Introduction
Gastric cancer is one of the most common malignant
tumors and occurs frequently in China with approximately 400,000
new cases each year, accounting for 42% of new cases of gastric
cancer worldwide (1). In addition,
its incidence shows an increasing trend year by year. Gastric
cancer has a high metastatic and recurrence rate, poor prognosis
and low 5-year survival rate (2).
Thus, investigations on reducing the postoperative metastatic and
recurrence rate has important clinical significance.
FOLFOX4 is the first-line regimen of postoperative
chemotherapy for gastric cancer, but it has great toxic side
effects, such as hematologic toxicity and peripheral neurotoxicity
(3). Consequently, how to modify
FOLFOX4 regimen, improvement of the chemotherapeutic efficacy,
reduction of toxic and side effects and the metastatic and
recurrence rate of gastric cancer, as well as the prevention of
drug resistance reversal are clinical issues that remain to be
resolved. Brucea javanica oil is the fatty oil extracted
from the dried and mature fruit of Brucea javanica, a kind
of quassia plant. Clinical studies have confirmed that it has a
good therapeutic effect on a variety of tumors, such as lung,
liver, breast cancer and colorectal cancer (4), and it can improve chemotherapy efficacy
and reduce the adverse reaction of chemotherapeutic drugs when
combined with them (5). Brucea
javanica exerts antitumor pharmacological effects through the
inhibition of tumor cell DNA production, tumor cell proliferation
and activity and drug resistance reversal of DNA topoisomerase II
(6).
In this study, the effects of FOLFOX4 regimen
combined with Brucea javanica emulsion and simple FOLFOX4
regimen on serum VEGF content and postoperative recurrence rate of
patients with gastric cancer were compared, to provide a clinical
basis for improving the FOLFOX4 regimen for postoperative
chemotherapy of gastric cancer.
Materials and methods
General materials
Sixty gastric ulcer patients treated from January
2013 to January 2014 in the Sun Yat-sen Memorial Hospital were
selected as the normal group. Of the 60 patients, 41 were males and
19 were females, with an average age of 49 years. A total of 150
patients with gastric cancer confirmed via pathological examination
were randomly divided into the control and experimental groups. Of
the 150 patients there were 75 patients in the control group,
including 42 males and 33 females with an average age of 52 years,
and there were 75 patients in the experimental group, including 40
males and 35 females with an average age of 55 years. The results
of blood routine, liver and kidney function and electrocardiogram
for all the patients before treatment were normal, and there was no
obvious metastasis in liver, spleen and pancreas in upper abdominal
ultrasonography and CT examination. According to the statistical
analysis, there were no significant differences in the general
materials of all the subjects, and they were comparable.
Patients in the control and experimental groups
provided written informed consent regarding surgery and
chemotherapy. Approval for the study was obtained from the Ethics
Committee of Sun Yat-sen Memorial Hospital.
Treatment methods
Patients in the normal group were treated with
regular treatment of ulcer, while patients in the control group
were treated with FOLFOX4 chemotherapy. On day 1 the patients were
treated with intravenous drip of 85 mg/m2 oxaliplatin +
500 ml 5% glucose solution once time based on the surface area
formula, and then intravenous drip of 200 mg/m2
leucovorin + 100 ml sodium chloride solution was applied, followed
by small amount of 5-fluorouracil via intravenous drip, and
intravenous drip of 600 mg/m2 5-fluorouracil + 250 ml 5%
glucose solution. The method used on day 2 was the same as that in
day 1 after the intravenous drip in day 1 was finished, and the
same dosage of leucovorin and 5-fluorouracil was given to the
patients. Subsequently, the administration methods in day 1 and 2
were repeated with an interval of 14 days: A total of 16 days as 1
cycle and 6 cycles as 1 course of treatment. The chemotherapy
treatment of the experimental group was the same as that of the
control group, but 30 ml Brucea javanica injection + 250 ml
sodium chloride solution were given via intravenous drip between
the administrations of leucovorin and 5-fluorouracil. The treatment
cycle was the same as that of the control group.
Main experimental equipment and
reagents
Equipment used included the Eppendorf pipetter
(Eppendorf, Hamburg, Germany), BIO-RAD550 microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and human VEGF
ELISA kit (HCB, Ontario, Canada).
Specimen collection
Fasting peripheral venous blood (5 ml) was collected
from all the subjects before treatment, after chemotherapy for 3
times and at 1 and 3 months after chemotherapy, followed by
centrifugation at 3,000 × g for 10 min to separate the serums. Then
the supernatant was placed in the refrigerator at −20°C for later
inspection.
Statistical analysis
SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) software
was used for the statistical analysis. The measurement data were
presented as mean ± standard deviation, and the t-test was used for
the pairwise comparison of sample means and Chi-square test was
used for the enumeration data. P<0.05 was considered to indicate
a statistically significant difference.
Results
Comparisons of VEGF content at
different time points
The serum VEGF contents of different patients for
the groups at 4 different time points are shown in Table I. The results showed that the VEGF
content of patients in the control or experimental group was
significantly higher than that in the normal group, and the
differences were statistically significant (P<0.05). There were
no significant differences between the experimental and control
groups before treatment and after chemotherapy for 3 times. At 1
month after chemotherapy, the VEGF content of patients in
experimental group was significantly lower than that in control
group, and the difference was statistically significant
(P<0.05). In addition, at 3 months after chemotherapy, the VEGF
content of patients in the experimental group was significantly
lower than that in the control group, and the difference was
statistically significant (P<0.05).
 | Table I.Comparisons of VEGF content in
different patients between groups (pg/ml). |
Table I.
Comparisons of VEGF content in
different patients between groups (pg/ml).
| Variables | Before operation | After chemotherapy
for 3 times | At 1 month after
chemotherapy | At 3 months after
chemotherapy |
|---|
| Normal group | 91.72±14.36 | 89.32±10.69 | 85.24±24.73 | 82.91±31.28 |
| Control group | 392.4±48.19 | 359.7±39.42 | 289.3±41.65 | 301.9±26.32 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 |
| Normal group | 91.72±14.36 | 89.32±10.69 | 85.24±24.73 | 82.91±31.28 |
| Experimental
group | 405.8±37.62 | 353.5±47.39 | 215.2±24.68 | 159.6±23.71 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 |
| Control group | 392.4±48.19 | 359.7±39.42 | 289.3±41.65 | 301.9±26.32 |
| Experimental
group | 405.8±37.62 | 353.5±47.39 | 215.2±24.68 | 159.6±23.71 |
| P-value | >0.05 | >0.05 | <0.05 | <0.05 |
Serum VEGF contents for the three
groups at different time points
The serum VEGF contents of different patients among
groups at 4 different time points are shown in Table II. The results showed that there were
no significant differences among the three groups (P>0.05) in
the VEGF contents before operation and after chemotherapy for 3
times. An increase in chemotherapy, resulted in VEGF contents in
the control and experimental groups being decreased and the
differences were statistically significant (P<0.05) after
chemotherapy for 3 times and at 1 month after chemotherapy. The
VEGF contents of patients in the control group had no significant
decrease at 1 and 3 months after chemotherapy, but those in the
normal and experimental groups were obviously decreased, and the
differences were statistically significant (P<0.05).
 | Table II.Comparisons of VEGF content in
different patients within the group (pg/ml). |
Table II.
Comparisons of VEGF content in
different patients within the group (pg/ml).
|
| Groups |
|---|
|
|
|
|---|
| Variables | Normal | Control | Experimental |
|---|
| Before operation | 91.72±14.36 | 392.4±48.19 | 405.8±37.62 |
| After chemotherapy
for 3 times | 89.32±10.69 | 359.7±39.42 | 353.5±47.39 |
| P-value | >0.05 | >0.05 | >0.05 |
| After chemotherapy
for 3 times | 89.32±10.69 | 359.7±39.42 | 353.5±47.39 |
| At 1 month after
chemotherapy | 85.24±24.73 | 289.3±41.65 | 215.2±24.68 |
| P-value | >0.05 | <0.05 | <0.05 |
| At 1 month after
chemotherapy | 85.24±24.73 | 289.3±41.65 | 215.2±24.68 |
| At 3 months after
chemotherapy | 82.91±31.28 | 301.9±26.32 | 159.6±23.71 |
| P-value | >0.05 | >0.05 | <0.05 |
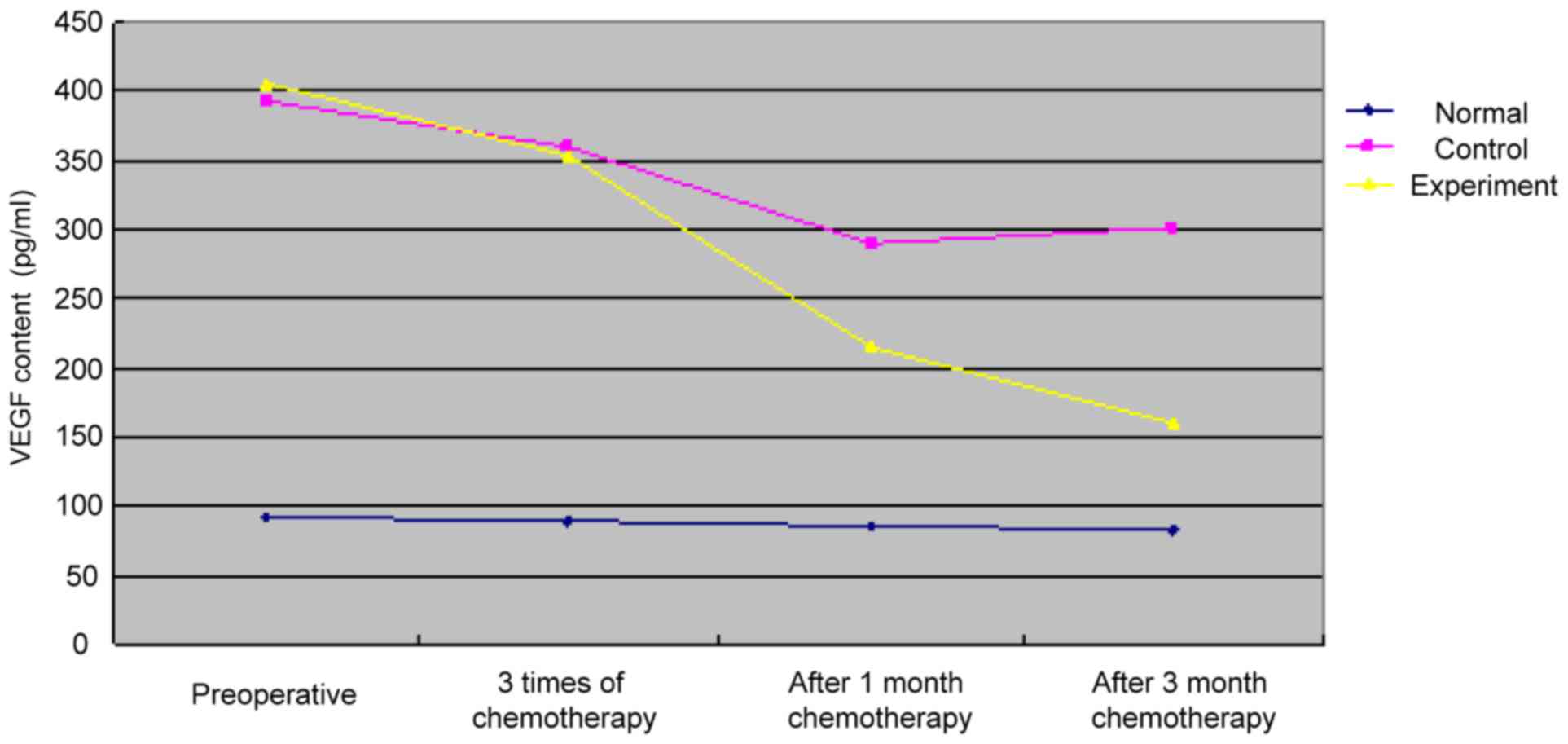
Tendency of VEGF contents in the three
groups at different time points
The VEGF contents of patients in the three groups at
different time points are shown in Fig.
1. The results showed that the VEGF content of patients in the
control group was gradually decreased with the advance of
chemotherapy, but slightly increased at 3 months after
chemotherapy. In addition, the VEGF content of patients in the
experimental group was also decreased gradually with the increase
of chemotherapy, and the decreasing rate was higher than that in
the control group at 1 month after chemotherapy.
Discussion
Gastric cancer is the most common malignant tumor
globally, and its morbidity and mortality rates rank second
worlwide (7). The incidence of
gastric cancer in different countries, ethnicities, genders and
ages is not the same. The number of male patients with gastric
cancer is more than that with female patients (about 2:1). Most
patients with gastric cancer are middle-aged and elderly
individuals aged 50–70 years, showing a young tendency with the
changes in lifestyle and diet (8).
Owning to its high incidence, metastatic and recurrence rates,
gastric cancer has become a serious health problem in China. The
occurrence and development of gastric cancer is a multifactor and
multi-step process. The proliferation and apoptosis of gastric
mucosal epithelial cells under the physiological condition is a
dynamic equilibrium process, which is controlled by a variety of
oncogenes, tumor suppressor genes and growth factors, such as
epidermal growth factor and VEGF. VEGF is closely related to tumor
growth, which can promote the growth of blood vessels,
proliferation of vascular endothelial cells, and migration of
vascular endothelial cells and change the permeability of blood
vessels (9). If gastric epithelial
cells proliferate excessively without apoptosis in pathological
conditions, gastric disease may develop into gastric cancer
(10). The present study, by
comparing the VEGF levels of patients in the normal, control and
experimental groups before treatment, found that the VEGF level in
patients with gastric cancer was significantly higher than that in
patients with gastric disease, and the difference was statistically
significant (P<0.05), indicating that the development of gastric
cancer is closely related to VEGF, and VEGF may be a target of
gastric cancer treatment.
At present, the only possible radical method of
gastric cancer is surgical resection and lymph node dissection
around the lesions (11). The
staging, infiltration degree and diffusion range of gastric cancer
determine the effect of gastric cancer surgery. The survival rate
can be improved after the surgical resection of partial stomach if
there is no metastasis of gastric cancer found in examination. The
purpose of chemotherapy is to reduce and remove the cancer foci and
prevent its metastasis and recurrence (12). There are lots of common chemotherapy
regimens for gastric cancer, one of which is FOLFOX4, including
three kinds of drugs: oxaliplatin, leucovorin and 5-fluorouracil.
Oxaliplatin is the third generation of platinum compound that plays
the anticancer effect via blocking the DNA replication and
transcription of cancer cells, and the curative effect is better
than cisplatin without cross-resistance (13). 5-Fluorouracil is the thymidylate
synthase inhibitor, and belongs to the cell cycle-specific drug,
which prevents cell division and proliferation via intervention in
nucleic acid metabolism of cancer cells. Calcium folinate can be
used as its sensitizer when combined with 5-fluorouracil. As with
chemotherapeutic drugs, the FOLFOX4 regimen, a kind of pure
chemical drug, has a certain cytotoxicity, hematologic toxicity and
peripheral neurotoxicity, and may also lead to drug resistance
reversal (14). However, the
participation of Chinese traditional medicine can play a
coordinating role to improve the efficacy of chemotherapy drugs,
reduce the toxic and side effects and prevent the multi-drug
resistance reversal and metastasis or recurrence of tumors.
Brucea javanica oil is the fat oil extracted from the peel
and seed of Brucea javanica via petroleum ether reflux,
containing the oil and various fatty acids, among which the oleic
acid and linoleic acid have strong anticancer activity because of
the specific affinity with the cancer cell membrane (15). It can achieve the anticancer effect in
a variety of ways, such as affecting the tumor cell cycle,
inhibiting DNA synthesis, inducing tumor cell apoptosis, inhibiting
cancer cell growth, improving immune function and reversing tumor
multi-drug resistance (16).
Brucea javanica emulsion can be used for the
treatment of a variety of cancers, such as colorectal, breast and
lung cancer. However, there are few reports about the treatment of
gastric cancer with Brucea javanica emulsion at present, and
the way to achieve the treatment purpose is not clear, either. In
the present study, the VEGF levels in the experimental and control
groups after chemotherapy for 3 times were compared and it was
found that there was no significant difference in VEGF content
between the two groups. Additionally, the advance of chemotherapy,
the comparisons of VEGF levels in patients between the experimental
and control groups at 1 month and 3 months after chemotherapy
showed that the levels in the experimental group were significantly
lower than those in the control group. It suggested that Brucea
javanica emulsion, as a kind of Chinese patent medicine, is
slow to take effect without obvious advantages compared with the
chemotherapeutic drug alone in the middle of chemotherapy. However,
the chemotherapeutic effect on gastric cancer was increased with
the passage of time after the Brucea javanica emulsion was
combined, indicating that Brucea javanica may improve the
curative effect of chemotherapy drugs by inhibiting the
angiogenesis of gastric cancer and proliferation of gastric cancer
cells, and promoting its apoptosis.
In summary, the FOLFOX4 regimen combined with
Brucea javanica emulsion in the treatment of patients with
gastric cancer after operation can significantly reduce the VEGF
content compared with the single administration of FOLFOX4, and the
difference is statistically significant (P<0.05), which
indicates that Brucea javanica emulsion may inhibit the
development of gastric cancer by inhibiting the angiogenesis of
gastric cancer, and the drug combination is helpful to improve the
curative effect of chemotherapy. Next, methyl thiazolyl tetrazolium
assay and flow cytometry can be used to detect the apoptosis, to
further study whether the antitumor effect of Brucea
javanica emulsion is realized by inhibiting the apoptosis of
gastric cancer cells. In conclusion, it was found in this study
that the effect of chemotherapy on patients with gastric cancer can
be improved by FOLFOX4 regimen combined with Brucea javanica
emulsion, and it is worthy of clinical recommendation.
References
|
1
|
Patru CL, Surlin V, Georgescu I and Patru
E: Current issues in gastric cancer epidemiology. Rev Med Chir Soc
Med Nat Iasi. 117:199–204. 2013.PubMed/NCBI
|
|
2
|
Schmidt T, Alldinger I, Blank S, Klose J,
Springfeld C, Dreikhausen L, Weichert W, Grenacher L, Bruckner T,
Lordick F, et al: Surgery in oesophago-gastric cancer with
metastatic disease: Treatment, prognosis and preoperative patient
selection. Eur J Surg Oncol. 41:1340–1347. 2005. View Article : Google Scholar
|
|
3
|
Jeon EK, Hong SH, Kim TH, Jung SE, Park
JC, Won HS, Ko YH, Rho SY and Hong YS: Modified FOLFIRI as
second-line chemotherapy after failure of modified FOLFOX-4 in
advanced gastric cancer. Cancer Res Treat. 43:148–153. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan Z, Zhang B, Huang Y, Qiu H, Chen P and
Guo GF: Involvement of autophagy inhibition in Brucea javanica oil
emulsion-induced colon cancer cell death. Oncol Lett. 9:1425–1431.
2015.PubMed/NCBI
|
|
5
|
Hu Y, Wan XJ, Pan LL, Zhang SH and Zheng
FY: Effects of Brucea javanica oil emulsion on human papilloma
virus type 16 infected cells and mechanisms research. Zhongguo
Zhong Xi Yi Jie He Za Zhi. 33:1545–1551. 2013.(In Chinese).
PubMed/NCBI
|
|
6
|
Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung
HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, et al: Everolimus
for previously treated advanced gastric cancer: Results of the
randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol.
31:3935–3943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamamoto D, Hamada Y, Okazaki S, Kawakami
K, Kanzaki S, Yamamoto C and Yamamoto M: Metastatic gastric tumor
from renal cell carcinoma. Gastric Cancer. 12:170–173. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chung HW, Lee SY, Han HS, Park HS, Yang
JH, Lee HH and So Y: Gastric cancers with microsatellite
instability exhibit high fluorodeoxyglucose uptake on positron
emission tomography. Gastric Cancer. 16:185–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wen J, Linghu EQ, Yang YS, Liu QS, Yang J
and Lu ZS: Associated risk factor analysis for positive resection
margins after endoscopic submucosal dissection in early-stage
gastric cancer. J BUON. 20:421–427. 2015.PubMed/NCBI
|
|
10
|
He YX, Song XH, Zhao ZY and Zhao H: HOXA13
upregulation in gastric cancer is associated with enhanced cancer
cell invasion and epithelial-to-mesenchymal transition. Eur Rev Med
Pharmacol Sci. 21:258–265. 2017.PubMed/NCBI
|
|
11
|
Ye M, Jin K, Xu G, Lin F, Zhou Q, Tao K
and Tao F: Short-and long-term outcomes after conversion of
laparoscopic total gastrectomy for gastric cancer: A single-center
study. J BUON. 22:126–133. 2017.PubMed/NCBI
|
|
12
|
Chung HW, Lee SY, Han HS, Park HS, Yang
JH, Lee HH and So Y: Gastric cancers with microsatellite
instability exhibit high fluorodeoxyglucose uptake on positron
emission tomography. Gastric Cancer. 16:185–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wen J, Linghu EQ, Yang YS, Liu QS, Yang J
and Lu ZS: Associated risk factor analysis for positive resection
margins after endoscopic submucosal dissection in early-stage
gastric cancer. J BUON. 20:421–427. 2015.PubMed/NCBI
|
|
14
|
He YX, Song XH, Zhao ZY and Zhao H: HOXA13
upregulation in gastric cancer is associated with enhanced cancer
cell invasion and epithelial-to-mesenchymal transition. Eur Rev Med
Pharmacol Sci. 21:258–265. 2017.PubMed/NCBI
|
|
15
|
Wu YC, Zhang YC, Dai GX, Wang LJ and
Jin-Kea YE: Clinical observation of Bruceolic oil emulsion combined
with FOLFOX4 in treating advanced carcinoma of stomach. Med Pharm J
Chin Peoples Liberation Army. 24:29–31. 2012.(In Chinese).
|
|
16
|
Xu W, Jiang X, Xu Z, Ye T and Shi Q: The
efficacy of Brucea javanica oil emulsion injection as adjunctive
therapy for advanced non-small-cell lung cancer: A meta-analysis.
Evid Based Complement Alternat Med. 2016:59285622016. View Article : Google Scholar : PubMed/NCBI
|