Introduction
Thyroid cancer is the most common type of endocrine
malignancy (1). The incidence of
thyroid cancer has been increasing rapidly globally. For example,
in the United States of America, thyroid cancer incidence rates
have increased by 211% between 1975 and 2013 (2,3). This
incidence of thyroid cancer is increasing, primarily due to a rise
in the incidence of papillary thyroid carcinoma (PTC), which
accounts for between 80 and 90% of all thyroid malignancies
(4,5).
Although the prognosis of patients with PTC is favorable, with 10-
and 15-year survival rates of >91 and >87%, respectively
(6,7),
lymph node metastasis in the neck is observed in between 20 and 90%
of all patients (8,9). Recurrence is observed in between 5 and
20% of patients who undergo a total thyroidectomy (10,11).
Therefore, investigations into understanding the underlying
molecular mechanisms of PTC are urgently required, in order to
develop effective diagnosis and therapeutic strategies to improve
patient prognosis.
MicroRNAs (miRNAs/miRs) are noncoding single
stranded RNAs that regulate gene expression at the
post-transcriptional level (12). A
previous study identified that miRNAs serve important functions in
tumorigenesis and may be applied as biomarkers in a variety of
cancer types (13). miR-144 has been
demonstrated to be downregulated and accompanied by suppressed
proliferation and invasion in non-small cell lung cancer, breast
cancer, hepatocellular carcinoma, prostate cancer, bladder cancer
and laryngeal squamous cell carcinoma (14–18). The
results of the present study further support these observations, as
miR-144 was identified to be significantly downregulated in PTC
tissues and cell lines. Low expression of miR-144 was associated
with increased tumor sizes in PTC via reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), and
the ectopic expression of miR-144 significantly suppressed the
proliferation of IHH4 cells. WW domain-containing transcription
regulator 1 (WWTR1) was overexpressed in PTC tissues and associated
with proliferation and invasion. Furthermore, WWTR1 was identified
as a target of miR-144.
Materials and methods
Human tissue samples
PTC and adjacent non-cancerous tissues were
collected from 63 patients (25 male, 38 female) who underwent
thyroid cancer resection surgery at the First Hospital of China
Medical University (Shenyang, China) between September 2009 and
January 2015. Patient characteristics are presented in Table I. All specimens were frozen in liquid
nitrogen immediately and stored at −80°C until use. A diagnosis of
PTC was histologically confirmed at the First Hospital of China
Medical University. The inclusion criteria were as follows: i) All
patients had received primary surgery; ii) PTC was pathologically
confirmed intraoperatively or postoperatively; and iii) none of the
patients recruited in the present study had undergone prior
oncological surgery or head and neck irradiation. The present study
was approved by the Ethics Committee of the First Hospital of China
Medical University and written informed consent was obtained from
all study participants.
 | Table I.Association between miR-144 expression
and clinicopathological features in PTC. |
Table I.
Association between miR-144 expression
and clinicopathological features in PTC.
| Characteristics | n | High expression, n
(%) | Low expression, n
(%) | P-value |
|---|
| Sex |
|
|
| 0.027a |
| Male | 25 | 8 (32.0) | 17 (68.0) |
|
|
Female | 38 | 23 (60.5) | 15 (39.5) |
|
| Age, years |
|
|
| 0.884 |
|
<45 | 36 | 18 (50.0) | 18 (50.0) |
|
| ≥45 | 27 | 13 (48.1) | 14 (51.9) |
|
| Extrathyroidal
extension |
|
|
| 0.701 |
| Yes | 30 | 14 (46.7) | 16 (53.3) |
|
| No | 33 | 17 (51.5) | 16 (48.5) |
|
| TNM staging |
|
|
| 0.503 |
| I–II | 38 | 20 (52.6) | 18 (47.4) |
|
|
III–IV | 25 | 11 (44.0) | 14 (56.0) |
|
| Lymph node
metastasis |
|
|
| 0.214 |
|
Yes | 47 | 25 (53.2) | 22 (46.8) |
|
| No | 16 | 6 (37.5) | 10 (62.5) |
|
|
Multicentricity |
|
|
| 0.383 |
|
Yes | 27 | 15 (55.6) | 12 (44.4) |
|
| No | 36 | 16 (44.4) | 20 (55.6) |
|
| Tumor size, cm |
|
|
|
<0.001a |
|
<2 | 24 | 19 (79.2) | 5 (20.8) |
|
| ≥2 | 39 | 12 (30.8) | 27 (69.2) |
|
Cells culture and transfection
Nthy-ori 3–1 normal human thyroid follicular
epithelial cells were obtained from The European Collection of
Authenticated Cell Cultures (Salisbury, UK); IHH4 cells were
obtained from the Health Science Research Resources Bank (Osaka,
Japan). The Nthy-ori 3–1 cells were maintained in RPMI-1640
(Hyclone, GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The IHH4 cells were maintained in a 1:1
mixture of RPMI-1640 and Dulbecco's modified Eagle's medium
supplemented with 10% FBS at 37°C, in a humidified atmosphere with
5% CO2. The miR-144 mimic and negative control (NC) were
purchased from GenePharma Co., Ltd. (Suzhou, China). The sequences
of the miR-144 mimics were as follows: Sense (S);
5′-UACAGUAUAGAUGAUGUACU-3′ and antisense (AS);
5′-UACAUCAUCUAUACUGUAUU-3′. The sequences of the negative control
(NC) were: S; 5′-UUCUCCGAACGUGUCACGUTT-3′ and AS;
5′-ACGUGACACGUUCGGAGAATT-3′. The pcDNA-WWTR1 and the pcDNA empty
vector were purchased from Shanghai GeneChem, Inc. (Shanghai,
China). IHH4 cells were transfected using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Proteins were extracted 48 h
post-transfection and used for western blot analysis.
Western blotting
Proteins were extracted from IHH4 cells, as well as
PTC and adjacent normal tissue samples from 15 patients (6 male, 9
female), using a Total Protein Extraction kit (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China). Ten patients were <45 years
old, 5 patients were >45 years old. These 15 patients were
selected based on identical inclusion criteria to the
aforementioned 63 patients. A separate study population was used as
the processing performed on the other tissue samples may have made
protein expression difficult to detect. Protein extracts were
quantified with a BCA protein assay (Beyotime Institute of
Biotechnology, Haimen, China). Proteins (20–30 µg/lane) were
separated by 10% SDS-PAGE and then electrophoretically transferred
to polyvinylidene difluoride membranes. The membranes were blocked
with 5% non-fat milk in Tris-buffered saline for 2 h at room
temperature, and then incubated with primary antibodies against
rabbit anti-WWTR1 (1:1,000; cat. no; 23306-1-AP; ProteinTech Group,
Inc., Chicago, IL, USA) and rabbit anti-GAPDH (1:1,000; cat. no;
10494-1-AP; ProteinTech Group, Inc.) overnight at 4°C. Following
incubation with a horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (1:10,000 dilution; cat. no; 7074;
Cell Signaling Technology, Inc., Danvers, MA, USA) for 2 h at room
temperature, the protein bands were visualized by chemiluminescence
(SuperSignal@ West Pico Chemiluminescent Substrate,
Thermo Fisher Scientific, Inc.) and quantified by Image J (version
no. 1.43, National Institutes of Health, Bethesda, MD, USA).
Total RNA isolation and RT-qPCR
Total RNA, including miRNA, was extracted from the
frozen PTC and adjacent non-cancerous tissue specimens collected
from 63 patients, as well as from Nthy-ori 3-1 and IHH4 cell lines,
using RNAiso (Takara Biotechnology Co., Ltd., Dalian, China). The
RR716 Reverse Transcription kit (Takara Biotechnology Co., Ltd.)
was used to reverse transcribe RNA into cDNA and the temperature
protocol for reverse transcription was 37°C for 60 min, and then
85°C for 5 sec. RT-qPCR was performed using SYBR® Premix
Ex Taq™ II (Takara Biotechnology Co., Ltd.) on a LightCycler 480
system (Roche Diagnostics, Indianapolis, IN, USA). The
thermocycling conditions included an initial denaturation step at
95°C for 30 sec, denaturation at 95°C for 5 sec and annealing at
60°C for 30 sec for 40 cycles, dissociation stage at 95°C for 60
sec, 55°C for 1 min, 95°C for 30 sec. All primers were purchased
from Sangon Biotech Co., Ltd. (Shanghai, China). The miR-144 primer
sequences were as follows: forward, 5′-CGGCGGTACAGTATAGATGATG-3′.
The reverse primer of miR-144 was supplied as part of the RR716
Reverse Transcription kit. The U6 primer sequences were as follows:
Forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′. The endogenous control for
normalization of the input RNA was U6. The double-standard curves
method was used to analyze the relative expression of miR-144
(19).
Cellular proliferation assay
Cellular proliferation was assessed using the Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). IHH4 cells (3×103 cells/well) were
seeded into a 96-well plate at a final volume of 100 µl and then
transfected with miR-144 mimics, negative control (NC) and
pcDNA-WWTR1, as aforementioned. The samples were detected at 0, 24,
48 and 72 h following gene transfection. CCK-8 solution (10 µl) was
added into each well and incubated for 3 h at 37°C. Absorbance was
measured at 450 nm to calculate the number of viable cells.
Colony formation assays
For the colony formation assay, IHH4 cells
transfected with miR-144 mimic and NC were seeded into 6-well
plates at 5×102/well and cultured for 2 weeks at 37°C,
in a humidified atmosphere with 5% CO2. Colonies were
washed with PBS, fixed in 4% methanol for 20 min at room
temperature and stained with 0.5% crystal violet for 20 min at room
temperature. The number of colonies (>50 cells/colony) was
counted (original magnification, ×40; Leica Microsystems, Wetzlar,
Germany). The images were captured using a digital camera (Nikon
D5300; Nikon, Toyko, Japan).
Bioinformatics analysis
The following software programs were used for the
prediction of putative miR-144 targets: TargetScan (www.targetscan.org, version no. 6.0–6.2) and miRWalk
(http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk/index.html).
The date of access was June 2014.
Statistical analysis
SPSS software (version 13.0; SPSS, Inc., Chicago,
IL, USA) was applied to perform statistical analyses. Data are
presented as the mean ± standard deviation, obtained from at least
three independent experiments. The χ2 test was applied
to assess the association between miR-144 expression and the
clinicopathological characteristics. A paired Student's t-test was
used to assess inter-group comparisons. Two receiver operating
characteristic curves (ROCs) were established to evaluate the
diagnostic value of miR-144 for benign or malignant status, and
larger tumors (≥2 cm). Youden's index was used to calculate the
threshold value to predict benign or malignant status, and larger
tumors (≥2 cm). P<0.05 was considered to indicate a
statistically significant difference.
Results
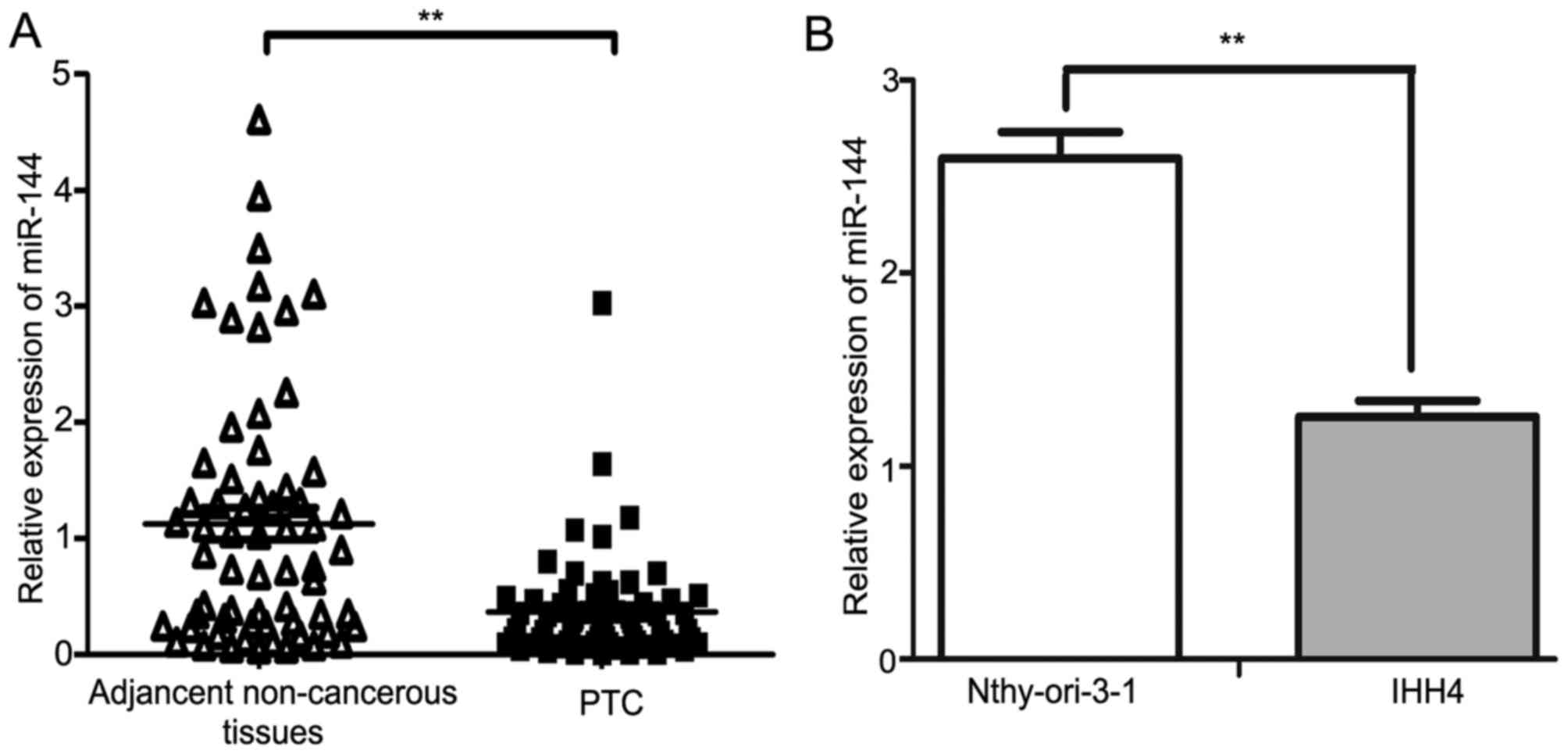
miR-144 was downregulated in human PTC
tissues and cell line
The expression of miR-144 in PTC tissues and matched
non-cancerous tissues from 63 patients were investigated using
RT-qPCR. The results demonstrated that miR-144 was significantly
downregulated in PTC tissues compared with in the adjacent
non-cancerous tissues (P<0.01; Fig.
1A). The expression of miR-144 in the IHH4 PTC cell line was
decreased compared with in the normal human Nthy-ori 3-1 thyroid
follicular epithelial cell line (P<0.01; Fig. 1B). The median fold-change value of
miR-144 between PTC tissues and the adjacent non-cancerous tissues
was 0.28, which was then used as a threshold value to separate the
results into two groups: High miR-144 expression group (≥0.28;
n=31); low miR-144 expression group (<0.28; n=32). The
downregulation of miR-144 was associated with larger tumor sizes
when the threshold value for tumor sizes was 2 cm (P<0.001),
whereas no significant difference was observed between miR-144
expression level and patient age, extrathyroidal extension, TNM
stage, lymph node metastasis or multicentricity (Table I). Patients were staged according to
the TNM staging system (7th edition) of the American Joint
Committee on Cancer (20).
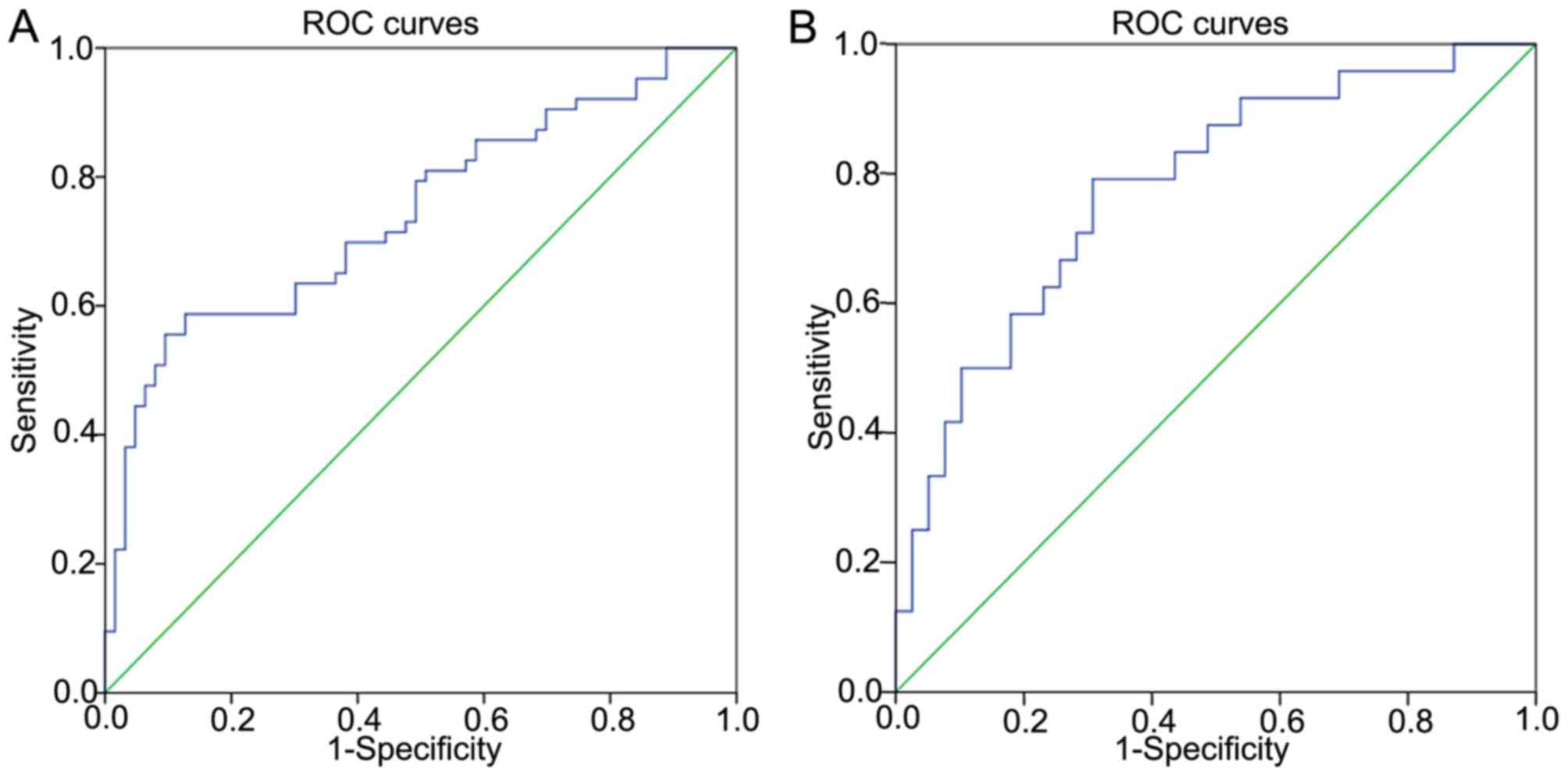
Diagnostic value of miR-144
The diagnostic value of miR-144 was also
investigated. ROCs curves were applied to evaluate whether miR-144
may serve as a biomarker to predict benign or malignant status. The
area under the curve (AUC) was 0.743 [95% confidence interval (CI),
0.657–0.829; P<0.001], suggesting that miR-144 may be applied as
a potential biomarker for PTC (Fig.
2A). The sensitivity was 58.7%, and the specificity was 87.3%
when the threshold value was 0.63. The threshold values for
predicting benign or malignant status refer to the relative
expression in PTC tissues and the adjacent non-cancerous tissues,
investigated by RT-qPCR. In addition, miR-144 was revealed to be a
potential diagnostic biomarker for whether the tumor size is ≥2 cm
(Fig. 2B). The AUC was 0.779 (95% CI,
0.661–0.896; P<0.001). The sensitivity was 79.2%, and the
specificity was 69.2% when the threshold value was 0.28. The
threshold values for predicting whether the tumor sizes ≥2 cm refer
to the fold-change between the PTC tissues and the adjacent
non-cancerous tissues, determined by RT-qPCR.
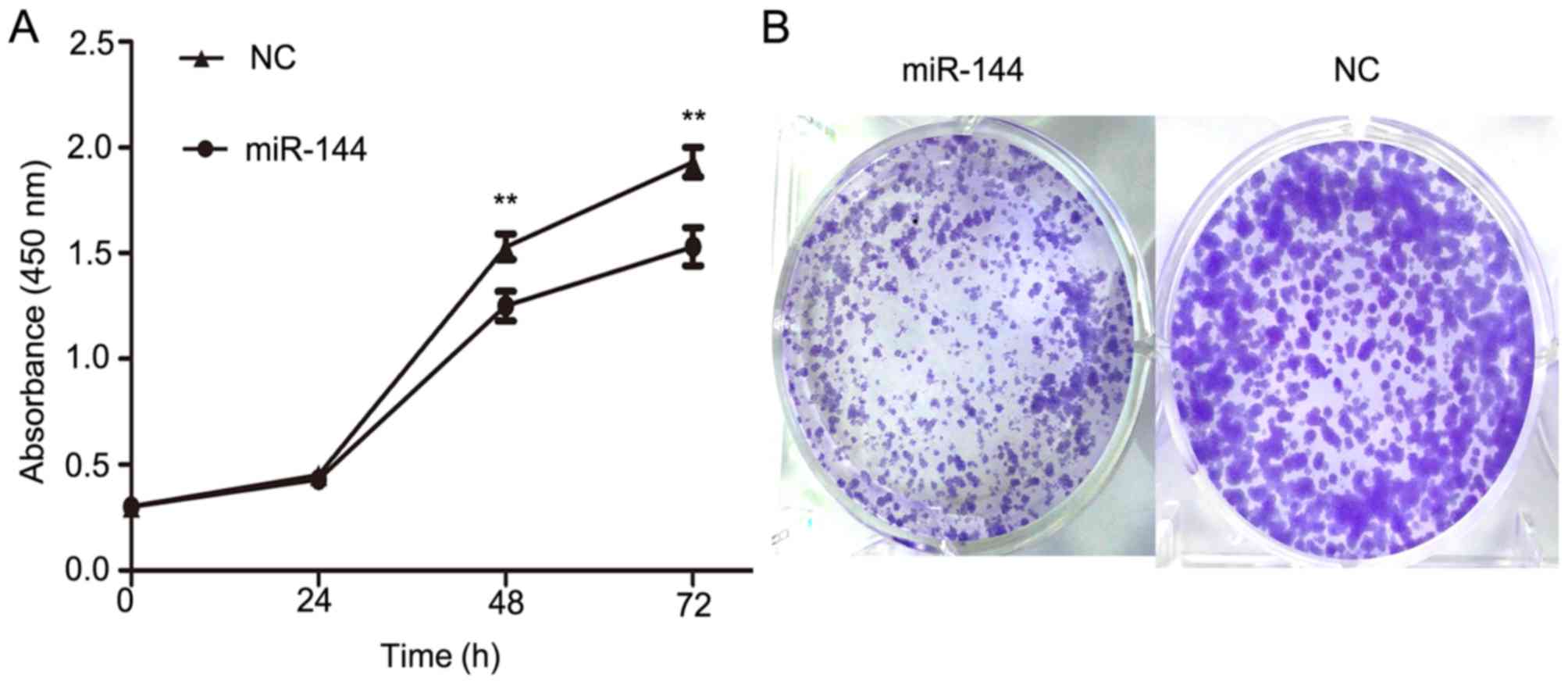
miR-144 inhibited proliferation in a
PTC cell line
To further evaluate the function of miR-144 on PTC
development, miR-144 was overexpressed in IHH4 cells using miR-144
mimics and a CCK-8 assay was used to determine its effects on
cellular proliferation. The overexpression of miR-144 in IHH4 cells
resulted in a significant decrease in cellular proliferation when
compared with the NC group (P<0.01; Fig. 3A). Furthermore, a colony formation
assay was conducted to provide further evidence of the effect on
the proliferation of IHH4 cells (Fig.
3B). The colony formation assay indicated that miR-144
overexpression significantly inhibited the ability of proliferation
in IHH4. These results suggested that miR-144 exhibits a
suppressive effect on tumor proliferation in PTC.
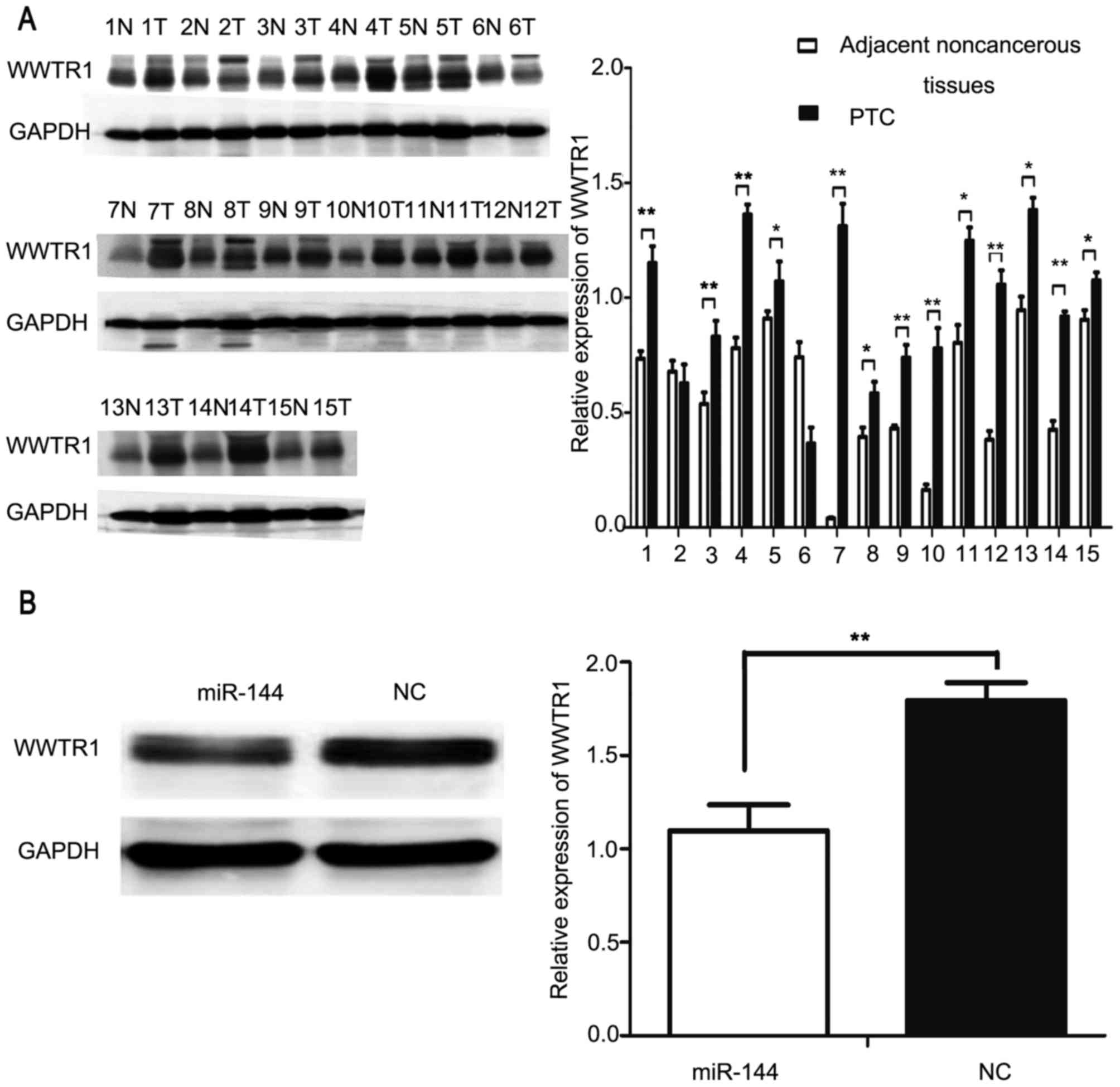
WWTR1 is a target of miR-144
TargetScan (www.targetscan.org) and StarBase (starbase.sysu.edu.cn) were used to identify the
potential targets of miR-144, and revealed that WWTR1 may be a
target of miR-144. Previous studies have identified that the
expression of WWTR1 is upregulated in PTC tissues as compared with
in the adjacent non-cancerous tissues (21,22). The
upregulation of WWTR1 was demonstrated to be positively associated
with tumor size and lymph node metastasis in PTC (22). Western blot analysis was used to
investigate the expression of WWTR1 in PTC and adjacent normal
tissues from 15 patients. The results of the present study
indicated that WWTR1 was significantly overexpressed in PTC tissues
compared with in the adjacent normal tissues in 13/15 patients
(P<0.05; Fig. 4A). Subsequently,
miR-144 and NC were transfected into IHH4 cells and western blot
analysis was applied to detect the protein expression of WWTR1. The
expression of WWTR1 was significantly decreased in the
miR-144-overexpressing groups compared with the NC group
(P<0.01; Fig. 4B).
Overexpression of WWTR1 impairs the
miR-144-induced inhibition of proliferation
A ‘rescue’ strategy was adopted in order to
investigate the functional relevance of WWTR1 targeting by miR-144
in IHH4 cells. pcDNA-WWTR1 was first transfected into IHH4 cells.
Then, miR-144 mimics were co-transfected into the cells with
pcDNA-WWTR1. CCK-8 was used to evaluate the proliferation of IHH4
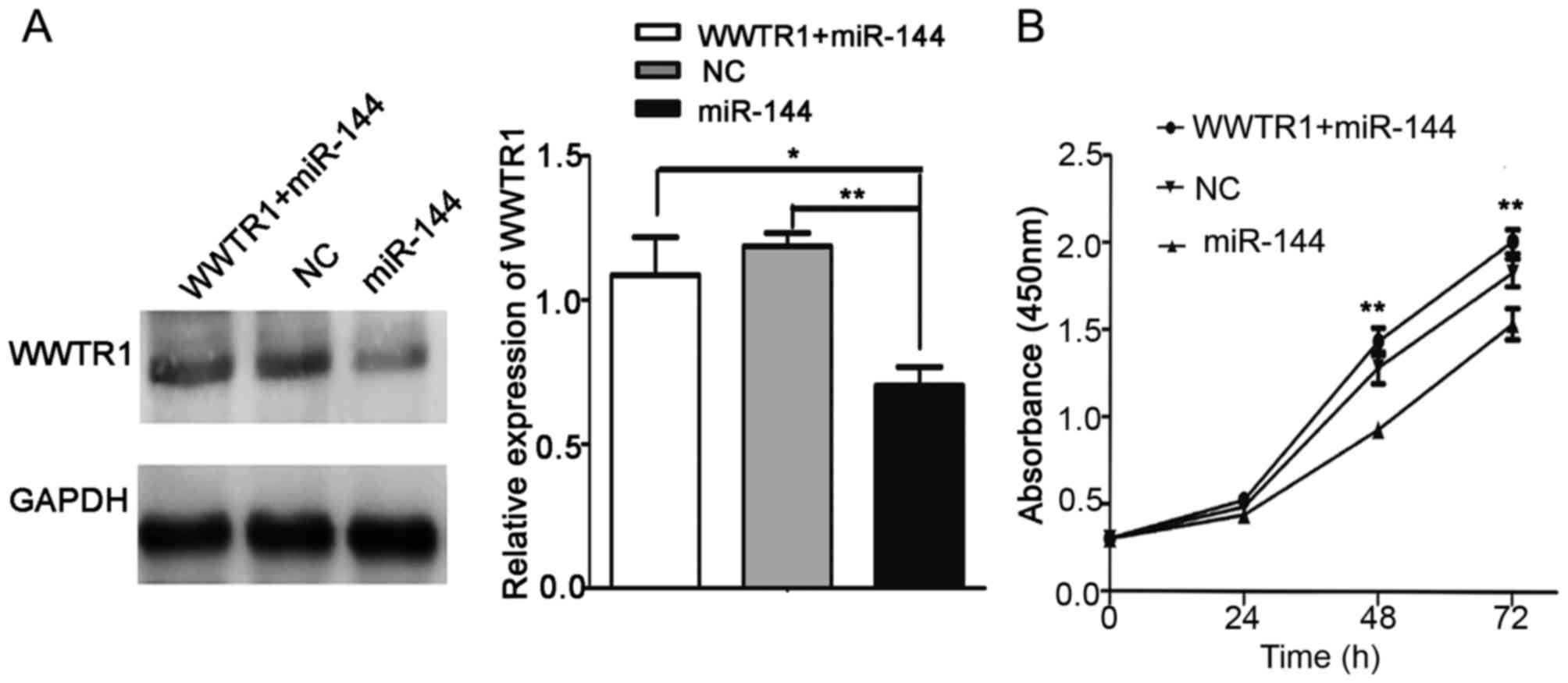
cells. As presented in Fig. 5A,
western blot analysis was used to analyze the expression of WWTR1
in the WWTR1+miR-144, NC and miR-144 groups. The CCK-8 assay
results indicated that pcDNA-WWTR1 significantly increased WWTR1
expression and rescued the inhibition of proliferation in the
presence of miR-144 mimics (P<0.01; Fig. 5B). These results suggested that WWTR1
may be a target of miR-144 in PTC.
Discussion
In the USA, the overall incidence of thyroid cancer
increased by 6.6% annually between 2000 and 2009; the highest
increase among all types of cancer (23,24).
Although PTC is a relatively indolent disease compared with
hepatocellular carcinoma and gastric cancer, with low mortality,
lymph node metastasis and recurrence frequently occur, leading to a
poor prognosis. Therefore, the development of novel strategies for
the diagnosis and treatment of PTC is urgently required (7).
miRNAs regulate the expression efficiency and
stability of mRNAs at the post-transcriptional level by primarily
binding to the 3′-untranslated region of target mRNAs, leading to
mRNA degradation or translation inhibition (25,26).
Increasing evidence has demonstrated the function of miRNAs in the
development of PTC. An miRNA-chromatin immunoprecipitation
microarray assay revealed a set of miRNAs that were upregulated in
PTC tissues compared with those in normal thyroid tissues,
including miR-21, miR-146, miR-221, miR-222, miR-155, miR-181a and
miR-181b, whereas miRNAs such as miR-26a-1, miR-219-5p and miR-345
were identified to be downregulated (27,28).
Unique miRNA profiles have demonstrated potential for the
development of cancer biomarkers and novel therapeutics. Decreased
miR-144 expression is demonstrated in numerous other types of
cancer, acting as a tumor suppressor; for example, by inhibiting
TP53-induced glycolysis regulatory phosphatase in lung cancer
(16), inhibiting hepatocellular
carcinoma by targeting E2F transcription factor 3 (17) and inhibiting enhancer of zeste homolog
2 in bladder cancer (18).
In the present study, miR-144 was revealed to
inhibit cellular proliferation by targeting WWTR1 in PTC. First,
the expression levels of miR-144 in PTC and corresponding adjacent
normal tissues were analyzed, and the results indicated that
miR-144 was significantly downregulated in PTC tissues compared
with the adjacent non-cancerous tissues and was negatively
associated with tumor size. miR-144 was also downregulated in PTC
cell lines. Secondly, miR-144 demonstrated potential as a biomarker
for diagnosing thyroid cancer (sensitivity, 58.7%; specificity,
87.3%). The expression fold-change of miR-144 between PTC tissues
and adjacent non-cancerous tissues was a predictive marker for
tumor sizes ≥2 cm (sensitivity, 79.2%; specificity, 69.2%). miR-144
may be applied as a biomarker in thyroid cancer, particularly for
tumor size. Thirdly, the function of miR-144 was explored in the
IHH4 PTC cell line. Cellular proliferation was investigated using a
CCK-8 and colony formation assays. The results indicated that
miR-144 significantly inhibited the proliferation ability of IHH4
cells. Subsequently, TargetScan and miRWalk were used to analyze
miR-144 target genes in PTC.
The transcriptional coactivator WWTR1 was initially
identified through its ability to interact with 14-3-3 proteins and
is a downstream member of the Hippo pathway (29). The Hippo pathway regulates cellular
proliferation, survival and differentiation in normal tissues and
cells. Aberrant activation of WWTR1 is also implicated in a variety
of cancer types, including breast cancer, hepatocellular carcinoma
and lung cancer (30,31). In addition, the overexpression of
WWTR1 has been demonstrated in PTC tissues, and is able to confer a
proliferation advantage to thyroid cells and to induce the
epithelial-to-mesenchymal transition (21,22). In
the present study, western blot analysis was applied to analyze the
expression of WWTR1. WWTR1 was significantly overexpressed in PTC
tissues compared with in the adjacent non-cancerous tissues.
Subsequently, miR-144 was overexpressed in IHH4 cells, and the
WWTR1 protein level was revealed to be significantly decreased in
the miR-144 overexpressed group compared with in the NC group.
Furthermore, IHH4 cells were co-transfected with pcDNA-WWTR1 and
miR-144, and it was demonstrated that the overexpression of WWTR1
was able to ‘rescue’ the inhibition of proliferation caused by
miR-144.
In conclusion, the results of the present study
demonstrated that miR-144 was frequently downregulated in PTC and
the expression of miR-144 was associated with larger tumor sizes.
miR-144 exhibited tumor suppressor activity by inhibiting IHH4 cell
proliferation via targeting WWTR1. These results assist in
improving understanding of the underlying molecular mechanisms in
PTC, as well as providing further avenues to investigate whether
miR-144 may be a potential biomarker and therapeutic target for
PTC.
Acknowledgements
The present study was supported by the Liaoning
BaiQianWan Talents Program (grant no. 2014921033), the Science and
Technology Project of Shenyang City (grant no. F16-205-1-41), the
Natural Science Foundation of Liaoning Province (grant no.
2015020536), the Liaoning Province PhD Start-up Fund (grant nos.
20141042 and 201501008) and the National Natural Science Foundation
of China (grant nos. 81402208 and 81502319).
References
|
1
|
Sun W, Lan X, Zhang H, Dong W, Wang Z, He
L, Zhang T and Liu S: Risk factors for central lymph node
metastasis in CN0 papillary thyroid carcinoma: A systematic review
and meta-analysis. PLoS One. 10:e01390212015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lundgren CI, Hall P, Dickman PW and
Zedenius J: Clinically significant prognostic factors for
differentiated thyroid carcinoma: A population-based, nested
case-control study. Cancer. 106:524–531. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim H, Devesa SS, Sosa JA, Check D and
Kitahara CM: Trends in thyroid cancer incidence and mortality in
the United States, 1974–2013. JAMA. 317:1338–1348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hundahl SA, Fleming ID, Fremgen AM and
Menck HR: A National Cancer Data Base report on 53,856 cases of
thyroid carcinoma treated in the U.S., 1985–1995 [see comments].
Cancer. 83:2638–2648. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao Y and Xing M: Recent incidences and
differential trends of thyroid cancer in the USA. Endocr Relat
Cancer. 23:313–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sciuto R, Romano L, Rea S, Marandino F,
Sperduti I and Maini CL: Natural history and clinical outcome of
differentiated thyroid carcinoma: A retrospective analysis of 1503
patients treated at a single institution. Ann Oncol. 20:1728–1735.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toniato A, Boschin I, Casara D, Mazzarotto
R, Rubello D and Pelizzo M: Papillary thyroid carcinoma: Factors
influencing recurrence and survival. Ann Surg Oncol. 15:1518–1522.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grebe SK and Hay ID: Thyroid cancer nodal
metastases: Biologic significance and therapeutic considerations.
Surg Oncol Clin N Am. 5:43–63. 1996.PubMed/NCBI
|
|
9
|
Kouvaraki MA, Shapiro SE, Fornage BD,
Edeiken-Monro BS, Sherman SI, Vassilopoulou-Sellin R, Lee JE and
Evans DB: Role of preoperative ultrasonography in the surgical
management of patients with thyroid cancer. Surgery. 134:946–955.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mazzaferri EL and Kloos RT: Clinical
review 128: Current approaches to primary therapy for papillary and
follicular thyroid cancer. J Clin Endocrinol Metab. 86:1447–1463.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hay ID, Thompson GB, Grant CS, Bergstralh
EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL,
et al: Papillary thyroid carcinoma managed at the Mayo Clinic
during six decades (1940–1999): Temporal trends in initial therapy
and long-term outcome in 2444 consecutively treated patients. World
J Surg. 26:879–885. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hobert O: Gene regulation by transcription
factors and microRNAs. Science. 319:1785–1786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosenfeld N, Aharonov R, Meiri E,
Rosenwald S, Spector Y, Zepeniuk M, Benjamin H, Shabes N, Tabak S,
Levy A, et al: MicroRNAs accurately identify cancer tissue origin.
Nat Biotechnol. 26:462–469. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu L, Yang Y, Hou J, Zhai C, Song Y, Zhang
Z, Qiu L and Jia X: MicroRNA-144 affects radiotherapy sensitivity
by promoting proliferation, migration and invasion of breast cancer
cells. Oncol Rep. 34:1845–1852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang SY, Lu ZM, Lin YF, Chen LS, Luo XN,
Song XH, Chen SH and Wu YL: miR-144-3p, a tumor suppressive
microRNA targeting ETS-1 in laryngeal squamous cell carcinoma.
Oncotarget. 7:11637–11650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen S, Li P, Li J, Wang Y, Du Y, Chen X,
Zang W, Wang H, Chu H, Zhao G and Zhang G: MiR-144 inhibits
proliferation and induces apoptosis and autophagy in lung cancer
cells by targeting TIGAR. Cell Physiol Biochem. 35:997–1007. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao T, Li H, Hu Y, Ma D and Cai X: miR-144
suppresses the proliferation and metastasis of hepatocellular
carcinoma by targeting E2F3. Tumour Biol. 35:10759–10764. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo Y, Ying L, Tian Y, Yang P, Zhu Y, Wang
Z, Qiu F and Lin J: miR-144 downregulation increases bladder cancer
cell proliferation by targeting EZH2 and regulating Wnt signaling.
FEBS J. 280:4531–4538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Larionov A, Krause A and Miller W: A
standard curve based method for relative real time PCR data
processing. BMC Bioinformatics. 6:622005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. Springer;
New York, NY: 2010
|
|
21
|
Liu C, Huang W and Lei Q: Regulation and
function of the TAZ transcription co-activator. Int J Biochem Mol
Biol. 2:247–256. 2011.PubMed/NCBI
|
|
22
|
de Cristofaro T, Di Palma T, Ferraro A,
Corrado A, Lucci V, Franco R, Fusco A and Zannini M: TAZ/WWTR1 is
overexpressed in papillary thyroid carcinoma. Eur J Cancer.
47:926–933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen AY, Jemal A and Ward EM: Increasing
incidence of differentiated thyroid cancer in the United States,
1988–2005. Cancer. 115:3801–3807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:pp. 2257–2261.
2006; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He H, Jazdzewski K, Li W, Liyanarachchi S,
Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, et al:
The role of microRNA genes in papillary thyroid carcinoma. Proc
Natl Acad Sci USA. 102:pp. 19075–19080. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pallante P, Visone R, Ferracin M, Ferraro
A, Berlingieri MT, Troncone G, Chiappetta G, Liu CG, Santoro M,
Negrini M, et al: MicroRNA deregulation in human thyroid papillary
carcinomas. Endocr Relat Cancer. 13:497–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Santinon G, Pocaterra A and Dupont S:
Control of YAP/TAZ activity by metabolic and nutrient-sensing
pathways. Trends Cell Biol. 26:289–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hansen CG, Moroishi T and Guan KL: YAP and
TAZ: A nexus for Hippo signaling and beyond. Trends Cell Biol.
25:499–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Santucci M, Vignudelli T, Ferrari S, Mor
M, Scalvini L, Bolognesi ML, Uliassi E and Costi MP: The Hippo
pathway and YAP/TAZ-TEAD protein-protein interaction as targets for
regenerative medicine and cancer treatment. J Med Chem.
58:4857–4873. 2015. View Article : Google Scholar : PubMed/NCBI
|