Introduction
Paclitaxel (PTX), an antineoplastic drug, is
commonly used as a first-line therapy for certain general types of
malignancy, including lung, breast and ovarian cancer. Furthermore,
low-dose PTX has been used to treat noncancer human diseases
(1), and the anticancer activity of
low concentrations of PTX has been investigated in specific tumor
types (2–4). PTX causes cell cycle arrest and induces
cell death in a concentration-dependent manner primarily by
stabilizing polymerized microtubules, and enhancing microtubule
assembly (5). PTX blocks
G0/G1 phases or prevents G2/M
phases of the cell cycle, causing cell death (6). The inhibitory effects of low-dose PTX on
the metastasis and progress of cancer primarily depends on blocking
angiogenesis and lymphangiogenesis (7). In addition, low-dose PTX has been
demonstrated to induce the upregulation of thrombospondin-1
expression and downregulation of vascular endothelial growth factor
expression in breast cancer (8). The
findings of these previous studies suggest that determining the
mechanism of a low concentration of PTX may aid in the effective
application of PTX in clinical practice.
MYC proto-oncogene bHLH transcription factor
(c-Myc), which belongs to the Myc gene family, is a pleiotropic
transcription factor that participates in numerous cellular
processes, including cell proliferation, apoptosis,
differentiation, metabolism, genome stability and DNA repair
(9). Thus far, ~20% of human cancer
types have been associated with c-Myc overexpression; c-Myc
overexpression is frequently observed in breast and cervix
carcinoma, small-cell lung cancer, osteosarcoma, and myeloid
leukemia (10). Aberrant c-Myc
expression is likely ascribable to direct gene alterations, which
are associated with tumorigenesis and sustained tumor growth
(11). Thus, the inhibition of c-Myc
has promise as a therapeutic strategy for treating human cancer
(12).
Colorectal carcinoma (CRC) is the third leading
cause of cancer-associated mortalities worldwide (13). Despite advances in CRC diagnosis and
treatment, 142,820 new CRC cases are diagnosed each year (14). Colorectal carcinogenesis is associated
with genetic abnormalities; for example, elevated c-Myc expression
has been identified in 44% of CRCs (15). Therefore, manipulation of genetic
abnormalities may be a promising approach for CRC treatment.
The anticancer activity of low-dose PTX has been
confirmed in certain types of cancer. However, no studies have
investigated the effect of low-dose PTX on CRC cells, and no
guidelines are available regarding the lowest effective
concentrations of PTX for inhibiting the cell cycle. The aim of the
present study was to evaluate whether low-dose PTX could
downregulate the expression of c-Myc and phosphorylated (P)-c-Myc,
thus inhibiting the cell cycle at the G0/G1
stage in CRC HCT116 and LOVO cells.
Materials and methods
Reagents and antibodies
PTX was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Antibodies directed against c-Myc (cat. no.
1472-1), P-c-Myc (cat. no. 1203-1), β-actin (cat. no. P30002) and
β-tubulin (cat. no. M30109) were obtained from Abcam (Cambridge,
MA, USA). Antibody directed against poly(ADP-ribose) polymerase
(PARP)-1 (cat. no. 2586S), were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin (Ig)G and
goat anti-mouse IgG antibodies (cat. nos. HAF007 and HAF008) were
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
β-actin (cat. no. A5441) was purchased from Sigma-Aldrich; Merck
KGaA. α-tubulin was purchased from ProteinTech Group, Inc. (cat.
no. 66031-1-lg; Chicago, IL, USA).
Cell lines and culture conditions
The cell lines LOVO, HCT116 and IEC-6 were purchased
from Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai,
China). LOVO and HCT116 cells were cultured in RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and Dulbecco's
modified Eagle's medium (DMEM)-F-12 (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% heat-inactivated
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 100
IU/ml penicillin (Solarbio, Beijing, China), respectively. IEC-6
cells were maintained in DMEM medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% heat-inactivated fetal
bovine serum and 100 IU/ml penicillin. All cells were seeded in
gelatin-coated 75-cm2 flasks and cultured in 10 ml of
medium at 37°C in a humidified atmosphere of 5% CO2 in
air.
Cell morphology observations
The exponentially growing cells were transferred to
12-well plates and cultured at 37°C in a 5% CO2
atmosphere. HCT116 and LOVO cells were treated with 0, 1 and 3 nM
PTX and then cultured at 37°C in a 5% CO2 atmosphere for
3 days. Images were captured using an Olympus IX 71 microscope
(magnification, ×100; Olympus Corporation, Tokyo, Japan) when the
cells reached 60–70% confluence.
MTT assay of cell survival
MTT colorimetric assay was used to determine the
cytotoxicity of PTX. HCT116 and LOVO cells were plated in 96-well
plates at densities of 1×103 and 2×103
cells/well, respectively, and were incubated with various
concentrations of PTX (0.1–30 nM) for 3 days. Untreated cells were
used as control groups. Then, 50 µl of a 1 mg/ml solution of the
MTT tetrazolium substrate (Sigma-Aldrich; Merck KGaA) in PBS was
added to each well, and the plates were incubated for an additional
4 h at 37°C. The resulting violet formazan precipitate was
solubilized by the addition of 100 µl of dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA). All the plates were agitated for 5 min
at room temperature and read immediately at 578 nm using a Bio-Rad
Model 550 microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The half-maximal inhibitory concentration
(IC50) values of the examined compounds on different
cell lines were obtained from the concentration-effect curves.
Flow cytometric analysis of the cell
cycle
The cells were plated at densities of
3×104 cells/well in 6-well plates and incubated for 1, 3
and 5 days with nutrient solution containing various concentrations
of PTX extracts (0.1–30 nM). The cells were collected by
centrifugation at 1,000 × g for 5 min at 4°C, fixed in cold 70%
ethanol and stored at −20°C. The cells were subsequently washed
with PBS, resuspended in cold PBS, and incubated with 10 mg/ml
RNase and 1 mg/ml propidium iodide (Sigma-Aldrich; Merck KGaA) at
37°C for 30 min. Flow cytometric analysis of DNA content was
performed using a flow cytometer (BD Biosciences, San Jose, CA,
USA). The percentages of cells in the different cell cycle phases
were determined using FlowJo software version 9.3.2 (BD
Biosciences, San Jose, CA, USA).
Protein extraction and
immunoblotting
Subsequent to treatment of the LOVO and HCT116 cells
with PTX at the indicated concentrations and times, the cells were
washed twice with PBS and collected by centrifugation at 200 × g
for 5 min at 4°C. Then, total/nuclear protein concentrations of
cell lysates were determined using a BCA Protein Assay kit
(Beyotime Institute of Biotechnology, Shanghai, China). Protein
samples (total protein, 150 µg; nuclear protein, 50 µg) were
separated using SDS-PAGE (10% gel) and transferred onto
polyvinylidene difluoride membranes. The membranes were incubated
in 5% bovine serum albumin buffer (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) for 30 min at room
temperature with gentle agitation to block nonspecific binding
prior to incubation with the diluted primary antibody (anti-c-Myc,
1:1,000; Abcam; anti-P-c-Myc, 1:500; Abcam; anti-PARP-1, 1:200;
Santa Cruz Biotechnology) overnight at 4°C. Subsequently, the
membranes were incubated with diluted anti-rabbit or anti-mouse
secondary antibody (1:5,000; R&D Systems, Inc.) for 90 min at
room temperature. The membranes were washed three times in PBS for
10 min each at room temperature and then the membranes were
developed using the ECL detection system (EasySee Western Blot kit;
Transgene SA, Strasbourg, France) and visualized with an Imaging
lab™ software version 4.0 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
Statistical comparisons were performed using one-way
analysis of variance followed by Scheffe's post hoc test using SPSS
22.0 software (IBM Corp., Armonk, NY, USA). Quantitative data are
presented as the mean of triplicate experiments ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of PTX treatment on cell
viability
As high concentrations of PTX exhibit high
cytotoxicity, the concentration that does not induce significant
cell death needed to be determined. This determination was
particularly important as cellular toxicity may interfere
significantly with cellular signaling outcomes and gene expression.
Therefore, cells were incubated for 3 days with PTX concentrations
ranging from 0.1 to 30 nM, at which point MTT assays were performed
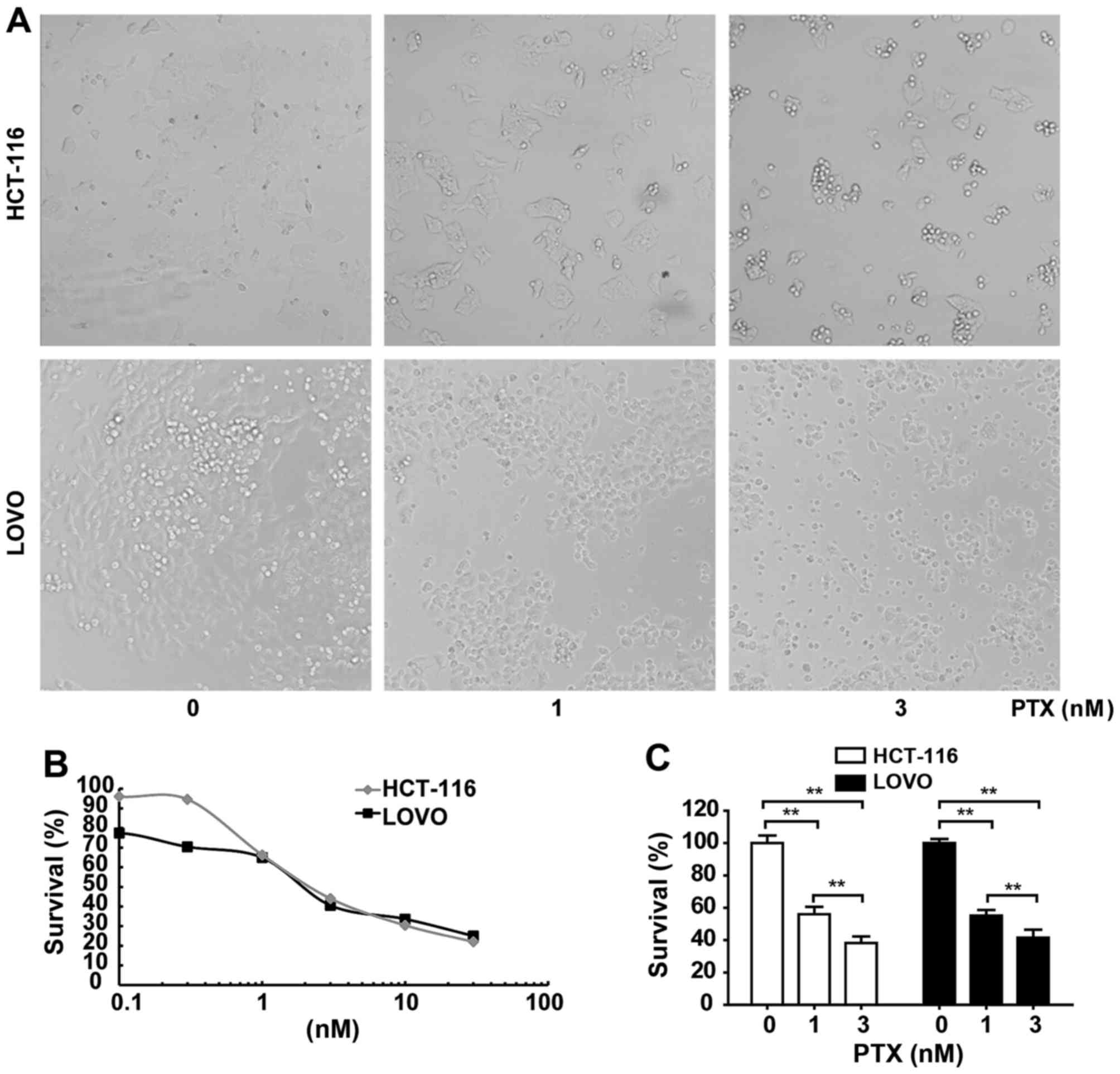
(Fig. 1). The survival rates of
HCT116 and LOVO cells indicated dose-dependent toxic effects of PTX
on these cells (Table I). The
IC50 values of PTX for HCT116 and LOVO cell lines were
2.46 and 2.24 nM, respectively (Fig.
1B). Cell viability reduced dose-dependently by 1 and 3 nM PTX
(Fig. 1C). Cell morphology
observations revealed that the CRC cell lines HCT116 and LOVO
exhibited sensitivity to 1, and 3 nM PTX as indicated by
significant decreases in cell survival compared with the control
(Fig. 1C). Subsequently, two
concentrations close to the IC50 values were used to
treat these cells and it was observed that cell lines exhibited
rounded, wrinkled and damaged morphologies with increasing
concentrations of PTX (Fig. 1A).
 | Table I.Survival rate of colon cancer cells
treated with PTX for 3 days. |
Table I.
Survival rate of colon cancer cells
treated with PTX for 3 days.
|
| Survival, % |
|---|
|
|
|
|---|
| PTX (nM) | HCT116 Cell | LOVO Cell |
|---|
| 0.00 | 100.0±4.6593 | 100.0±2.5036 |
| 0.03 | 84.78±2.4391 | 97.56±4.7486 |
| 0.10 | 70.83±3.8203 | 82.05±2.9887 |
| 0.30 | 66.22±4.3737 | 68.59±3.3481 |
| 1.00 | 56.04±4.5880 | 55.23±3.5017 |
| 3.00 | 38.20±4.0808 | 41.54±4.9635 |
| 10.00 | 23.90±3.2255 | 39.28±3.7560 |
| 30.00 | 19.08±1.1245 | 27.37±4.4055 |
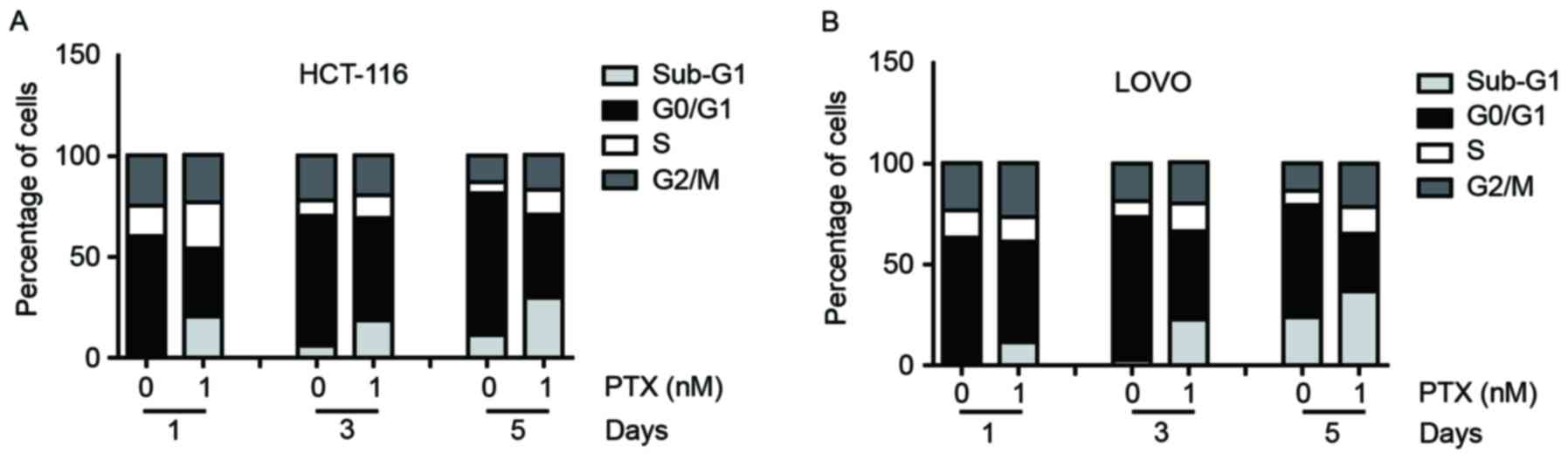
Effects of different concentrations
PTX on the cell cycle distribution of CRC cells
As is known, PTX can induce G2/M cell
cycle arrest in breast cancer (16).
To determine whether PTX has an effect on the cell cycle
distribution of colon cancer cells, flow cytometric analysis on the
cell cycle was performed. After the cells were treated with PTX (1
nM) for 1, 3 or 5 days, the proportion of HCT116 and LOVO cells in
the sub-G1 phases increased and the proportion of these
cells in G0/G1 phases decreased, whereas the
proportions of cells in S and G2/M phases only slightly
changed for both cell lines (Fig.
2).
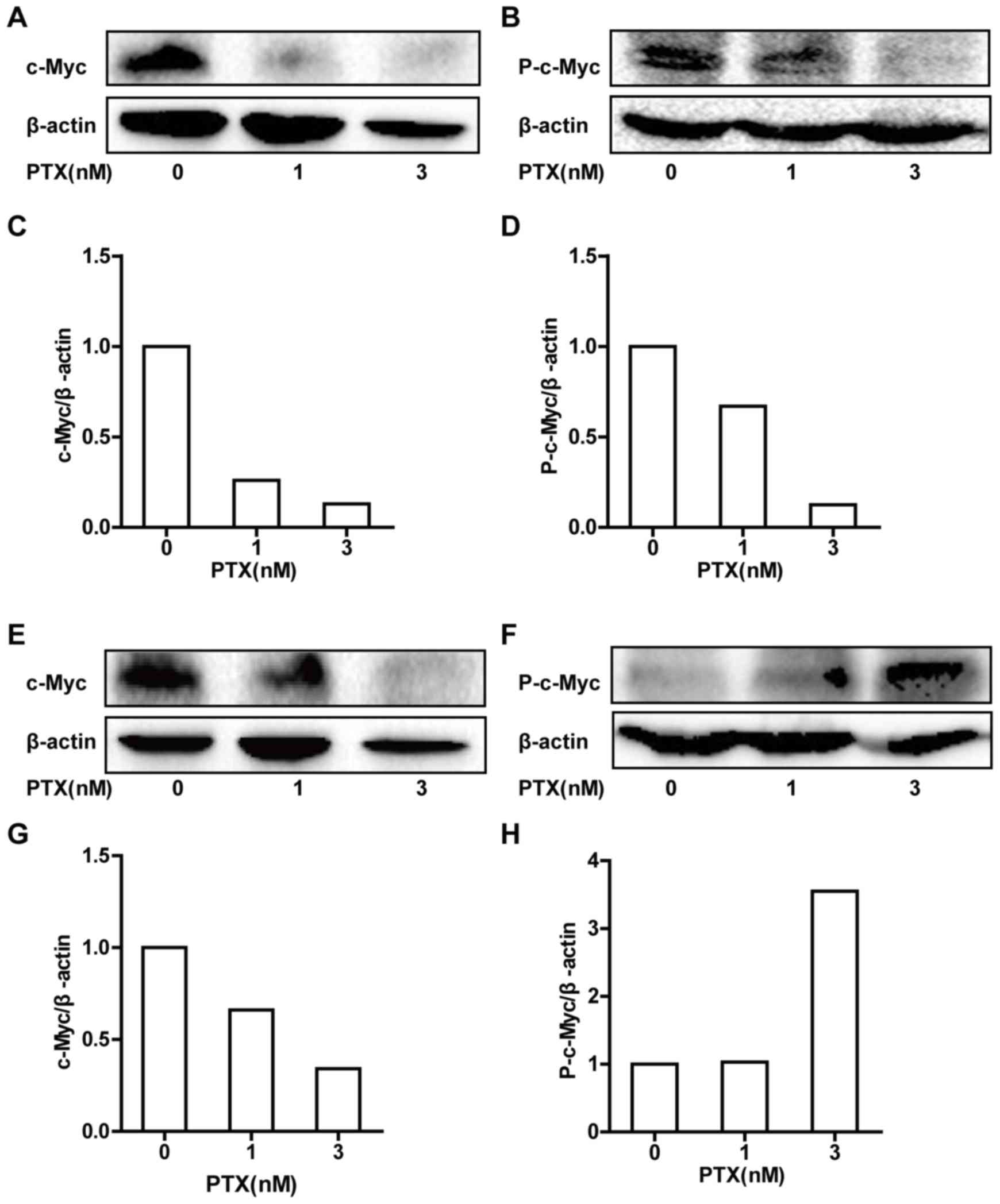
PTX regulates the total protein
expression of c-Myc and P-c-Myc in CRC cells
c-Myc drives cell cycle progression and is
extensively controlled through post-translational modifications,
with a major role for phosphorylated c-Myc. c-Myc phosphorylation
has been associated with protein stabilization and described to
occur as cells enter mitosis (17).
Thus, western blot analysis was performed to determine the total
protein expression levels of c-Myc and P-c-Myc (Fig. 3). PTX treatment of HCT116 cells
induced dose-dependent decreases in c-Myc and P-c-Myc total protein
expression levels (Fig. 3A-D). In
LOVO cells, PTX treatment induced a dose-dependent decrease in
c-Myc total protein expression, but a dose-dependent increase in
P-c-Myc total protein expression (Fig.
3E-H).
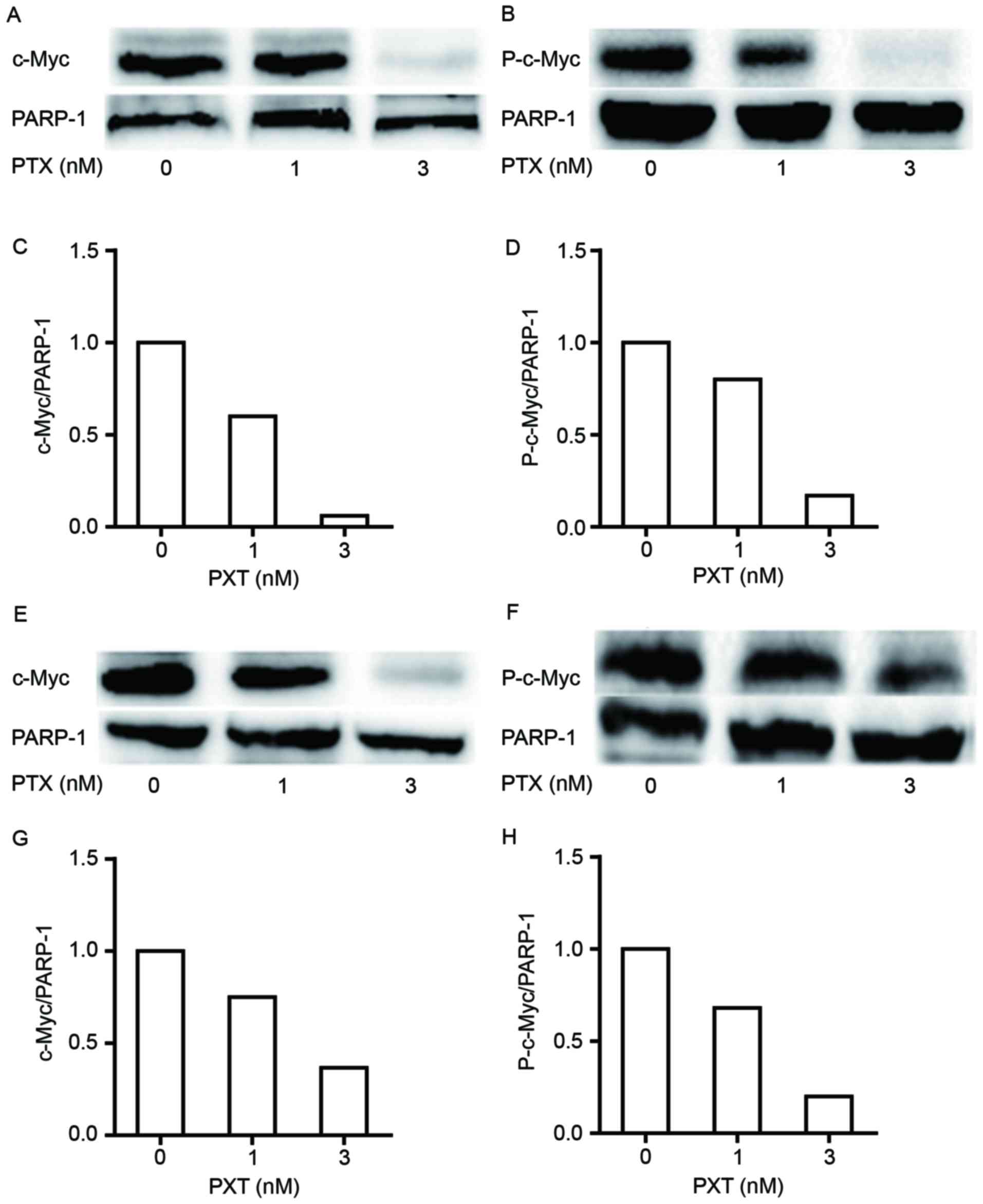
PTX regulates the nuclear protein
expression of c-Myc and P-c-Myc in CRC cells
c-Myc protein is regulated primarily by its
sequestration in nucleoli; its phosphorylated form accumulates in
the nuclei of tumor cells because of impaired ubiquitination by
proteasomes (18). Thus, the nuclear
protein expression of c-Myc and P-c-Myc in colon cancer cells
treated with the indicated concentrations of PTX was detected. It
was observed that the nuclear protein expression levels of c-Myc
and P-c-Myc were decreased by PTX in a dose-dependent manner in
HCT116 cells (Fig. 4A-D). The nuclear
protein expression levels of c-Myc and P-c-Myc were also decreased
in LOVO cells following PTX treatment (Fig. 4E-H).
PTX regulates the expression of
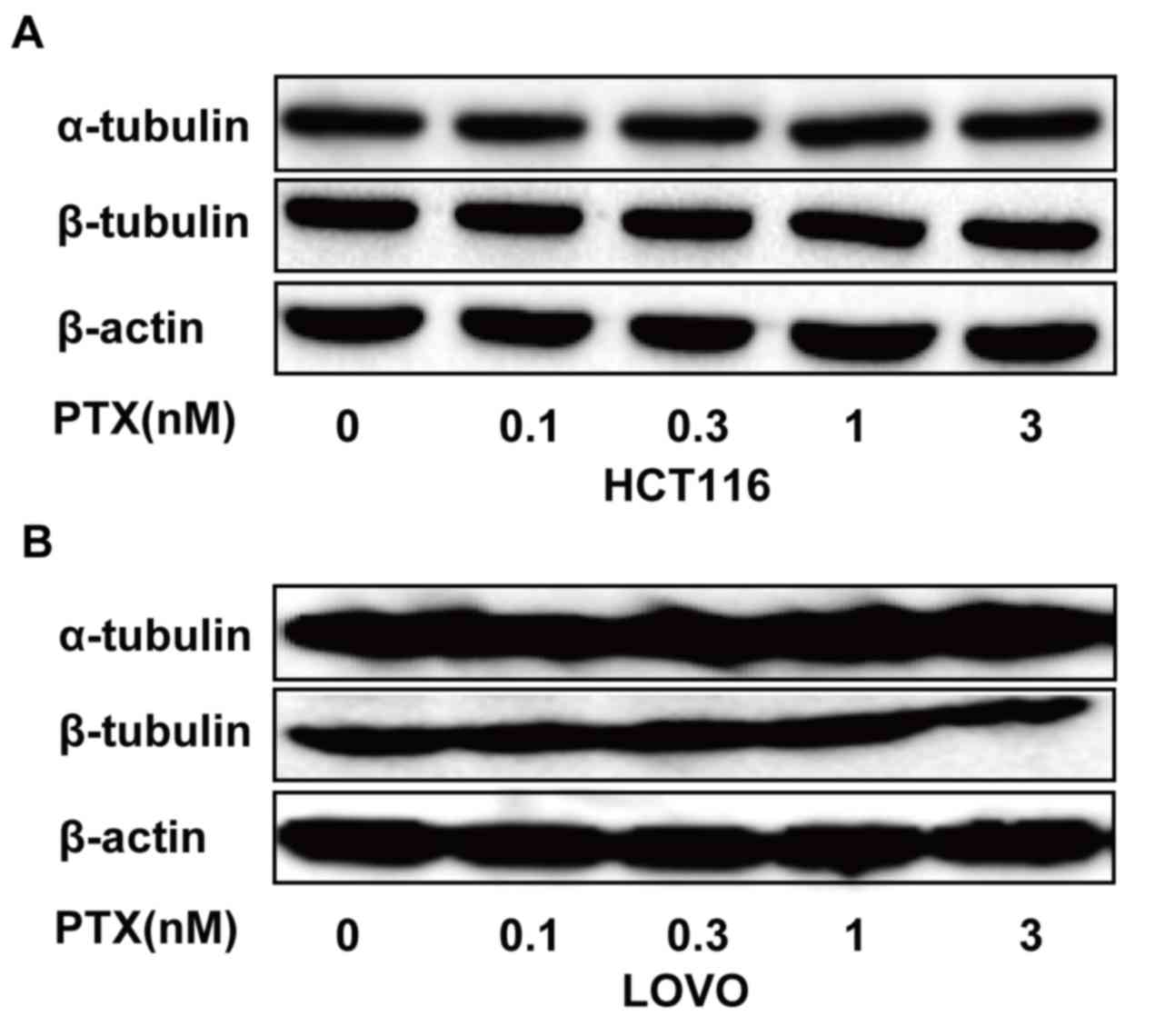
α-tubulin and β-tubulin in CRC cells
The anticancer activity of PTX primarily depends on
stabilizing polymerized microtubules and enhancing microtubule
assembly. Tubulin is a microtubule protein, the integrity of which
is essential for the separation and segregation of chromosomes
during cell division. High expression of tubulin reduces the
chemosensitivity of numerous cancer types to PTX (19). To determine whether low-dose PTX
affected the expression of α-tubulin and β-tubulin in HCT116, and
LOVO cells, these cells were treated with PTX at the indicated
concentrations and times, and α-tubulin and β-tubulin expression
was examined. It was revealed that α-tubulin and β-tubulin
expression did not differ in these cells following PTX treatment
(Fig. 5). A schematic representation
of the proposed mechanism of cell cycle regulation by PTX in the
CRC cell lines analyzed in the present study is demonstrated in
Fig. 6.
Discussion
PTX is one of the most effective cytotoxic agents
for the clinical treatment of cancer. However, its clinical benefit
is often limited by dose-dependent toxicity and drug resistance.
PTX generates antitumor activity by inhibiting cell proliferation
and inducing cell apoptosis, with unavoidable damage to normal
cells, severe anaphylactic hypersensitivity reactions, and
peripheral neuropathy (20).
Nevertheless, low-dose PTX halts the progress and metastasis of
cancer through its antiangiogenic activities rather than by
inducing tumor cell apoptosis (6). A
previous study reported that low-dose PTX induced
G0/G1 cell cycle arrest in esophageal
squamous cell carcinoma larynx carcinoma and ovarian cancer
(21). Consistently, the results of
the present study demonstrated that 1 nM PTX blocked
G0/G1 phases of the cell cycle, and induced
minimal apoptosis with less toxic effects in HCT116 and LOVO
cells.
Tubulins serve essential roles in the
chemosensitivity of cancer; their structural alterations could
specifically modify PTX sensitivity in vitro, and high
tubulin expression reduces the chemosensitivity of numerous types
of cancer to PTX (22). The
overexpression of tubulins has been reported in several types of
cancer cells including breast cancer and acute lymphoblastic
leukemia with PTX resistance (19).
In the current study, the response of CRC cells to low-dose PTX was
investigated. It was demonstrated that 0–0.3 nM PTX exhibited no
significant influence on the viability of HCT116 and LOVO cells,
whereas 1–30 nM PTX significantly affected cell viability, the
IC50 values of PTX for these cell lines were 2.46 and
2.24 nM, respectively. These results demonstrated that both cell
lines have high chemosensitivity towards low-dose PTX. It was also
demonstrated that α-tubulin and β-tubulin expression did not differ
in HCT116, and LOVO cells. These results suggest that low-dose PTX
does not affect the structure of tubulin proteins in these
cells.
A previous study has suggested that the use of PTX
in antitumor therapeutic strategies should be rationally based on
the molecular profile of the individual tumor by specifically
analyzing Myc expression levels (23). Other anticancer drugs, including
fluorouracil and niclosamide, have been reported to directly
downregulate c-Myc expression in human colon cancer KM12C cells and
human osteosarcoma cells, respectively (24,25). PTX
treatment of HT29-4D colon carcinoma cells, HL-60 promyelocytic
leukemic cells and ovarian cancer cells has been suggested to
indirectly downregulate c-Myc expression (26–28). In
the present study, it was demonstrated that PTX treatment
dose-dependently decreased the total/nuclear protein expression of
c-Myc in both cell lines, the total/nuclear protein expression of
P-c-Myc in HCT116 cells and the nuclear protein expression of
P-c-Myc in LOVO cells. In contrast, in LOVO cells, low-dose PXT
treatment dose-dependently increased the total protein expression
of P-c-Myc. In a previous study, PTX treatment induced c-Myc and
P-c-Myc redistribution in prostate carcinoma cell lines; these
proteins underwent reorganization, and were more homogeneously
diffused (29). Whether CRC cell
lines have different responses to PTX treatment is unknown and
requires further study.
The functions of c-Myc and P-c-Myc in cell growth,
and transformation have been investigated (29); extracellular regulated kinase 2 has
been revealed to phosphorylate c-Myc at threonine 58 (Thr58) and
serine (Ser62), and to stimulate the activity of cyclin
E/cyclin-dependent kinase 2 complexes (30). Janus kinase phosphorylates c-Myc at
Ser62 and Ser71, which are associated with cell proliferation, and
cell cycle regulation (31). The
anti-P-c-Myc antibody used in the present study only detects c-Myc
phosphorylated on Thr58 and Ser62. In conclusion, the findings of
the current study demonstrated that PTX affects c-Myc through
downregulating the expression of c-Myc and P-c-Myc in CRC cells. A
greater understanding of the mechanisms by which PTX regulates the
cell cycle may provide novel approaches for the treatment of
CRC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81160282 and
31360549), Major Program on Basic Research Projects of Yunnan
Province (grant no. 2014FC006), the Science Foundation Key Project
of Yunnan Province Department of Education (grant no. ZD2013003),
and Talent Project of Young and Middle-aged Academic Technology
Leadership in Yunnan Province (grant no. 2013HB073).
References
|
1
|
Zhang D, Yang R, Wang S and Dong Z:
Paclitaxel: New uses for an old drug. Drug Des Devel Ther.
8:279–284. 2014.PubMed/NCBI
|
|
2
|
Hirose A, Tajima H, Ohta T, Tsukada T,
Okamoto K, Nakanuma S, Sakai S, Kinoshita J, Makino I, Furukawa H,
et al: Low dose paclitaxel inhibits the induction of
epidermal-mesenchymal transition in the human cholangiocarcinoma
CCKS-1 cell line. Oncol Lett. 6:915–920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He Q, Li J, Yin W, Song Z, Zhang Z, Yi T,
Tang J, Wu D, Lu Y, Wang Z, et al: Low-dose paclitaxel enhances the
anti-tumor efficacy of GM-CSF surface-modified whole-tumor-cell
vaccine in mouse model of prostate cancer. Cancer Immunol
Immunother. 60:715–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsukada T, Fushida S, Harada S, Terai S,
Yagi Y, Kinoshita J, Oyama K, Tajima H, Ninomiya I, Fujimura T and
Ohta T: Low-dose paclitaxel modulates tumour fibrosis in gastric
cancer. Int J Oncol. 42:1167–1174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barbuti AM and Chen ZS: Paclitaxel through
the ages of anticancer therapy: Exploring its role in
chemoresistance and radiation therapy. Cancer (Basel). 7:2360–2371.
2015. View Article : Google Scholar
|
|
6
|
Bocci G, Di Paolo A and Danesi R: The
pharmacological bases of the antiangiogenic activity of paclitaxel.
Angiogenesis. 16:481–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tao WY, Liang XS, Liu Y, Wang CY and Pang
D: Decrease of let-7f in low-dose metronomic paclitaxel
chemotherapy contributed to upregulation of thrombospondin-1 in
breast cancer. Int J Biol Sci. 11:48–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Paolo A, Bocci G and Danesi R: The
preclinical bases of the rational combination of paclitaxel and
antiangiogenic drugs. Clin Cancer Drugs. 1:100–115. 2014.
View Article : Google Scholar
|
|
9
|
Meyer N and Penn LZ: Reflecting on 25
years with MYC. Nat Rev Cancer. 8:976–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pelengaris S, Khan M and Evan G: C-MYC:
More than just a matter of life and death. Nat Rev Cancer.
2:764–776. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen BJ, Wu YL, Tanaka Y and Zhang W:
Small molecules targeting c-Myc oncogene: Promising anti-cancer
therapeutics. Int J Biol Sci. 10:1084–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Zhao X, Fiskus W, Lin J, Lwin T,
Rao R, Zhang Y, Chan JC, Fu K, Marquez VE, et al: Coordinated
silencing of MYC-mediated miR-29 by HDAC3 and EZH2 as a therapeutic
target of histone modification in aggressive B-cell lymphomas.
Cancer Cell. 22:506–523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Douaiher J, Ravipati A, Grama B, Chowdhury
S, Alatise O and Are C: Colorectal cancer-global burden, trends,
and geographical variations. J Surg Oncol. 115:619–630. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goel A and Boland CR: Epigenetics of
colorectal cancer. Gastroenterology. 143:1442–1460. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li JP, Yang YX, Liu QL, Pan ST, He ZX,
Zhang X, Yang T, Chen XW, Wang D, Qiu JX and Zhou SF: The
investigational Aurora kinase A inhibitor alisertib (MLN8237)
induces cell cycle G2/M arrest, apoptosis, and autophagy via p38
MAPK and Akt/mTOR signaling pathways in human breast cancer cells.
Drug Des Devel Ther. 9:1627–1652. 2015.PubMed/NCBI
|
|
17
|
Bottone MG, Soldani C, Tognon G, Gorrini
C, Lazzè MC, Brison O, Ciomei M, Pellicciari C and Scovassi AI:
Multiple effects of paclitaxel are modulated by a high c-myc
amplification level. Exp Cell Res. 290:49–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sanders JA and Gruppuso PA: Nucleolar
localization of hepatic c-Myc: A potential mechanism for c-Myc
regulation. Biochim Biophys Acta. 1743:141–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie S, Ogden A, Aneja R and Zhou J:
Microtubule-binding proteins as promising biomarkers of Paclitaxel
sensitivity in cancer chemotherapy. Med Res Rev. 36:300–312. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Flatters SJ, Xiao WH and Bennett GJ:
Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful
peripheral neuropathy. Neurosci Lett. 397:219–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yun T, Liu Y, Gao D, Linghu E, Brock MV,
Yin D, Zhan Q, Herman JG and Guo M: Methylation of CHFR sensitizes
esophageal squamous cell cancer to docetaxel and paclitaxel. Genes
Cancer. 6:38–48. 2015.PubMed/NCBI
|
|
22
|
Wang S, Qiu J, Shi Z, Wang Y and Chen M:
Nanoscale drug delivery for taxanes based on the mechanism of
multidrug resistance of cancer. Biotechnol Adv. 33:224–241. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gatti G, Maresca G, Natoli M, Florenzano
F, Nicolin A, Felsani A and D'Agnano I: MYC prevents apoptosis and
enhances endoreduplication induced by paclitaxel. PLoS One.
4:e54422009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao HY, Ooyama A, Yamamoto M, Ikeda R,
Haraguchi M, Tabata S, Furukawa T, Che XF, Iwashita K, Oka T, et
al: Down regulation of c-Myc and induction of an angiogenesis
inhibitor, thrombospondin-1, by 5-FU in human colon cancer KM12C
cells. Cancer Lett. 270:156–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao Z, Nan G, Yan Z, Zeng L, Deng Y, Ye
J, Zhang Z, Qiao M, Li R, Denduluri S, et al: The anthelmintic drug
Niclosamide inhibits the proliferative activity of human
osteosarcoma cells by targeting multiple signal pathways. Curr
Cancer Drug Targets. 15:726–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilmes A, Chan A, Rawson P, Jordan T
William and Miller JH: Paclitaxel effects on the proteome of HL-60
promyelocytic leukemic cells: Comparison to peloruside A. Invest
New Drugs. 30:121–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
el Khyari S, Bourgarel V, Barra Y, Braguer
D and Briand C: Pretreatment by tubulin agents decreases C-MYC
induction in human colon carcinoma cell line HT29-D4. Biochem
Biophys Res Commun. 231:751–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu Q, Chen Z, Gong X, Cai Y, Chen Y, Ma X,
Zhu R and Jin J: β-Catenin expression is regulated by an
IRES-dependent mechanism and stimulated by paclitaxel in human
ovarian cancer cells. Biochem Biophys Res Commun. 461:21–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Supino R, Favini E, Cuccuru G, Zunino F
and Scovassi AI: Effect of paclitaxel on intracellular localization
of c-Myc and P-c-Myc in prostate carcinoma cell lines. Ann N Y Acad
Sci. 1095:175–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo
F, Lyssiotis CA, Aldape K, Cantley LC and Lu Z: ERK1/2-dependent
phosphorylation and nuclear translocation of PKM2 promotes the
Warburg effect. Nat Cell Biol. 14:1295–1304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Supino R and Scovassi AI: c-myc: A
double-headed Janus that regulates cell survival and death. Gene
Ther Mol Biol. 8:385–394. 2004.
|