Introduction
As the most common type of malignancy in females
worldwide, breast cancer has become the second leading cause of
cancer-associated mortalities (1).
Patients with breast cancer receive chemotherapy, including
anthracyclines and taxanes, which cause severe cumulative
toxicities and tolerability disturbance (2). Therefore, investigating alternative
therapeutic drugs for the treatment of breast cancer is required in
the coming years (3).
Accumulating evidence has demonstrated that certain
natural products provide a novel avenue for neoplastic therapy, due
to their extensive involvement in biological activities and
comparatively low toxicities (4). As
an effective component extracted from Chinese herbs, Sophora
flavescens, matrine [MT; dodecahydro-3a, 7a-diaza-benzo
(de)anthracen-8-one, molecular formula:
C15H24N2O], has revealed antitumor
effects by inhibiting proliferation via inducing apoptosis or
altering cell cycle arrest in numerous types of cancer cells
(5,6).
MT was also observed to suppress proliferation, invasion and
metastasis of breast cancer in vitro and in vivo
(7,8).
However, the underlying mechanisms of MT against breast cancer have
not been fully elucidated.
The Wnt/β-catenin signaling pathway serves a vital
role in the occurrence and development of various cancer types
(9,10). It has been reported that Wnt1 is
overexpressed in human breast cancer and upregulated in
leptin-induced tumor progression (11–13).
Radiation therapy may suppress self-renewal capacities of cancer
stem cells by inhibiting the cyclinD1/protein kinase B1/Wnt1
signaling pathway (11–13).
Dysregulated activation of the Wnt/β-catenin
signaling pathway has been surveyed in human breast cancer
(14). In order to investigate the
mechanisms underlying antitumor effects of MT against breast
cancer, the present study hypothesized that MT may suppress breast
cancer growth by downregulating the expression of vascular
endothelial growth factor (VEGF) via the Wnt/β-catenin signaling
pathway.
Materials and methods
Reagents, cell lines and animals
MT (purity, 99.12%) and doxorubicin (Dox) were
purchased from Ningxia Bauhinia Pharmaceutical Co., Ltd. (Ningxia,
China) and Pfizer, Inc. (New York, NY, USA), respectively.
The mouse 4T1 and human MCF-7 breast cancer cell
lines were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). Cancer cells were cultured in RMPI-1640
supplemented with 10% fetal bovine serum at 37°C in a humidified 5%
CO2 incubator. A total of 32 female BALB/c mice
(8-weeks-old; weight, 18–22 g) were obtained from the Laboratory
Animal Center, Shandong University (Shandong, China). The animals
were housed in a specified chamber with controlled air conditions
(temperature, 20–25°C, humidity, 50–65%) with free access to
sterile food and water.
MTT assay
The viability of 4T1 and MCF-7 cells was analyzed by
MTT assay. Cells (5×103/well) were cultured in 96-well
plates, and incubated for 24, 48 and 72 h with various
concentrations of MT (0.1, 0.2, 0.4, 0.8 and 1.6 mM), which were
prepared as previously described (2).
The cells were incubated for an additional 4 h following the
addition of MTT (20.0 ml/well). Then, the supernatants were
discarded and the crystals were dissolved in 150 µl dimethyl
sulfoxide. The absorbance was measured at 490 nm using a
microculture plate reader (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The viability (%) was calculated as the formula: (mean
absorbance in control wells - mean absorbance in test wells)/mean
absorbance in control wells × 100%.
Flow cytometric assay of
apoptosis
4T1 cells were treated with MT (0.2, 0.4, 0.8 mM)
for 48 h, harvested and the numbers of cells counted. Apoptosis was
evaluated by Annexin V-fluorescein isothiocyanate/propidium iodide
(PI), according to manufacturer's instructions (BD Pharmingen; BD
Biosciences, Franklin Lakes, NJ, USA) and analyzed using a FACScan
flow cytometer (BD FACSDiva software version 8.0.1; BD
Biosciences).
Animal experiments
The protocol for in vivo experiments was
approved by the Institutional Care and Use Committee of Shandong
University (permit no. 201402079) and performed according to the
Guide for the Care and Use of Laboratory Animals published by the
US National Institutes of Health (15). Tumors were established by injection of
a 4T1 cell suspension (100 µl, 5×106 cells/ml) into the
right side of the 4th mammary gland of mice. The tumor length (a)
and width (b) were evaluated every two days and the tumor volume
was calculated as the formula: V (mm3) = (a ×
b2)/2 (15). When the size
of tumors was ~100 mm3, mice were randomly assigned to
four groups and received treatment every two days seven times
(8): i) Control group (n=8), mice
received intraperitoneal (i.p.) injection of saline; ii) MT group
(50 mg/kg, n=8), mice received i.p. injection of MT at a dose of 50
mg/kg; iii) MT group (100 mg/kg, n=8), mice received i.p. injection
of MT at a dose of 100 mg/kg; and iv) Dox group (n=8), mice
received i.p. injection of Dox at a dose of 3 mg/kg. The mice were
sacrificed on day 21 and the tumors were removed rapidly and
weighed. Numerous tissues were fixed with 10% formaldehyde for
TdT-mediated dUTP nick-end labeling (TUNEL) and
immunohistochemistry (IHC), whereas others were rapidly stored at
−80°C for western blotting.
TUNEL assay
Tumor sections were stained with TUNEL system
(Promega Corporation, Madison, WI, USA), according to the
manufacturer's instructions. Briefly, the tumor tissues from the
four mouse groups were fixed in 10% formaldehyde at room
temperature overnight, and then were embedded in paraffin. The
sections were then deparaffinized with xylene, rehydrated and then
treated with 3% hydrogen peroxide to quench the endogenous
peroxidase activity. Subsequent antigen retrieval was performed by
heating in citrate buffer solution (0.01 M) using a microwave oven.
Sections were cut at 5 µm, dewaxed and rehydrated, washed with PBS,
reacted with proteinase K (2 µl 50X proteinase K and 98 µl PBS) at
room temperature for 20 min. Samples were labeled using TdT
reaction mix and incubated for 1 h at 37°C in a humidified chamber
(80–90%). Subsequently, 2X saline sodium citrate buffer (Ningxia
Bauhinia Pharmaceutical Co., Ltd.) was added for 15 min to stop the
reaction. Following hematoxylin staining, samples were mounted on
to neutral balsam medium. Apoptotic cells were identified using
dark brown nuclear staining (100 cells obtained from six random
microscopic fields in each group) using a Nikon Eclipse 90i
microscope (Nikon Corporation, Tokyo, Japan).
IHC assay
Tumor tissues from the four mouse groups were fixed
in 10% formaldehyde at room temperature overnight, and then were
embedded in paraffin. The sections were deparaffinized with xylene,
rehydrated and then treated with 3% hydrogen peroxide at room
temperature for 20 min to quench the endogenous peroxidase
activity. Subsequent antigen retrieval was performed by heating in
citrate buffer solution (0.01 M) using a microwave oven. Sections
were 5 µm thick and stained with a VEGF antibody (1:800, cat. no.
9698; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C
overnight according to the manufacturer's instructions.
Subsequently, sections were further incubated with
3,3′-diaminobenzidine tetrahydrochloride for 5 min at room
temperature. The sections were observed and captured with a Nikon
Eclipse 90i microscope (magnification, ×200; Nikon
Corporation).
Western blotting
Western blotting was performed as previously
described (15). Tumor tissues were
homogenized and the supernatants were harvested by centrifugation
(3,600 × g) at 4°C for 5 min. Proteins were extracted using
cytoplasmic extraction reagents (Beyotime Institute of
Biotechnology, Haimen, China). Protein concentration in the
supernatants was determined by bicinchoninic acid assay. A total of
20 µg protein was separated using SDS PAGE (10% gel) and
transferred onto polyvinylidene fluoride membranes. The membranes
were blocked in 5% skimmed milk in TBST at room temperature for 1.5
h with gentle agitation and then incubated with primary antibodies
at 4°C overnight, and secondary antibodies at 4°C for 1.5 h and
visualized. Triplicate experiments with triplicate samples were
performed. The primary antibodies were all obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA), including cleaved caspase-9
(cat. no. sc-24528, 1:1,000), cleaved caspase-3 (cat. no.
sc-113427, 1:1,000), cytochrome c (cat. no. sc-13561,
1:800), VEGF (cat. no. sc-81670, 1:1,000), Wnt1 (cat. no. sc-6266,
1:1,000), β-catenin (cat. no. sc-221398, 1:800), cyclin D1 (cat.
no. sc-70899, 1:800), c-Myc (cat. no. sc-4084, 1:800) and β-actin
(cat. no. sc-70319, 1:1,000). The secondary antibodies horseradish
peroxidase labeled goat anti-mouse IgG (H+L) (cat. no. A0216,
1:1,000) were obtained from Beyotime Institute of Biotechnology.
The bound antibodies were visualized using an enhanced
chemiluminescence reagent (EMD Millipore, Billerica, MA, USA) and
quantified by densitometry using ChemiDoc™ XRS+ image
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
expression levels of proteins were quantified using model GS
800™ Calibrated Imaging Densitometry (cat. no. 170-7980;
Bio-Rad Laboratories, Inc.). The signal strength of each target
protein signal was normalized against the corresponding β-actin
control.
Statistical analysis
One-way analysis of variance with Bonferroni
adjustments for post hoc tests was used and the statistical
analyses were performed with SPSS Windows version 32 statistical
software (IBM Corp., Armonk, NY, USA). P<0.05 and P<0.01 were
considered to indicate a statistically significant and highly
significant differences, respectively.
Results
MT inhibits the growth of breast
cancer cells in vitro
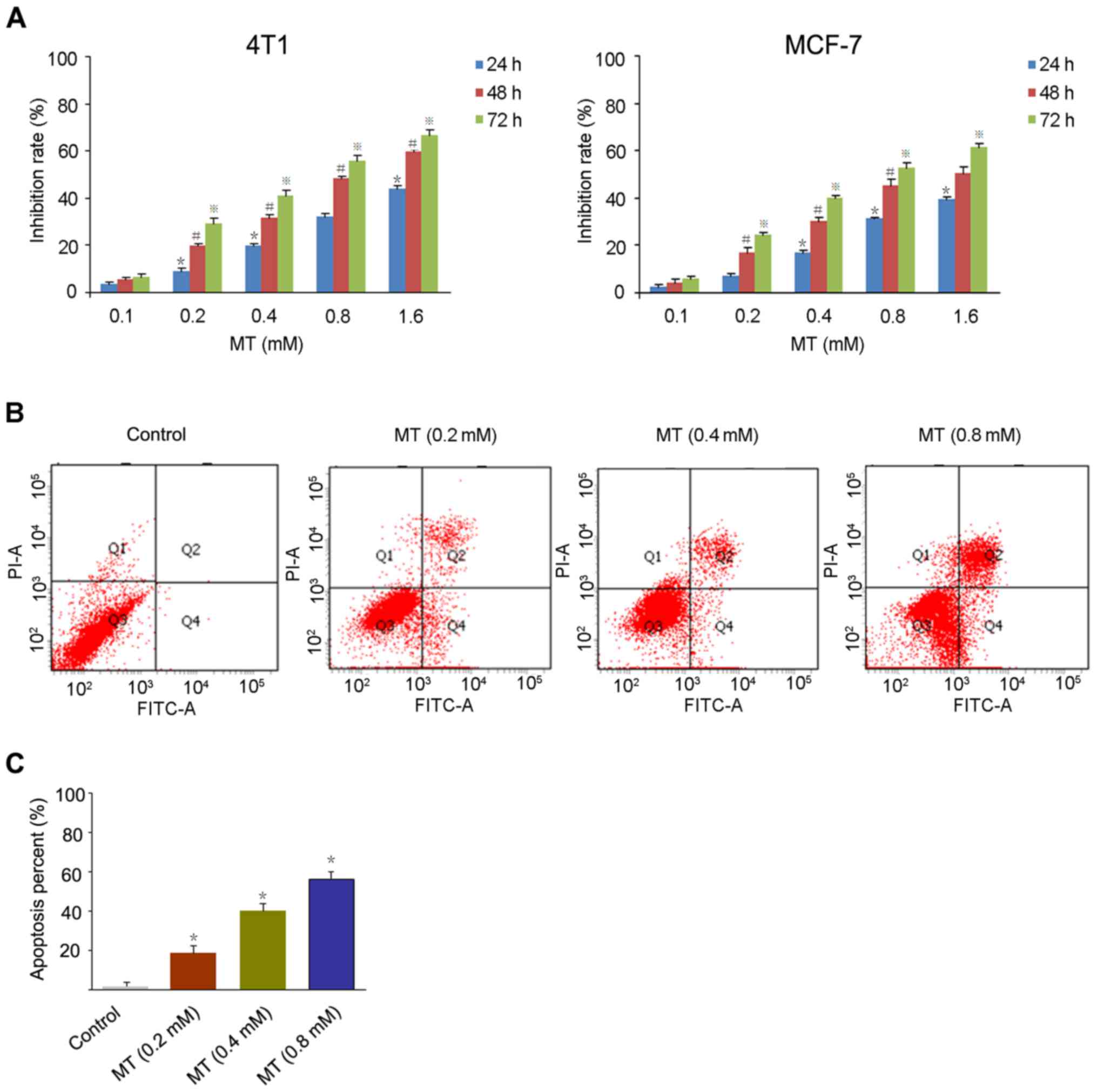
For the MTT assay, 4T1 and MCF-7 breast cancer cells
were treated with MT at a series of concentration (0.1, 0.2, 0.4,
0.8 and 1.6 mM) for 24, 48 and 72 h, respectively. As presented in
Fig. 1A, MT inhibited the growth of
4T1 and MCF-7 cells in a dose- and time-dependent manner with a
half-maximal inhibitory concentration (48 h) of 0.78, and 0.86 mM,
respectively.
In order to detect the apoptosis rate in cancer
cells induced by MT, the Annexin V/PI method was used to stain the
4T1 cells treated with 0.2, 0.4 and 0.8 mM MT for 48 h. Fig. 1B and C demonstrates a significant
increase in apoptotic cells following exposure to MT compared with
the control. The apoptosis rates of 4T1 cells were 20.1% (0.2 mM),
30.2% (0.4 mM) and 57.6% (0.8 mM), respectively.
MT suppresses the growth of 4T1 tumors
in vivo
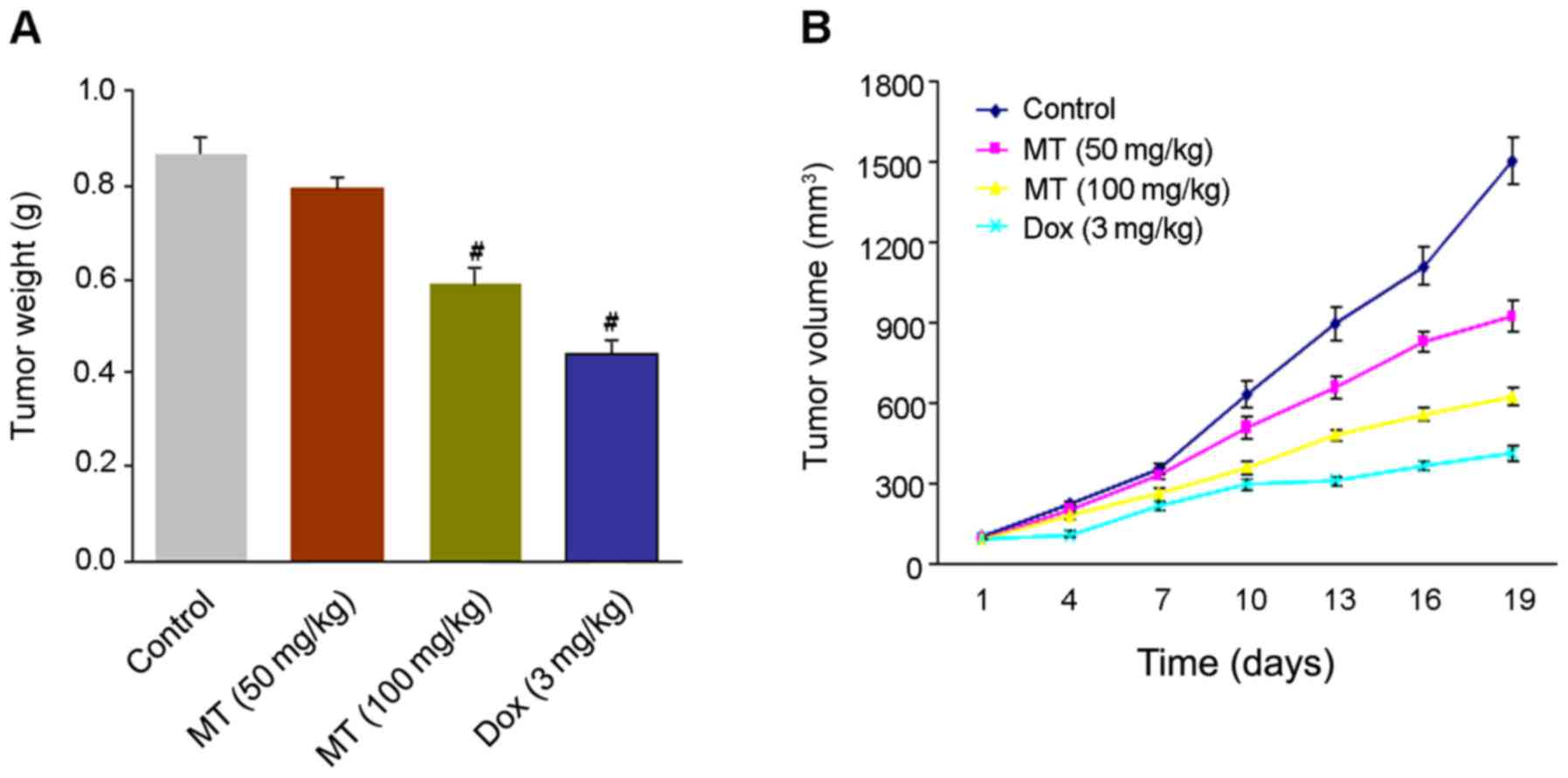
As presented in Fig.
2A, a gradual loss of tumor weight was detected in the four
groups. The weights of mice tumors treated with 100 mg/kg MT and 3
mg/kg Dox significantly decreased to 0.58, and 0.43 g compared with
the controls (0.86 g). The average tumor volumes were determined as
923.33 and 622.34 mm3 in 50 or 100 mg/kg MT compared
with 1,502.00 mm3 in the control. Notably, tumor volumes
represented a reduction to 412.36 mm3 with 3 mg/kg Dox
treatment. The results revealed a greater inhibitory effect on
tumor growth in MT (100 mg/kg) compared with the controls (Fig. 2B).
MT induces apoptosis in 4T1 tumors in
vivo
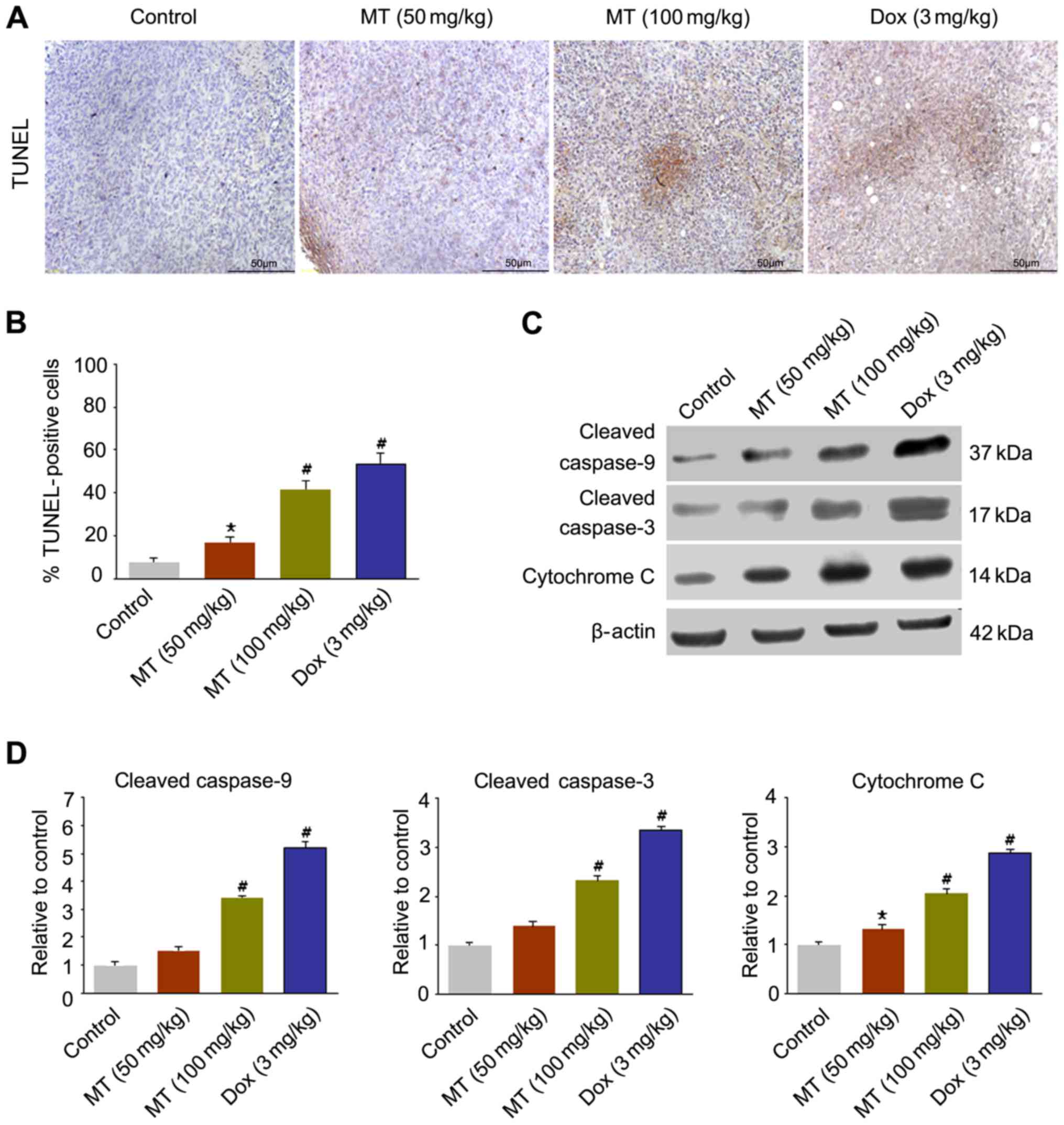
As presented in Fig. 3A
and B, the apoptotic cell proportion in TUNEL-stained samples
in four groups was determined. The 50 mg/kg MT treatment resulted
in apoptosis in 18.95% of the tumor cells, whereas 100 mg/kg MT and
3 mg/kg Dox treatments induced apoptosis in 41.62, and 52.03% of
cells (n=6), respectively.
In order to confirm the apoptosis changes, the
expression levels of apoptosis-associated factors were estimated in
tumors. The western blotting results demonstrated that the
expression levels of cleaved caspase-9, cleaved caspase-3 and
cytochrome c increased significantly in 100 mg/kg MT
compared with the controls (Fig. 3C and
D).
MT reduces angiogenesis in 4T1 tumor
cells in vivo
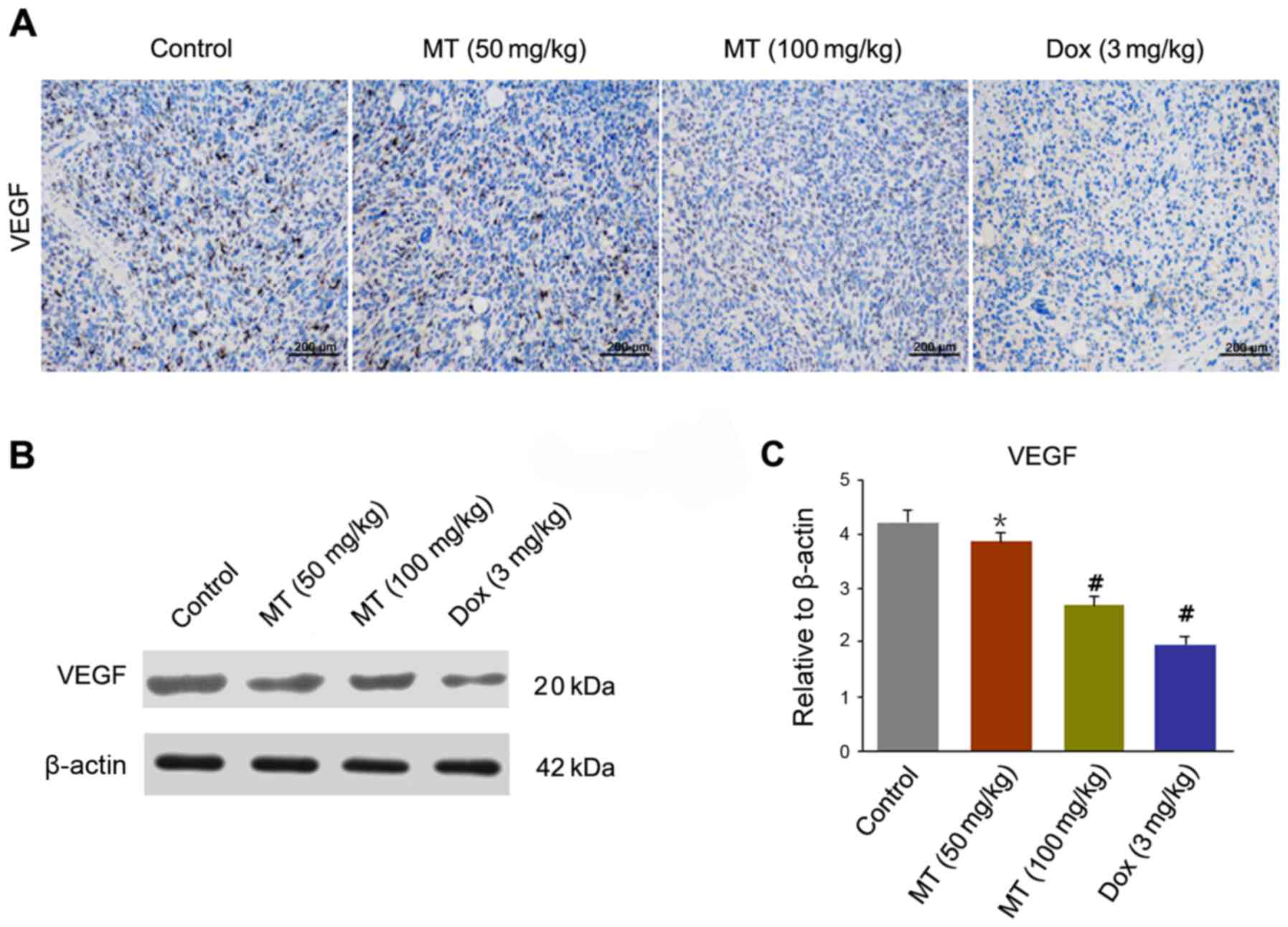
To observe microvascular expression of tumor cells,
the expression of VEGF in endothelial cells was determined by IHC
in harvested tumor tissues (Fig. 4A).
A significantly decreased level of VEGF staining was observed in
tumors treated with MT and Dox compared with the control. The
expression level of VEGF was significantly decreased in MT (50
mg/kg), MT (100 mg/kg) and Dox (3 mg/kg) treated cells, compared
with the controls (Fig. 4B and
C).
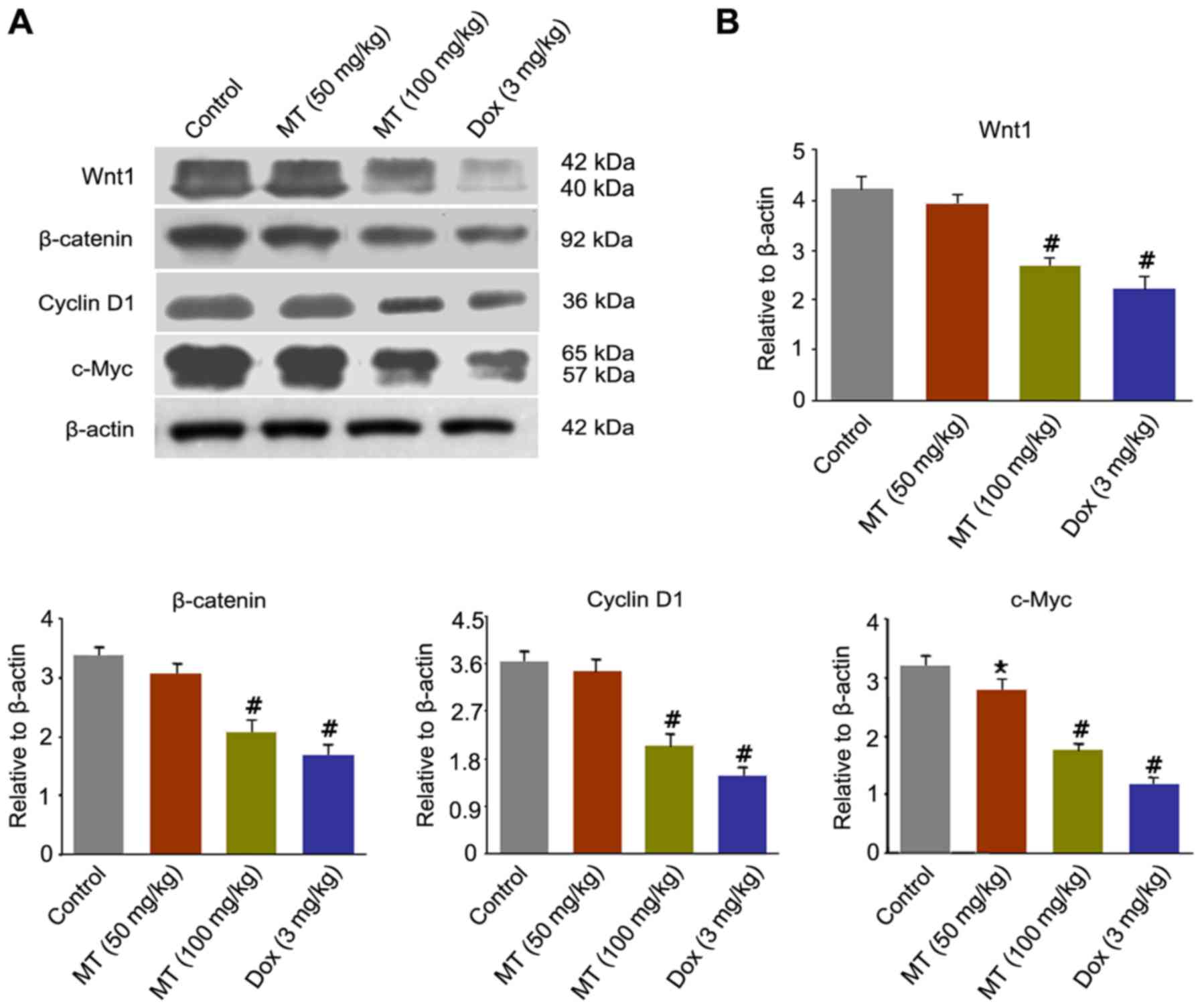
MT inhibits breast cancer growth by
downregulating Wnt/β-catenin signaling pathway in vivo
To clarify the underlying mechanisms responsible for
the antitumor effect of MT on breast cancer, the present study
analyzed alterations in numerous important proteins of the
Wnt/β-catenin signaling pathway, including Wnt1, β-catenin, cyclin
D1 and c-Myc. Fig. 5A revealed that
the expression levels of Wnt1, β-catenin, cyclin D1 and c-Myc were
markedly decreased in the treatment of MT and Dox compared with the
control. Following MT (100 mg/kg) exposure, the expression levels
of Wnt1, β-catenin, cyclin D1 and c-Myc significantly decreased by
33.3, 36.36, 44.44 and 43.75%, respectively, compared with the
control (Fig. 5B).
Discussion
Breast cancer is the most frequent type of
malignancy in females worldwide and affects 1.3 million individuals
(1). The prognosis of breast cancer
remains poor despite recent advances in the therapeutic regimens.
Therefore, studies aiming to identify a successful therapeutic
strategy should be a priority. MT was revealed to possess antitumor
function of inhibiting cell growth and proliferation in various
cancer cell lines, including hepatoma, acute myeloid leukemia,
gastric carcinoma, lung cancer and breast cancer (4,16–19). Numerous studies focused on the
antitumor activity of MT against breast cancer in vitro;
however, the mechanisms have not been fully elucidated (2,7). Thus, the
present study investigated the anti-breast-cancer effects of MT
in vitro and in vivo.
Uncontrolled proliferation serves an important role
in tumorigenesis, and the suppression of cancer cell proliferation
is a key aspect in tumor therapy (20). For the in vitro experiments,
the present study performed an MTT assay to evaluate cell viability
by treating cells with a series concentrations of MT (0.1–1.6 mM).
The results indicated that MT had a dose- and time-dependent
inhibition effect on growth of 4T1, and MCF-7 cells. Furthermore,
the 4T1 cells were more sensitive to MT compared with MCF-7 cells.
The pro-apoptotic effect of MT on 4T1 cells was confirmed by flow
cytometry. The results of the present study were in accordance with
previous studies, which demonstrated that MT significantly
inhibited the growth of breast cancer (11–13). For
the in vivo experiments, the 4T1-tumor bearing mice were
treated with MT (100 mg/kg) or Dox and demonstrated a significant
reduction in tumor weight and volume compared with the control.
However, the reduction in tumor weight and volume was insignificant
in the MT 50 mg/kg group. The TUNEL results and expression levels
of apoptotic-associated factors demonstrated the pro-apoptosis
activity of MT, and Dox against breast neoplasms.
Tumor growth is not only due to abnormal
proliferation, but also depends on a reduction in apoptosis
(21). Hence, the induction of
apoptosis is a major underlying mechanism in chemotherapy. Cellular
apoptosis involves the extrinsic pathway induced by the
death-receptor and the intrinsic pathway, also known as
mitochondrial pathway. In the intrinsic pathway, the release of
cytochrome c activates caspase-9, which subsequently induces
the activation of caspase-3. Activated caspase-3 serves a role in
the apoptotic pathway for the cleavage of various cellular targets
(22,23). The results of the present study
demonstrated that the expression levels of cleaved caspase-9,
cleaved caspase-3 and cytochrome c decreased significantly
in 100 mg/kg MT-treated mice compared with the control, which
implied that the mitochondrial pathway may be involved in
MT-induced apoptosis in 4T1-tumors.
Angiogenesis serves an important role in the
development of tumor growth (24). As
a crucial antigenic cytokine, VEGF is the major regulator of
endothelial cell survival (25). The
decrease of VEGF is considered to be a key prognostic indicator in
patients with breast cancer (26). In
the present study, the expression levels of VEGF were observed by
IHC and western blotting at a higher level in the controls, and
were downregulated significantly in MT-treated mice at a dose of
100 mg/kg. VEGF is a major downstream target of the Wnt/β-catenin
signaling pathway. The sustained activation of the Wnt/β-catenin
signaling pathway was observed in the development of numerous types
of cancer, including breast cancer. At normal levels, β-catenin is
degraded via the ubiquity pathway. When the oncogenes activated
Wnt, the β-catenin translocated to the nucleus in the formation of
polymers, resulting in regulating target gene expression levels,
including cyclin D1 and c-Myc (12–14).
It was demonstrated that the expression level of
Wnt1 mRNA was markedly upregulated in human breast cancer cells
(27). Furthermore, Wnt1 was able to
inhibit the processes of apoptosis by obstructing the release of
cytochrome c and restricting the activation of caspase-9
(28). Cyclin D1 increased the cell
proliferation in various type of cancer by promoting the transition
from G0/G1 to the S cell cycle phase
(29). c-Myc has been revealed to by
overexpressed in breast cancer, which is associated with poor
curative effect. Repressing c-Myc expression is a critical event in
the tumor suppressor, which is likely to be highly relevant in the
novel therapy designation (30,31). MT
was able to inhibit human pancreatic cancer cell migration via
downregulating the Wnt signaling pathway. Therefore, it is of
interest to investigate whether apoptosis is induced by MT via the
Wnt/β-catenin signaling pathway. In the present study, the high
expression levels of Wnt1, β-catenin, cyclin D1 and c-Myc implied
that the Wnt/β-catenin signaling pathway serves an important role
in breast cancer development, which is consistent with previous
studies. MT treatment (100 mg/kg) caused a marked reduction in the
expression levels of major proteins compared with the control,
which suggested that MT effectively inhibits breast cancer growth
by downregulating the Wnt/β-catenin signaling pathway and may be a
potential drug in cancer therapy.
Acknowledgements
The present study was supported by Chengde City
Science and Technology Research and Development Program (grant no.
201701A068).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li LQ, Li XL, Wang L, Du WJ, Guo R, Liang
HH, Liu X, Liang DS, Lu YJ, Shan HL and Jiang HC: Matrine inhibits
breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells.
Cell Physiol Biochem. 30:631–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vahdat LT, Pruitt B, Fabian CJ, Rivera RR,
Smith DA, Tan-Chiu E, Wright J, Tan AR, Dacosta NA, Chuang E, et
al: Phase II study of eribulin mesylate, a halichondrin B analog,
in patients with metastatic breast cancer previously treated with
an anthracycline and a taxane. J Clin Oncol. 27:2954–2961. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J,
Zhang S, Wu J, Yu K and Han Y: Matrine induces apoptosis in human
acute myeloid leukemia cells via the mitochondrial pathway and Akt
inactivation. PLoS One. 7:e468532012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li T, Wong VK, Yi XQ, Wong YF, Zhou H and
Liu L: Matrine induces cellanergy in human Jurkat T cells through
modulation of mitogen-activated protein kinases and nuclear factor
of activated T-cells signaling with concomitant upregulation of
anergy-associated genes expression. Biol Pharm Bull. 33:40–46.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang S, Cheng B, Li H, Xu W, Zhai B, Pan
S, Wang L, Liu M and Sun X: Matrine inhibits proliferation and
induces apoptosis of human colon cancer LoVo cells by inactivating
Akt pathway. Mol Biol Rep. 41:2101–2108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu P, Liu Q, Liu K, Yagasaki K, Wu E and
Zhang G: Matrine suppresses breast cancer cell proliferation and
invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology.
59:219–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Tan G, Jiang X, Qiao H, Pan S, Jiang
H, Kanwar JR and Sun X: Therapeutic effects of Matrine on primary
and metastatic breast cancer. Am J Chin Med. 38:1115–1130. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polakis P: The many ways of Wnt in cancer.
Curr Opin Genet Dev. 17:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smalley MJ and Dale TC: Wnt signalling in
mammalian development and cancer. Cancer Metastasis Rev.
18:215–230. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katoh M: Expression and regulation of WNT1
in human cancer: Up-regulation of WNTI by beta-estradiol in MCF-7
cells. Int J Oncol. 22:209–212. 2003.PubMed/NCBI
|
|
12
|
Sun H, Ding C, Zhang H and Gao J: Let-7
miRNAs sensitize breast cancer stem cells to radiation-induced
repression through inhibition of the cyclin D1/Akt1/Wnt1 signaling
pathway. Mol Med Rep. 14:3285–3292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Si W, Li Y, Shao H, Hu R, Wang W, Zhang K
and Yang Q: miR-34a inhibits breast cancer proliferation and
progression by targeting Wnt1 in Wnt/β-catenin signaling pathway.
Am J Med Sci. 352:191–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Li B, Zhou L, Yu S, Su Z, Song J,
Sun Q, Sha O, Wang X, Jiang W, et al: Prodigiosin inhibits
Wnt/β-catenin signaling and exerts anticancer activity in breast
cancer cells. Proc Natl Acad Sci USA. 113:pp. 13150–13155. 2016;
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Xue X, Zhang B, Jiang W, Cao H, Wang
R, Sun D and Guo R: The protective effects of paeonol against
epirubicin-induced hepatotoxicity in 4T1-tumor bearing mice via
inhibition of the PI3K/Akt/NF-kB pathway. Chem Biol Interact.
244:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X and Yu H: Matrine inhibits
diethylnitrosamine-induced HCC proliferation in rats through
inducing apoptosis via p53, Bax-dependent caspase-3 activation
pathway and down-regulating MLCK overexpression. Iran J Pharm Res.
15:491–499. 2016.PubMed/NCBI
|
|
17
|
Zhang JW, Su K, Shi WT, Wang Y, Hu PC,
Wang Y, Wei L, Xiang J and Yang F: Matrine inhibits the adhesion
and migration of BCG823 gastric cancer cells by affecting the
structure and function of the vasodilator-stimulated phosphoprotein
(VASP). Acta Pharmacol Sin. 34:1084–1092. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
An Q, Han C, Zhou Y, Li F, Li D, Zhang X,
Yu Z, Duan Z and Kan Q: Matrine induces cell cycle arrest and
apoptosis with recovery of the expression of miR-126 in the A549
non-small cell lung cancer cell line. Mol Med Rep. 14:4042–4048.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Li X, Bai M, Suo Y, Zhang G and Cao
X: Matrine inhibited proliferation and increased apoptosis in human
breast cancer MCF-7 cells via upregulation of Bax and
downregulation of Bcl-2. Int J Clin Exp Pathol. 8:14793–14799.
2015.PubMed/NCBI
|
|
20
|
Jiang H, Hou C, Zhang S, Xie H, Zhou W,
Jin Q, Cheng X, Qian R and Zhang X: Matrine upregulates the cell
cycle protein E2F-1 and triggers apoptosis via the mitochondrial
pathway in K562 cells. Eur J Pharmacol. 559:98–108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song M, Wu H, Wu S, Ge T, Wang G, Zhou Y,
Sheng S and Jiang J: Antibiotic drug levofloxacin inhibits
proliferation and induces apoptosis of lung cancer cells through
inducing mitochondrial dysfunction and oxidative damage. Biomed
Pharmacother. 84:1137–1143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shao H, Yang B, Hu R and Wang Y: Matrine
effectively inhibits the proliferation of breast cancer cells
through a mechanismrelated to the NF-κB signaling pathway. Oncol
Lett. 6:517–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu M, Xiusheng H, Xiao X and Wang Y:
Overexpression of miR-422a inhibits cell proliferation and invasion
and enhances chemosensitivity in osteosarcoma cells. Oncol Rep.
36:3371–3378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim D, Ku SH, Kim H, Jeong JH, Lee M, Kwon
IC, Choi D and Kim SH: Simultaneous regulation of apoptotic gene
silencing and angiogenic gene expression for myocardial infarction
therapy: Single-carrier delivery of SHP-1 siRNA and VEGF-expressing
pDNA. J Control Release. 243:182–194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo J, Zhong Y, Huang S, Li L, Zhang C and
Zou X: Ginkgolide B enhances the differentiation of preosteoblastic
MC3T3-E1 cells through VEGF: Involvement of the p38 MAPK signaling
pathway. Mol Med Rep. 14:4787–4794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ławicki S, Zajkowska M, Głażewska EK,
Będkowska GE and Szmitkowski M: Plasma levels and diagnostic
utility of VEGF, MMP-2 and TIMP-2 in the diagnostics of breast
cancer patients. Biomarkers. 24:157–164. 2017. View Article : Google Scholar
|
|
27
|
Avtanski DB, Nagalingam A, Kuppusamy P,
Bonner MY, Arbiser JL, Saxena NK and Sharma D: Honokiol abrogates
leptin-induced tumor progression by inhibiting Wnt1-MTA1-β-catenin
signaling axis in a microRNA-34a dependent manner. Oncotarget.
6:16396–16410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma X, Yan W, Dai Z, Gao X, Ma Y, Xu Q,
Jiang J and Zhang S: Baicalein suppresses metastasis of breast
cancer cells by inhibiting EMT via downregulation of SATB1 and
Wnt/β-catenin pathway. Drug Des Devel Ther. 10:1419–1441. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chu Q, Han N, Yuan X, Nie X, Wu H, Chen Y,
Guo M, Yu S and Wu K: DACH1 inhibits cyclin D1 expression, cellular
proliferation and tumor growth of renal cancer cells. J Hematol
Oncol. 7:732014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Janghorban M, Farrell AS, Allen-Petersen
BL, Pelz C, Daniel CJ, Oddo J, Langer EM, Christensen DJ and Sears
RC: Targeting c-MYC by antagonizing PP2A inhibitors in breast
cancer. Proc Natl Acad Sci USA. 111:pp. 9157–9162. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim JY, Valencia T, Abu-Baker S, Linares
J, Lee SJ, Yajima T, Chen J, Eroshkin A, Castilla EA, Brill LM, et
al: c-Myc phosphorylation by PKCζ represses prostate tumorigenesis.
Proc Natl Acad Sci USA. 110:pp. 6418–6423. 2013; View Article : Google Scholar : PubMed/NCBI
|