.
Materials and methods
Animals
C56BL/6 male mice weighing 25–27 gr were purchased
by the Hellenic Pasteur Institute. Mice were kept in stainless
cages at constant 22 to 24°C temperature and allowed free access to
food and water during the 24-h day/night cycle. All experimental
procedures were performed according to the guide for care and use
of laboratory animals (24), and
ethical approval and licensing (License reference no. 4956) were
provided by the competent National Veterinary Administration
Authorities according to Greek legislative (Decree no. 2015/92,
160/91) and European Communities Council directive (no.
86/609/EEC).
Experimental design
A total of 28 male mice were used. At the age of 14
days, mice were injected with a single i.p. injection of the
carcinogen N-nitrosodiethylamine (DEN; 5 mg/kg of BW) for the
induction of hepatocellular carcinoma (n=22). Ten
carcinogen-injected mice were further treated at the age of 9
months with daily i.p. anti-WNT-1 antibody (Abcam, Cambridge, UK;
50 mg/kg of bw) injections for ten consecutive days. Mice were
killed with an overdose of ketamine and xylazine during anaesthesia
at ten months of age (n=24) with the exception of four mice from
the DEN-treated experimental group that were killed at the age of
12 months (n=4) Blood was collected for ELISA and liver tissues
were fixed in neutral-buffered formalin 10% for histopathology and
immunohistochemistry (IHC).
Histopathology, IHC and
morphometry
Formalin-fixed livers were embedded in paraffin, cut
at 5 µm, and stained with hematoxylin and eosin or IHC. Primary
antibodies for IHC included rabbit antibodies against Ki-67, Cyclin
D1, Wnt-1, DKK1 (Abcam), cleaved caspase-3, NF-κB p65, c-Jun (Cell
Signaling Technology, Inc., Beverly, MA, USA), β-catenin (Thermo
Fisher Scientific/Lab Vision, Fremont, CA, USA), Frizzled 1/Wnt
receptor and FOXM1/HFH 11 (Bioss Inc., Woburn, MA, USA).
Heat-induced antigen retrieval was performed with citrate buffer,
pH 6, for c-Jun, Cyclin D1, cleaved caspase-3, NF-κB p65 and
β-catenin or with EDTA buffer, pH 8, for Ki-67, DKK1, FOXM1, Wnt-1
and Frizzled 1/Wnt receptor. Rabbit primary antibody binding was
detected with goat anti-rabbit polymer HRP (ZytoChem Plus, Berlin,
Germany). Color was developed with Diaminobenzidine
substrate-chromogen (Thermo Fisher Scientific/Lab Vision) and
tissues were counterstained with hematoxylin.
For quantitative histomorphometry, liver tumors in
HE-stained sections were subscribed and their area was
automatically measured in image pixels. The size of each tumor was
recorded. The total tumor area per total liver area ratio was also
calculated for each mouse liver section. IHC-positive cells or
pixels were counted in hepatocellular adenoma images of ×20
representative high power fields and results were recorded as
number of cells or pixels per image as previously described
(25). The ImageJ image processing
and analysis program (National Institutes of Health, Bethesda, MD,
USA) was used for all histomorphometrical assessments.
Blood serum ELISA
Hepatocyte growth factor receptor (C-MET/HGFR) and
Bcl-2 antagonist of cell death (BAD) serum levels were determined
using a quantitative sandwich enzyme immunoassay technique of
Cusabio Biotech Co., Ltd. (Wuhan, China). Standards and serum
samples diluted 1:2 in Sample Diluent and assayed in duplicate in a
96-well microplate, pre-coated with an antibody specific for
C-MET/HGFR and BAD, respectively. A 2-h incubation in 37°C was
followed by the addition of the appropriate antibody, 1-h
incubation in 37°C, washing, the addition of avidin conjugated
horseradish peroxidase (HRP), incubation of 1 h in 37°C, washing
and the addition of the appropriate substrate. Finally, the
reaction was stopped and within 5 min the optical density was
determined, using a microplate reader set to 450 nm. A standard
curve was created and the concentration of the samples was
calculated, taking into account the initial dilution of the
samples. The inter-assay and intra-assay precision for both assays
were <10% and <8%, respectively. The detection range for
C-MET/HGFR was 0.078-5 ng/ml and for BAD was 31.2–2,000 pg/ml.
Statistical analyses
Histomorphometry and serum protein measurements data
were compared between groups using Mann-Whitney U analysis.
Statistical significance was set at P<0.05. All analyses were
performed with the Graphpad Prism version 5.0 for windows (GraphPad
Software, San Diego, CA, USA). Data representation was done with
bar graphs depicting the mean and standard error of the parameter
assessed for each experimental group.
Results
Hepatic cell tumors in DEN-challenged
mice
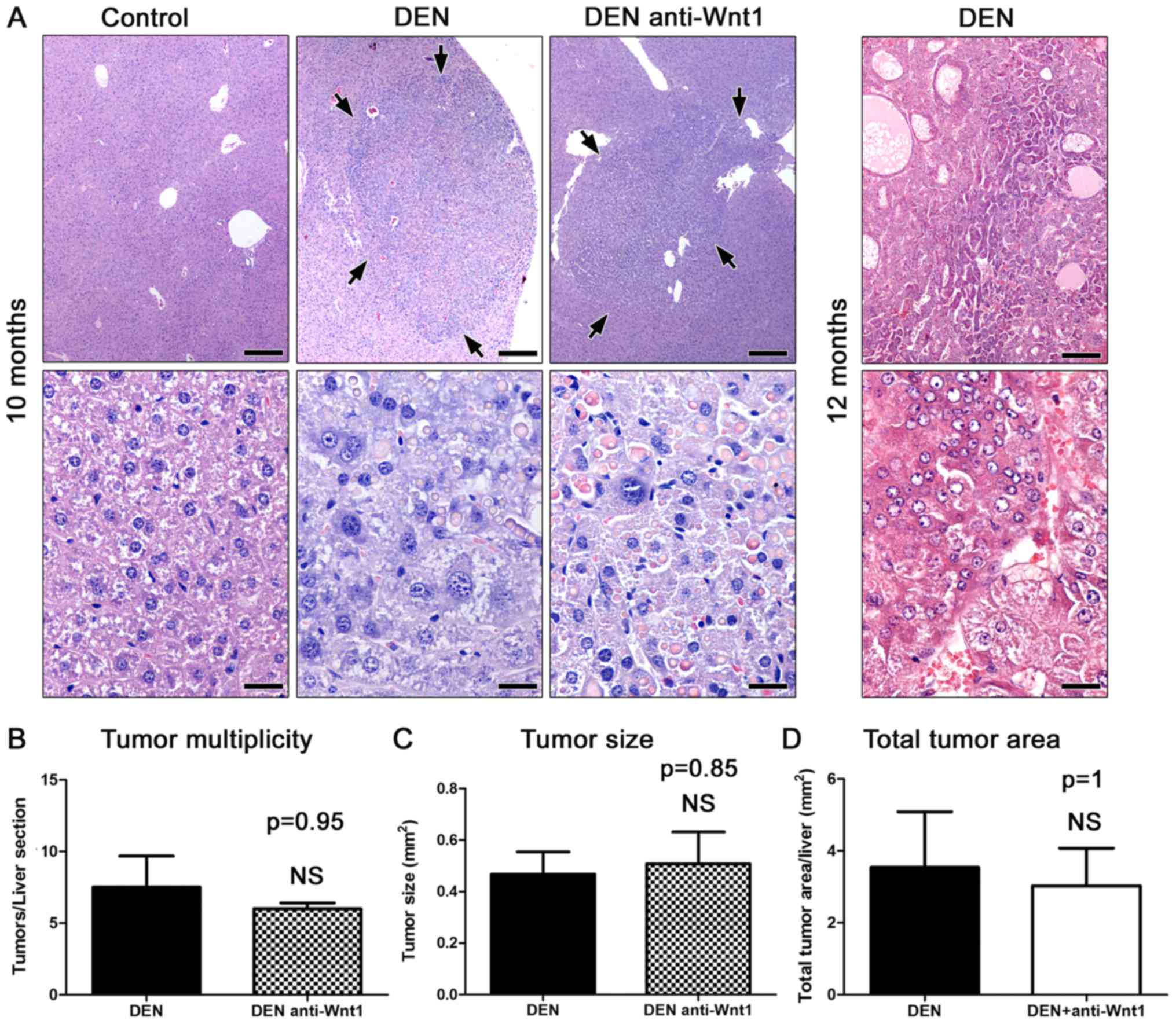
The carcinogen challenged male mice examined at 10
months after DEN administration (n=8) had multiple variably-sized
hepatic cell tumors. Histologically, the tumors appeared as sharply
demarcated, hypercellular, basophilic hepatic cell nodules. The
lesions often compressed the adjacent liver tissue areas in a
variable degree of severity. Consistently, the nodules showed loss
of normal lobular architecture. The larger ones had peripheral
liver plates that formed oblique angles with the corresponding
plates of normal adjacent parenchyma. Neoplastic cells showed mild
to moderate pleomorphism and atypia, and rare mitotic figures. In
their majority tumors contained large amounts of spherical
variably-sized eosinophilic hyaline inclusion bodies (Fig. 1A). According to recently published
histopathological classification criteria (26), the tumors were diagnosed as
hepatocellular adenomas rather than carcinomas, based on absent
frank malignancy features, such as necrosis and hemorrhage,
indistinct demarcation and trabeculae formation. Upon histological
examination, the untreated mice used as controls (n=6) had normal
livers.
To confirm the malignant potential of the
DEN-induced liver lesions in our experiments, four additional mice
(n=4) were examined at the more advanced time-point of 12 months
post carcinogen administration. As expected, the liver of all 4
mice had notable well-sized typical HCC lesions. The tumors were
highly infiltrative, had indistinct expanding borders and often
contained areas of hemorrhage and necrosis. The neoplastic cells
were highly pleomorphic and atypical. The degree of differentiation
varied from tumor to tumor ranging from well to poorly
differentiated. Several different histological types were
recognized, including trabecular, solid, acinar and clear cell HHC
(Fig. 1A).
DEN-induced liver tumor formation does
not require intact Wnt/β-catenin signaling
Accumulating data suggest that the Wnt/β-catenin
pathway is involved in liver carcinogenesis (9). To test whether an exogenous disruption
of the canonical Wnt/β-catenin signaling pathway might affect
DEN-induced carcinogenesis, we treated DEN-challenged mice with
neutralizing antibodies against Wnt-1 and examined their livers
histologically at 10 months after DEN administration (n=10). By
comparison with time-point-matched controls (n=8) the
Wnt-1-depleted mice did not show a statistically significant
difference in liver tumor multiplicity (Fig. 1B). Likewise, the size of liver tumors
induced in the two experimental groups of mice did not differ at
significant levels (Fig. 1A and B).
To further confirm these findings, we also analyzed the total tumor
area found in each liver histological section. As with previous
comparisons no significant differences were found (Fig. 1B). The liver tumors of Wnt-1-depleted
mice were comparable to control mouse tumors not only
quantitatively but qualitatively as well. Indeed, the liver tumors
of treated mice had the same typical histomorphological features of
hepatocellular adenomas as those found in otherwise untreated mice
at 10 months after DEN administration (Fig. 1A). These results suggest that lack of
Wnt-1 does not affect hepatocellular tumor formation in the DEN
mouse model of liver cancer.
Effects of Wnt-1 depletion on
proliferation and apoptosis of liver tumor cells
Proliferation and apoptosis are key events in tumor
evolution and progression. The Wnt/β-catenin pathway is involved
together with other important signaling pathways in both
proliferation and apoptosis of HCC cells (9). For that, we next sought to address
whether the depletion of Wnt-1 affected proliferation or apoptosis
in the hepatocellular adenomas found in the mice of our study.
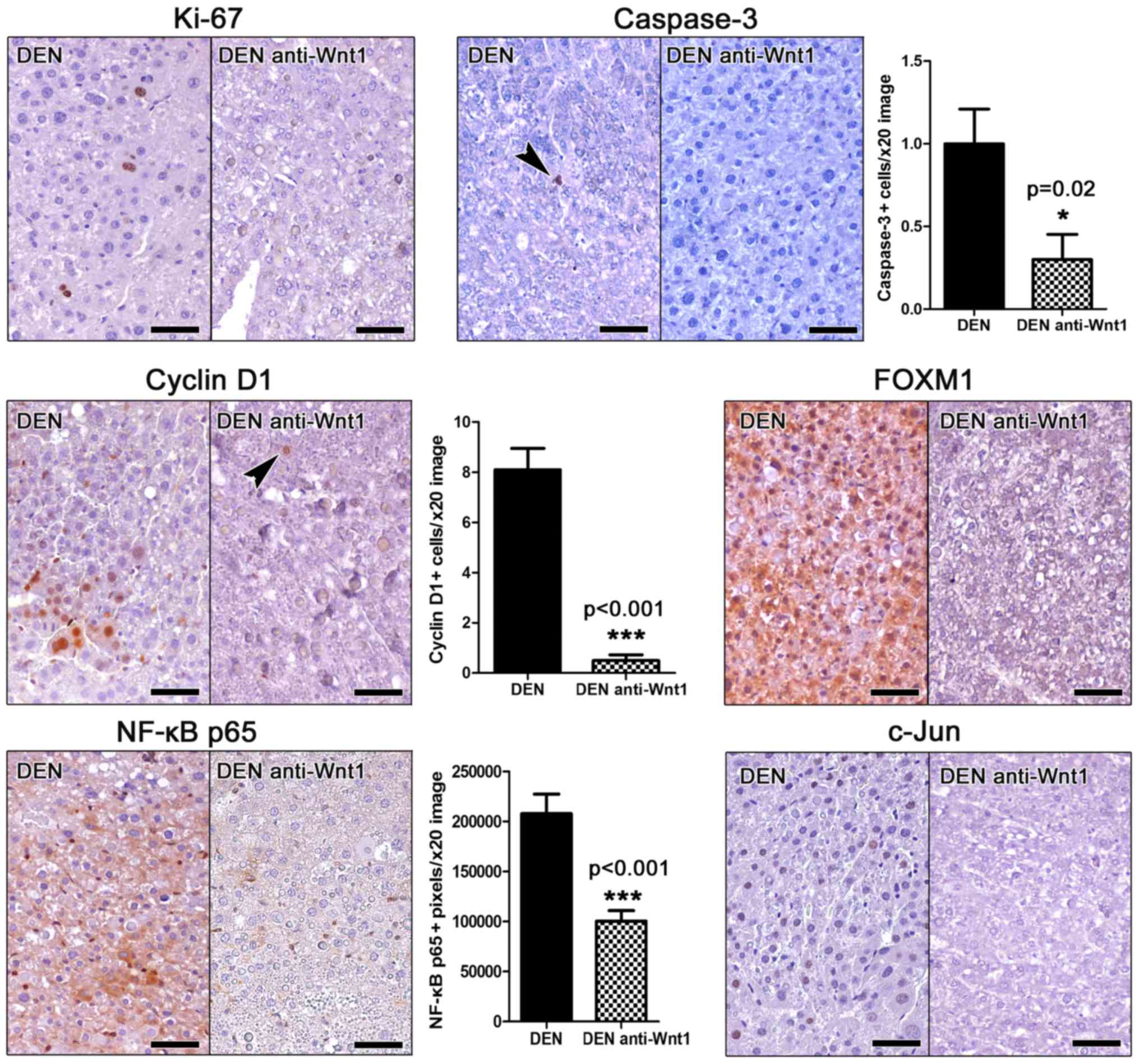
For assessing proliferation, we applied IHC to
detect the proliferation marker ki-67 in liver sections. Unlike
other mouse cancers stained simultaneously as stain controls, we
find that hepatocellular adenomas of DEN-challenged mice contained
very few (either none or up to six at the most per tumor)
Ki-67-positive neoplastic cells (Fig.
2). Interestingly, we were able to detect none ki-67-positive
neoplastic liver cell in the tumors of anti-Wnt-1-treated mice
(Fig. 2), whereas other types of
cells in the same liver sections showed Ki-67-positive
immunostaining (internal stain control).
Apoptosis in liver tumors was examined by means of
Caspase-3-specific IHC. Similarly to proliferation, apoptosis was
also in low levels, with occasional tumors cells showing Caspase-3
positive cytoplasmic signal. However, in Wnt-1-treated animal
tumors the presence of apoptotic cells was particularly rare by
comparison with liver tumors of untreated mice. To quantify this
result we next assessed morphometrically Caspase-3 positive tumor
cells. Indeed, we found that although in low numbers, the apoptotic
tumor cell counts of Wnt-1-treated mice were lower than non-treated
in statistically significant levels (Fig.
2).
These results suggest that DEN-induced liver tumor
cells have low proliferative and apoptotic activity. Also, that the
depletion of Wnt-1 suppresses further not only proliferation but
apoptosis as well in DEN-induced hepatocellular adenomas.
Effects of Wnt-1 depletion on selected
tumor markers
Having found that the intervention with neutralizing
anti-Wnt-1 antibodies affects the proliferation and apoptosis of
tumor cells, we next examined whether it modulated the expression
of selected relevant tumor markers.
Cyclin D1 is a key regulator of cell cycle. Its
upregulation is an early carcinogenesis event in many different
tumor types including hepatocellular neoplasms (11). Wnt-1 blockade has been shown to
suppress cyclin D1 expression in hepatocellular carcinoma cells
in vitro (22). By applying
Cyclin D1-specific IHC in mouse liver sections we found that
hepatocellular adenomas in DEN-treated mice contained several
Cyclin D1-positive tumor cells. Their anti-Wnt-1-treated
counterpart tumors, however, contained significantly less as
further confirmed by morphometric assessment (Fig. 2).
The expression of the proto-oncogene key cell cycle
regulator Forkhead box M1 (FOXM1) (27) was in accordance to ki-67 and Cyclin D1
results. Indeed, by IHC the hepatocellular adenomas in DEN-treated
mice showed ample FOXM1 expression. In contrast, adenomas of
Wnt-1-depleted mice had FOXM1 in practically non-detectable levels
(Fig. 2).
The activation of NF-κB and c-Jun signaling pathways
in tumor cells correlate with increased tumor malignancy in
hepatocellular tumors of both humans and mouse models (17). Using IHC and morphometric counts of
positively stained image pixels (NF-κB p65), we found that
hepatocellular adenomas of anti-Wnt-1-treated mice had
significantly less NF-κB p65expression compared to their matched
controls and c-Jun (Fig. 2). Also,
that they had absent c-Jun-positive cells, whereas the detection of
such cells in the tumors of control mice was consistent (Fig. 2).
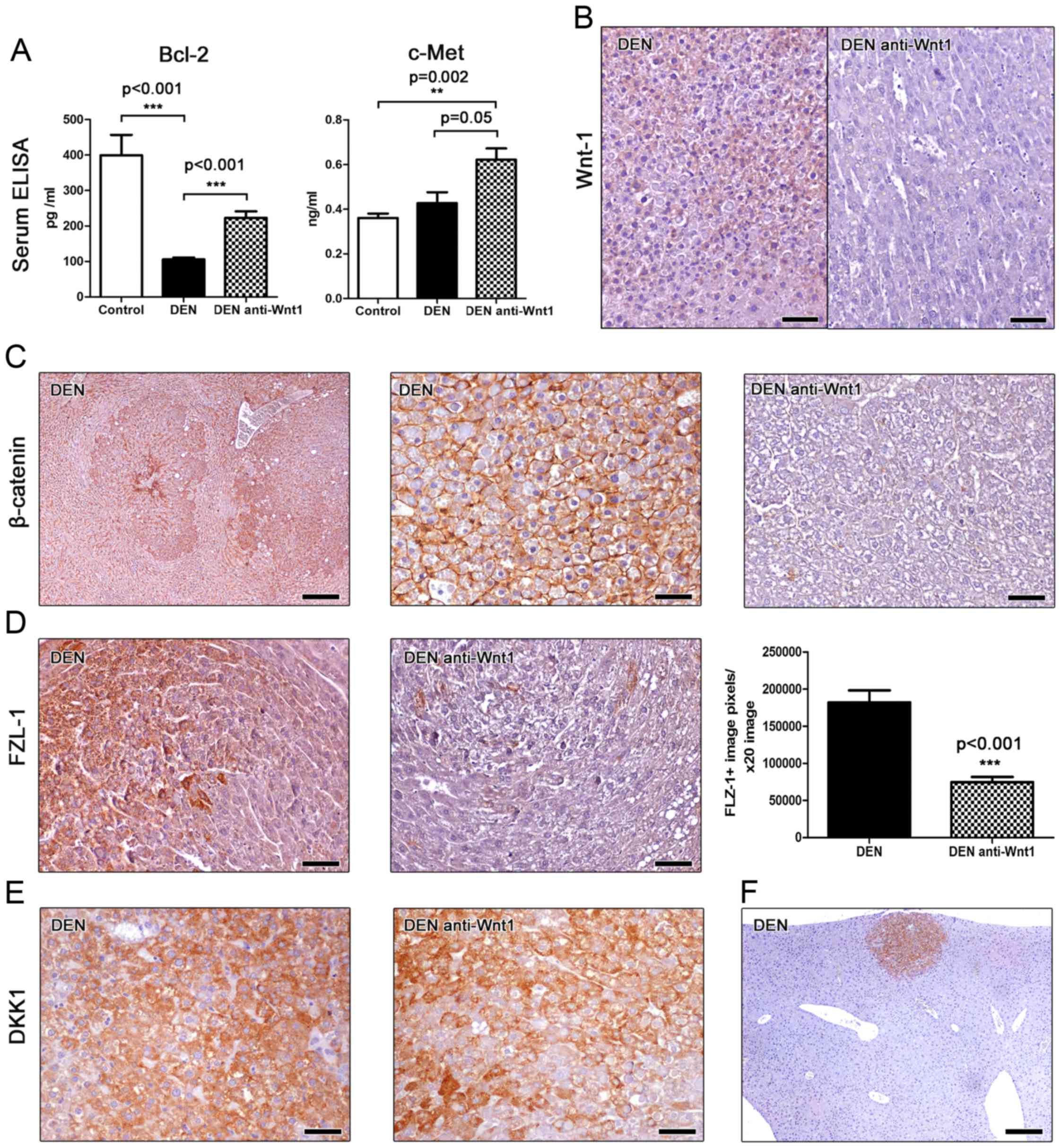
B-cell lymphoma 2 (Bcl-2) is a major anti-apoptotic
signaling protein affecting the development of many different
neoplasms, including hepatocellular cancer (28). For that, we next assessed Bcl-2 levels
in the serum of mice using ELISA. We found that mice bearing liver
tumors had significantly lower serum levels of Bcl-2 compared to
cancer-free controls. However, treatment with Wnt-1 neutralizing
antibodies had a statistically significant effect in increasing
circulating levels of Bcl-2 (Fig.
3A). This result suggests that Wnt-1 blockade increases
Bcl-2-associated anti-apoptotic signals in mice with DEN-induced
liver tumors.
By using ELISA, we also measured c-Met serum protein
levels in the mice of our study. c-Met, also called hepatocyte
growth factor receptor (HGFR), is a tyrosine-protein kinase
activator that is abnormally upregulated in liver cancer and has
been shown to correlate with poor prognosis (7). We found that serum c-met is upregulated
in mice with DEN-induced liver tumors by comparison with
cancer-free controls, with the difference reaching statistical
significance, however, only for the anti-Wnt-1-treated experimental
group. This group showed a considerable statistical significance
trend for higher c-met levels when compared to DEN-exposed mice
that received no further treatment (Fig.
3A). The circulating c-met level assessment results match our
histopathological findings showing that Wnt-1 blockade did not
suppress DEN-induced liver tumor formation in mice.
Effects of Wnt-1 depletion on selected
Wnt/β-catenin signaling molecules
Utilizing IHC we first tested the efficacy of our
depletion strategy in the mouse liver sections. Non-tumoral liver
tissue and hepatocellular adenomas of DEN-treated mice showed a
mild diffuse Wnt-1-specific staining. The same areas of the livers
of mice that were further subjected to Wnt-1 blockade, however,
showed absent immunohistochemical signal. This result suggests that
no particular Wnt-1 upregulation is observed in DEN-induced
hepatocellular adenomas compared to the non-tumoral liver tissue.
Also, that the depletion strategy we applied using anti-Wnt-1
antisera worked to bring down the presence of this protein in
immunohistochemically non-detectable levels (Fig. 3B).
This result matched similar outcomes of our staining
for β-catenin, which is a basic downstream target molecule of Wnt-1
protein (11). The livers of
DEN-treated control mice showed normal β-catenin staining.
Hepatocellular adenomas in these mice had a more pronounced
β-catenin staining compared to non-tumoral liver tissue (Fig. 3C). However, the liver tumor cells did
not show aberrant nuclear or cytoplasmic accumulation of β-catenin
(Fig. 3C). By contrast, livers from
Wnt-1-depleted mice had diminished presence of β-catenin
throughout, including both non-tumoral and tumor areas. (Fig. 3C).
The Frizzled-1 (FZL-1) wnt receptor is a basic
element of the β-catenin canonical signaling pathway. Upon binding
with Wnt-1 the activation of disheveled proteins inhibits
glycogen-synthase kinase-3 leading to an aberrant cytoplasmic and
nuclear accumulation of β-catenin (11). The anti-FLZ-1 receptor
immunohistochemical stain of DEN-treated mouse livers showed a
multifocal positivity of hepatocytes, by contrast to untreated
normal mouse livers, which had absent IHC signal. FLZ-1 receptor
expressing hepatocytes located mostly in (without completely
restricting to) the centrilobular zone of hepatic lobules.
Neoplastic cells of hepatocellular adenomas, however, showed a more
often and prominent positive immunohistochemical signal. The
anti-Wnt-1 treatment appeared to reduce FLZ-1 expression in both
non-tumoral and neoplastic areas of liver. To quantify FLZ-1
expression in hepatocellular adenomas we next performed
morhometrical counts. We found that tumors from Wnt-1-depleted mice
had significant less FLZ-1 compared to tumors from untreated
controls (Fig. 3D).
Dickkopf-related protein 1 (DKK1) inhibits the WNT
signaling pathway and reduces β-catenin expression (3). Immunohistochemically, DKK1 was found to
be consistently upregulated in all hepatocellular adenomas found in
both anti-Wnt-1-treated and untreated mice. The staining pattern
and density was comparable between the two groups (Fig. 3E). Interestingly, upregulation of DKK1
was clearly evident not only in large tumors but in small-sized
neoplastic and preneoplastic lesions as well (Fig. 3F). This result suggests that DKK1
upregulation is an early event in DEN-induced mouse liver
carcinogenesis. Also, that this upregulation remains unaffected in
the absence of Wnt-1.
Discussion
Recent evidence suggests that deregulations of the
Wnt/β-catenin signaling pathway contribute to HCC development and
growth (3,11–13).
Consequently, elements of this pathway started to emerge as
potential targets for improving outcomes of anti-HCC treatment
(9,10). In the light of this evidence, the
present paper examined the effects of Wnt-1 blockade in the
classical DEN-induced chemical carcinogenesis mouse model of HCC
(5). We found that the depletion of
Wnt-1 using neutralizing antisera for ten consecutive days at the
age of 9 months was particularly potent in suppressing the
expression of critical elements of the Wnt/β-catenin pathway and
selected tumor markers. Nonetheless, by examining mouse livers 20
days after the completion of treatment we found that tumor size and
multiplicity were not affected.
In our study, mice treated with the chemical
carcinogen DEN at 10 months of age had hepatocellular adenomas. The
malignant potential of these adenomas was confirmed, since four
mice that were kept for an additional period of two months
developed well-sized hepatocellular carcinomas. The occurrence,
growth and evolution of DEN-induced mouse hepatic proliferative and
neoplastic lesions depends on several factors including the dose
scheme and administration route of DEN and also age, strain and
gender of mice (1,2). Our finding of hepatocellular adenomas in
10-months-old C56BL/6 male mice that were treated with 5 mg/kg BW
of DEN matches the results of other published studies using mice of
comparable genetic backgrounds and the same gender and similar
experimental designs (6,29).
The hepatocellular adenomas found in the present
study had unusually low neoplastic cell proliferation and apoptosis
levels, which is not similar to what is typically observed in other
types of tumors seen in the liver or elsewhere. This unexpected
feature of DEN-induced hepatocellular adenomas, however, has been
reported by others before. Comparisons between different studies
using DEN and mice is difficult due to the usage of different
strains of mice, and the application of divergent experimental
designs and morphometrical approaches for accessing proliferation
and apoptosis. One study that is in many ways comparable to the
present one, however, reported that DEN-induced hepatocellular
proliferating lesions including adenomas have only 11 PCNA-positive
proliferating hepatocytes in every 100 abnormal hepatocytes counted
(29). The relatively low
proliferating index, which is a typical feature of human
hepatocellular adenoma as well (30),
probably reflects the low proliferation rate of hepatocytes that in
normal conditions rest in the G0 phase of the cell
cycle. It also explains the slow growth of hepatocellular tumors in
DEN-treated rodent models, that, as in the present study, are not
further manipulated to promote tumor initiation and growth
(1,2).
Similarly to proliferation, apoptosis in hepatocellular adenomas of
mice has also been reported to be as low as 0.02 to 0.44%
regardless of mouse strain and induction of carcinogenesis protocol
(29,31–33). The
results of caspase-3 specific IHC performed in the present study
further confirm that apoptosis in mouse hepatocellular adenomas is
rather rare.
The Wnt-1 glycoprotein is a secreted ligand of the
Wnt/β-catenin pathway (34). Its
binding to the transmembrane Frizzled (FZD) receptors of cells
activates the Wnt/β-catenin pathway leading to cytoplasmic
accumulation and nuclear translocation of the β-catenin protein
(11–13). Intranuclear β-catenin binds to T-cell
factor 4 (Tcf4) and thus activates an array of genes that regulate
fundamental cellular functions including proliferation, apoptosis,
differentiation and migration (3,9–13). The Wnt/β-catenin pathway plays a
significant role in embryonic liver development and post-natal
growth. In the adult normal liver, however, it remains inactive
with β-catenin restricting to the cell membrane of hepatocytes; its
activation, evidenced by cytoplasmic and nuclear stabilization of
β-catenin, occurs only in the case of liver regeneration and
disease, including hepatocellular cancer (11–13).
Along these lines, the depletion of Wnt-1 and the
immunohistochemical stain of selected Wnt/β-catenin pathway
proteins in the present study are informative at many different
levels. In the context of hepatocellular cancer, Wnt-1 blockade
testing is thus far restricted to cell culture studies (20,22,23) or
studies using HCC xenograft mouse models (20,22). To
the best of our knowledge, our study is the first examining the
effects of blocking Wnt-1 in the mouse liver using a spontaneous
HCC mouse model.
Using this model and neutralizing antibodies against
Wnt-1 for ten consecutive days we found diminished expression of
Wnt-1 in the liver of mice 20 days after the end of treatment. This
result confirms the efficacy of the depletion treatment applied. It
also demonstrates that in this experimental setting the rabbit
polyclonal IgG antibody used remained in recipient mouse
circulation in adequate numbers for Wnt-1 depletion for at least 20
days. This conclusion is further evidenced by the results of the
β-catenin-specific IHC stain we have applied. Indeed, anti-Wnt-1
treatment was potent enough to switch off β-catenin expression in
DEN-treated mouse livers, both in the hepatocellular adenomas and
in the remaining non-tumoral liver tissue.
In the untreated controls of our study the normal
liver showed a weak hepatocyte cell membrane β-catenin staining,
which was clearly fainter compared to what has been previously
described (11–13,35–37). Other
studies in adult mice have reported either a complete lack of
specific β-catenin IHC signal (38),
or a rather non-specific one along liver sinusoids (38,39). The
different primary antibodies and IHC protocols used in the various
studies could contribute to these discrepant results, but not
completely explain them. Especially, since in most of these studies
the IHC assays applied yielded convincing β-catenin-specific IHC
stain outcomes in mouse liver tumors (12,13,35–40).
Therefore, it is possible that the differences observed may reflect
inherent or temporal variances of the subclinical metabolic state
of the adult mice examined in each case. Indeed, liver metabolism
is influenced by genetic background, diet or even the gut
microbiome (8,12,13,35,37,41,42).
Interestingly, by comparison with the normal liver
of untreated controls, the non-tumoral liver tissue of DEN-treated
mice showed increased expression of β-catenin and FZL-1, but
unchanged expression of Wnt-1. The Wnt signaling inhibitor DKK1 had
absent expression in the normal liver of untreated controls and the
non-lesional liver of DEN-treated mice. In hepatocellular adenomas,
the expression of β-catenin and FLZ-1 were further increased, DKK1
expression emerged, but Wnt-1 remained unaffected. The increased
β-catenin in the hepatocellular adenomas observed in our study
restricted to the cell membranes of hepatocytes, which is
consistent with what others have observed in liver adenomas of mice
treated with DEN (39) or other
chemical carcinogens (43). By
contrast, hepatocellular adenomas of certain genetically engineered
mouse models of HCC contain cells with aberrant nuclear
localization of β-catenin (36).
The depletion of Wnt-1 in our study effectively
suppressed β-catenin and FLZ-1 but had no effect on DKK1 expression
and failed to reduce size and multiplicity of DEN-induced
hepatocellular adenomas. Although the role of Wnt-1, β-catenin and
FLZ-1 in the early preneoplastic stages of DEN-induced liver cancer
cannot be definitively excluded, our results do not confirm that
Wnt-1 blockade counteracts hepatocellular tumors, by contrast to
previous studies (20,22,23). It
should be noted, however, that those studies base their conclusions
on experiments using HCC cell cultures and tumor transplant mouse
models grafted with HCC rather than hepatocellular adenoma cells
(20,22,23).
β-catenin which is an important downstream molecule of Wnt-1-FLZ1
binding is mutated in mice treated with DEN and phenobarbital but
not in those treated with DEN alone (4,44).
According to another study, β-catenin mutations are absent in
DEN-treated mouse hepatocellular adenomas, but appear at the later
stage of HCC (39).
Cyclin D1 is a major cell cycle regulator and a
target molecule of the Wnt/β-catenin pathway and β-catenin nuclear
translocation (14). Cyclin D1 is a
proto-oncogene with important roles in the growth and evolution of
many types of cancer including HCC (11,13,45). In
the present study, hepatocellular adenomas showed no evidence of
β-catenin nuclear translocation. Nonetheless, they had neoplastic
cells with nuclear Cyclin D1 signal, which suggests that
DEN-induced hepatocellular tumors of mice at the stage of adenoma
do not require nuclear β-catenin for the initial stages of Cyclin
D1 upregulation. On the other hand, however, the disruption of the
Wnt/β-catenin pathway in the present study worked to suppress
Cyclin D1 in the mouse tumors, which is similar to what has been
frequently reported in studies using mouse models of HCC (4,13,20,22,36,46).
Another proto-oncogene key cell cycle regulator
examined in our study is FOXM1. This molecule that has also been
described to participate in apoptosis and DNA repair processes is
overexpressed in various types of cancers, including HCC (27). In line with these findings, we found
increased FOXM1 expression in DEN-induced hepatocellular adenomas
of mice. We also observed that Wnt-1 blockade suppressed FOXM1
expression in neoplastic hepatocytes. In other types of cancer,
such as glioma and lung, cervical and pancreatic cancers FOXM1 is
involved in the activation of Wnt pathway and the nuclear
translocation of β-catenin (19,47–49). In
our study, overexpression of FOXM1, however, did not coincide with
nuclear stabilization of β-catenin in neoplastic cells.
Furthermore, the Wnt pathway blockade-associated downregulation of
FOXM1 did not affect hepatocellular adenomas. Our result agrees
with findings in the DEN-phenobarbital mouse model of HCC
suggesting that this molecule has unremarkable effects on tumor
evolution (33). On the other hand
they contrast findings of others which use the same mouse model to
demonstrate that FOXM1 is essential for the development of HCC
(18).
NF-κB and c-Jun are important pleiotropic molecules
that regulate fundamental cell processes including proliferation
and apoptosis and their role in DEN-induced liver cancer has been
reported (17,50). Specifically, it has been shown that
the deletion of either IκB kinase β (IKKβ), which is required for
NF-κB activation (17), or c-Jun
(50) markedly affected the early
stages of tumor development and reduced chemical carcinogenesis. In
our study, however, the considerable reduction of both NF-κB and
c-Jun in hepatocellular adenomas after Wnt-1 depletion, had no
effect in hepatocellular adenomas. It is possible that a longer
period of Wnt-1 blockade may have been required for counteracting
hepatic tumors in this case.
The hepatocyte growth factor receptor (HGFR) c-Met
has been shown to promote all major events of human HCC evolution
including tumor cell growth and survival (7). Its tumor promoting role has also been
demonstrated in the DEN mouse model of liver cancer (51). In our study, we find that c-met is
increased in the blood serum of mice bearing hepatocellular
adenomas and that its increased serum levels are not affected by
anti-Wnt-1 treatment.
In previous studies using B6C3F1 mice and DEN, Lee
et al (29) have shown that
the anti-apoptotic molecule Bcl-2 is increased in hepatocellular
adenomas (29,52). In the serum of DEN-treated mice,
however, we found that Bcl-2 was significantly decreased in
comparison with cancer-free controls of the same age. Also, that
anti-Wnt-1 treatment worked to increase the circulating levels of
Bcl-2, which suggests increased anti-apoptotic signaling after
disruption of the Wnt pathway. This suggests a pro-apoptotic
function of Wnt-1, which differs from earlier findings of an in
vitro study that used cell cultures to show that Wnt-1
signaling inhibits apoptosis (53).
Overall, the results of our study show that
ani-Wnt-1 blockade affects an array of important regulators of
proliferation and apoptosis. Indeed, immunohistochemical assessment
of Ki-67 and caspase-3 showed that anti-Wnt-1 treatment suppressed
both proliferation and apoptosis. This anti-proliferative and
anti-apoptotic milieu, however, failed to reduce hepatocellular
adenomas. This result suggests, that, at least for counteracting
DEN-induced liver tumors of mice, reducing proliferation of
neoplastic hepatocytes does not suffice. It appears that in this
mouse model of cancer, hepatocellular apoptosis is equally
important to proliferation in the critical stage of adenoma to
carcinoma transition. Furthermore, the present study adds to
existing preclinical data aiming to explore the roles of
Wnt/β-catenin pathway in hepatocellular cancer. Further testing in
mice involving longer periods of anti-Wnt-1 treatment of liver
tumors compared to the 10-days-long period applied in the present
study may be useful along these lines. A considerable amount of
such data is needed before considering therapeutic interventions
based on the most fundamental and pleiotropic biological pathways,
such as the Wnt/β-catenin one.
Acknowledgements
This study was founded as Research Scholarship by
the Experimental-Research Center ELPEN. Also, we would like to
express our sincere thanks to Mr. E. Gerakis and Mr. S. Gerakis for
their assistance in the implementation of the experiments.
References
|
1
|
Santos NP, Colaço AA and Oliveira PA:
Animal models as a tool in hepatocellular carcinoma research: A
review. Tumor Biol. 39:10104283176959232017. View Article : Google Scholar
|
|
2
|
Heindryckx F, Colle I and Van Vlierberghe
H: Experimental mouse models for hepatocellular carcinoma research.
Int J Exp Pathol. 90:367–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giakoustidis A, Giakoustidis D, Mudan S,
Sklavos A and Williams R: Molecular signalling in hepatocellular
carcinoma: Role of and crosstalk among WNT/β-catenin, sonic
hedgehog, notch and dickkopf-1. Can J Gastroenterol Hepatol.
29:209–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim Y, Sills RC and Houle CD: Overview of
the molecular biology of hepatocellular neoplasms and
hepatoblastomas of the mouse liver. Toxicol Pathol. 33:175–180.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JS, Chu IS, Mikaelyan A, Calvisi DF,
Heo J, Reddy JK and Thorgeirsson SS: Application of comparative
functional genomics to identify best-fit mouse models to study
human cancer. Nat Genet. 36:1306–1311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldfarb S, Pugh TD, Koen H and He YZ:
Preneoplastic and neoplastic progression during
hepatocarcinogenesis in mice injected with diethylnitrosamine in
infancy. Environ Health Perspect. 50:149–161. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bupathi M, Kaseb A, Meric-Bernstam F and
Naing A: Hepatocellular carcinoma: Where there is unmet need. Mol
Oncol. 9:1501–1509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Monga SP: Role of Wnt/β-catenin signaling
in liver metabolism and cancer. Int J Biochem Cell Biol.
43:1021–1019. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pez F, Lopez A, Kim M, Wands JR, Caron De
Fromentel C and Merle P: Wnt signaling and hepatocarcinogenesis:
Molecular targets for the development of innovative anticancer
drugs. J Hepatol. 59:1107–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dahmani R, Just PA and Perret C: The
Wnt/β-catenin pathway as a therapeutic target in human
hepatocellular carcinoma. Clin Res Hepatol Gastroenterol.
35:709–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thompson MD and Monga SP: WNT/β-catenin
signaling in liver health and disease. Hepatology. 45:1298–1305.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nejak-Bowen KN and Monga SP: Beta-catenin
signaling, liver regeneration and hepatocellular cancer: Sorting
the good from the bad. Semin Cancer Biol. 21:44–58. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Monga SP: β-catenin signaling and roles in
liver homeostasis, injury and tumorigenesis. Gastroenterology.
148:1294–1310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shtutman M, Zhurinsky J, Simcha I,
Albanese C, D'Amico M, Pestell R and Ben-Ze'ev A: The cyclin D1
gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad
Sci USA. 96:pp. 5522–5527. 1999; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu
Y, Wang N, Niu Y, Wu Z, Zhou J, et al: Serum DKK1 as a protein
biomarker for the diagnosis of hepatocellular carcinoma: A
large-scale, multicentre study. Lancet Oncol. 13:817–826. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu B, Yang X, Xu Y, Yao G, Shu H, Lin B,
Hood L, Wang H, Yang S, Gu J, et al: Elevated expression of DKK1 is
associated with cytoplasmic/nuclear beta-catenin accumulation and
poor prognosis in hepatocellular carcinomas. J Hepatol. 50:948–957.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maeda S, Kamata H, Luo JL, Leffert H and
Karin M: IKK beta couples hepatocyte death to cytokine-driven
compensatory proliferation that promotes chemical
hepatocarcinogenesis. Cell. 121:977–990. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalinichenko VV, Major ML, Wang X,
Petrovic V, Kuechle J, Yoder HM, Dennewitz MB, Shin B, Datta A,
Raychaudhuri P and Costa RH: Foxm1b transcription factor is
essential for development of hepatocellular carcinomas and is
negatively regulated by the p19ARF tumor suppressor. Genes Dev.
18:830–850. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Li Y, Xue J, Gong A, Yu G, Zhou A,
Lin K, Zhang S, Zhang N, Gottardi CJ and Huang S: Wnt-induced
deubiquitination FoxM1 ensures nucleus β-catenin transactivation.
EMBO J. 35:668–684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang JG, Shi Y, Hong DF, Song M, Huang D,
Wang C and Zhao G: miR-148b suppresses cell proliferation and
invasion in hepatocellular carcinoma by targeting WNT1/β-catenin
pathway. Sci Rep. 5:80872015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Xu L, Liu P, Jairam K, Yin Y, Chen
K, Sprengers D, Peppelenbosch MP, Pan Q and Smits R: Blocking wnt
secretion reduces growth of hepatocellular carcinoma cell lines
mostly independent of β-catenin signaling. Neoplasia. 18:711–723.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei W, Chua MS, Grepper S and So SK:
Blockade of Wnt-1 signaling leads to anti-tumor effects in
hepatocellular carcinoma cells. Mol Cancer. 8:762009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahsani Z, Mohammadi-Yeganeh S, Kia V,
Karimkhanloo H, Zarghami N and Paryan M: WNT1 gene from wnt
signaling pathway is a direct target of mir-122 in hepatocellular
carcinoma. Appl Biochem Biotechnol. 181:884–897. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. 8th. National Academies Press
(US); Washington, DC: pp. 1182011
|
|
25
|
Ouzounidis N, Giakoustidis A, Poutahidis
T, Angelopoulou K, Iliadis S, Chatzigiagkos A, Zacharioudaki A,
Angelopoulos S, Papalois A, Papanikolaou V and Giakoustidis D:
IL-18 Binding protein ameliorates ischemia/reperfusion-induced
hepatic injury in mice. Liver Transpl. 22:237–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thoolen B, Maronpot RR, Harada T, Nyska A,
Rousseaux C, Nolte T, Malarkey DE, Kaufmann W, Küttler K, Deschl U,
et al: Proliferative and nonproliferative lesions of the rat and
mouse hepatobiliary system. Toxicol Pathol. 38 7 Suppl:5S–81S.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koo CY, Muir KW and Lam EWF: FOXM1: From
cancer initiation to progression and treatment. Biochim Biophys
Acta. 1819:28–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mott JL and Gores GJ: Piercing the armor
of hepatobiliary cancer: Bcl-2 homology domain 3 (BH3) mimetics and
cell death. Hepatology. 1–911. 2007.PubMed/NCBI
|
|
29
|
Lee GH, Ooasa T and Osanai M: Mechanism of
the paradoxical, inhibitory effect of phenobarbital on
hepatocarcinogenesis initiated in infant B6C3F1 mice with
diethylnitrosamine. Cancer Res. 58:1665–1669. 1998.PubMed/NCBI
|
|
30
|
Kanel GC: Hepatic Tumors BenignPathology
of Liver Diseases. John Wiley & Sons, Ltd.; Hoboken, NJ: pp.
266–88. 2017, View Article : Google Scholar
|
|
31
|
Bursch W, Chabicovsky M, Wastl U,
Grasl-Kraupp B, Bukowska K, Taper H and Schulte-Hermann R:
Apoptosis in stages of mouse hepatocarcinogenesis: Failure to
counterbalance cell proliferation and to account for strain
differences in tumor susceptibility. Toxicol Sci. 85:515–529. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chabicovsky M, Wastl U, Taper H,
Grasl-Kraupp B, Schulte-Hermann R and Bursch W: Induction of
apoptosis in mouse liver adenoma and carcinoma in vivo by
transforming growth factor-beta1. J Cancer Res Clin Oncol.
129:536–542. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kalinina OA, Kalinin SA, Polack EW,
Mikaelian I, Panda S, Costa RH and Adami GR: Sustained hepatic
expression of FoxM1B in transgenic mice has minimal effects on
hepatocellular carcinoma development but increases cell
proliferation rates in preneoplastic and early neoplastic lesions.
Oncogene. 22:6266–6276. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nusse R, Brown A, Papkoff J, Scambler P,
Shackleford G, McMahon A, Moon R and Varmus H: A new omenclature
for int-1 and related genes: The wnt gene family. Cell. 64:2311991.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang J, Mowry LE, Nejak-Bowen KN, Okabe H,
Diegel CR, Lang RA, Williams BO and Monga SP: β-catenin signaling
in murine liver zonation and regeneration: A Wnt-Wnt situation.
Hepatology. 60:964–976. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Calvisi DF, Factor VM, Loi R and
Thorgeirsson SS: Activation of beta-catenin during
hepatocarcinogenesis in transgenic mouse models: Relationship to
phenotype and tumor grade. Cancer Res. 61:2085–2091.
2001.PubMed/NCBI
|
|
37
|
Benhamouche S, Decaens T, Godard C,
Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, Kuo CJ, Kahn
A, Perret C and Colnot S: Apc tumor suppressor gene Is the
‘Zonation-Keeper’ of mouse liver. Dev Cell. 10:759–770. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Salleng KJ, Revetta FL, Deane NG and
Washington MK: The applicability of a human immunohistochemical
panel to mouse models of hepatocellular neoplasia. Comp Med.
65:398–408. 2015.PubMed/NCBI
|
|
39
|
Ogawa K, Yamada Y, Kishibe K, Ishizaki K
and Tokusashi Y: Beta-catenin mutations are frequent in
hepatocellular carcinomas but absent in adenomas induced by
diethylnitrosamine in B6C3F1 mice. Cancer Res. 59:1830–1833.
1999.PubMed/NCBI
|
|
40
|
Colnot S, Decaens T, Niwa-Kawakita M,
Godard C, Hamard G, Kahn A, Giovannini M and Perret C:
Liver-targeted disruption of Apc in mice activates beta-catenin
signaling and leads to hepatocellular carcinomas. Proc Natl Acad
Sci USA. 101:pp. 17216–17221. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sekine S, Lan BY, Bedolli M, Feng S and
Hebrok M: Liver-specific loss of beta-catenin blocks glutamine
synthesis pathway activity and cytochrome p450 expression in mice.
Hepatology. 43:817–825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tilg H, Cani PD and Mayer EA: Gut
microbiome and liver diseases. Gut. 65:2035–2044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Devereux TR, Anna CH, Foley JF, White CM,
Sills RC and Barrett JC: Mutation of beta-catenin is an early event
in chemically induced mouse hepatocellular carcinogenesis.
Oncogene. 18:4726–4733. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aydinlik H, Nguyen TD, Moennikes O,
Buchmann A and Schwarz M: Selective pressure during tumor promotion
by phenobarbital leads to clonal outgrowth of beta-catenin-mutated
mouse liver tumors. Oncogene. 20:7812–7816. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Diehl JA: Cycling to cancer with cyclin
D1. Cancer Biol Ther. 1:226–231. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu J, Dong A, Fernandez-Ruiz V, Shan J,
Kawa M, Martínez-Ansó E, Prieto J and Qian C: Blockade of Wnt
signaling inhibits angiogenesis and tumor growth in hepatocellular
carcinoma. Cancer Res. 69:6951–6959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Quan M, Cui J, Xia T, Jia Z, Xie D, Wei D,
Huang S, Huang Q, Zheng S and Xie K: Merlin/NF2 suppresses
pancreatic tumor growth and metastasis by attenuating the
foxm1-mediated wnt/β-catenin signaling. Cancer Res. 75:4778–4789.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Su J, Wu S, Wu H, Li L and Guo T: CD44 is
functionally crucial for driving lung cancer stem cells metastasis
through Wnt/β-catenin-FoxM1-twist signaling. Mol Carcinog.
55:1962–1973. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang T, Liu Z, Shi F and Wang J: Pin1
modulates chemo-resistance by up-regulating FoxM1 and the
involvements of Wnt/β-catenin signaling pathway in cervical cancer.
Mol Cell Biochem. 413:179–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Eferl R, Ricci R, Kenner L, Zenz R, David
JP, Rath M and Wagner EF: Liver tumor development: c-Jun
antagonizes the proapoptotic activity of p53. Cell. 112:181–192.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Horiguchi N, Takayama H, Toyoda M, Otsuka
T, Fukusato T, Merlino G, Takagi H and Mori M: Hepatocyte growth
factor promotes hepatocarcinogenesis through c-Met autocrine
activation and enhanced angiogenesis in transgenic mice treated
with diethylnitrosamine. Oncogene. 21:1791–1799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee GH: Correlation between Bcl-2
expression and histopathology in diethylnitrosamine-induced mouse
hepatocellular tumors. Am J Pathol. 151:957–961. 1997.PubMed/NCBI
|
|
53
|
Chen S, Guttridge DC, You Z, Zhang Z,
Fribley A, Mayo MW, Kitajewski J and Wang CY: Wnt-1 signaling
inhibits apoptosis by activating β-catenin/T cell factor-mediated
transcription. J Cell Biol. 152:87–96. 2001. View Article : Google Scholar : PubMed/NCBI
|