Introduction
In 1922, Galloway introduced the concept of
paraneoplastic glomerulopathy (1). In
1966, the association between malignant tumors and nephrotic
syndrome was initially identified (2). There is a strong association between
solid tumors and membranous nephropathy, Hodgkin's lymphoma and
minimal kidney disease (3). Lung,
gastric, breast and colorectal tumors are the most common types of
malignant tumor in association with renal glomerular diseases
(4). Immunoglobulin A (IgA)
nephropathy is the most common type of primary glomerulonephritis,
and represents a major health challenge worldwide (5,6). In the
present report, to the best of our knowledge, the second case of
breast cancer associated with IgA nephropathy within the last 30
years was described. A case of triple negative breast cancer
combined with proteinuria, pathologically validated focal
proliferative IgA nephropathy and Lee grading III was presented.
The present case was analyzed using relevant studies. The present
case study was approved by the Ethics Committee of The Fourth
Hospital of Hebei Medical University (Shijiazhuang, China) and the
patient provided informed written consent.
Case report
History of renal disease
A 31-year-old Chinese female was hospitalized at The
First Hospital of Hebei Medical University (Shijiazhuang, China) in
April 2012 experiencing fever (38–39°C; normal, 36.7–37.2°C), gross
hematuria and proteinuria (400 mg/24 h; normal, <150 mg/24 h),
and there were no abnormalities identified on physical examination;
thus, a diagnosis of chronic glomerulonephritis was validated.
Between June 2012 and March 2013, the patient administered
self-treatment using unknown traditional Chinese medicine and the
24-h proteinuria returned to the normal value (<150 mg/24 h).
During the patient's pregnancy in June 2013, the patient
experienced swollen limbs and increased blood pressure. The blood
pressure increased to 180/120 mmHg (normal, <130/90 mmHg) and
the highest proteinuria determined was 7 g/24 h (normal, <150
mg/24 h). All symptoms were relieved following the child's birth
and the proteinuria decreased to <2 g/24 h. Over frequent
monitoring (every 3 months for 2 years), no abnormalities were
observed. A renal biopsy was performed on the patient in December
2014, as the proteinuria increased to 2 g/24 h.
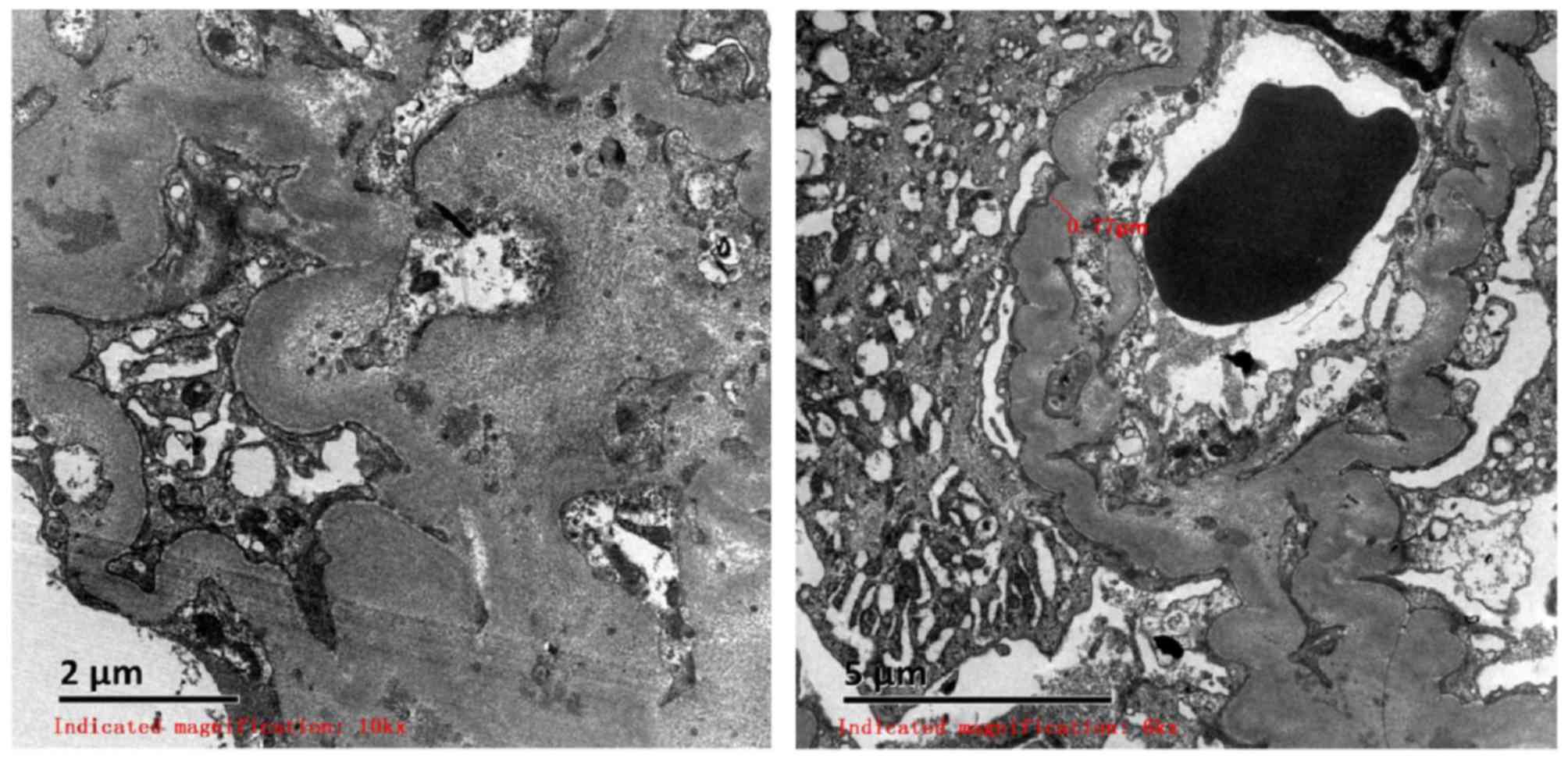
The renal biopsy (performed in December 2015) was
observed using scanning electron microscopy, which revealed
capillary endothelial cell vacuolar degeneration, presence of red
blood cells in the individual lumen, endothelial cells with no
hyperplasia and capillary loops under stress. In addition, it was
identified that the majority of the basement membrane was
thickened, corrugated and coiled, and the foot processes of
visceral epithelial cells were fused. Mesangial and stromal
hyperplasia was accompanied with a limited number of electron dense
deposits with high density. Furthermore, lymph and mononuclear cell
infiltration, partial renal tubular atrophy and renal interstitial
collagen fiber hyperplasia were observed. Red blood cells were
observed in the lumens of capillaries. In addition, arteriole walls
were thickened and a stenosis was identified in the lumen (Fig. 1).
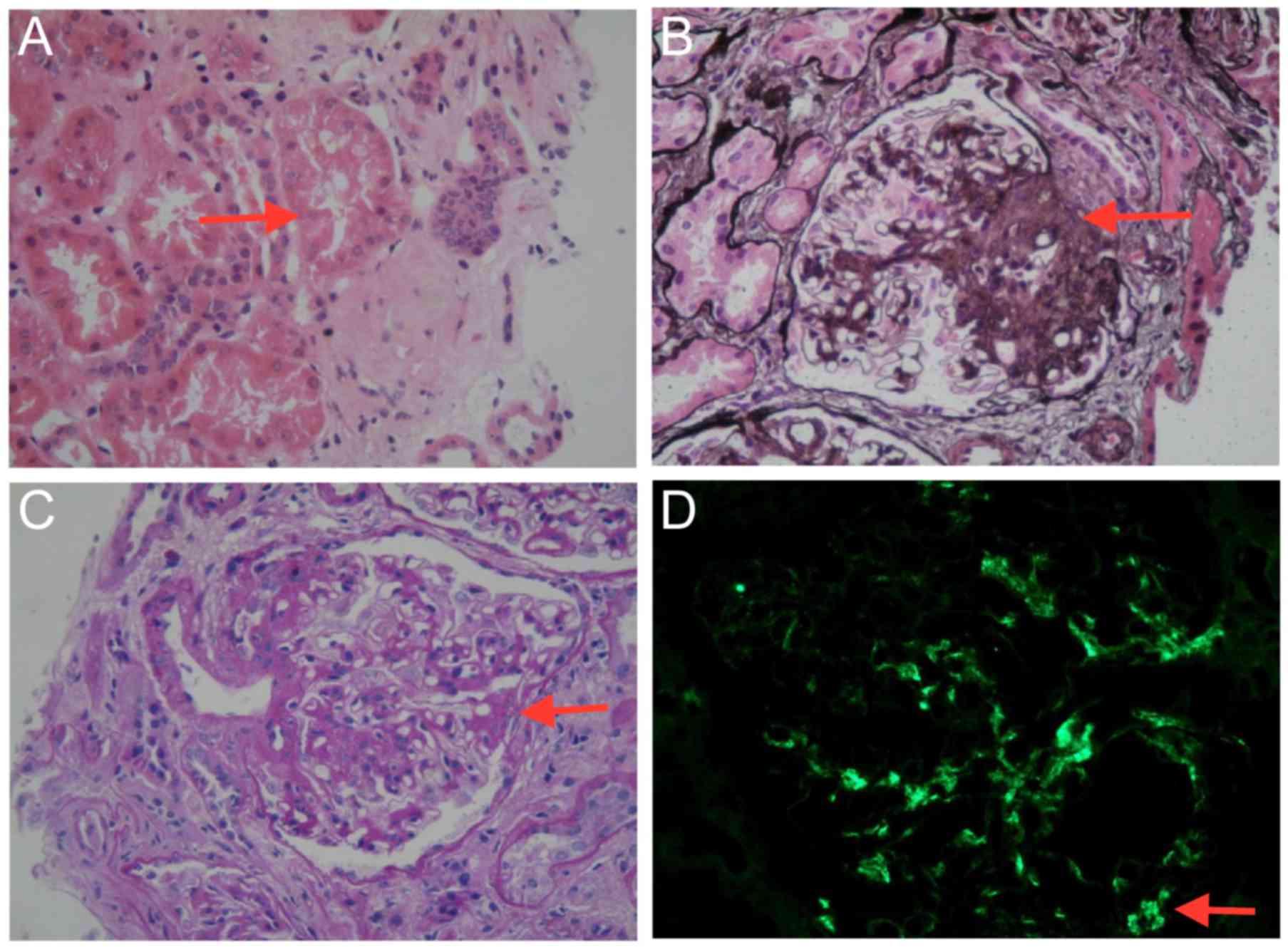
Optical microscopy performed on renal biopsy
sections (magnification, ×400) revealed eight glomeruli, one of
which was sclerotic (hematoxylin-eosin staining; Fig. 2A) and another one was segmental
sclerotic (periodic acid-silver methenamine; Fig. 2B). In the remaining six glomeruli,
glomerular mesangial cells were observed with mild-to-moderate
stromal hyperplasia (periodic acid-Schiff; Fig. 2C). Furthermore, focal segmental
exacerbation, vacuolization and granular degeneration of renal
tubular epithelial cells, focal atrophy, renal interstitial focal
necrosis and inflammatory cell infiltration were observed. The
results of immunofluorescent staining were as follows: IgA positive
(2+); and IgM, IgG, C3, C1q negative (Fig. 2D).
The patient was administered piperazine ferulate
tablets (200 mg, oral, three times a day), irbesartan (150 mg,
oral, once a day) and ‘corbrin capsule’, a traditional Chinese
medicine drug made from herbs (648 mg, oral, three times a day).
Subsequently, the 24-h proteinuria was controlled and levels
returned to normal (<150 mg/24 h).
History of breast cancer
A tumor was initially identified by the patient in
February 2015, with a diameter of 2 cm. The blood pressure of the
patient was 137/91 mmHg following hospitalization at The Fourth
Hospital of Hebei Medical University, but no other abnormalities
were identified during physical examination. The body mass index
(BMI) was calculated to be 32.95 kg/m2 (meaning she was
classified as obese) and the results of urinalysis were as follows:
Proteinuria, 1.0 g/l; the number of erythrocytes was 5 times higher
than normal; the number of leukocytes was within the normal range;
urinary cast examination was negative; serum albumin and serum
globulin ratio, 1.58 (normal value, 1.20–2.40); blood urea
nitrogen, 3.7 mmol/l; (normal value, 2.8–7.1 mmol/l); and serum
creatinine, 54.5 µmol/l (normal value, 44.2–132.6 µmol/l).
The patient accepted breast-conserving surgery and
sentinel lymph node biopsy in February 2015. The post-surgical
pathological manifestation revealed that the diameter of the left
breast tumor was 1.8 cm, and that the patient exhibited breast
metaplastic cancer without vascular tumor thrombus or metastasis of
sentinel lymph nodes with negative margins. Immunohistochemistry
results were as follows: Estrogen receptor, 0; progesterone
receptor, 0; human epidermal growth factor 2, 0; p53, 60%; Ki-67
protein, 50%; type II topoisomerase, 30%; cytokeratin+;
vimentin+; S-100 protein+; synaptophysin; and
cluster of differentiation 56−/+. The diagnosis of
triple negative breast cancer was validated and the tumor was
staged IA (T1cN0M0), according to the Tumor-Node-Metastasis staging
system (7). The patient consultation
at the Chinese Academy of Medical Sciences (Beijing, China),
revealed that the left breast exhibited an invasive breast cancer
with considerable necrosis. The patient was treated with paclitaxel
(90–120 mg/m2) for 12 weeks, followed by
cyclophosphamide treatment (200 mg/m2) for 12 weeks.
Diagnosis and treatment
The patient was initially treated with paclitaxel
liposome (180 mg, intravenously, day 1). Protein excretion
increased from 121 mg/24 h prior to treatment with paclitaxel to
679 mg/24 h, which is higher than the normal level (<150 mg/24
h), for half a month. The chemotherapy was suspended and
radiotherapy was administered. For the left breast and the organ at
risk, the following parameters were applied: Double lung V10 (i.e.,
the proportion of the lung receiving 10 Gy), ≤50%; V20, ≤25–28%;
and V30 ≤15–18%. The target dose was 90% CTV ≥50 Gy in 25
fractions, combining with 12-Mevβ electron beam; the dose was 1,600
cGy in 8 fractions. Following radiotherapy, seven cycles of
chemotherapy with paclitaxel liposome (180 mg, intravenously, day
1) were performed. The proteinuria (≤523.5 mg/24 h) was
occasionally higher than normal values during this period. At the
point of writing, no recurrence of tumors was observed.
Discussion
The majority of patients with carcinomas may be
complicated by glomerular diseases (4). The combined occurrence of cancer with
membranous nephropathy is common (8),
whereas tumors associated with IgA nephropathy have been rarely
studied. The present study identified only 10 cases of malignant
tumors with IgA nephropathy in a review of previous studies
performed in the last 30 years. To the best of our knowledge, the
first patient with breast cancer and atypical IgA nephropathy was
identified in 1986 (3). Other studies
included patients with IgA nephropathy with renal cell carcinoma
(5,9),
bronchial carcinoma (6), small cell
lung carcinoma (10,11), basaloid squamous cell carcinoma
(12), mesothelioma (13), rectal cancer (14) and gastric adenocarcinoma (15) identified in 1991, 1996, 1998, 2008,
2009, 2012 and 2013, respectively (Table
I).
 | Table I.Cases of cancer associated with IgA
nephropathy in the past 30 years. |
Table I.
Cases of cancer associated with IgA
nephropathy in the past 30 years.
| Case | Author (year) | Age, years | Sex | Tumor type | Nephrosis | IHC | Sequence of two
diseases | Symptoms | Therapy | Sequelae | (Refs.) |
|---|
| 1 | Yin et al
(1986) | 57 | F | Breast cancer | IgA nephropathy
(diffuse mesangial proliferative glomerulonephritis) | IgA deposits in
mesangial area | ST | Hypertension,
proteinuria | S | No further
examination | (3) |
| 2 | Tanaka et al
(1991) | 61 | M | Kidney cancer | IgA nephropathy | IgA, C3 and fibrin
deposits | ST | Proteinuria | S | Nephrosis
recovery | (5) |
| 3 | Schütte et al
(1996) | 45 | − | Bronchial cancer | IgA nephropathy | − | NC | − | − | − | (6) |
| 4 | Tomoda et al
(1998) | 66 | M | Small cell lung
cancer/gastric cancer | IgA nephropathy | − | CN | Edema,
proteinuria | S and C | Nephrosis
recovery | (10) |
| 5 | Lam et al
(1998) | 70 | M | Basaloid squamous
cell carcinoma of the oesophagus | IgA nephropathy
(mesangial proliferative glomerulonephritis) | IgA, C3 and IgM
deposits in mesangial area | NC | Erythra, proteinuria,
microscopic hematuria | S | − | (12) |
| 6 | Yacoub et al
(2008) | 55 | M | Small cell lung
cancer | IgA nephropathy | − | NC | Hypertension,
microscopic hematuria | S | Succumbed | (11) |
| 7 | Mimura et al
(2009) | 58, 66, 59 | M | Kidney cell cancer (3
cases) | IgA nephropathy | 1, IgA++,
IgG-, IgM− and C3+; 2, IgA++, IgG-,
IgM+ and C3+; 3, IgA++, IgG-, IgM−
and C3+ | 1, NC; 2, NC; 3,
S | All have microscopic
hematuria, proteinuria | 1, C and S; 2, C and
S; 3, S | 1 and 3, nephrosis
recovery; 2, renal failure | (9) |
| 8 | Fawole et al
(2012) | 65 | M | Mesothelioma | IgA nephropathy | IgA++,
C3++ | CN | Short breath, dry
cough, microscopic hematuria, proteinuria | CH and C | Succumbed | (13) |
| 9 | Yahata et al
(2013) | 68 | M | Rectal cancer | IgA
nephropathy | IgA+++,
C3+++, IgG+, IgM+ and C1q+ | CN | Hematuria,
proteinuria | S, CH and T | Nephrosis
recovery | (14) |
| 10 | Kocyigit et
al (2013) | 58 | M | Gastric cancer | IgA nephropathy
(focal segmental glomerulosclerosis) | IgA deposits | CN | abdominal
distention, loss weight | S, CH, R and C | Nephrosis
recovery | (15) |
In spite of the limited number of identified cases,
an association between solid tumors and IgA nephropathy has been
demonstrated. The reason for this association may be the following:
i) Detection bias as patients with membranous nephropathy are
likely to be screened for cancer; ii) similar demographic
characteristics of the population, as membranous nephropathy and
cancer exhibit a higher incidence in the elderly and/or smokers;
and iii) the majority of the drugs administered to treat glomerular
disease may be oncogenic, which may lead to subsequent malignancy
(4). Thus, these factors may lead to
the lack of information about the epidemiology of cancer-associated
membranous nephropathy.
The pathogenesis of tumor-associated IgA nephropathy
remains unknown. A previous study demonstrated that the abundance
of IgG deposits and the loss of glomerular foot processes may lead
to proteinuria and glomerular damage in tumor-bearing animals
(16). Previous studies using mouse
models revealed that minor glomerular abnormalities may be
associated with the immune response of malignant tumors (17,18).
Associated antigens were released from the surface of tumor cells,
thus stimulating the production of antibodies (19). Subsequently, antigen-antibody immune
complexes may be formed (19). The
most common type of antigen-antibody immune complex observed was
self-polymeric IgA 1 in IgA nephropathy, or as a joint auto-antigen
complement C3 (20). The IgG and/or
IgM antibody complexes may be formed and deposited in the
glomerular mesangium. The affinity of the latter for the
extracellular matrix may be increased, thus leading to glomerular
lesions (20).
Although membranous nephropathy possesses
similarities to solid tumors and there is a close association
between minimal change disease and Hodgkin's lymphoma, there are
numerous exceptions (21). Other
types of glomerular damage, including glomerulonephritis, IgA
nephropathy and rapidly progressive glomerulonephritis, may be
associated with solid tumors (21).
In addition, minimal nephrosis, membranous nephropathy,
mesangioproliferative glomerulonephritis, focal segmental
glomerulosclerosis and IgA nephropathy may be associated with
certain types of hematological malignancy (22).
In the present case study, the principal clinical
manifestation was hematuria and increased 24-hour proteinuria.
Immunofluorescence staining revealed IgA deposits and inflammatory
infiltration was observed in renal interstitial cells. Using our
present understanding of the pathogenesis of IgA nephropathy, the
present study hypothesized that the patient may exhibit a breast
cancer in the early stage of IgA nephropathy, without experiencing
specific symptoms. The antigen may be released from the breast
tumor cells and lead to continuous antigenemia. Subsequently, the
antigens may stimulate the production of antibodies and form immune
complexes which are deposited in the glomeruli, resulting in
glomerulonephritis. This process is likely to progress in the
subclinical phase of cancer. In spite of this, due to the lack of
tumor-associated antigen detection in the present and previous
studies, the specific antigens involved in IgA-associated breast
cancer remain unknown.
The identification of tumor-associated antigens in
the glomerular lesion is required for the diagnosis of
tumor-associated glomerular diseases. This diagnosis cannot be
validated in the present case study on the basis of the clinical
and pathological information, and may be due to the following
reasons: i) The patient cannot be diagnosed with breast
cancer-associated IgA nephropathy due to the absence of examination
results; ii) it remains unclear whether the patient exhibited
breast cancer prior to experiencing glomerular problems and whether
malignancy developed due to the renal disease; and iii)
tumor-associated glomerular disease can be taken into account only
when tumour and renal glomerular damage concurrence and other
factors which could cause glomerular damage are excluded. The
diagnosis could not be determined on the basis of the initial
symptoms experienced by the patient prior to kidney biopsy. The
patient's initial symptoms returned after 2 years; however,
although the IgA nephropathy was pathologically diagnosed, the
return of symptoms may be due to reasons other than breast cancer,
which were not eliminated.
The patient exhibited triple negative breast cancer
and IgA nephropathy, which may exhibit a poor prognosis. Triple
negative breast cancer is defined as the absence of estrogen
receptor, progesterone receptor and human epidermal growth factor
receptor 2 gene expression (23). The
principal characteristics of triple negative breast cancer are as
follows: i) More aggressive with higher probability of brain
metastases and recurrence during the first and third year following
diagnosis than other types of breast cancer; and ii) decreased
survival rate following the first metastatic event, compared with
other subtypes of breast cancer (23). The 5-year survival rate of patients
with triple-negative breast cancer was <15%, and
prognosis-associated factors include the tumor size and lymph node
status (23). Between 5 and 25% of
patients with IgA nephropathy may develop end-stage renal disease
within 10 years of the initial diagnosis. Severe obesity,
hypertension, renal injury and proteinuria are associated with the
prognosis of patients with breast cancer (24). The following parameters observed in
the present patient indicated a risk of recurrence of breast
cancer: BMI of 32.95 kg/m2, validation of triple
negative breast cancer diagnosis post-surgery, tumor size of 1.8 cm
and Ki-67 positive staining of 50%. The patient was administered
paclitaxel chemotherapy combined with radiotherapy, and regular
monitoring of renal functions and primary diseases were performed
during treatment. In addition, physical exercises and weight loss
were advised by a physician to minimize the controllable risk
factors, such as obesity or hypertension.
The IgA nephropathy may develop prior to, in
parallel with, or following malignancy'. Certain investigators
recommended that any patient with kidney lesions should be screened
for cancer (11). The occurrence of
malignant and membranous nephropathy should be well-documented in
medical studies, particularly those associated with cancer. The
strongest association was observed between membranous
glomerulonephritis and solid tumors (3). A previous study estimated that ~25%
patients >60 years of age with membranous glomerulonephritis
experienced an associated cancer and the overall incidence of
cancer in patients with membranous glomerulonephritis was 7.9%
(25). However, a previous study
revealed that 0.36% of patients with nephrotic syndrome exhibited
an underlying malignancy (26).
Additionally, it has been recommended that any patient with
nephrotic syndrome >40 years of age should be screened for
cancer (16). No associated guideline
has been published by the Scientific Association of Nephropathy
(4).
It was difficult to elucidate whether the
association between breast cancer and IgA nephropathy in the
present patient was a coincidence or an etiological association. In
addition, whether patients with IgA nephropathy should receive
cancer screening, and whether kidney disease is associated with
specific types of cancer remains unknown and requires additional
study.
With the approaching of precision medicine, genomics
will serve an essential role in diagnosis and disease therapy.
Drugs have been developed that target specific genes, enable
advancements in medical technology and improve the quality of lives
for patients. There is a limited number of studies that focus on
kidney-disease-associated cancer treatment, in spite of targeted
drugs being developed for other types of cancer. Further research
into cancer genomics and identification of targeted drugs is
urgent. Additional studies are required to determine the
association between IgA nephropathy and cancer, and the underlying
molecular mechanism. This would enable the genomics underlying IgA
nephropathy-associated cancer to be determined and novel
therapeutic strategies to be developed.
References
|
1
|
Galloway J: Remarks on Hodgkin's disease.
Br Med J. 2:1201–1208. 1922. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JC, Yamauchi H and Hopper J Jr: The
association of cancer and the nephritic syndrome. Ann Intern Med.
64:41–51. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yin P, Chen W and Chen G: Clinical report
of 4 cases of malignant tumor complicated with glomerular disease.
New Med. 7:354–355. 1986.
|
|
4
|
Pani A, Porta C, Cosmai L, Melis P, Floris
M, Piras D, Gallieni M, Rosner M and Ponticelli C: Glomerular
diseases and cancer: Evaluation of underlying malignancy. J
Nephrol. 29:143–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka K, Kanzaki H and Taguchi T: IgA
glomerulonephtitis in a patient with renal cell carcinoma. Nippon
Jinzo Gakkai Shi. 33:87–90. 1991.PubMed/NCBI
|
|
6
|
Schütte W, Ohlmann K, Koall W, Rösch B and
Osten B: Paraneoplastic IgA nephritis as the initial symptom of
bronchial carcinoma. Pneumologie. 50:494–495. 1996.(In German).
PubMed/NCBI
|
|
7
|
Bevers TB, Anderson BO, Bonaccio E, Buys
S, Daly MB, Dempsey PJ, Farrar WB, Fleming I, Garber JE, Harris RE,
et al: NCCN Clinical Practice Guidelines in Oncology: Breast Cancer
screening and diagnosis. J Natl Compr Canc Netw. 7:1060–1096. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cagnoli L: Solid tumors and paraneoplastic
glomerulonephritis. G Ital Nefrol. 27 Suppl 50:S51–S57. 2010.(In
Italian). PubMed/NCBI
|
|
9
|
Mimura I, Tojo A, Kinugasa S, Uozaki H and
Fujita T: Renal cell carcinoma in associated with IgA nephropathy
in the elderly. Am J Med Sci. 338:431–432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tomoda K, Hamada K, Fukuoka K, Tsukaguchi
K, Tsujimoto M, Mikasa K, Choh S, Yoneda T and Narita N: Small cell
lung cancer associated with nephritic syndrome: Remission after
chemotherapy. Nihon Kokyuki Gakkai Zasshi. 36:541–554. 1998.(In
Japanese). PubMed/NCBI
|
|
11
|
Yacoub G, Kosseifi SG, Shah LS, Byrd RP Jr
and Roy TM: IgA nephropathy and small cell lung carcinoma. Tenn
Med. 101:35–37. 2008.PubMed/NCBI
|
|
12
|
Lam KY, Law SY, Chan KW and Yuen MC:
Glomerulonephritis associated with basaloid squamous cell carcinoma
of the oesophagus. A possible unusual paraneoplastic syndrome.
Scand J Urol Nephrol. 32:61–63. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fawole A, Daw H, Taylor H and Rashidi A:
Immunoglobulin A nephropathy associated with mesothelioma. WMJ.
111:29–32. 2012.PubMed/NCBI
|
|
14
|
Yahata M, Nakaya I, Sakuma T, Sato H, Aoki
S and Soma J: Immunoglobulin a nephropathy with massive
paramesangial deposits caused by anti-vascular endothelial growth
factor therapy for metastatic rectal cancer: A case report and
review of the literature. BMC Res Notes. 6:4502013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kocyigit I, Dortdudak S, Eroglu E, Unal A,
Sipahioglu MH, Berk V, Tokgoz B and Oymak O: Immunoglobulin A
nephropathy could be a clue for the recurrence of gastric
adenocarcinoma. Nefrologia. 33:853–855. 2013.PubMed/NCBI
|
|
16
|
Takeda S, Chinda J, Murakami T, Numata A,
Iwazu Y, Akimoto T, Hamano Y, Muto S, Takahashi M and Kusano E:
Development of features of glomerulopathy in tumor-bearing rats: A
potential model for paraneoplastic glomerulopathy. Nephrol Dial
Transplant. 27:1786–1792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ronco PM: Paraneoplastic glomerulopathies:
New insights into an old entity. Kidney Int. 56:355–377. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Faria TV, Baptista MA, Burdmann EA and
Cury P: Glomerular deposition of immune complexes as a first
manifestation of malignant melanoma-a case report. Ren Fail.
32:1223–1225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Usalan C and Emri S: Membranoproliferative
glomerulonephritis associated with small cell lung carcinoma. Int
Urol Nephrol. 30:209–213. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stuchlová Horynová M, Raška M, Clausen H
and Novak J: Aberrant O-glycosylation and anti-glycan antibodies in
an autoimmune disease IgA nephropathy and breast adenocarcinoma.
Cell Mol Life Sci. 70:829–839. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bacchetta J, Juillard L, Cochat P and Droz
JP: Paraneoplastic glomerular diseases and malignancies. Crit Rev
Oncol Hematol. 70:39–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lien YH and Lai LW: Pathogenesis,
diagnosis and management of paraneoplastic glomerulonephritis. Nat
Rev Nephrol. 7:85–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dent RTM, Pritchard KI and Hanna WM:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schena FP: Ig-A nephropathyOxford textbook
of clinical nephrology. Davison AM, Grunfeld JP, Ponticelli C, Ritz
E, Wincarls C and Ypersele C: Oxford University Press; Oxford: pp.
469–502. 2005
|
|
25
|
Zech P, Colon S, Pointet P, Deteix P,
Labeeuw M and Leitienne P: The nephrotic syndrome in adults aged
over 60: Etiology, evaluation and treatment of 76 cases. Clin
Nephrol. 17:232–236. 1982.PubMed/NCBI
|
|
26
|
Kaplan BS, Klassen J and Gault MH:
Glomeruler injury in patients with neoplasia. Ann Rev Med.
27:117–125. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SM, Rao VM, Frankin WA, Schiffer MS,
Aronson AJ, Spargo BH and Katz AI: IgA nephropathy: Morphologic
predictors of progressive renal diease. Hum Pathol. 13:314–322.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Working Group of the International IgA
Nephropathy Network and the Renal Pathology Society, ; Cattran DC,
Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE,
Amore A, Barratt J, et al: The Oxford classification of IgA
nephropathy: rationale, clinicopathological correlations and
classification. Kidney Int. 76:534–545. 2009. View Article : Google Scholar : PubMed/NCBI
|