Introduction
The vast majority of oral cancers are squamous cell
carcinomas and include cancers in the tongue, cheeks, mouth floor,
lips and gingiva. This form of cancer usually originates from
normal oral mucosa, which progresses to dysplasia, then to squamous
cell carcinoma, and ultimately to metastatic carcinoma (1). Invasion and metastasis occur early in
oral cancer; therefore, regional lymph node metastasis is an
important factor in the high mortality of oral cancer. Many aspects
of the mechanism of lymphatic metastasis from oral cancer are still
unknown. Because of ethical reasons, it is very difficult to obtain
continuous carcinoma specimens of oral cancer from dysplasia to
metastasis from the same patients. Therefore, it is necessary to
establish an animal model in which the development and the
pathology of oral cancer echo those of the corresponding human
cancers, especially in relation to the origin of lymphatic
metastases.
Many studies indicate that the administration of
4-nitroquinoline-1-oxide (4NQO) to mice or rats effectively induces
oral cancer that resembles oral tumor growth in humans (2–4). However,
regional lymph node metastasis from oral cancer has barely been
examined in the 4NQO model. In a previous study, we have described
the development of a lymph node metastases from oral carcinoma
mouse model through the long-term administration of high-dose 4NQO
and a prolonged observation period (5). However, the molecular events that, in
this model, occur at the different stages of oral tumor
carcinogenesis have not yet been investigated. Furthermore, the
biological and molecular similarities of oral cancer lymph node
metastasis between this animal model and humans have not been
examined. Thus, in the present study, we try to expolre this point
by comparing the expressions of TGF (TGF)-β1, E-cadherin,
N-cadherin, TP53, RB1CC1 and HIF-1α in different stages of oral
squamous cell carcinoma (OSCC) in human and mouse.
The epithelial-mesenchymal transition (EMT) plays an
important role in the dissemination and metastasis of oral cancer:
It is characterized by the induction of a variety of cytokines and
chemokines, which destroy normal cell adhesion, lead to the loss of
cell-cell interaction, contribute to the recombination of
cytoskeleton, cause cell invasion and eventually result in the
dissemination and metastasis of oral cancer cells (6).
TGF-β1 plays a pivotal role in the activation of
EMT. TGF-β1 is a cytokine with multifunctional biological activity,
which can induce EMT by a variety of signal transduction mechanisms
(7). In addition, TGF-β1 is involved
in cell proliferation, differentiation, apoptosis, and plays an
important role in immune regulation. The change of the expression
and distribution of TGF-β1 in oral pre-cancerous lesions increase
the risk of cancer (8).
E-cadherin and N-cadherin are important members of
the family of cadherins, which are Ca2+-dependent cell
adhesive glycoproteins. E-cadherin is expressed in epithelial
tissues, while N-cadherin is expressed in neural tissues (9). During tumor invasion and metastasis, the
replacement of E-cadherin with N-cadherin results in the loss of
polarity and adhesion in epithelial cells; consequently, the
epithelial cells acquire the characteristics of mesenchymal cells,
and gain the ability to invade and metastasize. This change is an
important process in EMT; therefore, the cadherin switch is
considered a critical mechanism in tumor progression and metastasis
(10).
The occurrence of many tumors is related to the
activation of oncogenes, the inactivation of cancer suppressor
genes, or a combination of both. TP53 is a cancer suppressor
gene and is considered the defender of the genome. The inactivation
of TP53 (also called p53) causes the loss of p53 tumor suppressor
activity, promoting the malignant transformation of the cells. TP53
mutation is related to the pathologic grade, clinical stage and
lymph node metastasis of human oral squamous cell carcinoma (OSCC).
Changes in TP53 in cancer tissue are an independent factor for poor
prognosis in OSCC (11,12).
RB1-Inducible Coiled-coil 1 (RB1CC1) plays a
fundamental role in autophagosome formation (13). Studies had shown that autophagy can
improve the adaptability of tumor cells: Through degradation of
their components, normal cells can provide energy for the tumor
cells (14). The evolutionary
conserved protein encoded by RB1CC1 can interact with p53;
together, RB1CC1 and p53 regulate multiple signaling pathways in
the cells (13), thus controlling the
cell cycle and inhibiting cell proliferation (15). A study has pointed that RB1CC1 is
associated with early breast carcinogenesis (16).
Hypoxia is an important initiating factor in cancer.
In the initial stage of cancer, local hypoxia activates
hypoxia-inducible factor-1α (HIF-1α), which upregulates the
expression of the downstream vascular endothelial growth factor
(VEGF). VEGF induces the formation of blood and lymphatic vessels,
and promotes the rapid proliferation of cancer cells (17–19).
By detecting and comparing the expression of the
above described molecular biomarkers in normal oral mucosa,
dysplasia, OSCC and lymph node metastases samples from patients and
mice, the objective of this research had further demonstrated that
the mouse model we built closely mimics the human oral
carcinogenesis and lymphatic metastases, and to preliminarily
explore the function of these biomarkers in the development of oral
cancer.
Materials and methods
Patients
A total of 72 human samples (12 normal, 24
dysplastic, 24 OSCC and 12 lymph node-metastatic carcinoma) were
obtained from the patients of the stomatological hospital
affiliated to the Guangxi Medical University, from January 2012 to
June 2016. The patients aged between 27 and 78 years and were an
even mix of men and women. None of them received chemotherapy or
radiation therapy before sample collection. The normal tissues were
obtained from the gingiva, the dysplasia and OSCC tissues from the
tongue and the lymph node metastasis carcinoma tissues from the
neck lymph nodes of the patients. Informed consent was obtained
from all the participants. The Human Ethics Committee of Guangxi
Medical University, China, approved this study.
Animals
Mouse samples (9 normal, 20 dysplastic, 20 OSCC and
9 lymph node-metastatic carcinoma) were obtained from the Balb/c
mouse model lymphatic metastases from oral carcinoma, induced by
4-NQO. The normal, dysplasia and OSCC tissues were obtained from
the tongue and the lymph node metastasis carcinoma tissues were
obtained from the submandibular lymph nodes of the mice. The Animal
Ethics Committee of Guangxi Medical University, China, approved
this study.
Samples
Specimens were fixed in 10% formalin, embedded in
paraffin, and sectioned. Each specimen was stained with
hematoxylin-eosin (HE) or immunohistochemical staining.
Immunohistochemistry assay (IHC)
The antibodies used in the present study were as
follows: Mouse monoclonal antibodies for E-cadherin and p53
(ZSGB-Bio, China), rabbit polyclonal antibodies for TGF-β1 and
HIF-1α (Boster Biological Technology, China), mouse monoclonal
antibody for N-cadherin (Santa Cruz, USA), and rabbit polyclonal
antibodies for RB1CC1 (Proteintech Group, China). All antibodies
used in the present study were suitable for the detection of
proteins in both humans and mouse.
Immunohistochemistry was performed on paraffin
sections. Deparaffinized sections were pretreated with 0.4% pepsin
for 60 min at 37°C. Endogenous peroxidase activity was quenched by
treatment with 0.2% H2O2 for 3 h. The
sections were then incubated with the specific antibodies overnight
at 4°C. In addition, sections incubated with 0.01 mol/l phosphate
buffer saline (PBS) and tongue cancer tissues incubated with the
chosen antibodies were used as negative and positive controls,
respectively. The immunostaining was visualized with an SP kit
(ZSGB-Bio, China) using a diaminobenzidine-peroxidase substrate.
The sections were counterstained with Mayer's hematoxylin and
examined using the image analyzer of a light microscope (Leica
Leitz DMRB/E, Leica Microsystems, Wetzlar, Germany).
Evaluation of the IHC results
TGF-β1, N-cadherin, TP53, RB1CC1, HIF-1a are
expressed in the cytoplasm and E-cadherin is expressed in
cytomembrane. IHC staining of these six proteins in the cells was
scored subjectively under a light microscope and the percentage of
stained tumor cells was expressed according to a previous study
(20), with little modification as
follows: 0–10% of cells stained, score 0; 11–25% of cells stained,
score 1; 26–50% of cells stained, score 2; 51–100% of cells
stained, score 3. Cells scoring 0/1 were considered to be negative,
and those scoring 2/3 were considered to be positive.
Statistical analysis
The statistical analysis of the biological markers
was performed using the Chi-square test. The correlation analysis
was performed using the Spearman rank correlation test. The results
were considered statistically significant if P<0.05.
Results
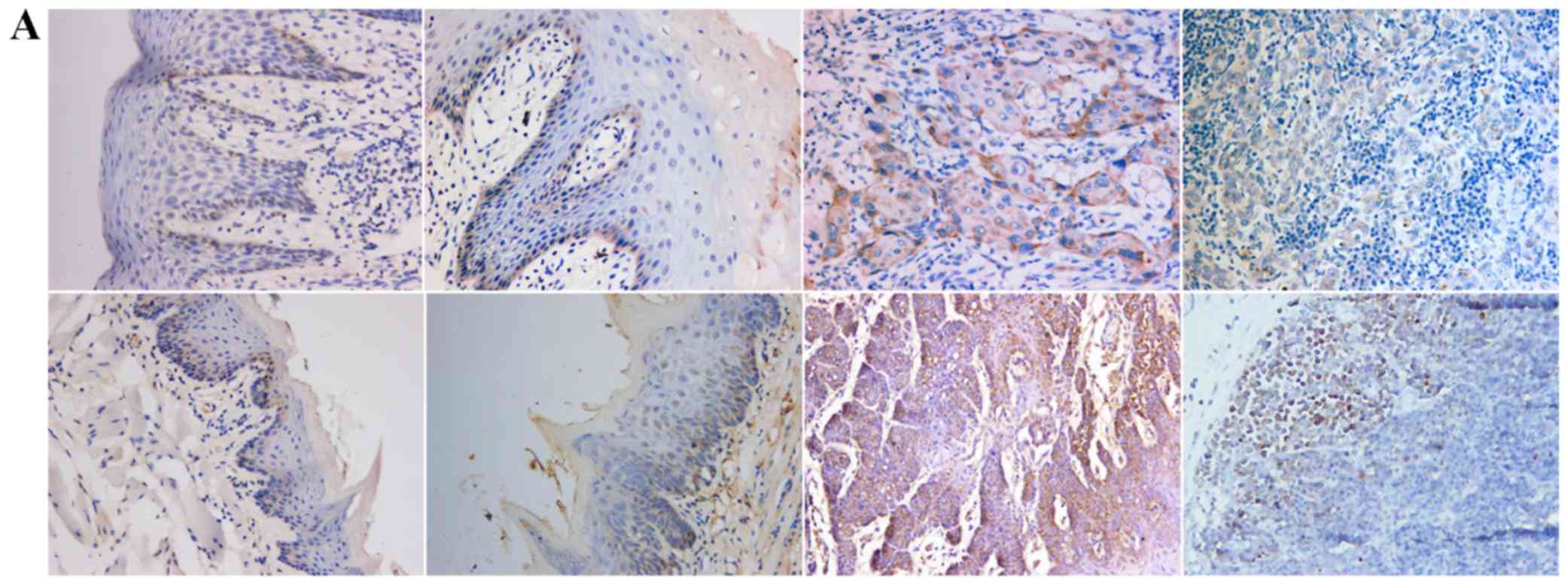
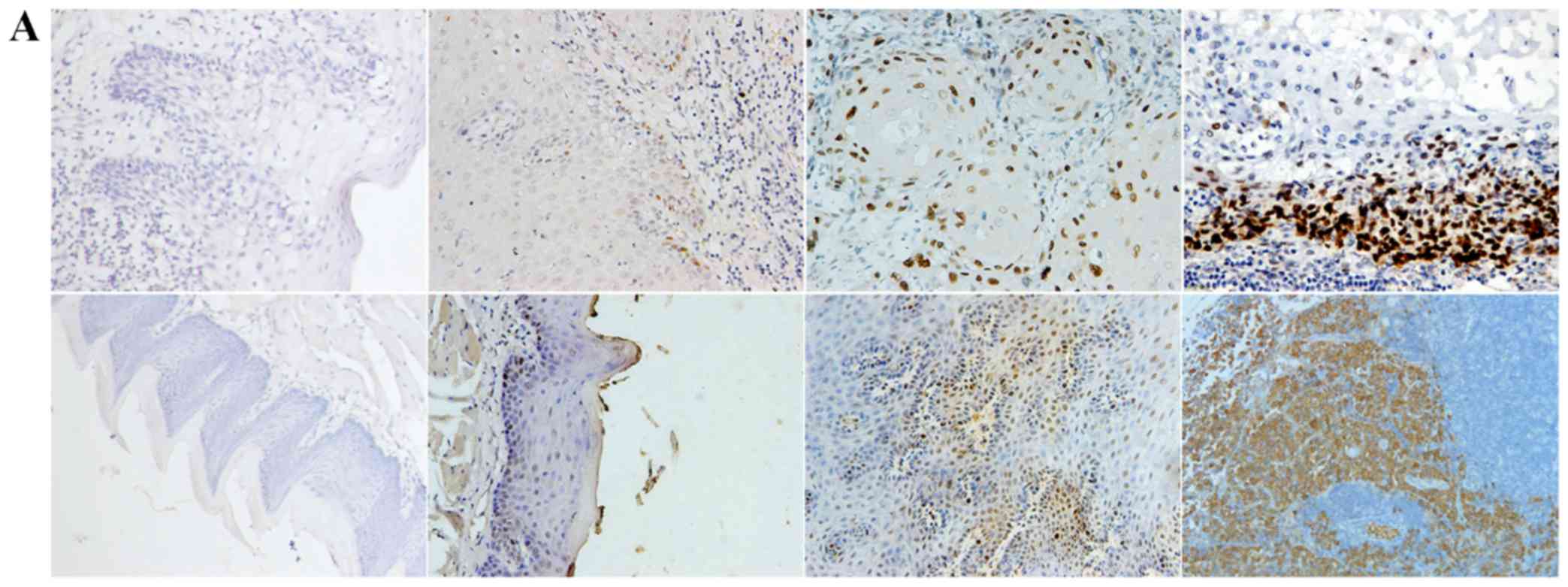
Positive expression of E-cadherin was mainly
observed in the cytomembrane, in both human and mouse samples
(Fig. 1A). Positive expression of
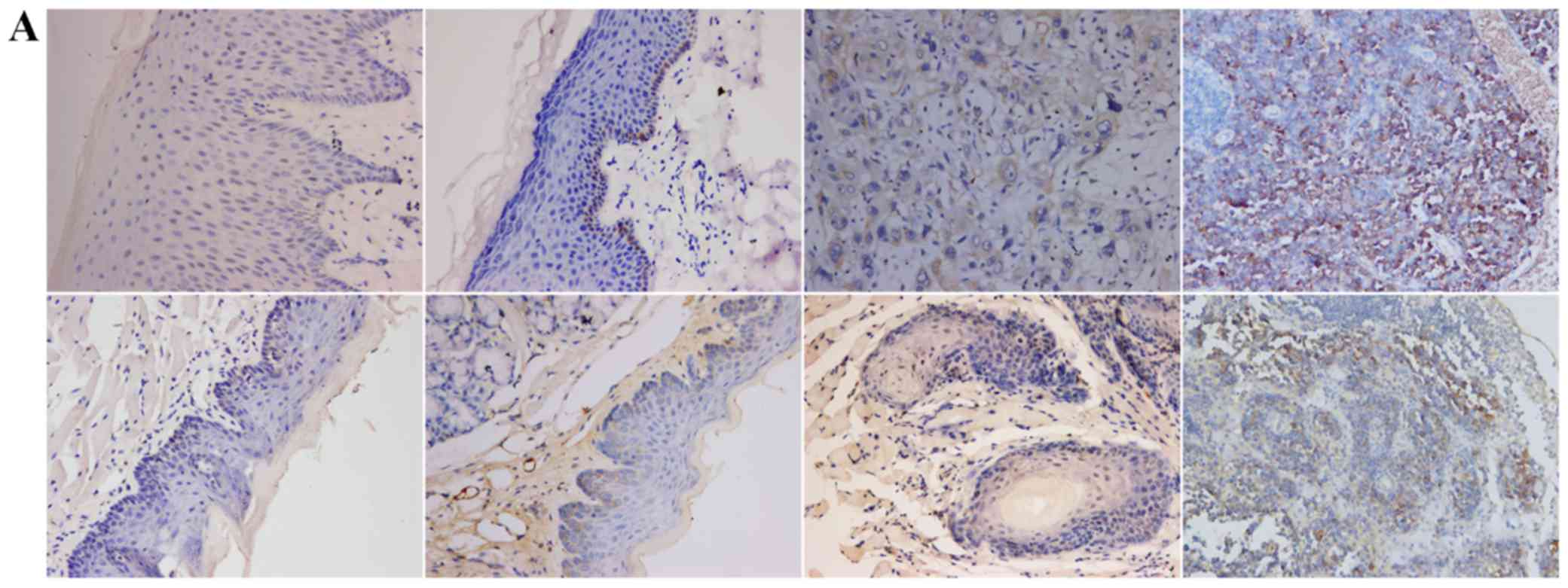
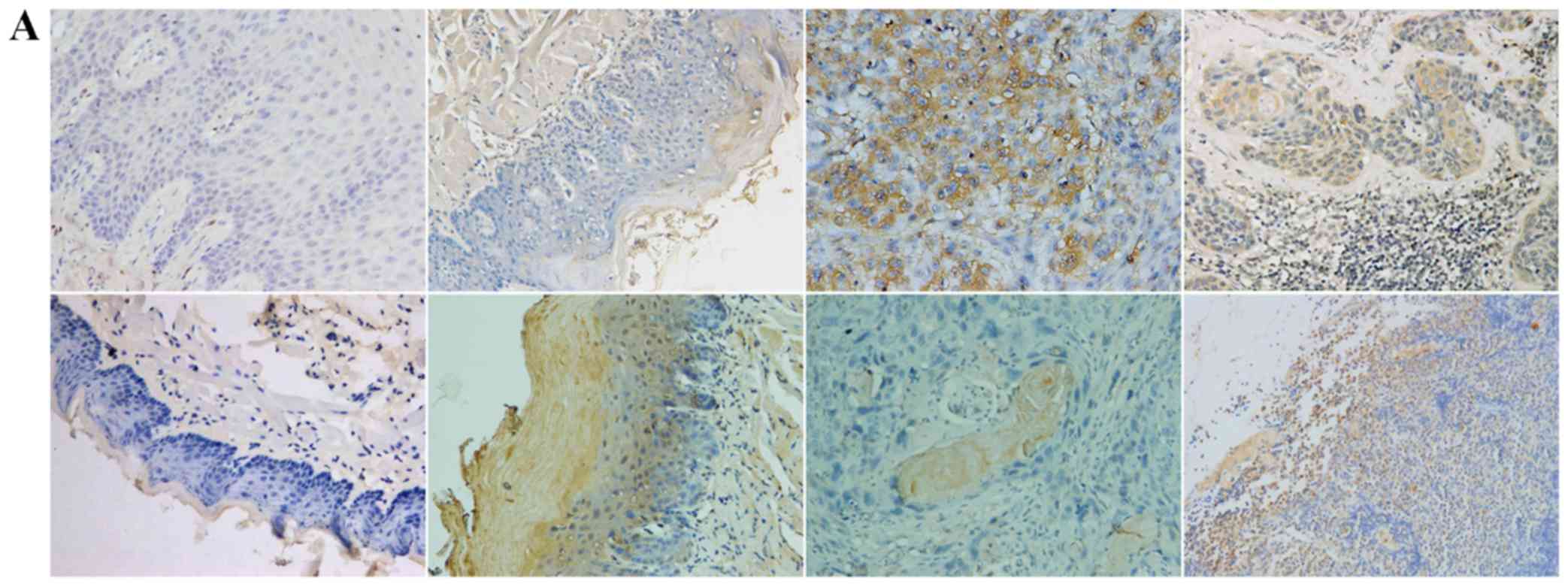
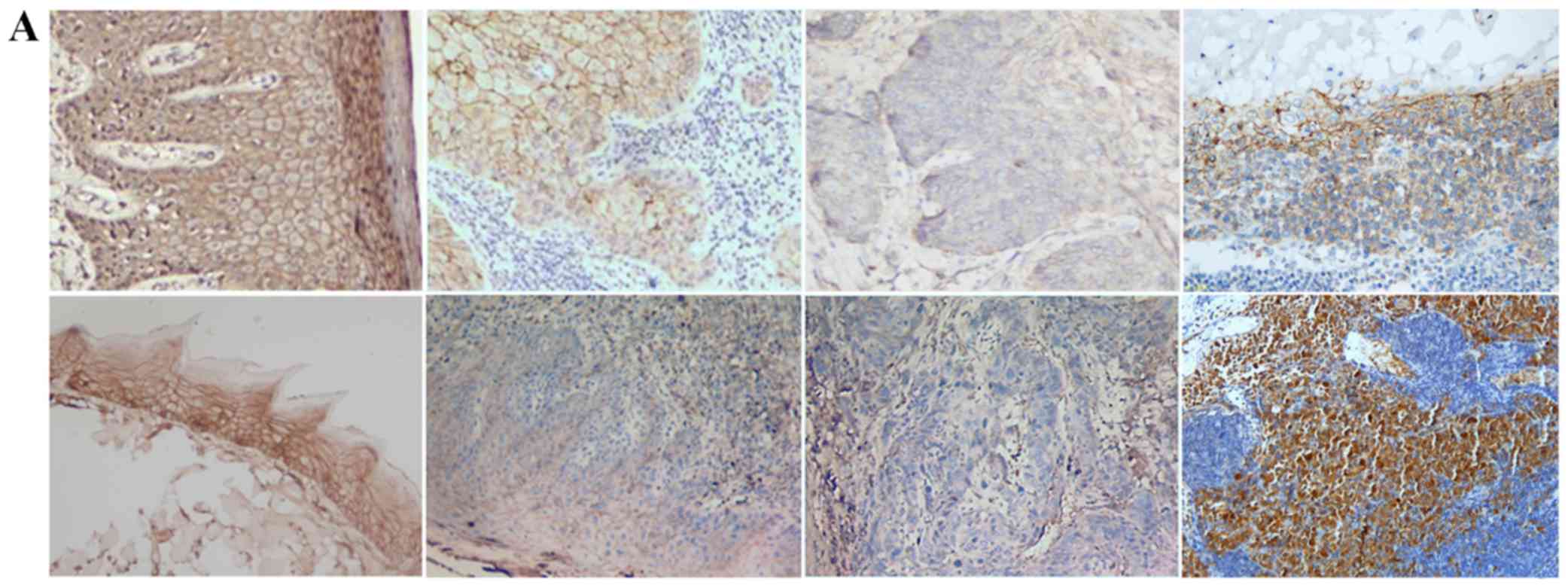
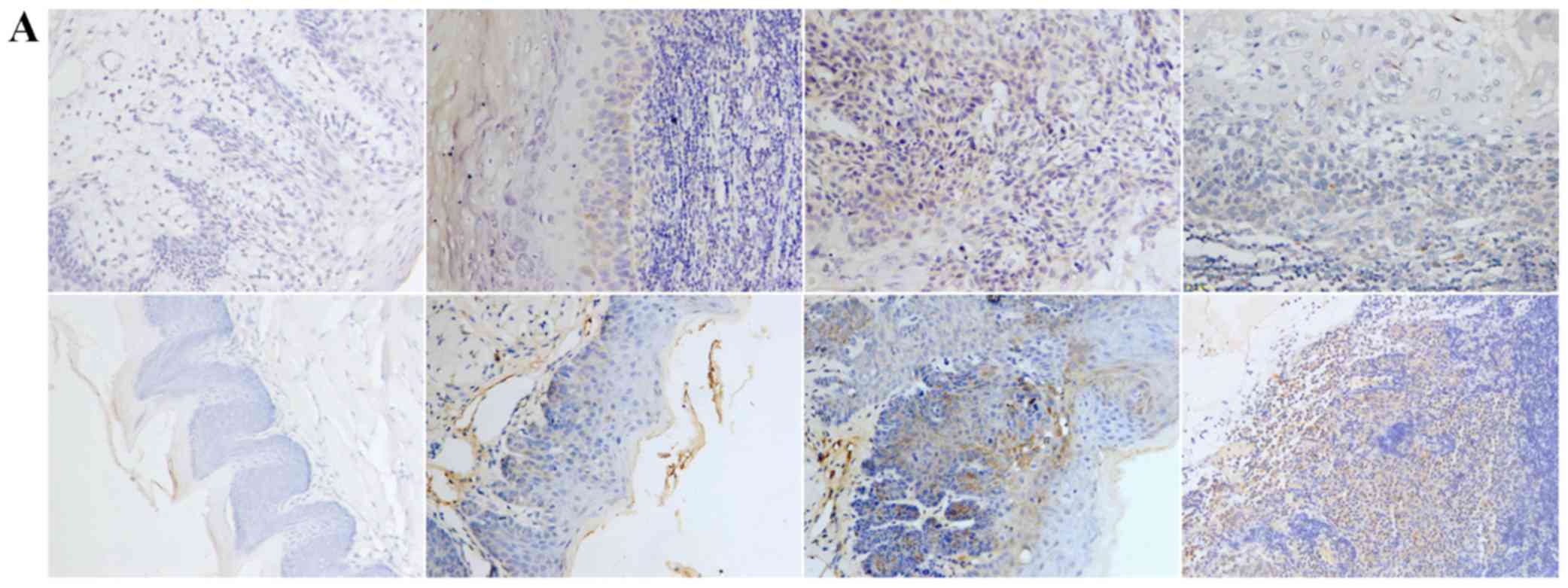
TGF-β1, N-cadherin, p53, RB1CC1 and HIF-1α was mainly detected in
the nucleus and the cytoplasm of cells, in both human and mouse
samples (Figs. 2–6A).
Moreover, the positive rates of the above mentioned
molecular biomarkers were analyzed and the results indicated that
there were no obvious differences between human and mouse samples
(P>0.05) (Figs. 1–6B-D). Figs. 1D
and 2D point to a significant
increase in the expression of TGF-β1 and N-cadherin in both human
and mouse samples in the progression from normal mucosa to lymph
node metastasis. Fig. 3D shows that
the expression of E-cadherin decreases in the progression from
normal mucosa to OSCC, but increases almost to its original amount
in both human and mouse lymph node metastases. Figs. 4D and 5D
indicates a significant increase in the expression of TP53 and
RB1CC1 in both human and mouse samples in the progression from
normal mucosa to lymph node metastasis. Additionally, the
expression of p53 positively correlates with that of RB1CC1 in both
human and mouse samples (r=0.971, P=0.029; r=0.97, P=0.03),
correlation analysis by Spearman rank correation test. Fig. 6D shows that the expression of HIF-1α
increases from normal mucosa to OSCC, but decreases in both human
and mouse lymph node metastases.
Discussion
Our study found that samples from the mouse model of
lymphatic metastases from oral cancer we built and human oral
cancer shared the similar expression in a wide variety of
biological molecular markers. Firstly, the expression of TGF-β1 at
different stages of oral cancer was similar in samples from
patients and from the mouse models, and it increased with the
progression of the cancer both cases. This finding corroborates a
study from Lu et al on head and neck squamous cell carcinoma
(21) and agrees with the notion that
TGF-β1 may promote EMT and accelerate tumor invasion and
metastasis. Secondly, with the progression of cancer, the
expression of E-cadherin decreased in mouse and human samples,
meanwhile, the expression of N-cadherin increased. Hazan et
al also reported the significant lack of E-cadherin in the most
terminal breast cancers and the increased expression of N-cadherin
(22). Studies on head and neck
cancer, colorectal cancer, pancreatic cancer and esophageal cancer
showed similar results (23–25). It is known that the switch from
E-cadherin to N-cadherin may cause the loss of adhesion between
cells, thus allowing tumor cells to leave the primary tumor and
migrate, and eventually induce metastases. Our study also found
that E-cadherin was re-expressed in both human and mouse lymph node
metastases, similarly to what has been observed in ovarian cancer
lymphatic metastases (26).
Therefore, we speculate that when the cancer cells reach the
targeted metastatic tissue, the re-expression of E-cadherin might
promote cancer cell colonization in the invaded tissue: Cancer
cells can, in such way, proliferate and invade to ultimately form
metastases. There might be a dynamic adjustment of protein
expression along with the change of microenvironment. In other
words, the expression of E-cadherin is time-dependent and
space-dependent. However, how this process starts and is fine-tuned
needs further exploration.
Thirdly, the expression of p53 and RB1CC1 in oral
mucosa dysplasia, OSCC and metastatic tissue from human and mouse
samples were higher than in oral normal tissue, and the expression
of p53 and RB1CC1 increased with progression of the cancer.
Nishioka et al found that, in human oral squamous cell
carcinoma, the expression of p53 increased with the progression of
OSCC (27). Suraneni et al
found that RB1CC1 was over-expressed in prostate cancer cells, and
increased with the malignant degree of the cancer (28). Wei and Guan found that RB1CC1 might
play a promoting role in early cancer in the MMTV-Pymt mouse model
of human breast cancer (29). The
studies above are consistent with our results. The possible
explanation is that p53 and RB1CC1 play a promoting role in the
procession of OSCC.
Our study also found that the expression of p53
correlates with that of RB1CC1. Morselli et al (30) found that p53 had a double effect on
autophagy. The fraction of p53 located in the nucleus stimulates
autophagy-inducing genes, while the fraction of p53 located in the
cytoplasm had the opposite effect. Wei et al found that
RB1CC1 regulated cell growth, division, proliferation, autophagy,
and apoptosis through the nuclear cytoplasmic shuttle mechanism and
multiple signaling pathways (31).
The mutual regulation between p53 and RB1CC1 may be the necessary
step to begin autophagy. When the tumor lacks nutrition, during its
rapid growth phase, autophagy might allow the tumor to grow more
quickly. Probably, the synergistic effect of p53 and RB1CC1 leads
to the development of OSCC.
Finally, we found that the expression of HIF-1α at
different stages of the oral cancer was similar in human and mouse
samples. The positive expression rate of HIF-1α raised from normal
to dysplastic oral mucosa, till reaching 100% in OSCC. Many studies
suggest that HIF-1α is over-expressed in a variety of malignant
human tumors (32). Bos et al
found that the increased expression of HIF-1α is an independent
factor predicting shorter prognosis in breast cancer (33). Aebersold et al also found that
94% of nasopharyngeal squamous carcinoma tissues had high
expression of HIF-1α (34). However,
the positive expression rate of HIF-1α in lymph node metastases
decreased in both human and mouse samples. The possible explanation
is that even though the primary cancer was anoxic, causing the
increased expression of HIF-1α, the metastases of the lymph nodes
might have had a good blood supply, therefore justifying the lower
expression of HIF-1α.
The results of our comparative study show that the
examined molecular markers in our mouse model of lymphatic
metastases from oral cancer and in samples from patients had
similar expression, and are probably the factors leading to the
activation of tumor, blood vessel formation, autophagy, EMT,
invasion and metastasis. In conclusion, the mouse model we built
may mimic human oral cancer and may be valuable to study the
mechanism of lymphatic metastases from oral carcinoma.
Acknowledgements
This study was supported by the National Nature
Science founding of China (grant nos. 81360407 and 81360403) and
the Guangxi Provincial Nature Science founding (grant no.
2013GXN5FAA019182).
References
|
1
|
Nauta JM, Roodenburg JL, Nikkels PG,
Witjes MJ and Vermey A: Comparison of epithelial dysplasia-the 4NQO
rat palate model and human oral mucosa. Int J Oral Maxillofac Surg.
24:53–58. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang XH, Knudsen B, Bemis D, Tickoo S and
Gudas LJ: Oral cavity and esophageal carcinogenesis modeled in
carcinogen-treated mice. Clin Cancer Res. 10:301–313. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fisker AV, Philipsen HP and Overvad K:
Dose-response relationship in complete oral 4NQO-carcinogenesis in
rats. Acta Pathol Microbiol Immunol Scand A. 95:281–288.
1987.PubMed/NCBI
|
|
4
|
Jiang C, Ye D, Qiu W, Zhang X, Zhang Z, He
D, Zhang P and Chen W: Response of lymphocyte subsets and cytokines
to Shenyang prescription in Sprague-Dawley rats with tongue
squamous cell carcinomas induced by 4NQO. BMC Cancer. 7:402007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Liang F, Yu D, Qing H and Yang Y:
Development of a 4-nitroquinoline-1-oxide model of lymph node
metastasis in oral squamous cell carcinoma. Oral Oncol. 49:299–305.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayashida T, Jinno H, Kitagawa Y and
Kitajima M: Cooperation of cancer stem cell properties and
epithelial-mesenchymal transition in the establishment of breast
cancer metastasis. J Oncol. 2011:5914272011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nawshad A, LaGamba D and Hay ED:
Transforming growth factor beta (TGFbeta) signalling in palatal
growth, apoptosis and epithelial mesenchymal transformation (EMT).
Archiv Oral Biol. 4:675–689. 2004. View Article : Google Scholar
|
|
8
|
Massagué J, Blain SW and Lo RS: TGFbeta
signaling in growth control, cancer, and heritable disorders. Cell.
103:295–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wheelock MJ and Johnson KR: Cadherins as
modulators of cellula phenotype. Annu Rev Cell Dev Biol.
19:207–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gravdal K, Halvorsen OJ, Haukaas SA and
Akslen LA: A switch from E-cadherin to N-cadherin expression
indicates epithelial to mesenchymal transition and is of strong and
independent importance for the progress of prostate cancer. Clin
Cancer Res. 13:7003–7011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bargonetti J and Manfredi JJ: Multiple
roles of the tumor suppressor p53. Curr Opin Oncol. 14:86–91. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tokino T and Nakamura Y: The role of
p53-target genes in human cancer. Crit Rev Oncol Hematol. 33:1–6.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei H, Wei S, Gan B, Peng X, Zou W and
Guan JL: Suppression of autophagy by FIP200 deletion inhibits
mammary tumorigenesis. Genes Dev. 25:1510–1527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lebovitz CB, Robertson AG, Goya R, Jones
SJ, Morin RD, Marra MA and Chandra B: Cross-cancer profiling of
molecular alterations within the human autophagy interaction
network. Autophagy. 11:1668–1687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei H, Wei S, Gan B, Peng X, Zou W and
Guan JL: Screening for microsatellite instability identifies
frequent 3′-untranslated region mutation of the RB1-inducible
coiled-coil 1 gene in colon tumors. PLoS One. 4:e77152009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmidt-Kittler O, Ragg T, Daskalakis A,
Granzow M, Ahr A, Blankenstein TJ, Kaufmann M, Diebold J, Arnholdt
H, Muller P, et al: From latent disseminated cells to overt
metastasis: Genetic analysis of systemic breast cancer progression.
Proc Natl Acad Sci USA. 100:pp. 7737–7742. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raica M, Cimpean AM and Ribatti D:
Angiogenesis in pre-malignant conditions. Eur J Cancer.
45:1924–1934. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Folkman J, Watson K, Ingber D and Hanahan
D: Induction of angiogenesis during the transition from hyperplasia
to neoplasia. Nature. 339:58–61. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jammal MP, Da Silva A, Filho AM, DE Castro
Côbo E, Adad SJ, Murta EF and Nomenili RS: Immunohistochemical
staining of tumor necrosis factor-α and interleukin-10 in benign
and malignant ovarian neoplasms. Oncol Lett. 9:979–983.
2015.PubMed/NCBI
|
|
21
|
Lu SL, Reh D, Li AG, Woods J, Corless CL,
Kulesz-Martin M and Wang XJ: Overexpression of transforming growth
factor beta1 in head and neck epithelia results in inflammation,
angiogenesis, and epithelial hyperproliferation. Cancer Res.
64:4405–4410. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hazan RB, Phillips GR, Qiao RF, Norton L
and Aaronson SA: Exogenous expression of N-cadherin in breast
cancer cells induces cell migration, invasion, and metastasis. J
Cell Biol. 148:779–790. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nguyen PT, Kudo Y, Yoshida M, Iizuka S,
Ogawa I and Takata T: N-cadherin expression is correlated with
metastasis of spindle cell carcinoma of head and neck region. J
Oral Pathol Med. 40:77–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakajima S, Doi R, Toyoda E, Tsuji S, Wada
M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, et al: N-cadherin
expression and epithelial-mesenchymal transition in pancreatic
carcinoma. Clin Cancer Res. 10:4125–4133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshinaga K, Inoue H, Utsunomiya T, Sonoda
H, Masuda T, Mimori K, Tanaka Y and Mori M: N-cadherin is regulated
by activin A and associated with tumor aggressiveness in esophageal
carcinoma. Clin Cancer Res. 10:5702–5707. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davidson B, Gotlieb WH, Ben-Baruch G,
Nesland JM, Bryne M, Goldberg I, Kopolovic J and Berner A:
E-cadherin complex protein expression and survival in ovarian
carcinoma. Gynecol Oncol. 79:362–371. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishioka H, Hiasa Y, Hayashi I, Kitahori
Y, Konishi N and Sugimura M: Immunohistochemical detection of p53
oncoprotein in human oral squamous cell carcinomas and
leukoplakias: Comparison with proliferating cell nuclear antigen
staining and correlation with clinicopathological findings.
Oncology. 50:426–429. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suraneni MV, Moore JR, Zhang D, Badeaux M,
Macaluso MD, Digiovanni J, Kusewitt D and Tang DG: Tumor
suppressive functions of 15-Lipoxygenase-2 and RB1CC1 in prostate
cancer. Cell Cycle. 13:1798–1810. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei H and Guan JL: Pro-tumorigenic
function of autophagy in mammary oncogenesis. Autophagy. 8:129–131.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morselli E, Shen S, Ruckenstuhl C, Bauer
MA, Mariño G, Galluzzi L, Criollo A, Michaud M, Maiuri MC, Chano T,
et al: P53 inhibits autophagy by interacting with the human
orthologue of yeast Atg17, RB1CC1/FIP200. Cell Cycle. 10:2763–2769.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei H, Wei S, Gan B, Peng X, Zou W and
Guan JL: Suppression of autophagy by FIP200 deletion inhibits
mammary tumorigenesis. Genes Dev. 25:1510–1527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
33
|
Bos R, van der Groep P, Greijer AE,
Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ and van
der Wall E: Levels of hypoxia-inducible factor-1alpha independently
predict prognosis in patients with lymph node negative breast
carcinoma. Cancer. 97:1573–1581. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aebersold DM, Burri P, Beer KT, Laissue J,
Djonov V, Greiner RH and Semenza GL: Expression of
hypoxia-inducible factor-1alpha: A novel predictive and prognostic
parameter in the radiotherapy of oropharyngeal cancer. Cancer Res.
61:2911–2916. 2001.PubMed/NCBI
|