Introduction
The incidence of adenocarcinoma of the
esophago-gastric junction (EGJ) is increasing in Western (1,2) and
Eastern (3–5) countries. By contrast, the prevalence of
squamous cell carcinoma (SCC) remains increased in Japan compared
with Western countries.
Siewert et al (6) proposed a classification system for
adenocarcinoma of the EGJ, and discussed the characteristics and
treatments of these according to the individual type of disease.
The authors proposed that patients with type I cancer should be
treated similarly to those with distal esophageal cancer, whereas
patients with type II and III cancer should undergo transhiatal
total gastrectomy, lower esophagectomy, lower mediastinal lymph
node dissection and extended (D2) lymph node dissection similar to
that performed in patients with gastric cancer. Conversely, a
previous study in which >50% of the patients had Siewert type II
adenocarcinoma of the EGJ identified that treatment with
esophagectomy was associated with improved patient outcomes
compared with treatment with gastrectomy (7). In Japan, Sasako et al (8) reported that a left thoracoabdominal
approach does not improve survival rate following an
abdominal-transhiatal approach and leads to increased morbidity in
patients with predominantly Siewert type II and III EGJ
adenocarcinoma. As aforementioned, the treatment strategies for
these types of cancer are so diverse that the optimal treatment
strategy has not yet been well established. Furthermore, the
aforementioned studies included only patients with adenocarcinoma
and did not include patients with SCC, although SCC occurs at a
high incidence in Eastern countries.
In Western countries, the standard treatment
modality, other than surgery, for patients with esophageal or EGJ
cancer is preoperative chemoradiotherapy (9–11). In
Japan, a randomized controlled trial by the Japan Clinical Oncology
Group (JCOG9204) which compared postoperative adjuvant chemotherapy
with cisplatin and 5-fluorouracil with surgery alone for treatment
of patients with esophageal SCC demonstrated superior disease-free
survival rates in the group of patients who received postoperative
chemotherapy (12). Furthermore,
another study by the same group (JCOG9907) demonstrated that
patients with esophageal SCC who received preoperative adjuvant
chemotherapy had superior overall survival rates compared with
those that received postoperative adjuvant chemotherapy (13). In the JCOG9204 study, locoregional
recurrences were observed in <50% of patients who experienced
recurrences. In Japan, the performance of aggressive surgeries for
the treatment of esophageal cancer is hypothesized to be one reason
for the lower rates of local recurrences and, therefore, the
eradication of systemic micrometastases by chemotherapy followed by
surgery is standard therapy in Japan. However, these treatment
strategies did not allow for appropriate control of distant
recurrences in locations including the liver.
In the present study, patients with EGJ cancer,
including those with SCC, were investigated to identify independent
prognostic factors and to clarify the patterns of recurrences for
those with poor prognoses. Additionally, a treatment strategy for
EGJ cancer is proposed.
Materials and methods
Definition of EGJ cancer
EGJ cancer was defined as cancer that invaded the
EGJ and exhibited tumor epicenters that were located ≤5 cm from the
EGJ. Tumors were classified as follows: Type I, tumor epicenters
located between 1 and 5 cm above the EGJ; type II, tumor epicenters
located between 1 cm above and 2 cm below the EGJ; and type III,
tumor epicenters located between 2 and 5 cm below the EGJ. The
locations of the epicenters were comprehensively evaluated on the
basis of results obtained by upper gastrointestinal series and
upper gastrointestinal endoscopy. In patients who received
preoperative chemotherapy, tumors were classified on the basis of
their characteristics prior to receiving chemotherapy.
Patients and clinicopathological
evaluations
Between January 1997 and December 2012, a total of
3,004 patients underwent surgery for esophageal or gastric cancer
at the Kitasato University School of Medicine (Sagamihara,
Kanagawa, Japan). Of these patients, 191 (6.4%) underwent surgical
resections for EGJ adenocarcinoma or SCC. Of these, 41 patients who
had undergone R1 or R2 resections were excluded. The medical
records of the remaining 150 patients who underwent curative (R0)
surgery were retrospectively reviewed in order to identify the
independent prognostic factors and clarify recurrence patterns. The
median follow-up period was 48 months (interquartile range, 26–68
months).
Tumor depths and lymph node metastases were
classified according to the International Union Against Cancer
Tumor Node and Metastases staging system, 7th edition (14).
Perioperative transfusion (POT) was defined as the
allogeneic blood transfusion performed during surgery or within the
first 2 postoperative days, as previously reported (15).
The present study was conducted in accordance with
The Declaration of Helsinki and was approved by the Research Ethics
Committee of the Kitasato University School of Medicine. The
requirement for informed consent was waived due to the
retrospective nature of the study.
Surgical procedures and morbidity
Surgical procedures were determined based on the
tumor locations and the lengths of esophageal invasions. A right
thoracic approach (RTA) was used to perform subtotal esophagectomy
and mediastinal lymph node dissections through right thoracotomy,
and gastric conduit reconstructions were performed through
laparotomy. A left thoracic approach (LTA) through left thoracotomy
and laparotomy and a transhiatal approach (THA) following
wide-splitting of the esophageal hiatus were used to perform total
gastrectomy, distal esophagectomy, D2 lymph node dissections
including splenectomy and lower mediastinal lymph node dissections.
For clinical T1 cancer, proximal gastrectomy and jejunal
interposition or esophago-gastric anastomosis were performed,
wherever possible. Video-assisted thoracoscopic esophagectomy was
performed through an RTA in only 5 patients; therefore, these
patients were included in the RTA group for analysis.
Surgical complications were classified by the
surgical approach using the Clavien-Dindo classification (16,17).
Chemotherapy
Of the patients who received preoperative
chemotherapy (n=29), 18 had marginally resectable disease and 11
had stage IV disease. For these patients, the combination of
docetaxel, cisplatin and S-1 (tegafur, gimeracil and oteracil
potassium) (DCS), cisplatin and 5-fluorouracil (CF), or cisplatin
and S-1 (CS) were commonly used for chemotherapy. The treatment
schedules for DCS, CF and CS have been described previously
(13,18,19). The
most commonly used regimen was DCS (in 11/18 patients with
marginally resectable disease and 3/11 patients with cStage IV
disease). All the patients who received DCS had adenocarcinoma. The
second most commonly used regimen was CF (in 5/18 patients with
marginally resectable disease). All the patients who received CF
had SCC.
A number of patients also received postoperative
adjuvant chemotherapy (n=56). The most commonly used regimen was
S-1 monotherapy (in 30/56 patients) (20,21). All
the patients who received S-1 monotherapy had adenocarcinoma.
Statistical analysis
Disease-specific survival (DSS) was measured from
the date of surgery or from the date of starting chemotherapy in
patients who received preoperative chemotherapy, to the date of
mortality from EGJ cancer or date of last follow-up. The causes of
mortality were identified from the hospital medical records.
Patients who succumbed to causes other than EGJ cancer were
regarded as censored at the time of mortality. Patients who were
alive at their last visit were also regarded as censored. Student's
t-test was used to analyze continuous variables and a χ2
test or Fisher's exact test was used to analyze categorical
variables. Survival rates were calculated by the Kaplan-Meier
estimator method (22). Univariate
analyses of prognostic factors for DSS were performed using
log-rank tests. Factors with P<0.10 on univariate analyses were
subjected to multivariate analysis using Cox's proportional hazards
model to identify independent prognostic factors. All calculations
were performed using JMP® 10 software (SAS Institute
Inc., Cary, NC, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics and stage
distributions
The characteristics of patients included in the
present study are listed in Table I.
The proportion of patients with type I, II and III cancer was 14%
(21/150), 62% (93/150) and 24% (36/150), respectively. The median
number of dissected lymph nodes was 37 (range, 5–96). A number of
patients received preoperative chemotherapy (n=29; 19%) and
pathologically complete responses in primary tumors (ypT0) were
obtained in 6 patients; 4 of these received DCS and 2 received
CS.
 | Table I.Patient characteristics (n=150). |
Table I.
Patient characteristics (n=150).
| Characteristic | No. | Proportion, % | Range |
|---|
| Median age,
years | 68 |
| 31–87 |
| Sex |
|
|
|
| Male | 124 | 83. |
|
|
Female | 26 | 17 |
|
| ASA |
|
|
|
| I | 47 | 31 |
|
| II | 91 | 61 |
|
|
III | 12 | 8 |
|
| Histological
type |
|
|
|
|
Adenocarcinoma | 130 | 87 |
|
|
Squamous cell carcinoma | 20 | 13 |
|
| Tumor type |
|
|
|
| I | 21 | 14 |
|
| II | 93 | 62 |
|
|
III | 36 | 24 |
|
| Tumor depth
(histological) |
|
|
|
|
(y)pT0 |
6 | 4 |
|
|
(y)pT1a |
7 | 5 |
|
|
(y)pT1b | 27 | 18 |
|
|
(y)pT2 | 19 | 13 |
|
|
(y)pT3 | 91 | 61 |
|
| Nodal stage
(histological) |
|
|
|
|
(y)pN0 | 62 | 41 |
|
|
(y)pN1 | 31 | 21 |
|
|
(y)pN2 | 31 | 21 |
|
|
(y)pN3 | 26 | 17 |
|
| Splenectomy |
|
|
|
|
Yes | 58 | 39 |
|
| No | 92 | 61 |
|
| POT |
|
|
|
|
Yes | 40 | 27 |
|
| No | 110 | 73 |
|
| Approach |
|
|
|
| Right
thoracic | 30 | 20 |
|
| Left
thoracic | 16 | 11 |
|
|
Transhiatal | 104 | 69 |
|
| Preoperative
chemotherapy |
|
|
|
|
Yes | 29 | 19 |
|
| No | 121 | 81 |
|
| Postoperative
chemotherapy |
|
|
|
|
Yes | 56 | 37 |
|
| No | 94 | 63 |
|
| Dissected lymph
nodes |
|
|
|
|
Median | 37 |
| 5–96 |
| Metastatic lymph
nodes |
|
|
|
|
Median |
1 |
| 0–25 |
Surgical approach, morbidity and
mortality
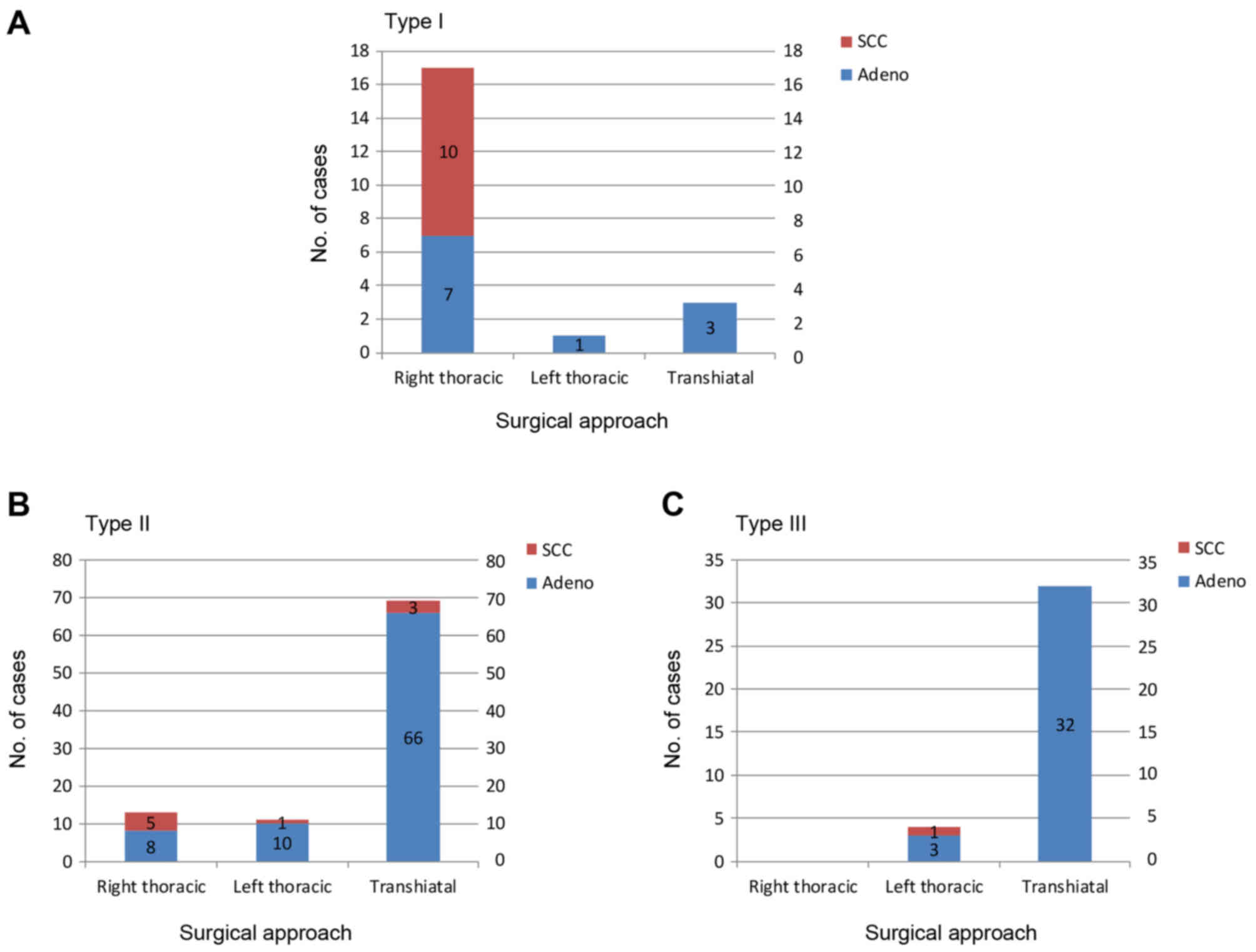
Surgery was performed via an RTA in 81% (17/21) of
the patients with type I tumors. A THA was used in 74% (69/93) and
89% (32/36) of the patients with type II and III tumors,
respectively (Fig. 1A-C).
The frequency of Clavien-Dindo Grade IIIa or IIIb
surgical complications was higher with the RTA, followed by the LTA
and the THA. Clavien-Dindo Grade IVb surgical complications were
identified in only 1 patient who underwent an RTA (Table II). In-hospital mortalities occurred
in 3 patients (2%). Of these, 1 had undergone an RTA and the others
had undergone a THA.
 | Table II.Morbidity classified by surgical
approach and Clavien-Dindo classification. |
Table II.
Morbidity classified by surgical
approach and Clavien-Dindo classification.
|
| THA (n=104) | LTA (n=16) | RTA (n=30) |
|---|
|
|
|
|
|
|---|
| Cause of
morbidity | IIIa | IIIb | IVa | IVb | V | IIIa | IIIb | IVa | IVb | IIIa | IIIb | IVa | IVb | V |
|---|
| Pulmonary
complicationsa | 1 (1) | 0 | 2 (2) | 0 | 1 (1) | 0 | 0 | 0 | 0 | 3 (10) | 1 (3) | 0 | 1 (3) | 0 |
| Anastomotic
leakage | 4 (4) | 2 (2) | 0 | 0 | 0 | 1 (6) | 0 | 0 | 0 | 6 (20) | 0 | 0 | 0 | 1 (3) |
| Recurrent nerve
palsy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (7) | 0 | 0 | 0 |
| Diaphragmatic
hernia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3) | 0 | 0 | 0 |
| Chylous
leakage | 1 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pancreatic
fistula | 7 (7) | 0 | 0 | 0 | 0 | 2 (13) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal
abscess | 3 (3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Small bowel
obstruction | 0 | 1 (1) | 0 | 0 | 0 | 0 | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cholecystitis | 0 | 0 | 0 | 0 | 0 | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wound
infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3) | 0 | 0 | 0 |
| Wound
dehiscence | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3) | 0 | 0 | 0 |
| Postoperative
hemorrhage | 1 (1) | 1 (1) | 0 | 0 | 1 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 17 (16) | 4 (4) | 2 (2) | 0 | 2 (2) | 4 (25) | 1 (6) | 0 | 0 | 9 (30) | 6 (20) | 0 | 1 (3) | 1 (3) |
Survival rate analysis
The 5-year DSS rate for all patients with EGJ cancer
was 72%. Patients with tumors classified as post-treatment primary
tumor stage 3 [(y)pT3] or higher had a 5-year DSS rate of 53% and
those with tumors classified as (y)pT0-2 had an 5-year DSS rate of
90%. Therefore, only patients with tumors classified as (y)pT3 or
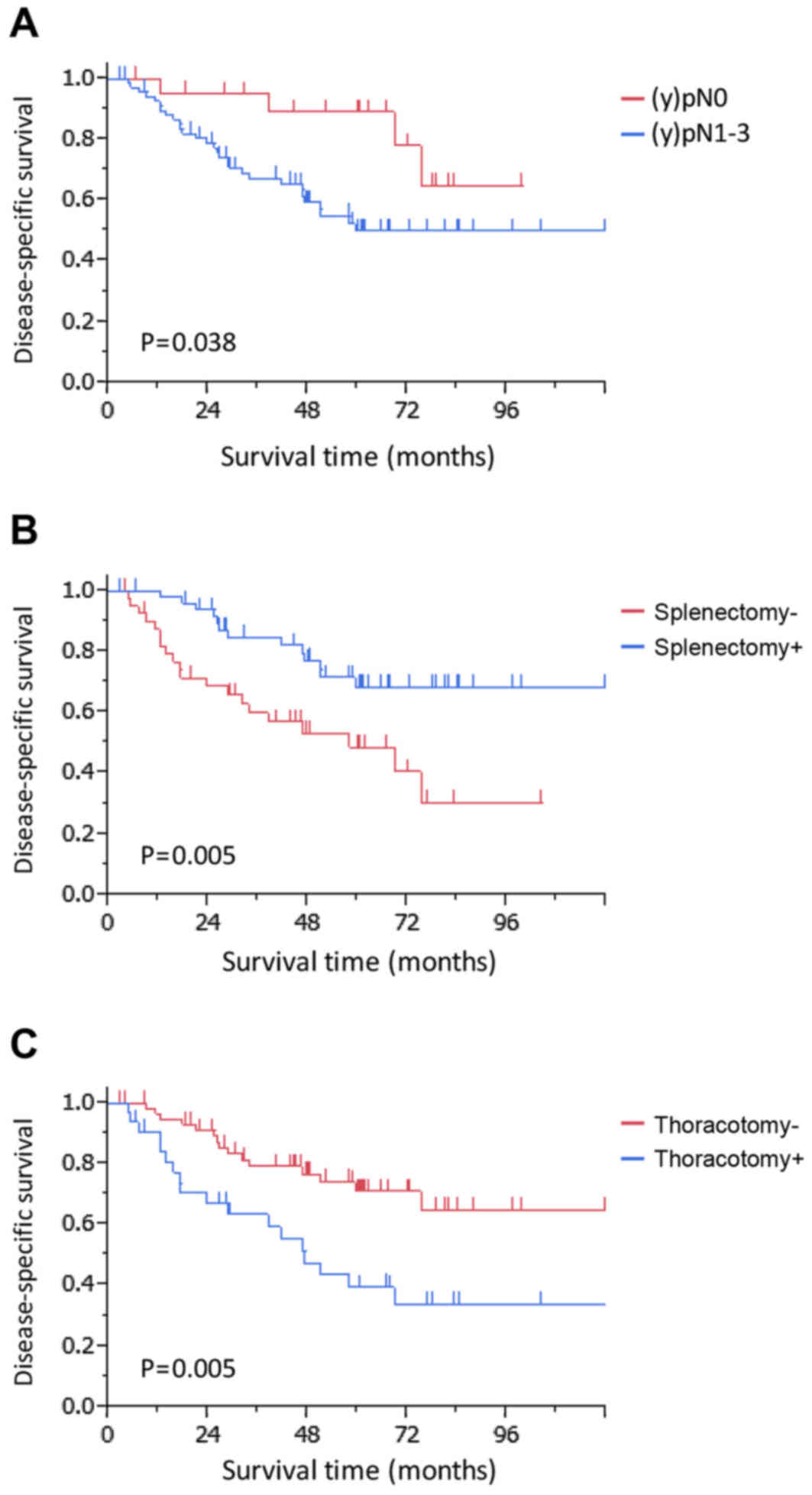
higher were analyzed further. In the univariate analysis, the
following factors were identified as potential predictors of poor
survival rate: a pathological nodal stage of post-treatment
regional lymph node stage 1–3 [(y)pN1-3] (P=0.038), splenectomy not
performed (P=0.005), thoracotomy performed (P=0.005) and POT
(P=0.093) (Table III). The
multivariate Cox's proportional hazards model revealed that a
pathological tumor nodal stage of (y)pN1-3 [hazard ratio (HR),
3.62; 95% confidence interval (CI), 1.39–12.36; P=0.006],
splenectomy not performed (HR, 2.40; 95% CI, 1.15–5.15; P=0.020)
and thoracotomy (HR, 2.07; 95% CI, 1.02–4.23; P=0.044) were
independent prognostic factors for poor DSS (Table III; Fig.
2).
 | Table III.Prognostic analysis in patients with
(y)pT3 EGJ cancer. |
Table III.
Prognostic analysis in patients with
(y)pT3 EGJ cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | Number | Proportion, % | 5-year DSS, % | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
≤65 | 51 | 56 | 59 | 0.46 |
|
|
|
|
<65 | 40 | 44 | 59 |
|
|
|
|
| Sex |
|
|
|
|
|
|
|
|
Male | 72 | 79 | 54 | 0.21 |
|
|
|
|
Female | 19 | 21 | 77 |
|
|
|
|
| Tumor type |
|
|
|
|
|
|
|
| I | 11 | 12 | 55 | 0.68 |
|
|
|
| II | 49 | 54 | 60 |
|
|
|
|
|
III | 31 | 34 | 59 |
|
|
|
|
| Esophageal
invasion, cm |
|
|
|
|
|
|
|
| ≤3 | 73 | 80 | 62 | 0.12 |
|
|
|
|
>3 | 18 | 20 | 46 |
|
|
|
|
| (y)pN |
|
|
|
|
|
|
|
|
(y)pN0 | 21 | 23 | 89 | 0.038a | 1.00 |
| 0.006a |
|
(y)pN1-3 | 70 | 77 | 50 |
| 3.62 | 1.39–12.36 |
|
| Histology |
|
|
|
|
|
|
|
|
Adeno | 77 | 85 | 58 | 0.52 |
|
|
|
|
SCC | 14 | 15 | 64 |
|
|
|
|
| Preoperative
chemotherapy |
|
|
|
|
|
|
|
|
Yes | 17 | 19 | 57 | 0.85 |
|
|
|
| No | 74 | 81 | 60 |
|
|
|
|
| Postoperative
chemotherapy |
|
|
|
|
|
|
|
|
Yes | 46 | 51 | 66 | 0.34 |
|
|
|
| No | 45 | 49 | 53 |
|
|
|
|
| POT |
|
|
|
|
|
|
|
|
Yes | 28 | 31 | 43 | 0.093 | 1.28 | 0.61–2.59 | 0.50 |
| No | 63 | 69 | 66 |
| 1.00 |
|
|
| Splenectomy |
|
|
|
|
|
|
|
|
Yes | 50 | 55 | 68 | 0.005a | 1.00 |
| 0.020a |
| No | 41 | 45 | 49 |
| 2.40 | 1.15–5.15 |
|
| Thoracotomy |
|
|
|
|
|
|
|
|
Yes | 32 | 35 | 40 | 0.005a | 2.07 | 1.02–4.23 | 0.044a |
| No | 59 | 65 | 71 |
| 1.00 |
|
|
Combination analysis of independent
prognostic factors in patients with tumors classified as (y)pT3 or
higher
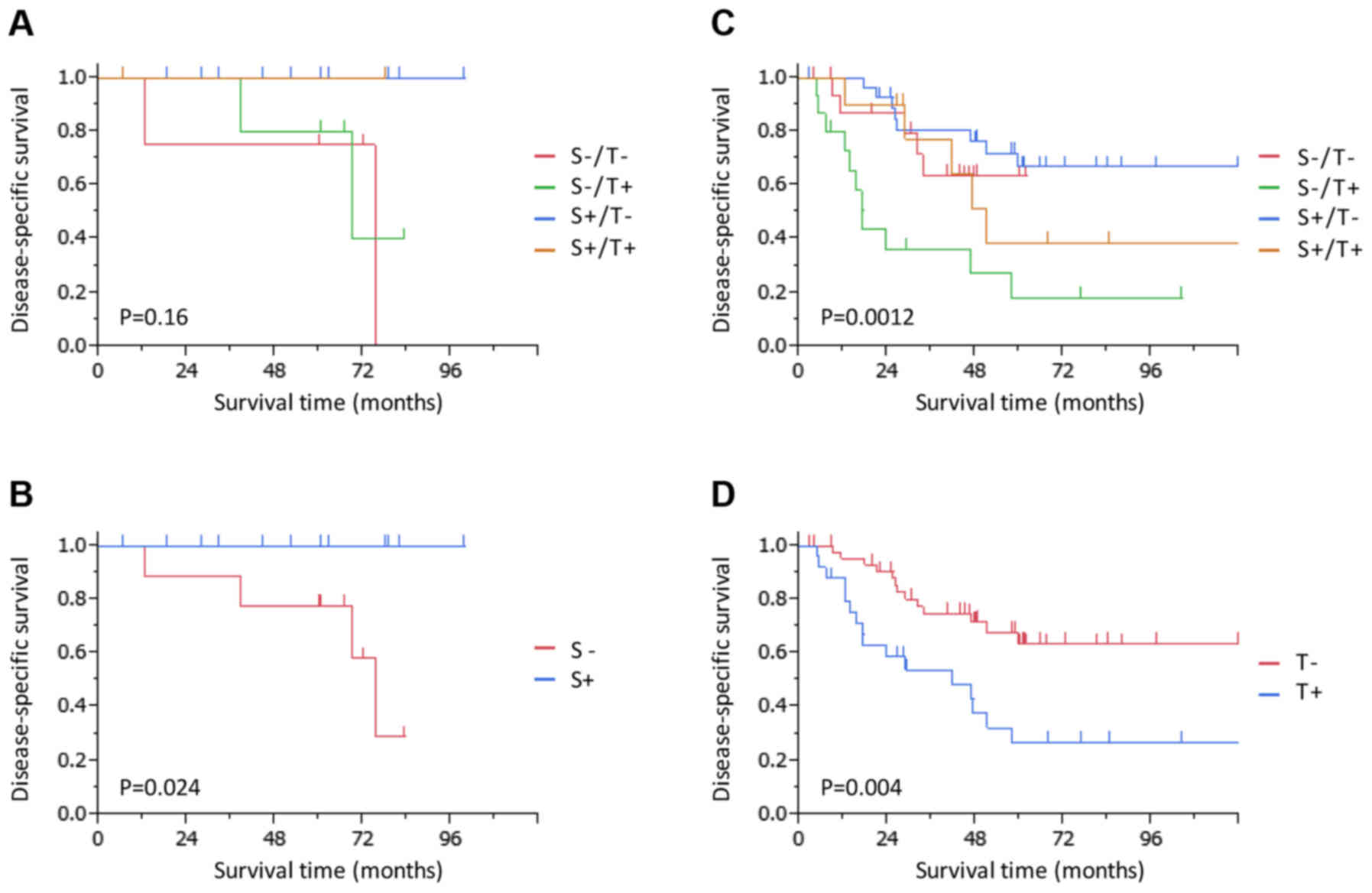
Combination analyses were conducted using
independent prognostic factors, namely, thoracotomy and
splenectomy, according to the (y)pN stage. In the patients with
(y)pN0 tumors, the 5-year DSS rate is presented according to their
status of treatment with thoracotomy and splenectomy (Fig. 3A). Notably, the 5-year DSS rate was
100% in patients who underwent splenectomy (Fig. 3B); the DSS was significantly improved
for patients who underwent splenectomy compared with for those who
did not (P=0.024). On the other hand, in the patients with (y)pN1-3
tumors, the 5-year DSS rate of patients who underwent thoracotomy
without undergoing splenectomy was as low as 18% (Fig. 3C); the DSS was significantly worse for
patients who underwent thoracotomy compared with for those who did
not (P=0.004; Fig. 3D).
Initial recurrence site in patients
with tumors classified as (y)pT3 or higher
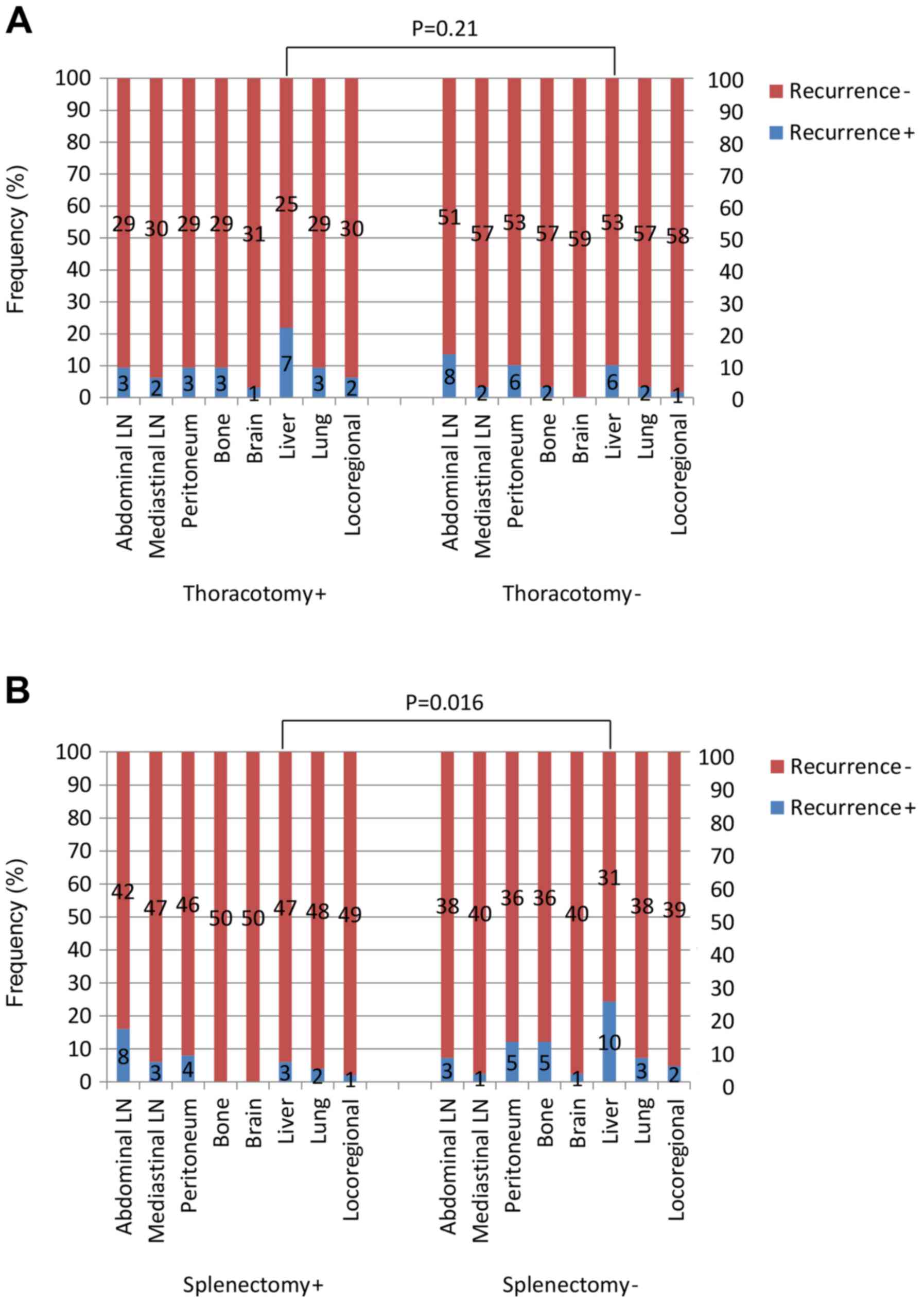
The proportion of patients whose tumors recurred
initially at locoregional sites was only 6% (5/91). Patients who
underwent thoracotomy and splenectomy and those who did not undergo
either procedure were analyzed further for their initial sites of
tumor recurrence. The proportion of patients with initial liver
recurrences tended to be larger in the group of patients who
underwent thoracotomy compared with those that underwent laparotomy
only (P=0.21; Fig. 4A). The
corresponding proportion of patients was significantly larger in
the group that did not undergo splenectomy compared with the group
that did (P=0.016; Fig. 4B). In the
patients with adenocarcinoma, the proportion of patients with
initial liver recurrences was larger among those who underwent
thoracotomy compared with those who underwent laparotomy only (19
vs. 10%; P=0.29). This corresponding proportion was also
significantly larger in the group of patients that did not undergo
splenectomy compared with the group that did (24 vs. 6%; P=0.024;
data not shown).
Sub-analysis of patients with tumors
classified as (y)pT3 or higher with initial recurrence sites in the
liver
Patients with initial recurrences in the liver were
analyzed further. Patients with type I cancer tended to exhibit
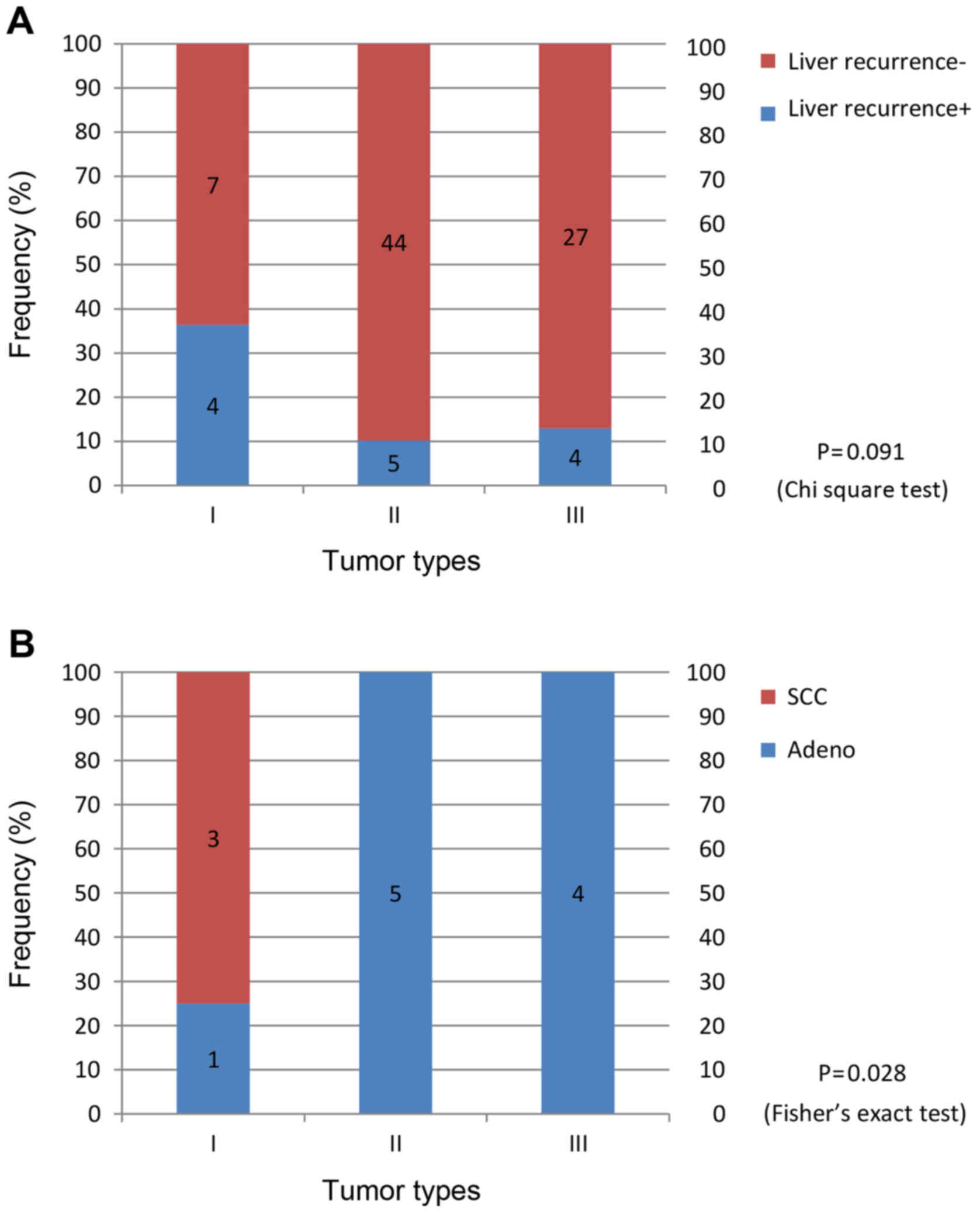
initial recurrences in the liver (P=0.091; Fig. 5A). Of the 4 patients with type I
cancer who had initial recurrences in the liver, 3 had SCC
(Fig. 5B). Of the 7 patients in the
thoracotomy group who experienced initial recurrences in the liver,
3 patients had type I SCC (Table
IV). In the group that did not undergo splenectomy, 3/10
patients who had initial recurrences in the liver had type I SCC.
These 3 patients were the same individuals aforementioned (Table IV) and only 1 of them had received
preoperative chemotherapy (CF).
 | Table IV.List of patients with Siewert type I
squamous cell carcinoma who had initial recurrence in the liver
following thoracotomy. |
Table IV.
List of patients with Siewert type I
squamous cell carcinoma who had initial recurrence in the liver
following thoracotomy.
| Age, years | Sex | (y)pT | (y)pN | (y)p stage TNM
7th | Preoperative
chemotherapy | Esophageal
invasion, mm | PM, mm | POT | Splenectomy | Adjuvant
regimen |
|---|
| 58 | M | 3 | 3 | IIIC | N | 51 | 78 | N | N | 5FU, cisplatin,
MMC |
| 74 | M | 3 | 3 | IIIC | N | 60 | 87 | Y | N | Radiation |
| 66 | M | 3 | 0 | IIA | CF | 40 | 85 | N | N | N |
Discussion
The prognostic analyses of EGJ cancer identified
that undergoing thoracotomy and splenectomy may affect the DSS in
patients. Patients who underwent thoracotomy and did not undergo
splenectomy tended to experience initial recurrences in the liver.
In addition, patients whose tumors initially recurred in the liver
tended to have type I SCC. The patients with type I SCC usually
underwent thoracotomy, not splenectomy, and data revealed that such
patients are prone to tumor recurrences in the liver. This may
indicate that type I SCC possesses biological behavior differing
from that of other EGJ cancer. In the institute where the present
study was conducted, type I SCC is included with thoracic
esophageal SCC, with respect to therapeutic strategy. Nevertheless,
2/3 type I SCC which exhibited recurrence in the liver were not
treated with preoperative chemotherapy. Thus, potent preoperative
chemotherapy may be required for patients with type I SCC to
improve their survival rates. However, in patients with
adenocarcinoma, the same effects of thoracotomy and splenectomy
were observed, in terms of initial liver recurrences.
Thoracotomy was one of the independent factors for
poor prognosis. The incidence rate of liver recurrences in patients
who underwent thoracotomy tended to be increased compared with the
corresponding rate in patients who did not undergo thoracotomy. In
patients undergoing surgery for non-small cell lung cancer,
compared with video-assisted thoracic surgery (VATS), thoracotomy
is reportedly associated with more marked decreases in the numbers
of circulating natural killer cells and more marked increases in
the plasma levels of matrix metalloproteinase-9 (23). In an experimental model, the excessive
surgical stress of undergoing a thoracolaparotomy was revealed to
markedly enhance tumor metastasis, by a mechanism that involved
immunosuppression (24). The results
of the present study may also reflect a subset of
immunosuppression. VATS may compensate for this drawback of
thoracotomy.
Splenectomy was also identified to be an independent
prognostic factor. However, the treatment of gastric cancer or EGJ
cancer with splenectomy is controversial. Numerous studies on
treatment of cancer with splenectomy have been published describing
the high morbidity rates and low survival rate benefits of such
treatment (25). However, Huang et
al (26) reported that
splenectomy is beneficial for splenic hilar lymph node dissections
in patients with advanced proximal gastric cancer and is associated
with improved survival rates. In the present study, 4 patients
exhibited metastatic splenic hilar lymph nodes. Of these, 1
patient, whose tumor had widely invaded the greater curvature of
the stomach, survived for 5 further years without experiencing any
recurrence of the tumor.
Splenectomy has been reported to serve an important
role in the antitumor immune response in an experimental model
(27–29). Higashijima et al (28) reported that splenectomy enhances liver
metastasis through an increase in forkhead box P3 mRNA in the
liver. On the other hand, Sonoda et al (29) reported that the metastatic lung
nodules were significantly smaller in size and number in the
subject group that underwent splenectomy compared with the control
group in a mouse metastasis model and concluded that this occurred
due to splenectomy-induced decreases in serum levels of vascular
endothelial growth factor and basic fibroblast growth factor. In
the present study, patients with (y)pT3N0 EGJ cancer who underwent
splenectomy had improved DSS compared with those who did not
undergo splenectomy. Splenectomy may exert a negative impact on the
recurrence of tumors by enhancing antitumor immunity. Furthermore,
patients with (y)pT3N1-3 EGJ cancer who underwent thoracotomy
without splenectomy had poorer prognostic outcomes (5-year DSS,
18%), which may be the result of the negative impact on antitumor
immunity caused by the combination of undergoing thoracotomy
without undergoing splenectomy. Patients who underwent thoracotomy
had significantly more severe complications compared with those who
did not; therefore, the immunosuppression caused by the
complications may be associated with the negative impact on
survival rate.
In Western countries, the standard therapy for
patients with esophageal or EGJ cancer is preoperative
chemoradiotherapy (9–11). However, the rate of locoregional
recurrences in patients who received preoperative chemoradiotherapy
was reported to be as high as 12.3 and 5.2% in patients with SCC
and adenocarcinoma, respectively (10). The use of chemotherapeutic drugs,
including carboplatin and paclitaxel, or 5-fluorouracil and
cisplatin did not appear to limit hematogenous recurrences. On the
other hand, locoregional recurrences were observed in only 6% of
patients in the series, although none of the patients received
preoperative chemoradiotherapy. In patients with EGJ
adenocarcinoma, the most frequent type of recurrence was reported
to be hematogenous spread to the liver and lungs (30,31). In
Korea, a randomized controlled trial evaluating the addition of
radiation therapy to postoperative chemotherapy with capecitabine
plus cisplatin for gastric or EGJ tumors following D2 dissections
did not identify any significant benefits with the inclusion of
radiation in the therapeutic regimen (32). That study indicated that the addition
of radiation to preoperative or postoperative chemotherapy, which
is commonly performed in Western countries, may not be effective
against EGJ cancer in Eastern countries where the majority of
surgeons are able to perform extensive lymph node dissections that
lead to good local control of tumors. Therefore, rather than
chemoradiotherapy, more aggressive preoperative chemotherapy and R0
surgery with adequate lymph node dissections was able to influence
improved oncological outcomes for patients with EGJ cancer.
The combination DCF has been used to treat
unresectable and recurrent SCC of the esophagus and is well
tolerated with high response rates (33,34).
Recently, preoperative chemotherapy using DCF was reported to have
increased survival rate benefits for patients with resectable and
advanced esophageal cancer (35). In
that study, significantly fewer distant metastases were observed in
the patients treated with DCF (23%; 7/30 patients) compared with
those treated with CF (52%; 13/25 patients; P=0.048). In the
present study, preoperative chemotherapy with DCS was preferred in
patients with EGJ adenocarcinoma. However, there is little evidence
supporting the use of DCS as preoperative chemotherapy for patients
with esophageal or esophago-gastric cancer (in adenocarcinoma and
SCC) and certain patients were not able to receive oral drugs,
including S-1, due to the stenosis caused by their EGJ cancer.
Therefore, preoperative chemotherapy using DCF may be a reasonable
treatment strategy for patients with EGJ cancer.
In the present study, patients with (y)pT0-2 tumors
were identified to have a 5-year DSS rate of 90%. However, 8
patients experienced recurrences and 3 had complete responses of
their primary tumors to preoperative chemotherapy. Of the 3
patients, 2 had initial recurrences in the brain. In the present
study, 29 patients underwent preoperative chemotherapy and 6
experienced complete responses of their primary tumors to
chemotherapy, i.e., of the 6 patients with complete responses of
primary tumors to preoperative chemotherapy, 3 experienced
recurrences and 2 experienced initial recurrences in the brain.
Patients who experience complete responses of their primary tumors
or metastatic lymph nodes to preoperative chemotherapy have been
reported to exhibit improved prognoses (36,37).
However, in cases where patients experience micrometastases in the
brain, these metastases may survive even if the cells themselves
are susceptible to chemotherapeutic agents as the blood-brain
barrier precludes the entry of chemotherapeutic agents into the
brain. Thus, recurrences in the brain become evident. Therefore, in
patients with complete responses to preoperative chemotherapy, the
regulation of brain metastases is important.
The present study has several important limitations.
First, the analysis was based on retrospective data collected at a
single center and the number of patients included was low.
Secondly, treatment strategies were not necessarily specified. For
example, an LTA has rarely been used since the results of the
JCOG9502 study were reported (8). The
regimens and indications for preoperative chemotherapy were also
heterogeneous. Thirdly, the choice of surgical approach and
performance of splenectomy included numerous selection biases.
In conclusion, splenectomy and thoracotomy may
critically affect prognoses in patients with (y)pT3 EGJ cancer
classified as (y)pN0 and (y)pN1-3 respectively, and the prognostic
differences may result from a distinct distribution of liver
recurrences. These results also suggested that careful attention
must be paid to improving surgical maneuvers, by means including
the use of less invasive surgery and splenectomy, to improve
prognoses in EGJ cancer with pT3 invasion.
References
|
1
|
Devesa SS, Blot WJ and Fraumeni JF Jr:
Changing patterns in the incidence of esophageal and gastric
carcinoma in the United States. Cancer. 83:2049–2053. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pohl H and Welch HG: The role of
overdiagnosis and reclassification in the marked increase of
esophageal adenocarcinoma incidence. J Natl Cancer Inst.
97:142–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kusano C, Gotoda T, Khor CJ, Katai H, Kato
H, Taniguchi H and Shimoda T: Changing trends in the proportion of
adenocarcinoma of the esophagogastric junction in a large tertiary
referral center in Japan. J Gastroenterol Hepatol. 23:1662–1665.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blaser MJ and Saito D: Trends in reported
adenocarcinomas of the oesophagus and gastric cardia in Japan. Eur
J Gastroenterol Hepatol. 14:107–113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamashita K, Sakuramoto S, Nemoto M,
Shibata T, Mieno H, Katada N, Kikuchi S and Watanabe M: Trend in
gastric cancer: 35 years of surgical experience in Japan. World J
Gastroenterol. 17:3390–3397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rüdiger Siewert J, Feith M, Werner M and
Stein HJ: Adenocarcinoma of the esophagogastric junction: Results
of surgical therapy based on anatomical/topographic classification
in 1,002 consecutive patients. Ann Surg. 232:353–361. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barbour AP, Rizk NP, Gonen M, Tang L,
Bains MS, Rusch VW, Coit DG and Brennan MF: Adenocarcinoma of the
gastroesophageal junction: Influence of esophageal resection margin
and operative approach on outcome. Ann Surg. 246:1–8. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sasako M, Sano T, Yamamoto S, Sairenji M,
Arai K, Kinoshita T, Nashimoto A and Hiratsuka M; Japan Clinical
Oncology Group (JCOG9502), : Left thoracoabdominal approach versus
abdominal-transhiatal approach for gastric cancer of the cardia or
subcardia: A randomised controlled trial. Lancet Oncol. 7:644–651.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Hagen P, Hulshof MC, van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al: Preoperative
chemoradiotherapy for esophageal or junctional cancer. N Engl J
Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zanoni A, Verlato G, Giacopuzzi S,
Weindelmayer J, Casella F, Pasini F, Zhao E and de Manzoni G:
Neoadjuvant concurrent chemoradiotherapy for locally advanced
esophageal cancer in a single high-volume center. Ann Surg Oncol.
20:1993–1999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tepper J, Krasna MJ, Niedzwiecki D, Hollis
D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D and Mayer
R: Phase III trial of trimodality therapy with cisplatin,
fluorouracil, radiotherapy, and surgery compared with surgery alone
for esophageal cancer: CALGB 9781. J Clin Oncol. 26:1086–1092.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ando N, Iizuka T, Ide H, Ishida K, Shinoda
M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N, et al:
Surgery plus chemotherapy compared with surgery alone for localized
squamous cell carcinoma of the thoracic esophagus: A Japan Clinical
Oncology Group Study-JCOG9204. J Clin Oncol. 21:4592–4596. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ando N, Kato H, Igaki H, Shinoda M, Ozawa
S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumours. 7th. Wiley-Blackwell; New
York, NY: 2009
|
|
15
|
Weitz J, D'Angelica M, Gonen M, Klimstra
D, Coit DG, Brennan MF and Karpeh MS: Interaction of splenectomy
and perioperative blood transfusions on prognosis of patients with
proximal gastric and gastroesophageal junction cancer. J Clin
Oncol. 21:4597–4603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clavien PA, Barkun J, de Oliveira ML,
Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J,
Slankamenac K, Bassi C, et al: The Clavien-Dindo classification of
surgical complications: Five-year experience. Ann Surg.
250:187–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koizumi W, Nakayama N, Tanabe S, Sasaki T,
Higuchi K, Nishimura K, Takagi S, Azuma M, Ae T, Ishido K, et al: A
multicenter phase II study of combined chemotherapy with docetaxel,
cisplatin, and S-1 in patients with unresectable or recurrent
gastric cancer (KDOG 0601). Cancer Chemother Pharmacol. 69:407–413.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): a phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sasako M, Sakuramoto S, Katai H, Kinoshita
T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and
Ohashi Y: Five-year outcomes of a randomized phase III trial
comparing adjuvant chemotherapy with S-1 versus surgery alone in
stage II or III gastric cancer. J Clin Oncol. 29:4387–4393. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
23
|
Ng CS, Wan IY and Yim AP: Impact of
video-assisted thoracoscopic major lung resection on immune
function. Asian Cardiovasc Thorac Ann. 17:426–432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirai T, Matsumoto H, Yamashita K, Urakami
A, Iki K, Yamamura M and Tsunoda T: Surgical oncotaxis-excessive
surgical stress and postoperative complications contribute to
enhancing tumor metastasis, resulting in a poor prognosis for
cancer patients. Ann Thorac Cardiovasc Surg. 11:4–6.
2005.PubMed/NCBI
|
|
25
|
Goto H, Tokunaga M, Sugisawa N, Tanizawa
Y, Bando E, Kawamura T, Niihara M, Tsubosa Y and Terashima M: Value
of splenectomy in patients with Siewert type II adenocarcinoma of
the esophagogastric junction. Gastric Cancer. 16:590–595. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang CM, Wang JB, Lu HS, Zheng CH, Li P,
Xie JW and Zhang XF: Prognostic impact of splenectomy on advanced
proximal gastric cancer with No. 10 lymph node metastasis. Chin Med
J (Engl). 122:2757–2762. 2009.PubMed/NCBI
|
|
27
|
Shiratori Y, Kawase T, Nakata R, Tanaka M,
Hikiba Y, Okano K, Matsumura M, Niwa Y, Komatsu Y, Shiina S, et al:
Effect of splenectomy on hepatic metastasis of colon carcinoma and
natural killer activity in the liver. Dig Dis Sci. 40:2398–2406.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Higashijima J, Shimada M, Chikakiyo M,
Miyatani T, Yoshikawa K, Nishioka M, Iwata T and Kurita N: Effect
of splenectomy on antitumor immune system in mice. Anticancer Res.
29:385–393. 2009.PubMed/NCBI
|
|
29
|
Sonoda K, Izumi K, Matsui Y, Inomata M,
Shiraishi N and Kitano S: Decreased growth rate of lung metastatic
lesions after splenectomy in mice. Eur Surg Res. 38:469–475. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wayman J, Bennett MK, Raimes SA and
Griffin SM: The pattern of recurrence of adenocarcinoma of the
oesophago-gastric junction. Br J Cancer. 86:1223–1229. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamashita H, Katai H, Morita S, Saka M,
Taniguchi H and Fukagawa T: Optimal extent of lymph node dissection
for Siewert type II esophagogastric junction carcinoma. Ann Surg.
254:274–280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee J, Lim DH, Kim S, Park SH, Park JO,
Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, et al: Phase III trial
comparing capecitabine plus cisplatin versus capecitabine plus
cisplatin with concurrent capecitabine radiotherapy in completely
resected gastric cancer with D2 lymph node dissection: The ARTIST
trial. J Clin Oncol. 30:268–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takahashi H, Arimura Y, Yamashita K,
Okahara S, Tanuma T, Kodaira J, Hokari K, Tsukagoshi H, Shinomura Y
and Hosokawa M: Phase I/II study of
docetaxel/cisplatin/fluorouracil combination chemotherapy against
metastatic esophageal squamous cell carcinoma. J Thorac Oncol.
5:122–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamasaki M, Miyata H, Tanaka K, Shiraishi
O, Motoori M, Peng YF, Yasuda T, Yano M, Shiozaki H, Mori M, et al:
Multicenter phase I/II study of docetaxel, cisplatin and
fluorouracil combination chemotherapy in patients with advanced or
recurrent squamous cell carcinoma of the esophagus. Oncology.
80:307–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ui T, Fujii H, Hosoya Y, Nagase M, Mieno
MN, Mori M, Zuiki T, Saito S, Kurashina K, Haruta H, et al:
Comparison of preoperative chemotherapy using docetaxel, cisplatin
and fluorouracil with cisplatin and fluorouracil in patients with
advanced carcinoma of the thoracic esophagus. Dis Esophagus.
28:180–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Korst RJ, Kansler AL, Port JL, Lee PC,
Kerem Y and Altorki NK: Downstaging of T or N predicts long-term
survival after preoperative chemotherapy and radical resection for
esophageal carcinoma. Ann Thorac Surg. 82:480–485. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Noble F, Nolan L, Bateman AC, Byrne JP,
Kelly JJ, Bailey IS, Sharland DM, Rees CN, Iveson TJ, Underwood TJ,
et al: Refining pathological evaluation of neoadjuvant therapy for
adenocarcinoma of the esophagus. World J Gastroenterol.
19:9282–9293. 2013. View Article : Google Scholar : PubMed/NCBI
|