Introduction
The progression, invasion and metastasis of
malignant tumor cells are associated with abnormal cell growth and
loss of cell-cell adhesion (1).
Caveolae have a specialized function in signal transduction,
cell-cell stabilization and maintain interactions with cell matrix
(2). Caveolin-1 is a pivotal scaffold
protein of caveolae. As a marker protein of caveolae, caveolin-1
interacts with a number of signaling molecules to regulate cell
proliferation, differentiation, apoptosis and cell adhesion by
controlling the distribution of signaling protein in subcellular
compartments and the activation status of these proteins (3,4). However,
the functions of caveolin-1 in pathogenesis, progression, invasion
and metastasis of malignancies are controversial. A number of
studies have demonstrated that caveolin-1 may serve as a tumor
suppressor in stomach cancer (5–7).
All-trans retinoic acid (ATRA) is a derivative of
vitamin A and has been used in oncotherapy. ATRA was first
identified to exhibit anti-leukemic effects, with other studies
demonstrating that ATRA also exhibits inhibitory effects on solid
tumors, including gastric cancer (8–11). In
order to determine the molecular mechanisms of ATRA on gastric
cancer, the present study investigated whether ATRA served a
suppressing tumor effect by regulating cell-cell adhesion potential
and then regulating the proliferation of gastric cancer cells.
In the present study, ATRA was identified to
significantly inhibit the proliferation of the gastric cancer cell
line SGC7901. In addition, translocalization of caveolin-1 to the
cell membrane in SGC7901 cells was promoted by ATRA treatment.
Furthermore, ATRA treatment was able to affect the level of
phosphorylation of extracellular signal-regulated kinase (ERK).
When treated with the specific antagonist against ERK, the effect
of ATRA treatment on caveolin-1 localization in SGC7901 cells was
increased, whereas the effect of ATRA treatment was reversed by
treatment with a specific agonist to ERK/mitogen-activated protein
kinase (MAPK). These results suggested that the treatment with ATRA
may adjust the membrane localization of caveolin-1 and inhibit the
growth of SGC7901 gastric cancer cells. In addition, the
aforementioned effects of ATRA in SGC7901 cells may be mediated by
the ERK/MAPK signaling pathway.
Materials and methods
Cell culture
The gastric cancer cell line SCG7901 (American Type
Culture Collection, Manassas, VA, USA) was cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (Clark Bioscience, Richmond, VA, USA), 100 U/ml penicillin
and 100 µg/ml streptomycin and maintained in an incubator with a
humidified atmosphere of 5% CO2 at 37°C. Upon reaching
80–90% confluence, the cells were trypsinized and sub-cultured.
Reagents
DMEM was purchased from Gibco (Thermo Fisher
Scientific, Inc. Waltham, MA, USA). ATRA was purchased from
Sigma-Aldrich (Merck KGaA; Darmstadt, Germany), dissolved in
dimethyl sulfoxide (DMSO) and stored at a concentration of 10
mmol/l. The antibodies were obtained from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). The remaining reagents were purchased from
Sigma-Aldrich (Merck KGaA).
Cell proliferation assay using
MTT
An MTT assay was performed to assess the effect of
ATRA at different concentrations on SGC7901 cell proliferation
according to standard protocol (12)
in 96-well plates. SGC7901 cells (5,000 cells/well) were seeded
into the wells and cultured. After reaching ~50–60% confluence, 5,
10, 25, 50 and 100 µmol/l ATRA was added to the wells,
respectively. The SGC7901 cells that were not treated with ATRA or
DMSO were used as untreated cells (untreated cells). The cells
treated with corresponding concentration of DMSO (1%) were used as
vehicle controls. A total of 20 µl MTT (1 mg/ml) was added to each
well following incubation at 37°C for 24, 48 or 72 h, respectively.
Following an additional 4 h incubation at 37°C, the medium was
removed, and the crystals generated by cellular reduction were
solubilized by adding 100 µl DMSO. Following a 10 min incubation
with agitation at 37°C, the optical density (OD) was measured using
a universal microplate reader (ELx800; BioTek Instruments, Inc.,
Winooski, VT, USA) at a wavelength of 570 nm. The inhibition ratio
was calculated as (OD of the control-OD of the unknown sample)/(OD
of the control-OD of the blank control)×100%. Each drug
concentration was analyzed in four replicates, and the experiment
was repeated in three times.
Immunofluorescence assay for labeling
of caveolin-1 in distinct subcellular compartments
The cells (2×104 cells) were seeded onto
sterile coverslips in a 12-well plate and were fixed with 4%
paraformaldehyde for 20 min at room temperature when the cells grew
to form a confluent monolayer following treatment with 10 µmol/l
ATRA with or without phorbol 12-myristate 13-acetate (PMA, 1
µmol/l) and PD98059 (10 µmol/l) for 72 h at 37°C. Subsequently, the
cells were incubated with 5% bovine serum albumin (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) in phosphate-buffered saline (pH
7.4) for blocking for 2 h at room temperature and then were
incubated overnight at 4°C with the primary antibody against
caveolin-1 (dilution, 1:50; cat no. sc-53564), followed by
visualization with goat anti-mouse fluorescein
isothiocyanate-coupled secondary antibody (dilution, 1:500; cat no.
A0568) for 2 h at room temperature in a dark box. Cover slips were
mounted, and the cells were imaged with a fluorescence
microscope.
Analysis of caveolin-1 protein levels
at distinct subcellular compartments using western blotting
Cell extracts were collected following treatment
with 10 µmol/l ATRA for 72 h at 37°C. For collection of cytoplasmic
protein, the cells were lysed in lysis buffer [20 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 2 mM
MgCl2, 1 mM EDTA, 2 mM DTT, 5 µg/ml leupeptin, 1 mM
phenylmethanesulfonyl fluoride; pH 7.4] and then centrifuged at
16,000 × g for 20 min at 4°C to collect the supernatant. The
membrane protein was obtained using Membrane Protein Extraction
kit, according to the manufacturer's protocol. Total Protein
Extraction kit was used to extract the total cellular protein,
according to the manufacturer's protocol. The concentration of all
the extracted protein was determined using the BCA method. Then the
protein (30 µg/lane) was separated using SDS-PAGE (12.5% gel) and
transferred to polyvinylidene difluoride membranes. Following
blocking in 5% skimmed milk at room temperature for 2 h, the
membranes were incubated with the primary antibody against
caveolin-1 (dilution, 1:500; cat no. sc-53564) at 4°C overnight,
followed by incubation at room temperature for 2 h with horseradish
peroxidase-conjugated secondary antibodies (dilution, 1:20,000;
goat anti-mouse IgG; cat no. AP124P). Protein-antibody complexes
were visualized by enhanced chemiluminescent detection system
according to the manufacturer's protocol. β-actin (dilution,
1:1,000; cat. no. sc-47778, incubate at 4°C overnight) was used as
the reference gene. The quantity of caveolin-1 relative to that of
the reference protein β-actin was calculated with Quantity One
(version, 4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Western blot analysis for the
detection of ERK signaling pathway activity
SGC7901 cells grown in 6-well cell culture plates
were treated at 37°C with 10 µmol/l ATRA for 5, 15, 30 and 45 min,
1, 2, 4 or 24 h. Then, the cells were lysed with lysis buffer and
the protein was collected, quantified and analyzed as
aforementioned. The primary antibodies used were antibodies against
phosphorylated (p)ERK (dilution, 1:500; cat no. sc-7383, 4°C
overnight) or ERK (dilution, 1:500; cat no. sc-514302, 4°C
overnight). Goat anti-mouse HRP-IgG was used as mentioned above
(dilution, 1:20,000; cat no. AP124P, 2 h at room temperature).
Statistical analyses
Statistical analyses were performed using SPSS
(version 16.0; SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance was used to analyze the difference among the distinct
groups of gastric cancer cells. Multiple comparison between the
groups was performed using the Student-Newman-Keuls method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ATRA inhibits the proliferation of
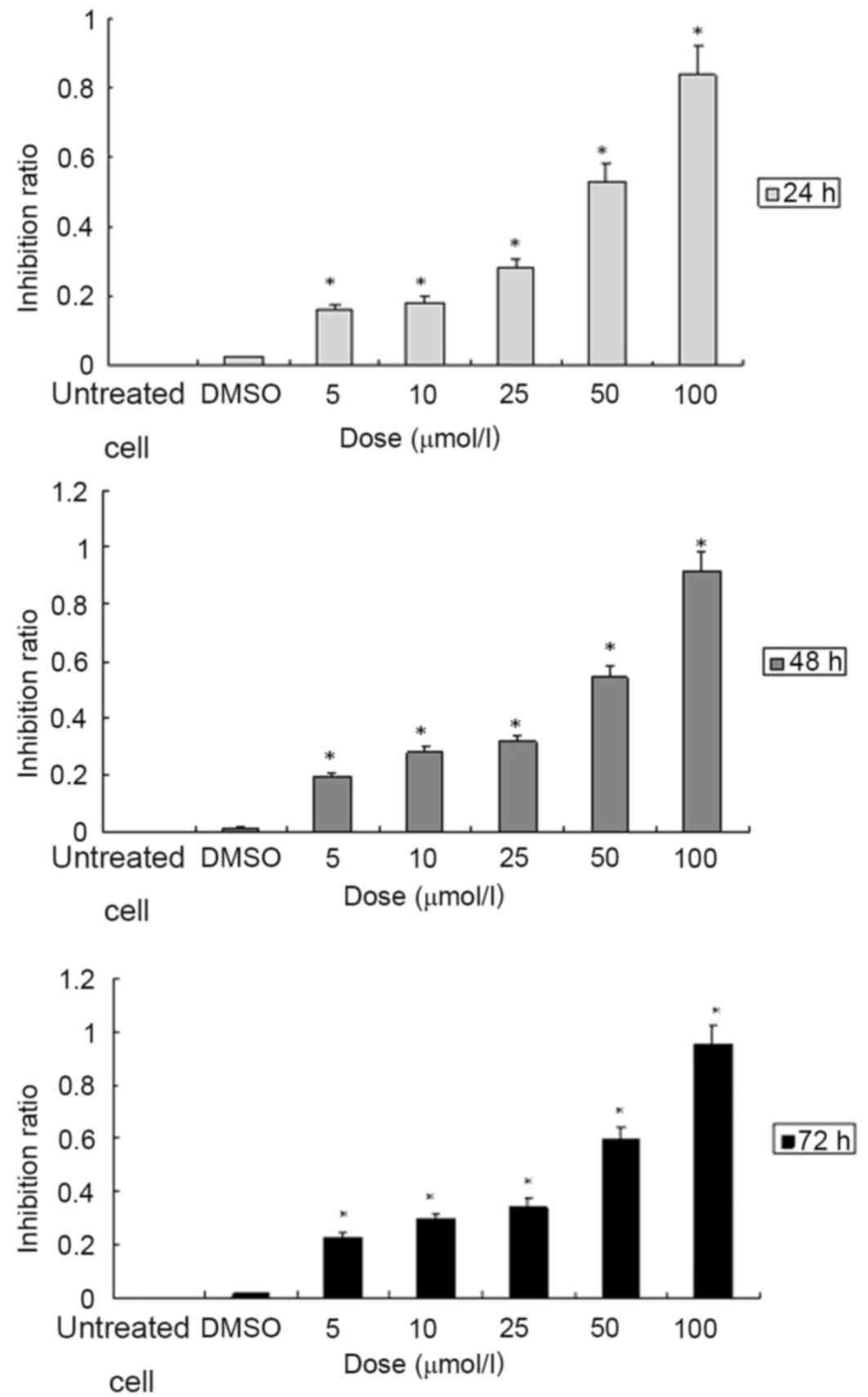
SGC7901 cells in a dose- and time-dependent manner
The cells in the 96-well culture plate were treated
with ATRA at various concentrations (5, 10, 25, 50 and 100 µmol/l)
for 24, 48 or 72 h, respectively. Cell viability was determined
with a colorimetric MTT assay. The results indicated that treatment
with ATRA was able to significantly inhibit the proliferation of
SGC7901 cells when compared to the vehicle control group. When the
cells were treated with ATRA at various concentrations of 5, 10,
25, 50 and 100 µmol/l for 24, the % inhibition values were 16.07,
18.02, 28.01, 53.04 and 83.93% respectively. For 48 h treatment,
the % inhibition ratios were 19.25, 28.28, 31.60, 54.26 and 91.31,
respectively. The % proliferation-inhibition were 22.88, 29.77,
34.35, 59.68 and 95.36% when treated for 72 h (Fig. 1; Tables
I–III). In accordance with
these results, 10 µmol/l ATRA was used to treat SGC7901 cells for
72 h in the subsequent experiments.
 | Table I.Inhibitory effect of ATRA at various
concentrations on the proliferation of SGC7901 cells at 24 h. |
Table I.
Inhibitory effect of ATRA at various
concentrations on the proliferation of SGC7901 cells at 24 h.
| Groups | OD570 | % Inhibition |
|---|
| Control | 0.212 |
|
| Vehicle control | 0.191 | 2.47 |
| ATRA, µmol/l |
|
|
| 5 | 0.186a | 16.07 |
| 10 | 0.183a | 18.02 |
| 25 | 0.167a | 28.01 |
| 50 | 0.126a | 53.04 |
| 100 | 0.076a | 83.93 |
 | Table III.Inhibitory effect of ATRA at various
concentrations on the proliferation of SGC7901 cells at 72 h. |
Table III.
Inhibitory effect of ATRA at various
concentrations on the proliferation of SGC7901 cells at 72 h.
| Groups | OD570 | % Inhibition |
|---|
| Control | 0.337 |
|
| vehicle control | 0.335 | 1.45 |
| atra, µmol/l |
|
|
| 5 | 0.271a | 22.88 |
| 10 | 0.252a | 29.77 |
| 25 | 0.238a | 34.35 |
| 50 | 0.166a | 59.68 |
| 100 | 0.063a | 95.36 |
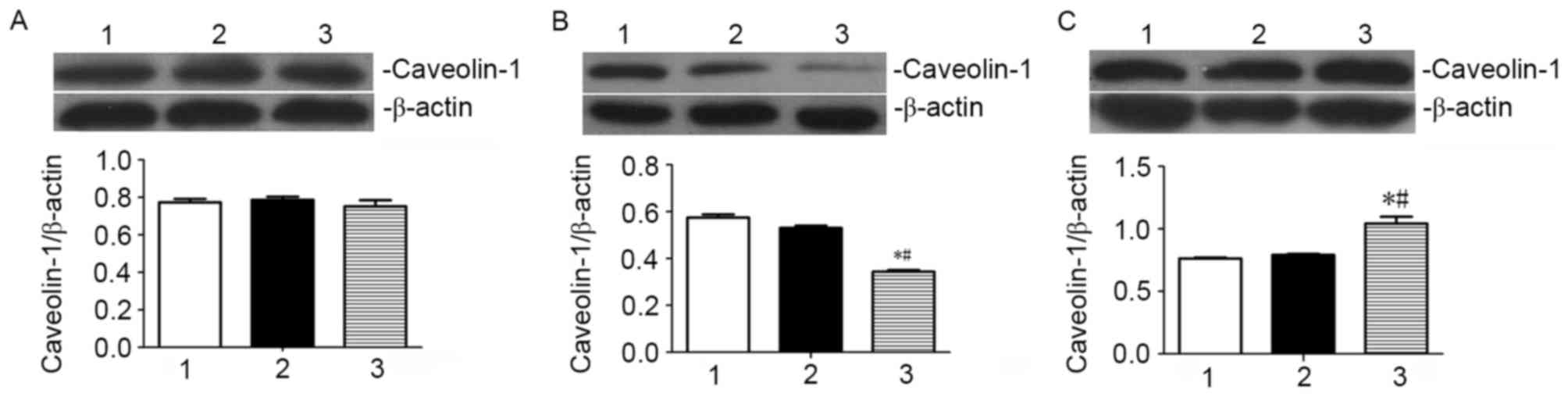
ATRA preferentially promotes the
translocation of caveolin-1 to the cell membrane in SGC7901
cells
Following treatment of SGC7901 cells with 10 µmol/l
ATRA for 72 h, caveolin-1 localization and expression were
determined by specific immunofluorescence labeling and western blot
assay, respectively. As presented in Fig.
2, the fluorescent staining of caveolin-1 was identified in the
cytoplasm and the cell membrane of the SGC7901 cells. Staining for
caveolin-1 in the cell membrane was markedly increased, whereas the
staining in the plasma was decreased when the cells were treated
with ATRA for 72 h when compared to untreated cells and vehicle
control. These results indicate that the cellular localization of
caveolin-1 in SGC7901 cells is affected by ATRA treatment. Western
blot analysis revealed that there was no significant change in the
total levels of caveolin-1 when SGC7901 cells were ATRA (Fig. 3). The levels of caveolin-1 in the
cytosolic protein fraction in SGC7901 cells were significantly
increased compared with the vehicle control and untreated cells. By
contrast, the levels of caveolin-1 in the cytosolic fraction in
ATRA-treated SGC7901 cells were significantly decreased compared
with the vehicle control and untreated cells (Fig. 3). These results indicate that ATRA
treatment promotes the localization of caveolin-1 in the cell
membrane, whereas ATRA treatment has no effects on the total
expression levels of caveolin-1 in SGC7901 gastric cancer
cells.
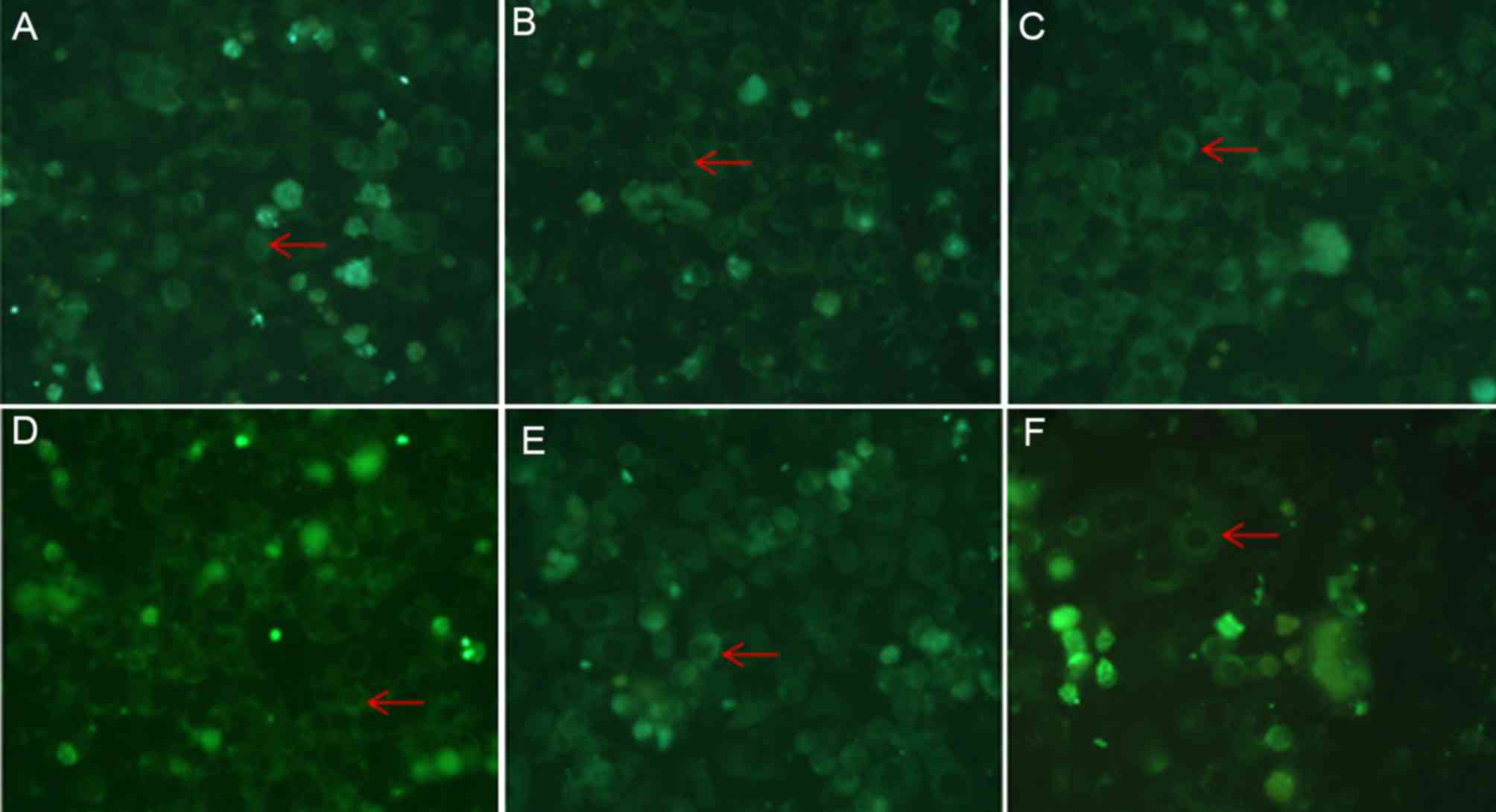
ATRA meditates its effects on the
localization of caveolin-1 by inhibiting the activity of the
ERK/MAPK signaling pathway
The SGC7901 cells were treated with 10 µmol/l ATRA
for 5, 15, 30 and 45 min, 1, 2, 4 or 24 h, and the activity of the
ERK signaling pathway was investigated by analyzing the
phosphorylation levels of ERK. As presented in Fig. 4, the levels of pERK started to
decrease when the cells were treated for 45 min compared with
untreated cells, and the levels began to restore when the cells
were treated longer than 2 h. The observations led to the
hypothesis that ATRA may meditate its effects on the regulation of
caveolin-1 expression and localization via the ERK/MAPK signaling
pathway. To investigate this hypothesis, a specific inhibitor
(PD98059) and activator (PMA) of the ERK/MAPK signaling pathway
were added to the cell culture medium either in combination with
ATRA or alone. Immunofluorescence assay revealed that the membrane
localization of caveolin-1 was markedly increased in the cells that
were treated with a combination of ATRA and PD98059 (a specific
inhibitor of the ERK/MAPK signaling pathway), compared with the
cells that were treated with ATRA or PD98059 alone. On the other
hand, caveolin-1 aggregation induced by ATRA was attenuated in the
PMA-treated cells compared with ATRA treated cells (Fig. 5).
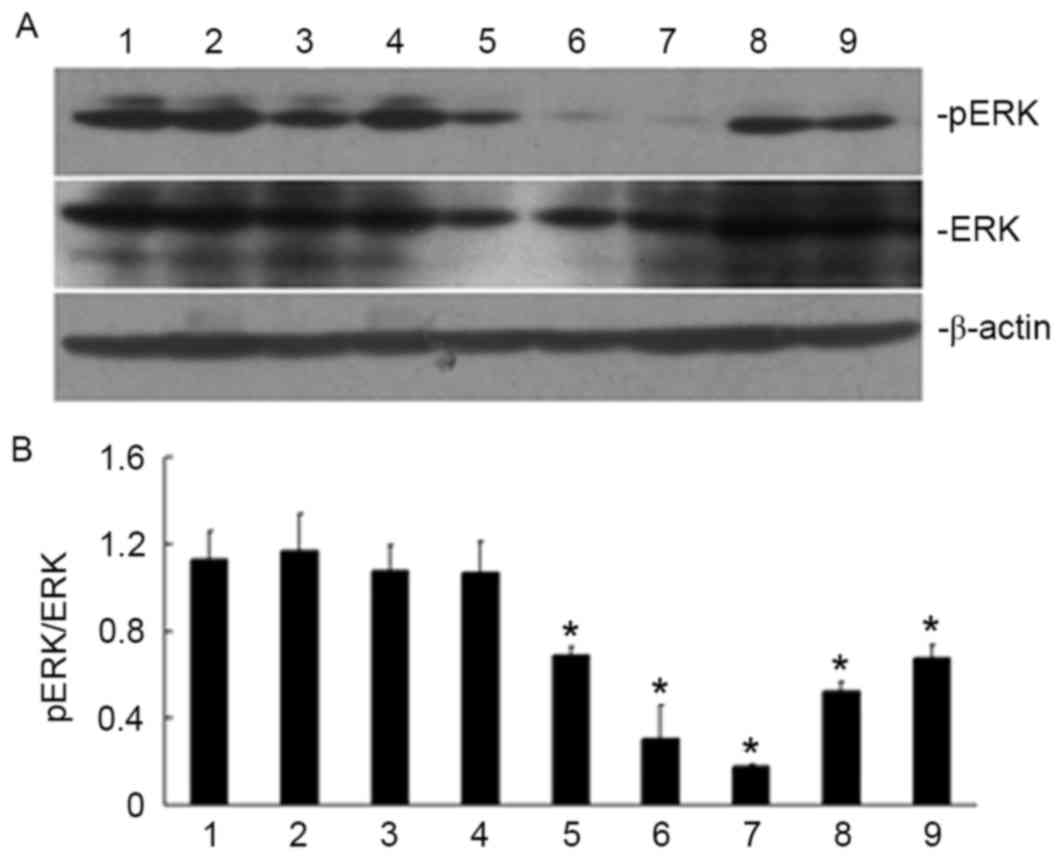 | Figure 4.Effect of ATRA treatment duration on
ERK phosphorylation in SGC790 1 gastric cancer cells. Treatment
with ATRA was able to inhibit ERK phosphorylation when the cells
were treated for 30 min. (A) Specific bands for pERK and ERK in
SGC7901 cells are shown in the western blots. (B) The ratio of pERK
and ERK under various treatment conditions. Lanes: 1, untreated
cells; 2, vehicle control; 3, ATRA-treated for 5 min; 4, ATRA
treatment for 15 min; 5, ATRA treatment for 30 min; 6, ATRA
treatment for 45 min; 7, ATRA treatment for 1 h; 8, ATRA treatment
for 2 h; 9, ATRA treatment for 4 h. *P<0.05, vs. untreated cells
and vehicle control. ATRA, all-trans retinoic acid; ERK,
extracellular signal-regulated kinase; p, phosphorylated. |
Discussion
Gastric cancer is one of the most common types of
malignant tumor in China and is the leading cause of
cancer-associated mortality (13). As
with other malignant tumors, multi-step processes and genetic
alterations are proposed to be involved in the development of
gastric cancer (14). Among these
diverse processes, dysfunction of caveolae has been identified to
be a critical factor involved in tumor development, invasion and
metastasis (3).
Caveolae are membrane microdomains consisting of
three major structural proteins, namely caveolin-1, −2 and −3
(15). Caveolin-1 is a principal
protein for constructing caveolae and exerts roles in multiple
cellular processes that are involved in tumor genesis signaling
(4,16). However, the tumor-associated functions
of caveolin-1 are not fully clarified and are dependent on the type
of tissue and stage of cancer (3). In
gastric cancer, caveolin-1 has been identified as a tumor
suppressor. A decrease or loss of caveolin-1 expression was
demonstrated to be implicated in the pathogenesis of oncogenic
transformation of cells (5,6). Decreased expression of caveolin-1 was
associated with increased occurrence of gastric cancer (5). Caveolin-1 expression resulted in cell
cycle arrest at the G0-G1 phase of the cell cycle, thereby
attenuating cell growth (17,18). In addition, silencing of cavelin-1 in
primary tumors promotes cell proliferation and enables excessive
clonal expansion of tumor cells (19,20).
Therefore, caveolin-1 may be regarded as a prognostic biomarker for
early detection, prediction and as a potential therapeutic target
of gastric adenocarcinoma (21).
ATRA is derived from vitamin A and has been used in
oncotherapy, particularly in the treatment of acute leukemia
(8). In addition, previous studies
have suggested that ATRA also regulate multiple cancer-associated
processes in solid cancer cells, including cell proliferation,
apoptosis, differentiation, migration and metastasis (22–24). The
current study aimed to investigate the effects of ATRA treatment on
the gastric cancer cell line SGC7901 and to determine whether
caveolin-1 is involved in the effects of ATRA.
Cell proliferation assay revealed that in the
SGC7901 cell line, treatment with ATRA led to a time- and
dose-dependent decrease in cell proliferation (Fig. 1). The inhibition % at 10 µmol/l was
18.02, 28.28 and 29.77% when the cells were treated with ATRA for
24, 48 and 72 h, respectively. In the subsequent experiments of the
present study, the SGC7901 cells were treated with ATRA at the
concentration of 10 µmol/l ATRA for 72 h.
Specific immunofluorescent staining was used to
localize caveolin-1 in SGC7901 cells. The results of this assay
demonstrated that caveolin-1 was located in the cytoplasm and on
the cell membrane of the SGC7901 cells. ATRA-treated cells
exhibited significantly increased membrane localization of
caveolin-1 accompanied by decreased cytosolic localization,
compared with the untreated controls (Fig. 2). To verify the results of the
immunofluorescence experiments, western blot analysis was performed
to detect the total levels of caveolin-1, and membrane and
cytosolic protein fractions in SGC7901 cells. In the western blot
assay, the quantity of caveolin-1 relative to that of the reference
protein β-actin was calculated with Quantity One. A significantly
increased level of caveolin-1 was observed in the cell membrane
protein fraction in ATRA-treated cells, compared with the untreated
control and vehicle control (Fig.
3C). At the same time, a significant decrease in the level of
caveolin-1 in the cytosolic protein fraction was observed in the
ATRA-treated cells compared with the untreated control and vehicle
control (Fig. 3B). However, there was
no noticeable difference in the total levels of caveolin-1 in
ATRA-treated cells compared with control cells (Fig. 3A). Consistent results were observed
from the immunofluorescence and western blot assays. The results of
the present study suggest that ATRA is able to specifically promote
the localization of caveolin-1 at the cell membrane, however ATRA
has no effect on the total levels of caveolin-1.
The treatment of the SGC7901 cells with ATRA was
able to inhibit the ERK/MAPK signaling pathway by decreasing the
phosphorylation of ERK (Fig. 4). It
was hypothesized that ATRA may regulate the localization of
caveolin-1 by inhibiting the ERK/MAPK signaling pathway. To
investigate the hypothesis, PMA (a specific agonist of the ERK/MAPK
signaling pathway) and PD98059 (a inhibitor of the ERK/MAPK
signaling pathway) were used to treat the SGC7901 cells, and
immunofluorescent staining of caveolin-1 was performed. The results
revealed that the membrane localization of caveolin-1 was increased
when the cells were treated with the specific inhibitor PD98059
alone compared with untreated cells, and the effect was more
apparent when the cells were treated with a combination of ATRA and
PD98059 (Fig. 5C and D). The specific
agonist PMA had the opposite effects compared with treatment with
PD98059 alone. Treatment with PMA was able to attenuate
ATRA-induced increase of membrane localization of caveolin-1
(Fig. 5E and F). These results may
suggest that ATRA serves a function in adjusting the cellular
localization of caveolin-1 via the ERK/MAPK signaling pathway.
In conclusion, the results of the present study
indicate that treatment with ATRA was able to inhibit the
proliferation of SGC7901 gastric cancer cells and the inhibitory
effects of ATRA may be partially mediated by increasing caveolin-1
membrane localization. In addition, the effects of ATRA treatment
in SGC7901 cells are partly mediated by inhibiting the activation
of ERK.
Acknowledgements
The present study was supported by the Fund for
Young Talents in College of Anhui Province (grant no. 2012SQRL067),
National Natural Science Foundation of China (grant no. 81201907),
National Natural Science Foundation of China (grant no. 81272399)
and Research Fund for Doctor in Anhui Medical University (grant no.
XJ201229).
References
|
1
|
Kamińska K, Szczylik C, Bielecka ZF,
Bartnik E, Porta C, Lian F and Czarnecka AM: The role of the
cell-cell interactions in cancer progression. J Cell Mol Med.
19:283–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kovtun O, Tillu VA, Ariotti N, Parton RG
and Collins BM: Cavin family proteins and the assembly of caveolae.
J Cell Sci. 128:1269–1278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng JP and Nichols BJ: Caveolae: One
function or many? Trends Cell Biol. 26:177–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fridolfsson HN, Roth DM, Insel PA and
Patel HH: Regulation of intracellular signaling and function by
caveolin. FASEB J. 28:3823–3831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun GY, Wu JX, Wu JS, Pan YT and Jin R:
Caveolin-1, E-cadherin and β-catenin in gastric carcinoma,
precancerous tissues and chronic non-atrophic gastritis. Chin J
Cancer Res. 24:23–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu L, Qu X, Li H, Li C, Liu J, Zheng H and
Liu Y: Src/caveolin-1-regulated EGFR activation antagonizes
TRAIL-induced apoptosis in gastric cancer cells. Oncol Rep.
32:318–324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao X, He Y, Gao J, Fan L, Li Z, Yang G
and Chen H: Caveolin-1 expression level in cancer associated
fibroblasts predicts outcome in gastric cancer. PLoS One.
8:e591022013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schenk T, Stengel S and Zelent A:
Unlocking the potential of retinoic acid in anticancer therapy. Br
J Cancer. 111:2039–2045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma HS, Greenblatt SM, Shirley CM, Duffield
AS, Bruner JK, Li L, Nguyen B, Jung E, Aplan PD, Ghiaur G, et al:
All-trans retinoic acid synergizes with FLT3 inhibition to
eliminate FLT3/ITD+ leukemia stem cells in vitro and in vivo.
Blood. 127:2867–2878. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dutta A, Sen T and Chatterjee A: All-trans
retinoic acid (ATRA) downregulates MMP-9 by modulating its
regulatory molecules. Cell Adh Migr. 4:409–418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ju J, Wang N, Wang X and Chen F: A novel
all-trans retinoic acid derivative inhibits proliferation and
induces differentiation of human gastric carcinoma xenografts via
up-regulating retinoic acid receptor β. Am J Transl Res. 7:856–865.
2015.PubMed/NCBI
|
|
12
|
Scudiero DA, Shoemaker RH, Paull KD, Monks
A, Tierney S, Nofziger TH, Currens MJ, Seniff D and Boyd MR:
Evaluation of a soluble tetrazolium/formazan assay for cell growth
and drug sensitivity in culture using human and other tumor cell
lines. Cancer Res. 48:4827–4833. 1988.PubMed/NCBI
|
|
13
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin K, Li T, van Dam H, Zhou F and Zhang
L: Molecular insights into tumour metastasis: Tracing the dominant
events. J Pathol. 241:567–577. 2016. View Article : Google Scholar
|
|
15
|
Fine SW, Lisanti MP, Galbiati F and Li M:
Elevated expression of Caveolin-1 in adenocarcinoma of the colon.
Am J Clin Pathol. 115:719–724. 2011. View Article : Google Scholar
|
|
16
|
Zhang Y, Hu XJ, Zhang LL, Sun LP, Yuan Y,
Qu XJ and Liu YP: Interaction among Caveolin-1 genotypes
(rs3807987/rs7804372), H. pylori infection, and risk of gastric
cancer in a Chinese population. Tumour Biol. 35:1511–1516. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burgermeister E, Xing X, Röcken C, Juhasz
M, Chen J, Hiber M, Mair K, Shatz M, Liscovitch M, Schmid RM and
Ebert MP: Differential expression and function of caveolin-1 in
human gastric cancer progression. Cancer Res. 67:8519–8526. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galbiati F, Volonté D, Liu J, Capozza F,
Frank PG, Zhu L, Pestell RG and Lisanti MP: Caveolin-1 expression
negatively regulates cell cycle progression by inducingG(0)/G(1)
arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol Biol Cell.
12:2229–2244. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Williams TM and Lisanti MP: The Caveolin
genes: From cell biology to medicine. Ann Med. 36:584–595. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hitkova I, Yuan G, Anderl F, Gerhard M,
Kirchner T, Reu S, Röcken C, Schäfer C, Schmid RM, Vogelmann R, et
al: Caveolin-1 protects B6129 mice against Helicobacter pylori
gastritis. PloS Pathog. 9:e10032512013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kannan A, Krishnan A, Ali M, Subramaniam
S, Halagowder D and Sivasithamparam ND: Caveolin-1 promotes gastric
cancer progression by up-regulating epithelial to mesenchymal
transition by crosstalk of signalling mechanisms under hypoxic
condition. Eur J Cancer. 50:204–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chu JH, Gao ZH and Qu XJ: Down-regulation
of sphingosine kinase 2 (SphK2) increases the effects of
all-trans-retinoic acid (ATRA) on colon cancer cells. Biomed
Pharmacother. 68:1089–1097. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun R, Liu Y, Li SY, Shen S, Du XJ, Xu CF,
Cao ZT, Bao Y, Zhu YH, Li YP, et al: Co-delivery of
all-trans-retinoic acid and doxorubicin for cancer therapy with
synergistic inhibition of cancerstem cells. Biomaterials.
37:405–414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin E, Chen MC, Huang CY, Hsu SL, Huang
WJ, Lin MS, Wu JC and Lin H: All-trans retinoic acid induces DU145
cell cycle arrest through Cdk5 activation. Cell Physiol Biochem.
33:1620–1630. 2014. View Article : Google Scholar : PubMed/NCBI
|