Introduction
Surgical liver resection has become a primary method
for treating liver cancer in previous decades; however, a shortage
in the postoperative residual liver volume has been an important
factor in the surgical resection of liver cancer, and postoperative
liver failure is one of the major complications (1–3) with this
surgery. Numerous studies have reported that percutaneous selective
portal vein embolization (PVE) prior to liver resection may
effectively induce compensatory hyperplasia of the left hepatic
lobe, and reduce the incidence of postoperative liver failure
(4–7).
There is a consensus that PVE may lead to left hepatic lobe
compensatory hyperplasia in patients with liver cancer and no liver
cirrhosis (8–11).
In China, the majority of patients with primary
liver cancer have associated liver cirrhosis following chronic
hepatitis B, and their liver function reserve is poor (12). Postoperative patients typically
experience liver failure, which may result in mortality (13). A previous study revealed that there
are fewer liver cirrhosis effects on compensatory hypertrophy of
the left lobe subsequent to PVE (14). Therefore, the purpose of the present
study was to investigate the value of preoperative PVE and the
effects of liver cirrhosis on left hepatic lobe hyperplasia, which
may have important clinical significance for the correct
implementation of PVE and two-step hepatectomy for liver cancer. A
total of 21 patients with liver cancer successfully underwent a
right hepatectomy following PVE. Combined with postoperative
pathological data, the effects of liver cirrhosis on the
compensatory hypertrophy of future liver remnants (FLRs) subsequent
to PVE were determined.
Patients and methods
Patient population
A total of 21 patients who underwent a hepatic
resection following PVE without major complications between January
2010 and December 2012 were identified and retrospectively
evaluated. This group included 20 males and 1 female with a mean
age of 52.1±11.3 years (range, 22–64). The results of tests for the
hepatitis B surface antigen were positive in 16 patients. There
were 14 patients with a single lesion and 7 patients with multiple
lesions in the right lobe of the liver. The mean maximum diameter
of the tumors was 8.6±2.4 cm (range, 4.7–12.8). All patients were
divided into non-cirrhosis (n=9, fibrosis score <3) and
cirrhosis (n=12, fibrosis score ≥3) groups, according to the
classification of Knodell et al (15); preoperative imaging studies, including
ultrasonography (US), computed tomography (CT) or magnetic
resonance imaging (MRI) and postoperative pathological data were
obtained for the patients (16). The
two groups were compared with baseline clinicopathological
characteristics obtained prior to PVE (Table I). The present study was approved by
the Ethics Research Committee of the First Affiliated Hospital,
School of Medicine, Zhejiang University (Hangzhou, China), and they
agreed that written informed consent was not necessary due to the
retrospective nature of the present study. All data were anonymized
and de-identified prior to analysis.
 | Table I.Patient clinicopathological
characteristics prior to PVE. |
Table I.
Patient clinicopathological
characteristics prior to PVE.
| Characteristics | Non-cirrhosis group
(n=9) | Cirrhosis group
(n=12) | P-value |
|---|
| Sex
(male/female) | 9/0 | 11/1 | 1.000 |
| Age (years) | 49.3±14.0 | 54.3±8.8 | 0.337 |
| Weight (kg) | 64.3±12.1 | 62.4±7.8 | 0.664 |
| Tumor size (cm) | 9.4±2.1 | 8.0±2.6 | 0.188 |
| Tumor multiplicity
(single/multiple) | 6/3 | 8/4 | 1.000 |
| Pathological type
(hepatocellular carcinoma/cholangiocarcinoma) | 7/2 | 10/2 | 1.000 |
| HBsAg (+/-) | 6/3 | 10/2 | 0.611 |
| ALT (U/l) | 43.67±18.96 | 49.00±29.40 | 0.641 |
| AST (U/l) | 50.78±23.16 | 56.25±38.41 | 0.710 |
| TB (µmol/l) | 14.44±6.46 | 19.17±9.93 | 0.231 |
| Prothrombin time
(sec) | 12.29±1.80 | 12.38±0.88 | 0.875 |
| Child-Pugh class
(A/B/C) | 9/0/0 | 12/0/0 | n/a |
| TACE session
(1/2/3) | 7/1/01 | 7/2/03 | 0.630 |
| FLR
(cm3) | 447.9±86.7 | 412.4±61.3 | 0.285 |
| FLR/weight (%) | 0.70±0.07 | 0.66±0.10 | 0.374 |
Transcatheter arterial
chemoembolization
Transarterial chemoembolization (TACE) was performed
2–4 weeks prior to PVE. Under fluoroscopic surveillance, a
conventional superior mesenteric and a common hepatic arteriography
were initially performed to assess the hepatic arterial anatomy,
tumor burden, vascularity and portal circulation on venous-phase
films. Subsequently, the tip of the catheter was selectively placed
in the right hepatic artery and cisplatin and hydroxycamptothecin
were slowly infused for 15 min into the hepatic artery. The infused
dose of cisplatin and hydroxycamptothecin were both 2 mg/kg body
weight. Subsequently, a mixture of 10–15 ml iodized oil (Lipiodol
Ultrafluid; Guerbet Laboratories, Paris, France) and 20 mg
epirubicin/pirarubicin was injected into the tumor-feeding arteries
under fluoroscopic surveillance, followed by embolization with
gelatin sponge particles (Gelfoam; Upjohn Laboratories, Kalamazoo,
MI, USA).
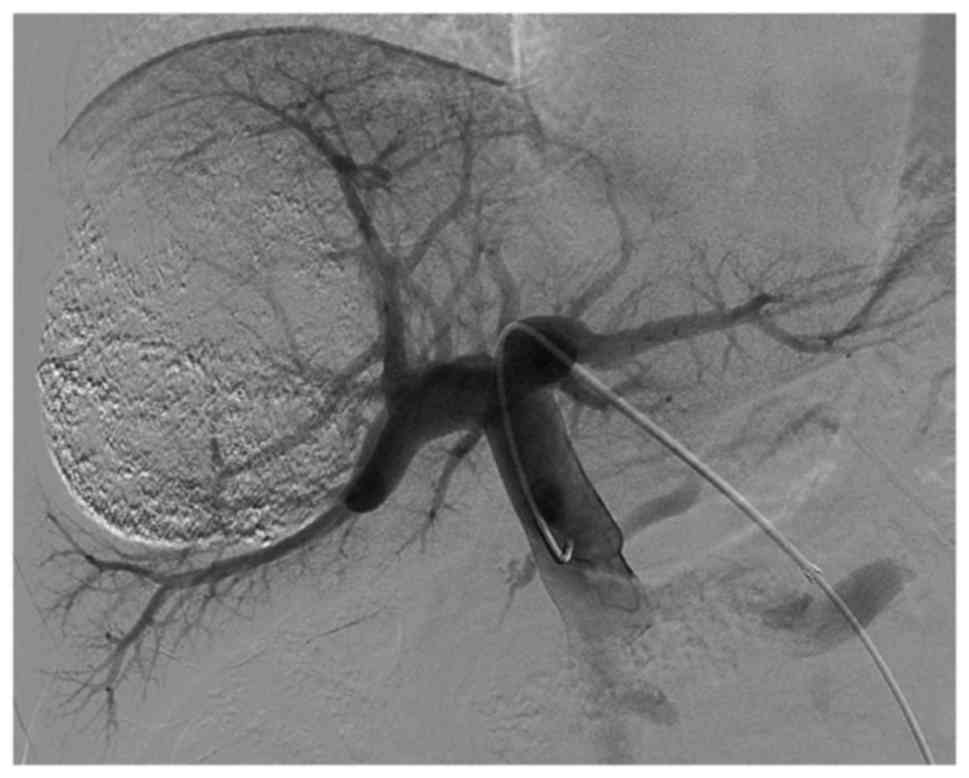
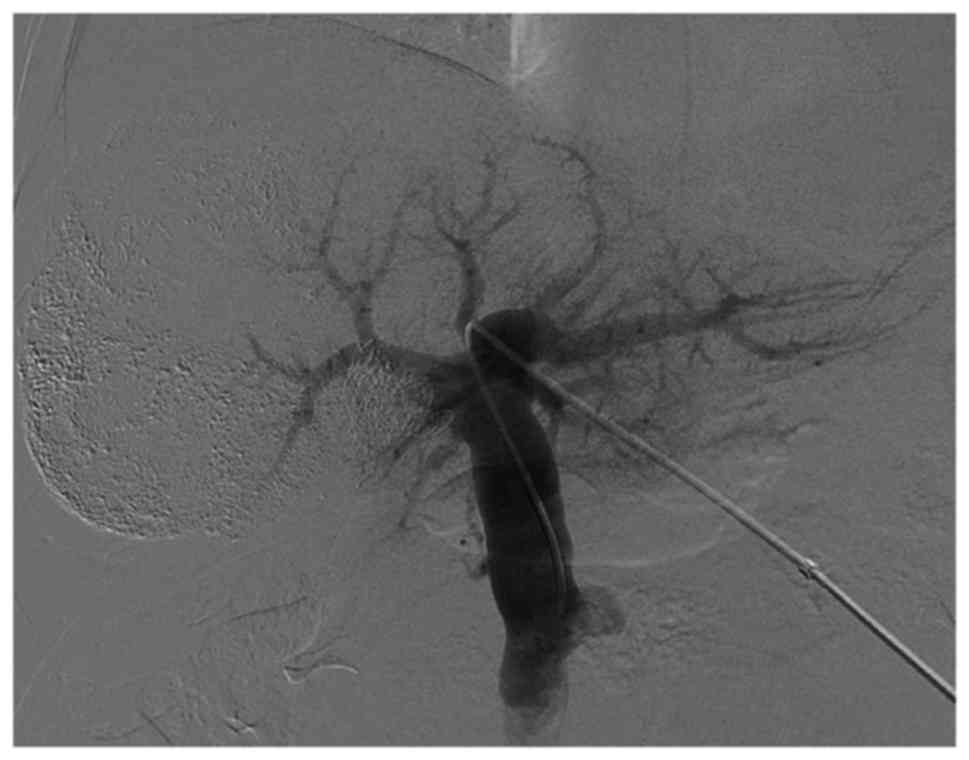
Right PVE
PVE was performed 2–4 weeks following TACE,
subsequent to the recovery of liver function (17). Under ultrasound guidance, the
secondary branch of the left portal vein was percutaneously
punctured with an 18-gauge PTC needle (Kyowa Hakko Co., Ltd.,
Nagano, Japan), and a 5F sheath (Terumo Co., Ltd., Tokyo, Japan)
was introduced. Following portal venography through a 5F C2
angiographic catheter (Cordis Corporation, Miami Lakes, FL, USA),
the right portal venous branches were embolized with coils using a
5F SIM2 catheter (Cordis Corporation). The right branch in
proximity to the portal vein trunk (1 cm) was retained for surgical
separation and stitching of the right portal vein. Repeated portal
venography following embolization was performed in order to confirm
complete right portal vein occlusion. Finally, the punctured
passage was embolized using coils to prevent intraperitoneal
hemorrhaging.
Main outcome evaluations
Liver function tests, including prothrombin time
(PT) and serum levels of total bilirubin (TB), aspartate
aminotransferase (AST) and alanine aminotransferase (ALT), were
performed prior to and following PVE, and prior to surgery. Hepatic
contrast-enhanced CT [Brilliance-iCT, version 4.1.6.00230; Phillips
Medical Systems (Cleveland), Inc., Cleveland, OH, USA] was
performed prior to and 4–6 weeks subsequent to PVE in order to
evaluate the degree of hypertrophy, and volumetric data was
obtained from the portal phase images. Volumetric evaluations were
performed using a CT analysis system for the entire liver as well
as the left liver lobe (segments I–IV). The FLR volume was
considered to represent the left hepatic lobe and caudate lobe as
the portal vein was not embolized.
Complications following PVE included intraperitoneal
hemorrhage, liver failure [defined by a prothrombin time of <50%
of normal and a serum bilirubin level of >50 µmol/l on
postoperative day 5 (18)],
intraperitoneal hemorrhaging, gastrointestinal bleeding and biliary
fistula.
Statistical analysis
Data analysis was performed using the statistical
package SPSS for Windows® (version 13.0; SPSS, Inc.,
Chicago, IL, USA). Data are expressed as the mean ± standard
deviation. Student's t-test, Fisher's exact test, paired sample
t-test, independent sample t-test and the χ2 test were
applied, where appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
All patients successfully underwent TACE treatment
prior to PVE; specifically, 14, 3 and 4 patients underwent 1, 2 and
3 sessions, respectively. PVE was successfully performed in all
patients 2–4 weeks following the final TACE treatment (portal vein
angiography prior to and subsequent to PVE is presented in Figs. 1 and 2).
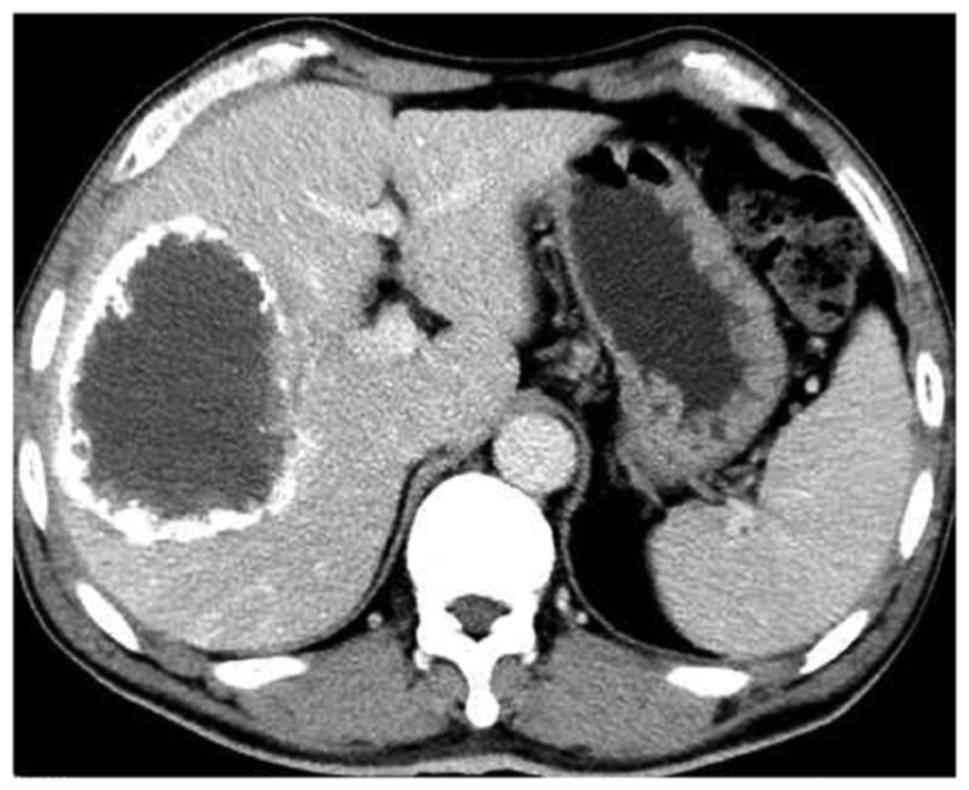
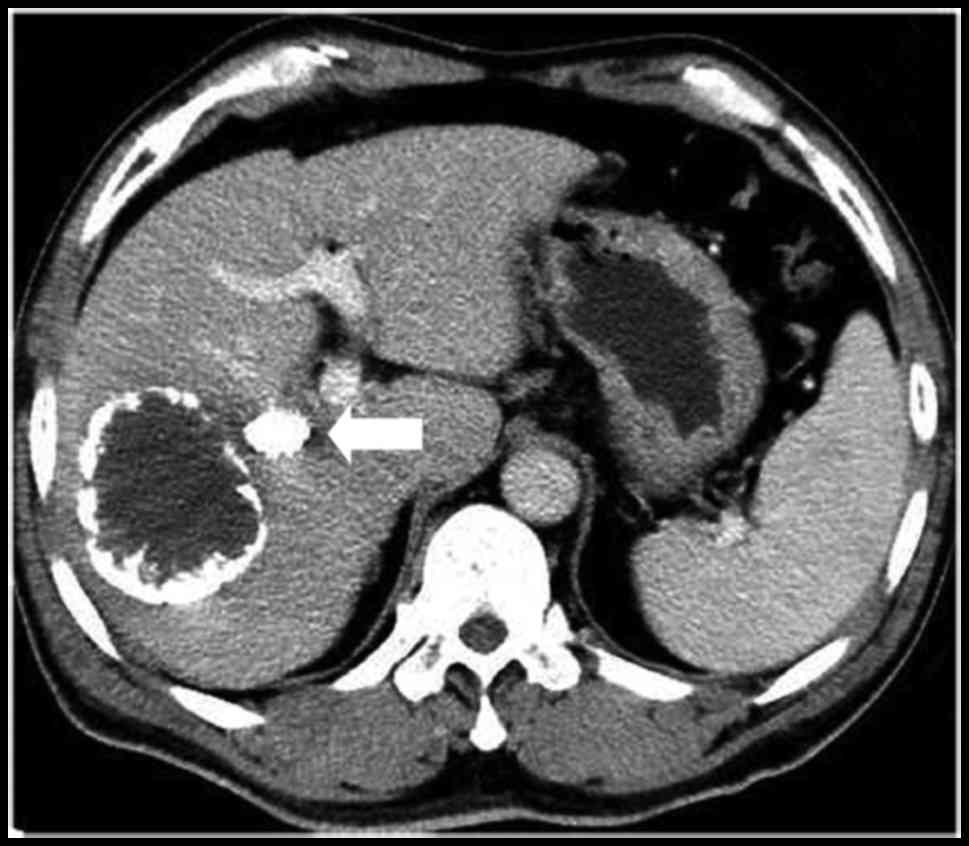
The mean absolute volume of the FRL was calculated prior to and 4–6
weeks following the PVE, and increased from 427.6±73.5 to
582.6±98.3 cm3 (+37.3%), which was a statistically
significant difference (P<0.001). The CT scan prior to and
following PVE is presented in Figs. 3
and 4.
In the univariate analysis, no significant
differences were identified in the following factors between the
groups: Sex, age, weight, size and number of tumors, pathology,
hepatitis B surface antigen, ALT, AST, TB, PT, Child-Pugh class,
TACE sessions, FLR and FLR/weight (P>0.05; Table I). Comparative results of the two
patient groups are summarized in Table
II. Following PVE (4–6 weeks), the left liver volume, as
evaluated by enhanced CT, demonstrated that PVE induced significant
compensatory hypertrophy whether in the non-cirrhosis group
(P=0.002) or cirrhosis group (P<0.001); however, no significant
difference was identified between the two groups, with respect to
left liver volume enlargement 4–6 weeks following PVE
(P=0.373).
 | Table II.Comparison of the FLR volume prior to
and following PVE. |
Table II.
Comparison of the FLR volume prior to
and following PVE.
|
| FLR comparison |
|
|---|
|
|
|
|
|---|
| Variable | Non-cirrhosis group
(n=9) | Cirrhosis group
(n=12) | P-value |
|---|
| FLR prior to PVE
(cm3) | 447.9±86.7 | 412.4±61.3 | 0.285 |
| FLR following PVE
(cm3) | 627.2±116.0 | 549.2±70.2 | 0.070 |
| Mean increase in
FLR (cm3) | 179.3±145.6 | 136.8±62.6 | 0.373 |
| Mean increased
percentage of FLR (%) | 45.6±47.3 | 31.1±16.1 | 0.331 |
| Each group
comparison (before and after PVE) (P-value) | 0.002 | <0.001 |
|
No severe complications, including intraperitoneal
hemorrhage, gastrointestinal bleeding, biliary fistula or liver
failure, developed one week subsequent to PVE in any of the
patients. The results from the liver function tests prior to and
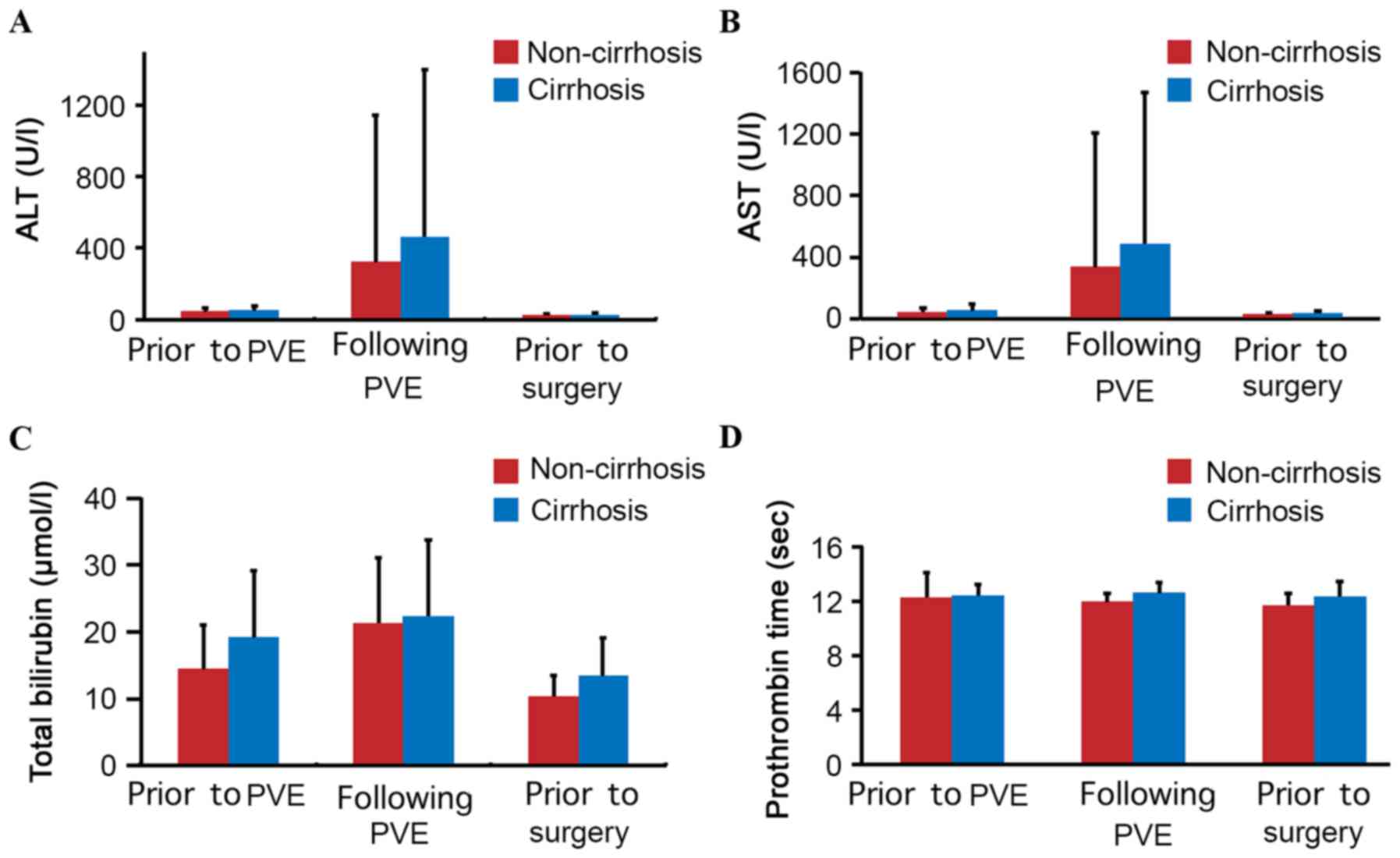
following PVE, and prior to surgery, for the two patient groups are
summarized in Fig. 5A-D. The ALT,
AST, TB and PT results were similar in the groups (P>0.05).
Discussion
Radical hepatic resection is frequently
contraindicated in a number of patients with liver cancer due to an
increased risk for postoperative hepatic failure (1–3). PVE prior
to liver resection has been proposed in order to induce atrophy of
embolized lobes with compensatory hypertrophy of non-embolized
FLRs, therefore preventing postoperative liver failure and
improving the two-step resection rate of liver cancers (19–23). In
the present study, surgical resection was not recommended for
patients with large liver tumors (multiple right hepatic tumors
were present in seven patients). Following PVE, the volume of the
left liver (FLR) increased from 427.6±73.5 to 582.6±98.3
cm3. The mean increase in the percentage of the FLR
volume induced by PVE was 37.3±33.1%, and the two-step resection
rate for hepatic cancer was 100%, thereby indicating that PVE is
effective for patients with liver cancer with or/and without liver
cirrhosis.
For patients with liver cirrhosis, Farges et
al (24) reported in a
prospective study that patients with liver cirrhosis prior to
partial hepatectomy benefited from PVE, and recommended performing
preoperative PVE in patients with right hepatic cancer and liver
cirrhosis as a routine preoperative preparation. Ogata et al
(17) demonstrated that TACE combined
with PVE may effectively induce hypertrophy of FLRs in patients
with chronic liver disease and improve the three-year disease-free
survival rate. Liu et al (14)
suggested that PVE may be used as an indication for half-liver
resection in patients with chronic liver injury (including
cirrhosis and chronic hepatitis). In the present study, the mean
percentage increase in FLR volume was significantly different in
each group prior to and following PVE. However, no significant
difference was identified between the two groups subsequent to PVE,
demonstrating that the impact of cirrhosis on the increased liver
volume following PVE was minor, but in the case selection process,
the selection bias induced by the degree of cirrhosis in chronic
liver disease patients may not be excluded. For example, the
increased number of patients with a fibrosis score of 3 in the
cirrhosis group led to the conclusion that liver cirrhosis has no
obvious effects on liver lobe hyperplasia following PVE.
Regarding embolization materials, previous studies
reported the use of anhydrous ethanol (25), gelatin sponges (26), isobutyl-2-cyano acrylate adhesive glue
(27), coils (28), lipiodol (29), polyvinyl alcohol (PVA) particles
(30), fibrin glue (22) or the combined use of two distinct
embolic agents (31). Each embolic
agent induced compensatory hypertrophy of non-embolized FLRs to
varying degrees; however, there is no consensus regarding the best
type of embolic material (27,29,30,32).
Madoff et al (32) revealed
that the mean percentage of FLR volume increased by 41.1% in the 4
weeks following PVE, using coils and PVA particles as embolization
materials. Ji et al (29) used
anhydrous ethanol and lipiodol as embolization materials to
increase the FLR volume by 27% in the 4 weeks subsequent to PVE.
The study by Corrêa et al (30) revealed that the left liver volume
increased by 23% one month following PVE, which was attributed to
the use of PVA particles. Giraudo et al (27) used isobutyl-2-cyanoacrylate adhesive
glue and lipiodol as embolization agents, resulting in an FLR
increase of 47.7±31.9% in the 4–8 weeks subsequent to PVE. In the
present study, coils were used as embolization agents, which led to
embolisms that thoroughly and maximally protected liver function.
The effects of PVE on the FLR volume were significant 4–6 weeks
following PVE, and no severe complications were observed, including
the appearance of liver failure, severe abdominal cavity hemorrhage
and gastrointestinal bleeding.
The present study also had limitations. The
cirrhosis cases were not further divided into fibrosis score 3 and
4 groups according to the degree of liver cirrhosis; therefore,
there may have been selection bias. In addition, the sample size
was small and considering further groupings according to the degree
of liver cirrhosis may decrease the sample size of each group;
therefore, there may be an increase in statistical type II error
(33), making the attainment of
negative results increasingly likely. Future studies should be
performed with larger sample sizes in order that cirrhosis cases
may be further grouped based on the degree of cirrhosis. The
effects of liver cirrhosis on the compensatory hypertrophy of FLRs
following PVE should be further clarified, and may aid doctors in
selecting appropriate patients for PVE treatment. For the optimal
timing of hepatectomy subsequent to PVE, further study is required
to identify the ideal embolic materials for PVE.
In conclusion, preoperative PVE may be safely and
effectively performed in order to increase the rate of hypertrophy
of FLRs and the probability of resection in patients with hepatic
cancer with or without liver cirrhosis, which may have extensive
value in clinical applications.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81371658 and
81172315/H1617), the National Major Projects of Infectious Diseases
of China (grant no. 2012ZX10002-017), the National Clinical Key
Subject Construction Project (General Surgery) of China (grant no.
2014ZJ01) and the Medical Health Fund of Zhejiang Province (grant
no. 2013KYB097).
References
|
1
|
Ribero D, Abdalla EK, Madoff DC, Donadon
M, Loyer EM and Vauthey JN: Portal vein embolization before major
hepatectomy and its effects on regeneration, resectability and
outcome. Br J Surg. 94:1386–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jarnagin WR, Gonen M, Fong Y, DeMatteo RP,
Ben-Porat L, Little S, Corvera C, Weber S and Blumgart LH:
Improvement in perioperative outcome after hepatic resection:
Analysis of 1,803 consecutive cases over the past decade. Ann Surg.
236:397–407. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Melendez J, Ferri E, Zwillman M, Fischer
M, DeMatteo R, Leung D, Jarnagin W, Fong Y and Blumgart LH:
Extended hepatic resection: A 6-year retrospective study of risk
factors for perioperative mortality. J Am Coll Surg. 192:47–53.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hwang S, Lee SG, Ko GY, Kim BS, Sung KB,
Kim MH, Lee SK and Hong HN: Sequential preoperative ipsilateral
hepatic vein embolization after portal vein embolization to induce
further liver regeneration in patients with hepatobiliary
malignancy. Ann Surg. 249:608–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abulkhir A, Limongelli P, Healey AJ,
Damrah O, Tait P, Jackson J, Habib N and Jiao LR: Preoperative
portal vein embolization for major liver resection: A
meta-analysis. Ann Surg. 247:49–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Covey AM, Brown KT, Jarnagin WR, Brody LA,
Schwartz L, Tuorto S, Sofocleous CT, D'Angelica M, Getrajdman GI,
DeMatteo R, et al: Combined portal vein embolization and
neoadjuvant chemotherapy as a treatment strategy for resectable
hepatic colorectal metastases. Ann Surg. 247:451–455. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoo H, Ko GY, Gwon DI, Kim JH, Yoon HK,
Sung KB, Kim N and Lee J: Preoperative portal vein embolization
using an amplatzer vascular plug. Eur Radiol. 19:1054–1061. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fusai G and Davidson BR: Strategies to
increase the resectability of liver metastases from colorectal
cancer. Dig Surg. 20:481–496. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fusai G and Davidson BR: Management of
colorectal liver metastases. Colorectal Dis. 5:2–23. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jaeck D, Bachellier P, Nakano H,
Oussoultzoglou E, Weber JC, Wolf P and Greget M: One or two-stage
hepatectomy combined with portal vein embolization for initially
nonresectable colorectal liver metastases. Am J Surg. 185:221–229.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Azoulay D, Castaing D, Smail A, Adam R,
Cailliez V, Laurent A, Lemoine A and Bismuth H: Resection of
nonresectable liver metastases from colorectal cancer after
percutaneous portal vein embolization. Ann Surg. 231:480–486. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luk JM and Liu AM: Proteomics of
hepatocellular carcinoma in Chinese patients. OMICS. 15:261–266.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hemming AW, Reed AI, Howard RJ, Fujita S,
Hochwald SN, Caridi JG, Hawkins IF and Vauthey JN: Preoperative
portal vein embolization for extended hepatectomy. Ann Surg.
237:686–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Peng YH, Yang ZH, Wang G and Tang
P: Preoperative portal vein embolizations increase the resectable
rate of primary liver cancer. Chin J Gen Surg. 19:582–583. 2004.(In
Chinese).
|
|
15
|
Knodell RG, Ishak KG, Black WC, Chen TS,
Craig R, Kaplowitz N, Kiernan TW and Wollman J: Formulation and
application of a numerical scoring system for assessing
histological activity in asymptomatic chronic active hepatitis.
Hepatology. 1:431–435. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schuppan D and Afdhal NH: Liver cirrhosis.
Lancet. 371:838–851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogata S, Belghiti J, Farges O, Varma D,
Sibert A and Vilgrain V: Sequential arterial and portal vein
embolizations before right hepatectomy in patients with cirrhosis
and hepatocellular carcinoma. Br J Surg. 93:1091–1098. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balzan S, Belghiti J, Farges O, Ogata S,
Sauvanet A, Delefosse D and Durand F: The ‘50–50 criteria’ on
postoperative day 5: An accurate predictor of liver failure and
death after hepatectomy. Ann Surg. 242:824–829. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cotroneo AR, Innocenti P, Marano G,
Legnini M and Iezzi R: Pre-hepatectomy portal vein embolization:
Single center experience. Eur J Surg Oncol. 35:71–78. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siriwardana RC, Lo CM, Chan SC and Fan ST:
Role of portal vein embolization in hepatocellular carcinoma
management and its effect on recurrence: A case-control study.
World J Surg. 36:1640–1646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu H and Fu Y: Portal vein embolization
before major hepatectomy. World J Gastroenterol. 11:2051–2054.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagino M, Kamiya J, Nishio H, Ebata T,
Arai T and Nimura Y: Two hundred forty consecutive portal vein
embolizations before extended hepatectomy for biliary cancer:
Surgical outcome and long-term follow-up. Ann Surg. 243:364–372.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vauthey JN, Pawlik TM, Abdalla EK, Arens
JF, Nemr RA, Wei SH, Kennamer DL, Ellis LM and Curley SA: Is
extended hepatectomy for hepatobiliary malignancy justified? Ann
Surg. 239:722–732. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farges O, Belghiti J, Kianmanesh R,
Regimbeau JM, Santoro R, Vilgrain V, Denys A and Sauvanet A: Portal
vein embolization before right hepatectomy: Prospective clinical
trial. Ann Surg. 237:208–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Igami T, Ebata T, Yokoyama Y, Sugawara G,
Takahashi Y and Nagino M: Portal vein embolization using absolute
ethanol: Evaluation of its safety and efficacy. J Hepatobiliary
Pancreat Sci. 21:676–681. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nanashima A, Sumida Y, Abo T, Nonaka T,
Takeshita H, Hidaka S, Sawai T, Yasutake T, Sakamoto I and Nagayasu
T: Clinical significance of portal vein embolization before right
hepatectomy. Hepatogastroenterology. 56:773–777. 2009.PubMed/NCBI
|
|
27
|
Giraudo G, Greget M, Oussoultzoglou E,
Rosso E, Bachellier P and Jaeck D: Preoperative contralateral
portal vein embolization before major hepatic resection is a safe
and efficient procedure: A large single institution experience.
Surgery. 143:476–482. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geisel D, Malinowski M, Powerski MJ,
Wüstefeld J, Heller V, Denecke T, Stockmann M and Gebauer B:
Improved hypertrophy of future remnant liver after portal vein
embolization with plugs, coils and particles. Cardiovasc Intervent
Radiol. 37:1251–1258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji W, Li JS, Li LT, Liu WH, Ma KS, Wang
XT, He ZP and Dong JH: Role of preoperative selective portal vein
embolization in two-step curative hepatectomy for hepatocellular
carcinoma. World J Gastroenterol. 9:1702–1706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Corrêa D, Schwartz L, Jarnagin WR, Tuorto
S, DeMatteo R, D'Angelica M, Allen P, Brown K and Fong Y: Kinetics
of liver volume changes in the first year after portal vein
embolization. Arch Surg. 145:351–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bellemann N, Stampfl U, Sommer CM, Kauczor
HU, Schemmer P and Radeleff BA: Portal vein embolization using a
Histoacryl/Lipiodol mixture before right liver resection. Dig Surg.
29:236–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Madoff DC, Hicks ME, Abdalla EK, Morris JS
and Vauthey JN: Portal vein embolization with polyvinyl alcohol
particles and coils in preparation for major liver resection for
hepatobiliary malignancy: Safety and effectiveness study in 26
patients. Radiology. 227:251–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giuffrida MA: Type II error and
statistical power in reports of small animal clinical trials. J Am
Vet Med Assoc. 244:1075–1080. 2014. View Article : Google Scholar : PubMed/NCBI
|