Introduction
Glioma is a common type of primary brain tumor,
making up ~30% of all brain and central nervous system tumors and
80% of all malignant brain tumors (1,2). Therapy
for the treatment of gliomas consists of tumor resection, followed
by radiotherapy and chemotherapy that usually involves
O6-alkylating agents (3–5). Despite
improvements in the standard of care, the median overall survival
(OS) time for patients with glioblastoma multiforme (GBM) has only
marginally improved over the past decade, and remains approximately
one year (3,4). Thus, therapeutic approaches to cure this
malignancy are urgently required to advance treatment.
Temozolomide (TMZ), an alkylating agent with simple
oral administration, is used in combination with and following
radiotherapy (5,6). Due to the overexpression of
phosphorylated protein kinase B (p-AKT), an increasing number of
TMZ-resistant cases have been reported clinically (7). AKT is a major downstream target of
growth factor receptor tyrosine kinases that signal via
phosphoinositide 3-kinase (PI3K) (8,9).
Activation of AKT has been demonstrated to be associated with
increased tumorigenicity and invasiveness (10). Inhibitors of the PI3K/AKT signaling
pathway, including NVP-BEZ235 and GDC-0941, have been identified to
enhance the cytotoxicity of TMZ (7,11,12).
The excessive growth and metastasis of malignant
gliomas may be controlled by a recombinant chlorotoxin-like toxin
in the venom of the scorpion Buthus martensii Kirsch
(BmK CT) fusion protein (13).
Similarly, the significance of BmK CT had been well
documented as a novel blocker of chloride channels and matrix
metallopeptidase 2 (14,15). Based on the aforementioned preclinical
studies, the combination of BmK CT and TMZ may be an
effective treatment for patients with GBM. Therefore, BmK CT
combined with TMZ in glioma treatment was deemed to be worthy of
further study.
In the present study, the antitumor effect of
glutathione S-transferase (GST)-BmK CT combined with TMZ was
investigated using the human malignant glioma U251 cell line. The
molecular mechanisms underlying this synergistic effect were also
investigated.
Materials and methods
Materials and protein preparation
The gene sequence of BmK CT was amplified by
polymerase chain reaction (PCR) from the vector pRSETc-BmK
CT (constructed in Molecular Biology Laboratory, Institute of
Biotechnology, Shanxi University, Taiyuan, China) and cloned into
pGEX-6p-1 (GE Healthcare Life Sciences, Shanghai, China) to
construct the expression vector. The forward primer was
5′-CGCGGATCCATGTGCGGTCCGTGCTTC-3′ (BamHI enzyme site), and
the reverse primer was 5′-CCGCTCGAGTCAGATACGGTTGCACAG-3′
(XhoI enzyme site). PCR was performed using Taq DNA
polymerase (cat no. RR001A; Takara Biotechnology Co., Ltd., Dalian,
China) and the thermocycling conditions were as follows: 95°C for 5
min, 40 cycles at 95°C for 30 sec, 63°C for 30 sec and 72°C for 30
sec, followed by 72°C for 5 min. The product of the PCR was fused
with the GST gene under the regulation of the tac promoter (Ptac).
The expression of Ptac was proportional to the concentration of
isopropyl β-D-1-thiogalactopyranoside (IPTG). The recombinant
plasmid pGEX-6p-1-BmK CT was transformed into E. coli
BL21 (DE3). The recombinant GST-BmK CT fusion protein was
induced using 0.4 mM IPTG at 37°C for 4 h and purified using a GSH
affinity chromatography column (GE Healthcare Life Sciences)
according to manufacturer's protocols. Minipreparation of GST
protein was performed and the protein was purified using a GSH
affinity chromatography column via the transformation of empty
plasmid pGEX-6p-1 into E. coli BL21 (DE3), and was then used
as a control sample in the present study. The expression and
purification of GST and GST-BmK CT proteins were analyzed
using 10% SDS-PAGE gel. The U251 human GBM cell line was kindly
provided by Dr Tao Hu (Department of Neurosurgery, Shanxi
Provincial People's Hospital, Taiyuan, China).
Cell culture
Cell cultures were prepared and maintained according
to standard cell culture procedures. The human glioma cell line
U251 was cultured in complete medium, consisting of Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing high glucose and pyruvate,
supplemented with 10% fetal bovine serum (FBS Premium; PAN-Biotech
GmbH, Aidenbach, Germany), 2 mM glutamine, 100 U ml−1
penicillin G and 100 ng ml−1 streptomycin. Cells were
maintained at 37°C in a humidified 5% CO2 atmosphere.
The cells were dissociated using TE (0.25% trypsin and 0.02% EDTA
solution; Gibco; Thermo Fisher Scientific, Inc.). For the apoptotic
experiments, cells were dissociated using 0.25% Trypsin and EDTA
free solution (Gibco; Thermo Fisher Scientific, Inc.).
Drug treatment
Cells were harvested and 5×104 cells were
plated in each well of 12-well plate and after 24 h later, cells
were treated with 1.12 µM GST, 1.12 µM GST-BmK CT and 0.1 mM
TMZ (Merck & Co., Inc., Whitehouse Station, NJ, USA).
BmK CT was a homolog of chlorotoxin (CTX). CTX could inhibit
U251 cell transwell migration at the inhibition rate of 56.26%
under 1 µM (16). Based on the
aforementioned study, the concentration of BmK CT used in
the present study was set at 1.12 µM. The half maximal inhibitory
concentration (IC50) value of TMZ, defined as the
concentration that reduces the global growth of cells by 50%, was
previously determined to be ~0.2 mM (17). Therefore, the concentration of TMZ
used in the present study was set at 0.1 mM, which was the same as
published data of TMZ used for glioma cells (17,18). TMZ
stocks were prepared by dissolving the drug in dimethyl sulfoxide
and stored at 4°C. Following treatment, cells were gently washed
with phosphate buffer saline (PBS), incubated in fresh media at
37°C, and harvested at various time periods.
Cell viability assay
Cell viability was assessed using the MTT method. A
total of 1×103 cells were seeded into each well of a
96-well culture plate. In brief, U251 cells were treated with GST,
GST-BmK CT and TMZ, and incubated for 1, 2, 3, 4 and 5 days.
The liquid was discarded and 100 µl sanlian dissolved liquid (10%
SDS, 5% isobutyl alcohol, 0.01 M HCl) was added to dissolve the
purple formazan. The absorbance at 570 nm was measured using a
microplate reader following incubation. Subsequently, a calibration
curve was prepared using the data obtained from the wells that
contained known numbers of viable cells. The experiment was
performed in six replicates and repeated three times.
Cell cycle assay
U251 cells were plated at a density of
5×104 cells per well in 12-well plates. After 96 h of
treatment with GST or GST-BmK CT in the presence or absence of TMZ,
cells were harvested and fixed in 70% ethanol at −20°C overnight.
Subsequently, the cells were washed and resuspended in 100 µl PBS
containing 5 µg/ml propidium iodide (PI; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and 100 µg/ml RNase A (Fermentas; Thermo Fisher
Scientific, Inc.) for 1 h at room temperature in the dark. Each
group of 2×104 cells were subsequently analyzed using a
FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA); data were analyzed using FlowJo software (version 7.6; FlowJo
LLC, Ashland, OR, USA). Cell cycle status was determined by
measuring the cellular DNA content, following staining with PI.
Apoptosis assay
A total of 5×104 U251 cells were plated
onto 12-well plates and treated with GST-BmK CT (1.12 µM),
TMZ (100 µM), and the combination of the two, respectively. The
apoptosis ratio was analyzed 96 h post-treatment using a StarGlow
Annexin V-fluorescein isothiocyanate (FITC)/PI Apoptosis Detection
kit (cat no. c203-05; GenStar BioSolutions Co., Ltd., Beijing,
China). Annexin V-FITC and PI double staining was performed at room
temperature for 5 min and was used to evaluate the percentage of
apoptotic cells. Annexin V− and PI− cells
were used as the controls. Annexin V+ and PI−
cells were designated as those in early-stage apoptosis, Annexin
V− and PI+ cells were designated as necrotic,
and Annexin V+ and PI+ cells as late-stage
apoptosis. The stained cells were quantified using a FACSCalibur
flow cytometer (BD Biosciences) and analyzed using FlowJo software.
Tests were replicated in triplicate, and repeated three times.
RNA isolation and reverse
transcription-quantitative (RT-q) PCR
Total mRNA was isolated from tumor cells using
TRIzol™ Reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. cDNA was synthesized
using the PrimeScript® First Strand cDNA Synthesis kit
(cat no. D6110A; Takara Biotechnology Co., Ltd., Dalian, China).
The transcription conditions were as follows: 37°C for 15 min, 85°C
for 5 sec and 4°C for 5 min. qPCR was performed using the CFX96
Real-Time PCR Detection System (cat no. 185-5195; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) with the SYBR®
PrimeScript™ RT-PCR kit (cat no. DDR083A; Takara Biotechnology Co.,
Ltd.). The thermocycling conditions were as follows: 95°C for 5
min, followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec.
The 2−ΔΔCq method was used to quantify gene expression
(19). Transcript levels were
normalized to those of GAPDH. The primers sequences for RT-qPCR
were as follows: PTEN forward, 5′-CCTTCTCCATCTCCTGTGTAATCAA-3′ and
reverse, 5′-GTTGACTGATGTAGGTACTAACAGCAT-3′; and GAPDH forward,
5′-AACGGGAAGCTTGTCATCAATGGAAA-3′ and reverse,
5′-GCATCAGCAGAGGGGGCAGAG-3′.
Western blot analysis
U251 cells were treated with GST, GST-BmK CT
or TMZ for 1, 2, 4, 8 and 16 h. Subsequently, the cultured cells
were washed twice in PBS after treatment at the indicated time
points (0, 1, 2, 4, 8 and 16 h) prior to lysis using the
M-PER® Mammalian Protein Extraction Reagent (cat no.
75801; Thermo Fisher Scientific, Inc.) supplemented with a
proteinase inhibitor cocktail (cat no. 04693116001; Roche
Diagnostics GmbH, Mannheim, Germany), phosphatase inhibitor
cocktail (cat no. 04906845001; Roche Diagnostics GmbH) and
phenylmethylsulfonyl for 30 min on ice. The concentration of
protein was quantitated using a BSA kit (cat no. QJ223202; Thermo
Fisher Scientific, Inc.). An equal amount (20 µg/lane) of protein
lysates was resolved to 10% SDS-PAGE gel. Proteins were transferred
onto polyvinylidene difluoride membranes with a 0.45 µm pore size
(EMD Millipore, Billerica, MA, USA), which were then blocked with
5% bovine serum albumin at room temperature for 1 h and probed with
primary antibodies at 4°C overnight. The following primary
antibodies, all of which were diluted at 1:1,000, were used in the
present study: Anti-GST (cat no. D110271; Sangon Biotech),
anti-β-actin (cat no. LK9001L; Tianjin SunGene Biotech Co., Ltd.,
Tianjin, China), anti-AKT (cat no. 2920S; Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-p-AKT (cat no. 4060S;
Cell Signaling Technology, Inc.), anti-B cell lymphoma 2 (Bcl-2;
cat no. 3498S; Cell Signaling Technology, Inc.), anti-Bcl-2
associated X protein (Bax; cat no. 2772S; Cell Signaling
Technology, Inc.), anti-caspase-9 (cat no. ab202068; Abcam,
Cambridge, UK) and anti-caspase-3 (cat no. 9664S; Cell Signaling
Technology, Inc.). They were subsequently incubated with a
secondary horseradish peroxidase-conjugated goat anti-rabbit IgG
antibody (cat no. 31210; dilution, 1:5,000) or a goat anti-mouse
IgG antibody (cat no. 31431; dilution, 1:5,000; both Thermo Fisher
Scientific, Inc.) at room temperature for 1 h. Results were
visualized using the SuperSignal West Dura chemiluminescent
substrate (cat no. QJ220977; Thermo Fisher Scientific, Inc.).
ImageJ software (National Institutes of Health, Bethesda, MD, USA)
was used to quantify the band density.
Statistical analysis
Data are presented as the mean ± standard deviation.
Experiments were repeated three or six times. Statistical analyses
were performed using GraphPad Prism 5.0 (GraphPad Software, Inc.,
La Jolla, CA, USA). Two-way analysis of variance, followed by a
Bonferroni's post hoc test, and the Student's t-test were applied
to calculate the statistical difference as appropriate. P<0.05
was considered to indicate a statistically significant
difference.
Results
Construction and purification of
recombinant GST-BmK CT
The gene BmK CT was fused with GST via the
BamHI and XhoI sites under the regulation of Ptac
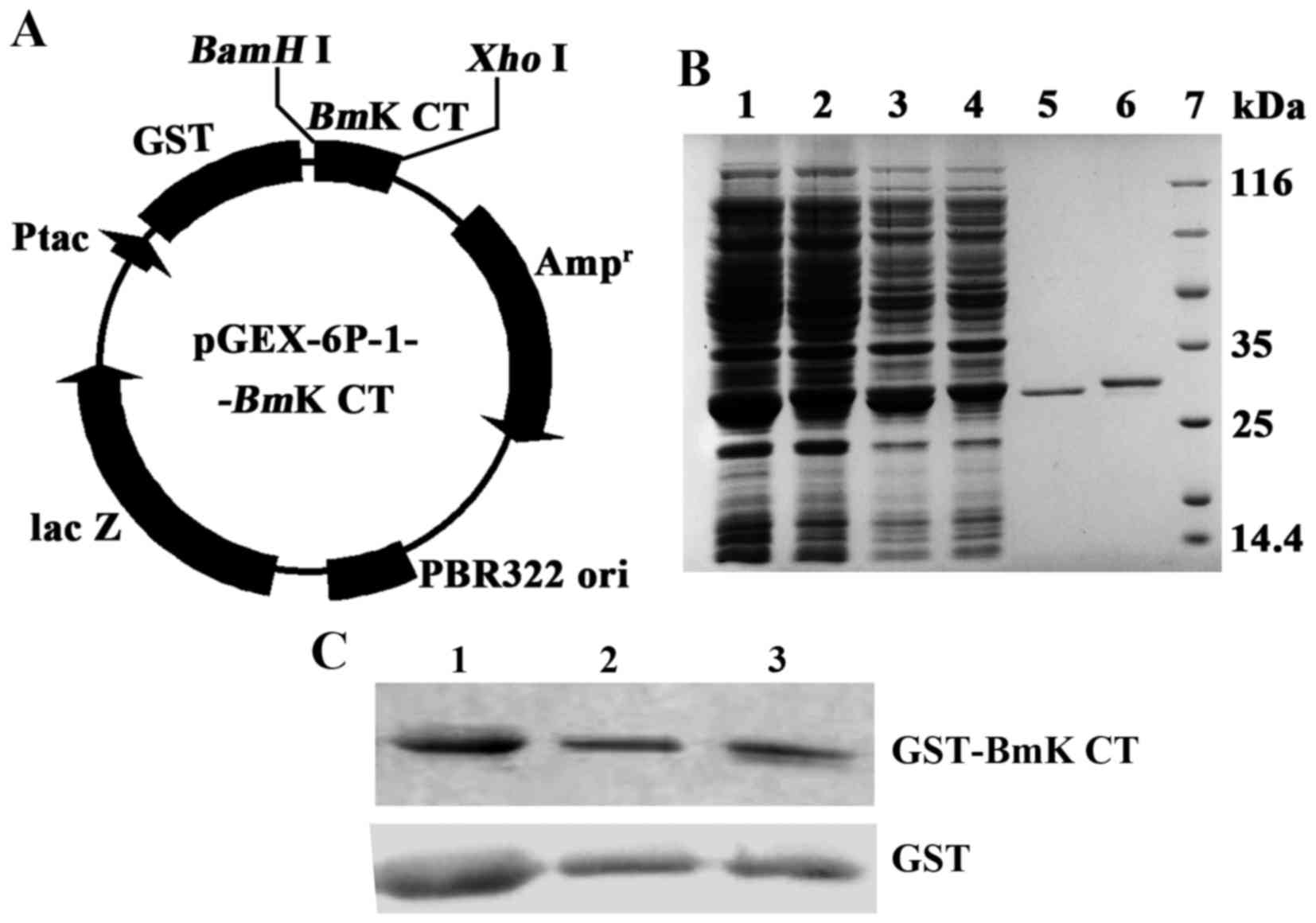
(Fig. 1A). The pGEX-6p-1-BmK
CT was transfected into E. coli BL21 (DE3). Then the
recombinant protein GST-BmK CT was expressed via induction
with IPTG. The recombinant protein was collected and concentrated
into PBS. A BCA kit was used to determine the concentration of
protein. The GST-BmK CT protein concentration was 6.62 mg/ml
(224 µM). GST was produced via the same strategy and the
concentration was adjusted to 224 µM. The proteins were diluted to
1.12 µM in complete medium. The expressed proteins of BL21
(DE3)/pGEX-6P-1 or BL21(DE3)/pGEX-6P-1-BmK CT, the proteins
bound to glutathione sepharose beads, and purified GST or
GST-BmK CT were analyzed using 10% SDS-PAGE gel (Fig. 1B) and immunologically identified using
western blot analysis (Fig. 1C).
GST-BmK CT increases the inhibitive
effect of TMZ on U251 cell viability
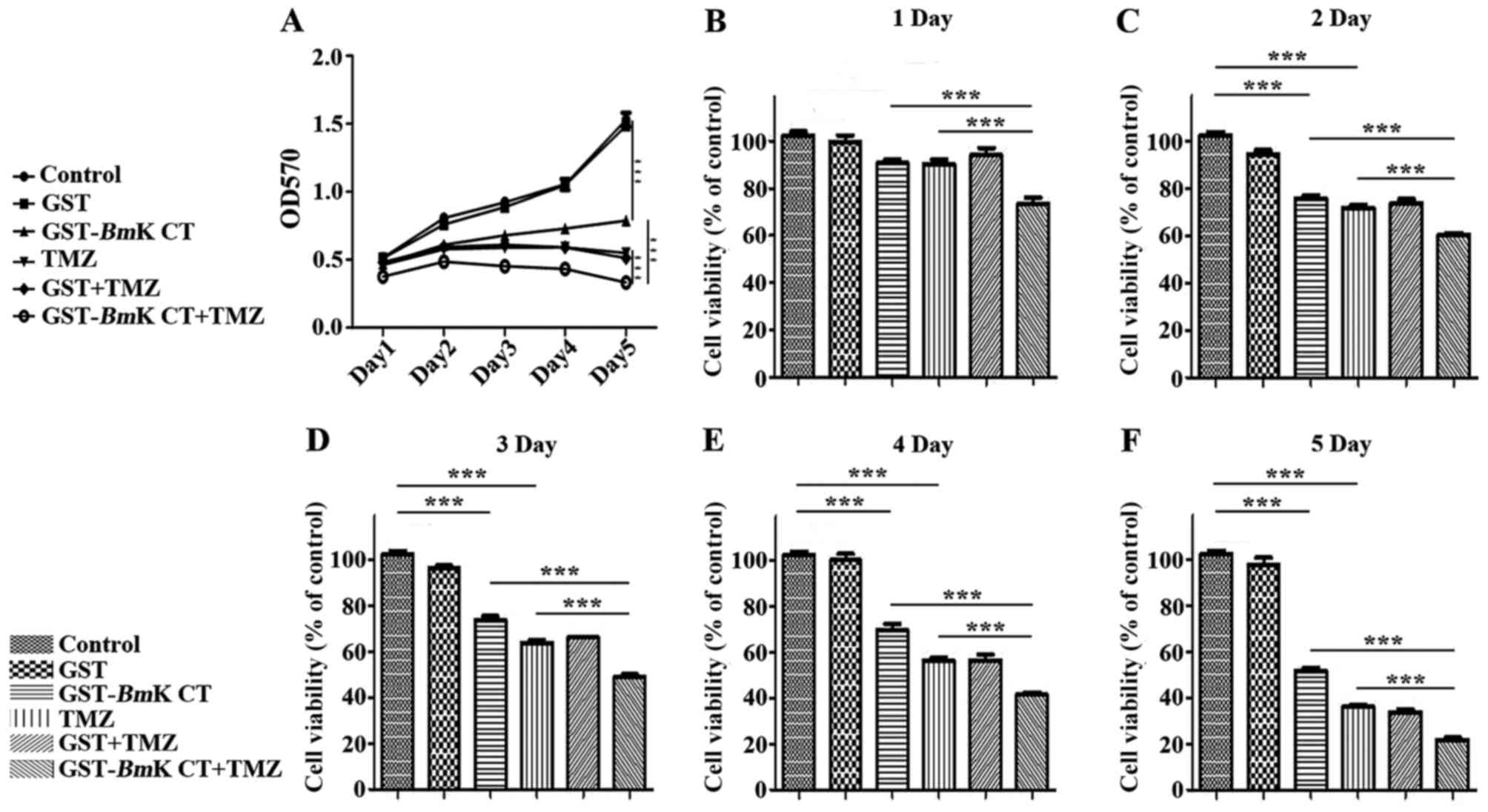
The number of GST and GST-BmK CT-treated
cells following TMZ administration were detected using an MTT assay
at 1, 2, 3, 4 and 5 days post treatment. In this assay, GST protein
demonstrated no effect on the proliferation of U251 cells, as
compared with the control group. However, GST-BmK CT protein
and TMZ inhibited the growth of U251 cells in a time-dependent
manner, compared with the control group (P<0.001). Furthermore,
there was an increased inhibitory effect when GST-BmK CT was
combined with TMZ compared with the effects of individual
treatments (P<0.001) (Fig. 2A-F).
The MTT assay revealed that TMZ treatment decreased cell viability
to 36±1.8% of the control. When TMZ was combined with BmK
CT, cell viability was further inhibited to 22±2.0% of the control
after 5 days (Fig. 2F). These results
demonstrated that TMZ combined with BmK CT inhibited the
viability of U251 cells by an increased degree (Fig. 2), compared with TMZ single treatment,
decreasing the IC50 value from a previously reported 0.2
mM (17) to 0.1 mM. This suggested
that GST-BmK CT protein and TMZ synergistically (20) inhibit U251 glioma cell growth.
GST-BmK CT enhances TMZ-induced cell
cycle arrest in U251 cells
To study the anti-proliferative mechanism of
BmK CT combined with TMZ, the effect of this combination
treatment on the glioma cell cycle was investigated. U251 cells
were cultured with 1.12 µM GST or GST-BmK CT in the presence
or absence of 100 µM TMZ for 96 h, following which the cell cycle
status was analyzed using flow cytometry. As presented in Fig. 3, BmK CT treatment resulted in
cell cycle arrest in the S phase, an effect which did not occur in
the control and GST treatment groups. Treatment with TMZ induced
G2/M phase arrest. Notably, markedly prolonged S and
G2/M phase arrest durations were observed when
BmK CT and TMZ were used in combination (Fig. 3).
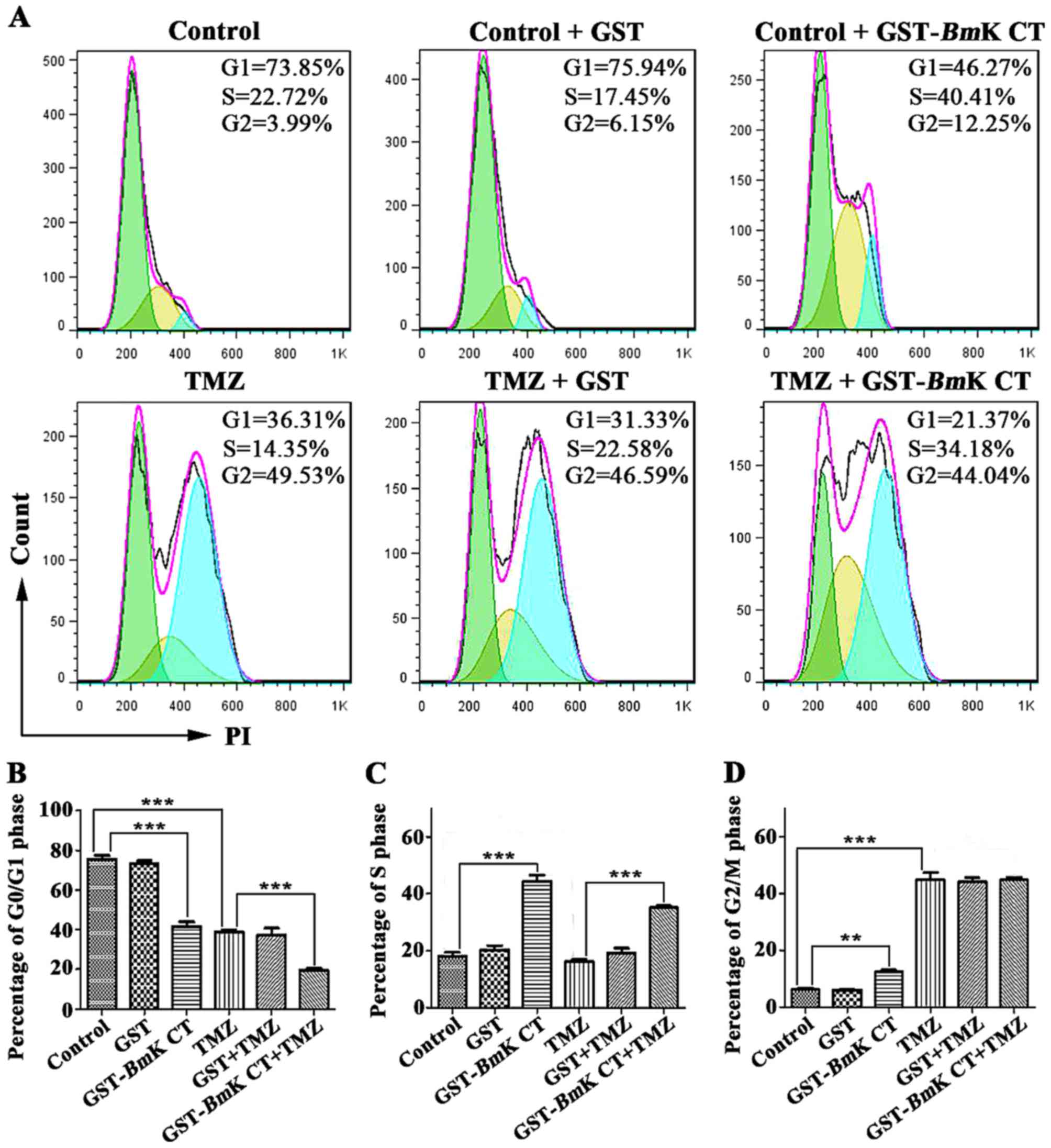 | Figure 3.Cell cycle arrest in U251 cells
treated with GST-BmK CT, TMZ, and the combination treatment.
(A) Representative results of the percentage of cells in the
G0/G1, S, or G2/M phases among
U251 cells exposed to GST, GST-BmK CT, TMZ or their
combination for 96 h, as detected by flow cytometry. The total
number of cells per group exposed to flow cytometry was 20,000.
Histograms indicate the percentage of U251 cells in the (B)
G0/G1, (C) S, and (D) G2/M phases
in three independent experiments. The data are presented as the
average of triplicate results from a representative experiment.
Bars represent the standard deviation. **P<0.005, ***P<0.001.
GST-BmK CT, Glutathione S-transferase fused with Buthus
martensii Kirsch chlorotoxin-like toxin; TMZ, temozolomide; PI,
propidium iodide. |
GST-BmK CT improves TMZ-induced U251
cell apoptosis
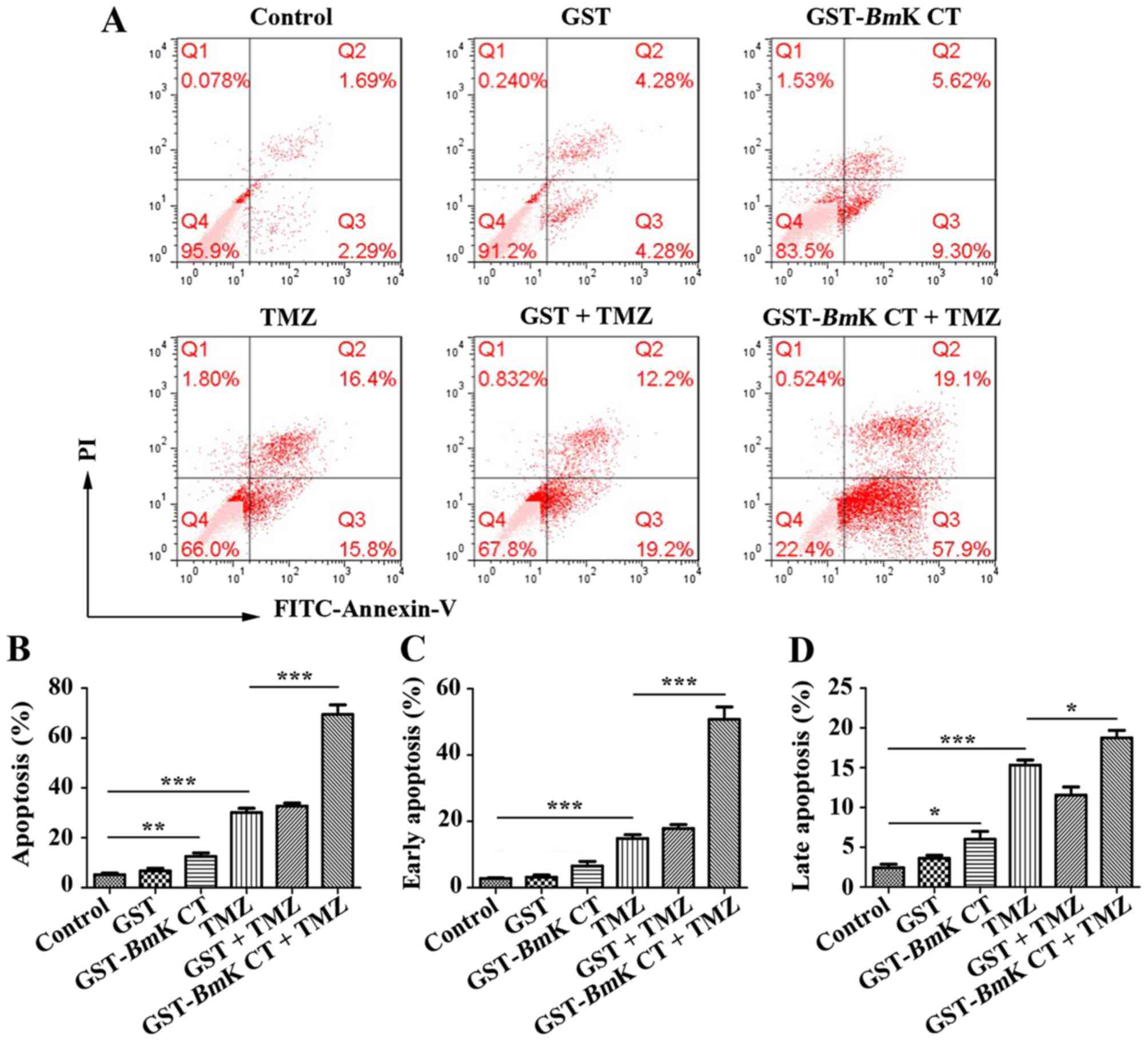
To assess the pro-apoptotic effect of BmK CT
combined with TMZ, U251 cells were treated with BmK CT in
the presence or absence of TMZ. Apoptotic cells were measured using
an Annexin-V/PI staining assay. As presented in Fig. 4A, U251 cells treated with
GST-BmK CT protein or TMZ exhibited an increased rate of
apoptosis, compared with the control. In particular, there was a
significant increase in the number of apoptotic cells in the
combined GST-BmK CT and TMZ treatment group (Fig. 4B). By analyzing the proportions of
early- and late-apoptotic cells, GST-BmK CT protein combined
with TMZ were identified to have an increased effect on the number
of early-apoptotic cells compared with TMZ alone, which was
increased by ~2.5-fold (Fig. 4C and
D).
GST-BmK CT protein induces U251 cell
apoptosis by downregulating p-AKT
AKT phosphorylation triggers resistance to TMZ and
upregulates the expression of various anti-apoptosis-associated
proteins (7). To understand the
mechanisms underlying the apoptosis induced by the combined
treatment, the levels of AKT phosphorylation and
apoptosis-associated proteins were investigated using western blot
analysis. The results demonstrated that in GST-BmK CT
treated U251 cells for 16 h, the phosphorylation of AKT was
significantly decreased compared with the control (0 h; Fig. 5A; P<0.005), while the
phosphorylation of AKT in GST-treated or TMZ-treated U251 cells did
not change (Fig. 5B and C). In
addition, the levels of Bax (Fig.
5D), cleaved caspase-3 and cleaved caspase-9 (Fig. 5E) were increased in GST-BmK CT
treated U251 cells compared with the control (0 h). The expression
level of Bcl-2 was revealed to be decreased in the BmK CT
treatment group (Fig. 5D).
Furthermore, GST-BmK CT increased the transcription of
phosphatase and tensin homolog (PTEN), which is a major negative
regulator of the AKT signaling pathway (21) (Fig. 5F);
however TMZ did not perform the same function (Fig. 5G).
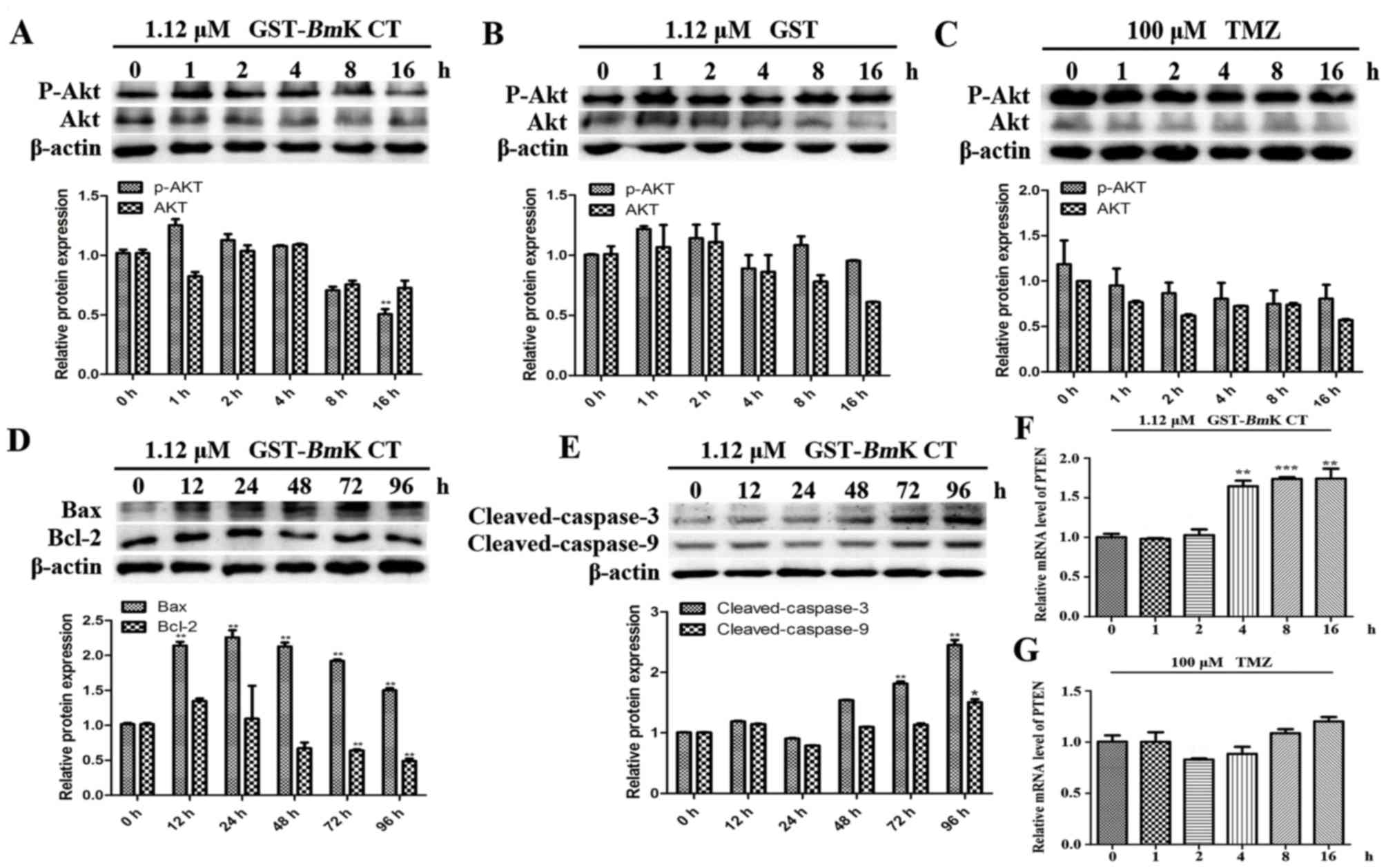 | Figure 5.BmK CT induced U251 cell
apoptosis through downregulating p-AKT. AKT and p-AKT levels were
measured in U251 cells treated with (A) 1.12 µM GST-BmK CT,
(B) 1.12 µM GST or (C) 100 µM TMZ for 0, 1, 2, 4, 8 or 16 h, using
western blotting and the densitometric quantification of AKT and
p-AKT was normalized to β-actin. (D) Western blot analysis of
Bcl-2, Bax and (E) caspase-3/9 levels and the quantification was
normalized to β-actin in the U251 cells stimulated with
GST-BmK CT (1.12 µM) for 0, 12, 24, 48, 72 or 96 h. For
caspase-3/−9, the antibody was directed against the active
fragment. The relative mRNA expression levels of PTEN were assayed
by quantitative polymerase chain reaction following the treatment
of U251 cells with (F) 1.12 µM GST-BmK CT or (G) 100 µM TMZ
for 0, 1, 2, 4, 8 or 16 h. Data are presented as the mean ±
standard deviation of three replicates. *P<0.05, **P<0.005,
***P<0.001 vs. untreated cells. GST-BmK CT, Glutathione
S-transferase fused with Buthus martensii Kirsch
chlorotoxin-like toxin; TMZ, temozolomide; Bcl-2, B cell lymphoma
2; Bax, Bcl-2 associated X protein; PTEN, phosphatase and tensin
homolog; ns, not significant; AKT, protein kinase B. |
GST-BmK CT synergistically enhances
the apoptosis-inducing effects of TMZ in U251 cells via the
downregulation of p-AKT
To investigate the contribution of BmK CT to
the effects of TMZ, parallel studies were performed, in which U251
cells were treated with BmK CT and TMZ for different lengths
of time. As presented in Fig. 6A, the
transcription PTEN was increased compared with the control (0 h)
when the cells were stimulated by GST-BmK CT protein
combined with TMZ. The results revealed that this drug combination
may enhance the suppressive effect on AKT phosphorylation (Fig. 6B) and the upregulation of
pro-apoptotic proteins in U251 cells (Fig. 6C), compared with the control (0 h).
Taken together, these results suggest that BmK CT enhances
the apoptosis sensitivity of TMZ-treated U251 glioma cells by
downregulating p-AKT.
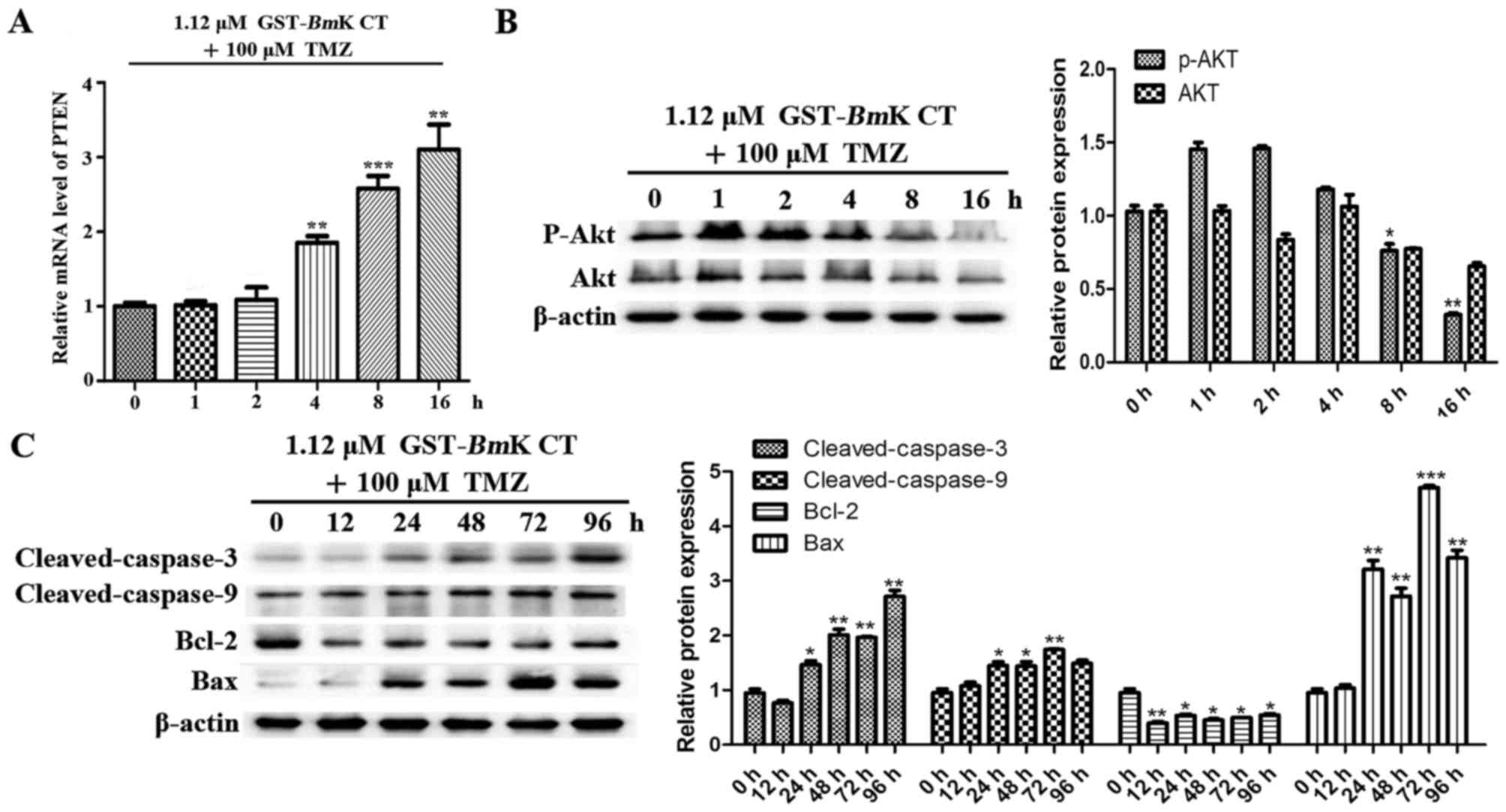 | Figure 6.BmK CT enhanced the
TMZ-sensitivity and the apoptotic response of U251 cells by
downregulating AKT phosphorylation. (A) The relative mRNA
expression levels of PTEN were investigated using quantitative
polymerase chain reaction following the combination treatment of
U251 cells with 1.12 µM GST-BmK CT and 100 µM TMZ in a
time-dependent manner. (B) Western blot analysis was performed to
evaluate the levels of p-AKT, AKT and (C) various apoptotic
proteins in BmK CT and TMZ combination-treated U251 cells.
The bands were quantified respectively. Data are presented as the
mean ± standard deviation of three replicates. *P<0.05,
**P<0.005, ***P<0.001 vs. untreated cells. GST-BmK CT,
Glutathione S-transferase fused with Buthus martensii Kirsch
chlorotoxin-like toxin; TMZ, temozolomide; Bcl-2, B cell lymphoma
2; Bax, Bcl-2 associated X protein; PTEN, phosphatase and tensin
homolog; ns, not significant; AKT, protein kinase B. |
Discussion
Accumulating evidence has demonstrated that GBM is
resistant to TMZ (7,11,12,22,23).
A possible reason for this is that GBM may affect AKT activity and
protect against drug-induced cytotoxicity (22,23). In
addition, clinical evidence has identified that primary or acquired
resistance to TMZ is a major therapeutic problem (11). Therefore, a combination therapy that
enhances the efficacy of TMZ is required.
BmK CT is a short chain peptide consisting of
35 amino acid residues with four disulfide bridges, and
specifically targets glioma cells and inhibits glioma cell growth
without any notable toxicity to normal astrocytes (13). In the present study, the synergistic
effect and mechanism of BmK CT involved in enhancing cell
sensitivity to TMZ-induced apoptosis was investigated using
malignant glioma U251 cells. The absence of structural
characterization of recombinant GST-BmK CT, which contains a 36-mer
peptide, is a limitation of the present study.
The results of the present study demonstrated that
BmK CT enhances the sensitivity of malignant glioma U251
cells to TMZ-induced apoptosis via the AKT signaling pathway;
inhibiting the growth, invasion and migration of glioma cells. It
was identified that BmK CT alone induced S phase arrest;
however, when combined with TMZ, the treatment significantly
induced G2/M arrest and cell death. These data revealed
that BmK CT arrested the cell cycle at the G2/M
phase following TMZ-induced DNA damage, in addition to blocking
effects at the S/G2 transition. Although it is a protein
oligopeptide that has been extensively studied, whether it may be
an agent with improved ability to cause glioma cell apoptosis when
combined with TMZ requires further study. In the present study, the
MTT, apoptosis and flow cytometry assays demonstrated that
BmK CT combined with TMZ significantly enhanced apoptotic
activity and attenuated cell growth.
The results of the present study also demonstrated
that the BmK CT protein may inhibit AKT activity and
increase U251 glioma cell sensitivity to TMZ. Activation of
caspase-9 and caspase-3 was significantly increased in the U251
cells treated with BmK CT for 96 h. In addition,
GST-BmK CT was revealed to increase the transcription of
PTEN. Although BmK CT was demonstrated to act
synergistically with TMZ in inhibiting U251 glioma cell
proliferation and generating the highest apoptotic rates, the use
of only the U251 cell line is a limitation of the present study.
The feasibility of the BmK CT with TMZ combination treatment
requires further study in other cell lines.
In conclusion, the results of the present study
demonstrated that BmK CT was able to enhance the
cytotoxicity of TMZ by downregulating the levels of p-AKT. The
results also suggest that BmK CT combined with TMZ is a
promising approach for glioma therapy.
Acknowledgements
The authors wish to thank Dr Yan Wang of Stanford
University (Stanford, CA, USA) for assistance with editing. The
present study was supported by the National Natural Science
Foundation of China (grant no. 31400765), the Shanxi Province
Science Foundation for Youths (grant no. 201601D202064), and the
Scientific and Technological Innovation Programs of Higher
Education Institutions in Shanxi (grant no. 2015117).
References
|
1
|
Cai J, Zhu P, Zhang C, Li Q, Wang Z, Li G,
Wang G, Yang P, Li J, Han B, et al: Detection of ATRX and
IDH1-R132H immunohistochemistry in the progression of 211 paired
gliomas. Oncotarget. 7:16384–16395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li QJ, Cai JQ and Liu CY: Evolving
molecular genetics of glioblastoma. Chin Med J (Engl). 129:464–471.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang T, Mao Y, Ma W, Mao Q, You Y, Yang
X, Jiang C, Kang C, Li X, Chen L, et al: CGCG clinical practice
guidelines for the management of adult diffuse gliomas. Cancer
Lett. 375:263–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chamberlain MC: Temozolomide: Therapeutic
limitations in the treatment of adult high-grade gliomas. Expert
Rev Neurother. 10:1537–1544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu Z, Xie G, Zhou G, Cheng Y, Zhang G, Yao
G, Chen Y, Li Y and Zhao G: NVP-BEZ235, a novel dual PI3K-mTOR
inhibitor displays anti-glioma activity and reduces chemoresistance
to temozolomide in human glioma cells. Cancer Lett. 367:58–68.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
West KA, Castillo SS and Dennis PA:
Activation of the PI3K/Akt pathway and chemotherapeutic resistance.
Drug Resist Updat. 5:234–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: Implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang H, Lin H, Zhang X and Li J:
Resveratrol reverses temozolomide resistance by downregulation of
MGMT in T98G glioblastoma cells by the NF-κB-dependent pathway.
Oncol Rep. 27:2050–2056. 2012.PubMed/NCBI
|
|
11
|
Shi F, Guo H, Zhang R, Liu H, Wu L, Wu Q,
Liu J, Liu T and Zhang Q: The PI3K inhibitor GDC-0941 enhances
radiosensitization and reduces chemoresistance to temozolomide in
GBM cell lines. Neuroscience. 346:298–308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi EJ, Cho BJ, Lee DJ, Hwang YH, Chun
SH, Kim HH and Kim IA: Enhanced cytotoxic effect of radiation and
temozolomide in malignant glioma cells: Targeting PI3K-AKT-mTOR
signaling, HSP90 and histone deacetylases. BMC Cancer. 14:172014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan S, Sun Z, Jiang D, Dai C, Ma Y, Zhao
Z, Liu H, Wu Y, Cao Z and Li W: BmKCT toxin inhibits glioma
proliferation and tumor metastasis. Cancer Lett. 291:158–166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu YJ, An N, Chan KG, Wu YB, Zheng SH and
Liang AH: A model of BmK CT in inhibiting glioma cell migration via
matrix metalloproteinase-2 from experimental and molecular dynamics
simulation study. Biotechnol Lett. 33:1309–1317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu YJ, Yin LT, Liang AH, Zhang CF, Wang W,
Chai BF, Yang JY and Fan XJ: Therapeutic potential of
chlorotoxin-like neurotoxin from the Chinese scorpion for human
gliomas. Neurosci Lett. 412:62–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soroceanu L, Manning TJ Jr and Sontheimer
H: Modulation of glioma cell migration and invasion using Cl(−) and
K(+) ion channel blockers. J Neurosci. 19:5942–5954.
1999.PubMed/NCBI
|
|
17
|
Wang P, Ye JA, Hou CX, Zhou D and Zhan SQ:
Combination of lentivirus-mediated silencing of PPM1D and
temozolomide chemotherapy eradicates malignant glioma through cell
apoptosis and cell cycle arrest. Oncol Rep. 36:2544–2552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Batista LF, Roos WP, Kaina B and Menck CF:
p53 mutant human glioma cells are sensitive to UV-C-induced
apoptosis due to impaired cyclobutane pyrimidine dimer removal. Mol
Cancer Res. 7:237–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Au JL and Wientjes MG: Comparison
of methods for evaluating drug-drug interaction. Front Biosci
(Elite Ed). 2:241–249. 2010.PubMed/NCBI
|
|
21
|
Cantley LC and Neel BG: New insights into
tumor suppression: PTEN suppresses tumor formation by restraining
the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA.
96:pp. 4240–4245. 1999; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sarkaria JN, Kitange GJ, James CD, Plummer
R, Calvert H, Weller M and Wick W: Mechanisms of chemoresistance to
alkylating agents in malignant glioma. Clin Cancer Res.
14:2900–2908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gallia GL, Tyler BM, Hann CL, Siu IM,
Giranda VL, Vescovi AL, Brem H and Riggins GJ: Inhibition of Akt
inhibits growth of glioblastoma and glioblastoma stem-like cells.
Mol Cancer Ther. 8:386–393. 2009. View Article : Google Scholar : PubMed/NCBI
|