Introduction
Retroperitoneal soft-tissue sarcomas located in the
retroperitoneum/intra-abdominal regions account for 10–15% of all
soft-tissue sarcoma cases. Liposarcomas, which are the most common
histotype, account for 20–45% of the cases of
retroperitoneum/intra-abdominal sarcoma, and 20% of the cases of
liposarcomas are primary retroperitoneal liposarcomas (PRPLS)
(1–3).
PRPLS often originates from adipose tissue around the kidney. Due
to the huge spaces in and the loose structure of the
retroperitoneum, it is difficult to diagnose and treat owing to a
lack of typical clinical symptoms. The sarcoma grows to a large
size and often exhibits tumor invasion of adjacent organs by the
time of diagnosis (4). The tumor
usually presents as a round or oval lobulated mass, and surrounding
those masses, there exist satellite lesions. Due to this, the tumor
often invades proximal tissues and blood vessels. In previous
years, the rate of morbidity of PRPLS has gradually increased.
PRPLS tumors have a high rate of local recurrence but rarely result
in distant metastases (5). Compared
with other malignancies, numerous patients with PRPLS have
succumbed to mortality due to the effects of local recurrence
(5). This is unsurprising due to the
large tumor size and its proximity to vital structures, which limit
the potential to achieve negative surgical margins in surgery.
Chemotherapy and radiotherapy are ineffective for the majority of
PRPLS cases, with a chemotherapy response rate of <10% (6,7). Despite
the large tumor size and the difficult nature of surgery due to the
proximity to vital structures, complete tumor resection remains the
most effective treatment for the majority of patients with PRPLS
(8). Immunohistochemistry is
considered to be the most important diagnostic method for PRPLS.
Evidence of the presence of PRPSL is the existence of signet-ring
lipoblastomas or dendritic blood capillaries in tumor cells
(9). At present, numerous immune
markers are utilized in order to diagnose and identify diseases
(10–12). However, it remains unclear whether the
common PRPLS markers (S-100, vimentin and Ki-67) are specific for
different types of PRPLS and whether increased Ki-67 expression is
associated with disease-specific survival (DSS).
The present study was designed to investigate the
immunohistochemical features and effects of surgical treatment of
PRPLS, to retrospectively analyze DSS using clinical cases of PRPLS
from a single institution, and to use the information gained to
elucidate key prognostic factors that may predict which patients
with PRPLS will benefit from surgical treatment.
Materials and methods
Patients
Between 1st January, 2005 and 31st March, 2015, a
group of 174 patients (including 82 males and 92 females, with ages
ranging from 16 to 81 years old and a median age of 51.3 years)
with liposarcoma who were treated at Southwest Hospital (Chongqing,
China) were identified. Of these, 99 patients were diagnosed with
retroperitoneal liposarcoma, and 65 were diagnosed with PRPLS.
Follow-up, via outpatient re-examination, or postal or telephone
correspondence, was recorded for all 65 PRPLS patients until
October 2015, with the follow-up time ranging from 3 to 114 months.
Of these 65 patients, 51 patients received a complete primary tumor
resection, for which the gross margins were ensured to be negative
during the surgery. The remaining 14 patients received palliative
surgery or biopsy. Contiguous organ resection was performed in 32
patients in order to achieve complete resection, with 22 patients
exhibiting primary local recurrence following complete resection.
The 65 patients diagnosed with PRPLS, including the 51 that
received complete primary tumor resection, formed the basis of the
present study (Table I).
 | Table I.Patients included in the present
study. |
Table I.
Patients included in the present
study.
| Patient group | Patients, n |
|---|
| All liposarcomas
treated | 174 |
| All retroperitoneal
liposarcomas treated | 99 |
| All primary
retroperitoneal liposarcomas treated | 65 |
| Completely
resected | 51 |
| Palliative surgery
or biopsy | 14 |
| Contiguous organ
resection | 32 |
| Primary local
recurrence following complete resection | 22 |
| Reoperation
following primary local recurrence | 16 |
Pathology
According to the criteria of the World Health
Organization (WHO) Classification of Tumors of Soft Tissue and
Bone, PRPLS tumors are widely accepted to be classified into one of
the five following histological subtypes, based on morphological
features and cytogenetic aberrations: Well differentiated,
de-differentiated, myxoid, round cell and pleomorphic (13,14).
Well-differentiated PRPLS was defined as retroperitoneal fatty
tumors containing mature adipocytes with occasional atypical cells
and irregular hyperchromatic nuclei. Lesions with regions of
non-lipogenic spindle cell sarcoma arising within a fatty tumor
were labeled as de-differentiated PRPLS. Myxoid PRPLS was composed
of adipocytes with different differentiation and a variable number
of small lipoblasts in a prominent myxoid stroma, with or without
delicate arborizing vasculature. Tumors with similar morphological
small round- or oval-shaped cells and scattered adipocytes were
determined to be round-cell PRPLS. Pleomorphic spindle and giant
cells, as well as sheets of pleomorphic lipoblasts, were classified
as pleomorphic PRPLS. The well-differentiated subtype accounts for
56% of PRPLS cases, with de-differentiated, myxoid and round-cell
subtypes accounting for 37, 5 and 2% of cases, respectively. As
certain myxoid liposarcomas exhibit morphological similarities to
round-cell liposarcomas and are similar in terms of histological
progression, these two types are difficult to distinguish from each
other. Additionally, in previous studies, mixed-type PRPLS was
defined by the WHO as including two or more subtypes of liposarcoma
(15,16). Thus, the present study allocated
patients with myxoid and round cell PRPLS into one group, and
patients with pleomorphic/mixed-type PRPLS into another group.
Although there are five PRPLS subtypes, histological grade may be
divided into two grades (high and low), defined by histological
subtype (17). The majority of
mixed-type PRPLS cases include de-differentiated or pleomorphic
PRPLS. Thus, the high-grade tumors include de-differentiated,
pleomorphic and mixed-type cells, whereas the low-grade tumors
consist of well-differentiated and myxoid/round cell types.
Immunohistochemistry
The tumor samples of 65 patients were fixed in 4%
formaldehyde for 24 h at room temperature, hydrated and dehydrated
with differing concentrations of ethanol (75, 85, 95 and 100%) and
100% xylene embedded in paraffin, cut into 4 µm sections and
stained using the hematoxylin and eosin (H&E) method for 24 h
at room temperature. Immunohistochemistry was performed by the
streptavidin-peroxidase method (18)
using antibodies against S-100-α2 (cat. no. bs-7873R; Bioss
Antibodies, Inc., Woburn, MA, USA), vimentin (catalog no. 5741;
Cell Signaling Technology, Inc., Danvers, MA, USA) and Ki-67
(catalog no. bs-2130R; Bioss Antibodies, Inc.). Positive expression
was defined as brown staining in the cytosol, cytomembrane or
nuclei of the tumor cells. A total of 65 H&E stained samples
for S-100, vimentin and Ki-67 respectively were separated into
group 1, group 2 and group 3 randomly to test for their specificity
for the tumor. The Ki-67 proliferation index was also assessed
using a scale reflecting the positive proportion of a total of
1,000 cells in 10 random high-power fields, as follows: +, ≥0 and
<10%; ++, ≥10 and <20%; +++, ≥20 and <30%; ++++, ≥30 and
<40%; and +++++, ≥40% positively stained cells.
Statistical analysis
The endpoints of the present study were upon patient
mortality or the date of last follow-up. DSS was defined as the
time from the primary treatment (time of diagnosis or time of
primary resection for the 65-patient analysis) to the time of
mortality or last follow-up. The endpoint for DSS was confirmed
disease-associated mortality (27 of the 65 patients). The survival
rate was calculated as the percentage of surviving patients among
the 65 patients at the end of the follow-up period. A Wilcoxon
signed-rank test was used to determine whether two dependent
samples were selected from populations with the same distribution,
and a Spearman's rank correlation was used to compare the
correlation between two variables.
The tumor burden was defined as the diameter of the
maximum dimension upon cross-sectional imaging of a single mass or
the largest mass removed from the abdominal cavity, rather than
other dispersive or small masses removed during primary resection.
Adjacent visceral structures that were removed during resection,
including those in the kidney, liver and intestines, were included
in the measure of tumor burden. The 51 patients who underwent
complete primary tumor resection were divided into three groups on
the basis of tumor size, from small to large, as follows: Group A,
≤10 cm; group B, >10 cm and ≤20 cm; and group C, >20 cm.
The organs removed during the resection of adjacent
organs, including the kidney, adrenal glands, ureter, spleen,
pancreas, gall bladder, epityphlon, colon, omentum and mesentery,
were recorded and organ identity was confirmed by postoperative
pathological examination.
Clinical, pathological and therapeutic variables,
including pathological subtype, histological grade, tumor burden,
complete primary tumor resection, adjacent organ resection and the
tumor margins, were examined, and the association of these factors
with survival outcome were analyzed using the Kaplan-Meier method
and log-rank test. Other univariate analysis and statistical
associations were examined using the χ2 test, Wilcoxon
signed-rank test and Spearman's rank correlation. Multivariate
factors, including age at presentation and gender, were analyzed
using the Cox proportional hazards regression model.
Results
Patient, primary tumor and
treatment-associated variables
Between January 2005 and March 2015, 65 patients
were diagnosed with PRPLS and 51 patients underwent complete
primary tumor resection at Southwest Hospital. The distribution of
clinical and pathological characteristics among patients is
illustrated in Table II. Of the 65
patients, 14 received palliative surgery or biopsy alone, due to
the invasion of adjacent major organs or vessels by the tumor. At
the endpoint of the study, 5 of these 14 patients survived. Of the
51 patients that received complete primary tumor resection, the
median survival time was 43.3 months. Tumor samples from 25
patients were determined to be of the well-differentiated
histological subtype, 10 were de-differentiated, 8 were
myxoid/round cell and 8 were pleomorphic/mixed-type (Table II). Thus, 18 tumors were high grade
and the remaining 33 tumors were low grade. Following surgery, the
gross resection margins were negative for tumor tissue in all 51
patients. To achieve complete resection, 32 patients received
adjacent organ resection, with 15 patients exhibiting
tumor-positive microscopic margins. Following complete primary
resection, 22 patients developed local recurrence and the median
time to recurrence was 29.2 months (range, 3–60 months). Of these,
16 patients underwent further surgery, 4 patients received adjuvant
chemotherapy or radiation, and 2 patients declined further
treatment. The characteristics of the 65 patients with PRPLS are
presented in Table II.
 | Table II.Characteristics of 65 patients with
primary retroperitoneal liposarcoma. |
Table II.
Characteristics of 65 patients with
primary retroperitoneal liposarcoma.
| Variables | Patients, n | Median survival
time, monthsa | Survival rate,
% |
|---|
| Follow-up | 65 | 38.1±25.9 | 58.5 |
| Primary tumor
complete resection | 51 | 43.3±26.8 | 64.7 |
| Palliative surgery
or biopsy | 14 | 19.4±6.8 | 35.8 |
| Histological
subtype |
|
|
|
|
Well-differentiated | 25 | 51.2±27.9 | 84.0 |
|
De-differentiated | 10 | 20.7±12.8 | 50.0 |
|
Myxoid/round cell subtype | 8 | 59.9±25.2 | 62.5 |
|
Pleomorphic/mixed-type | 8 | 30.1±12.6 | 25.0 |
| Histological
grade |
|
|
|
|
High | 18 | 24.9±13.2 | 38.9 |
|
Low | 33 | 53.3±27.2 | 78.8 |
| Contiguous organ
resection | 32 | 42.7±30.1 | 53.1 |
| Primary local
recurrence | 22 | 29.2±14.2 | 36.4 |
Immunohistochemical analysis
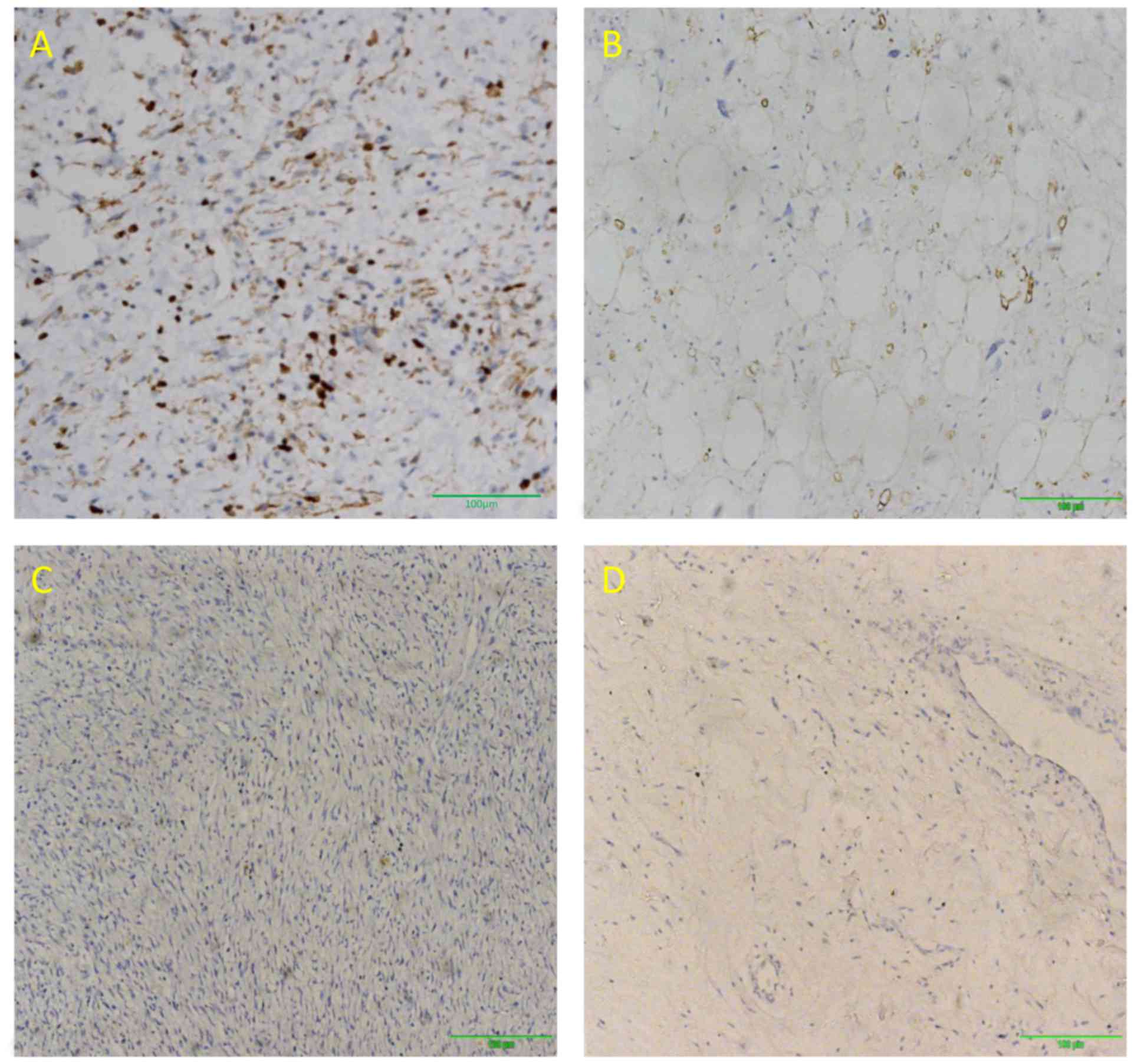
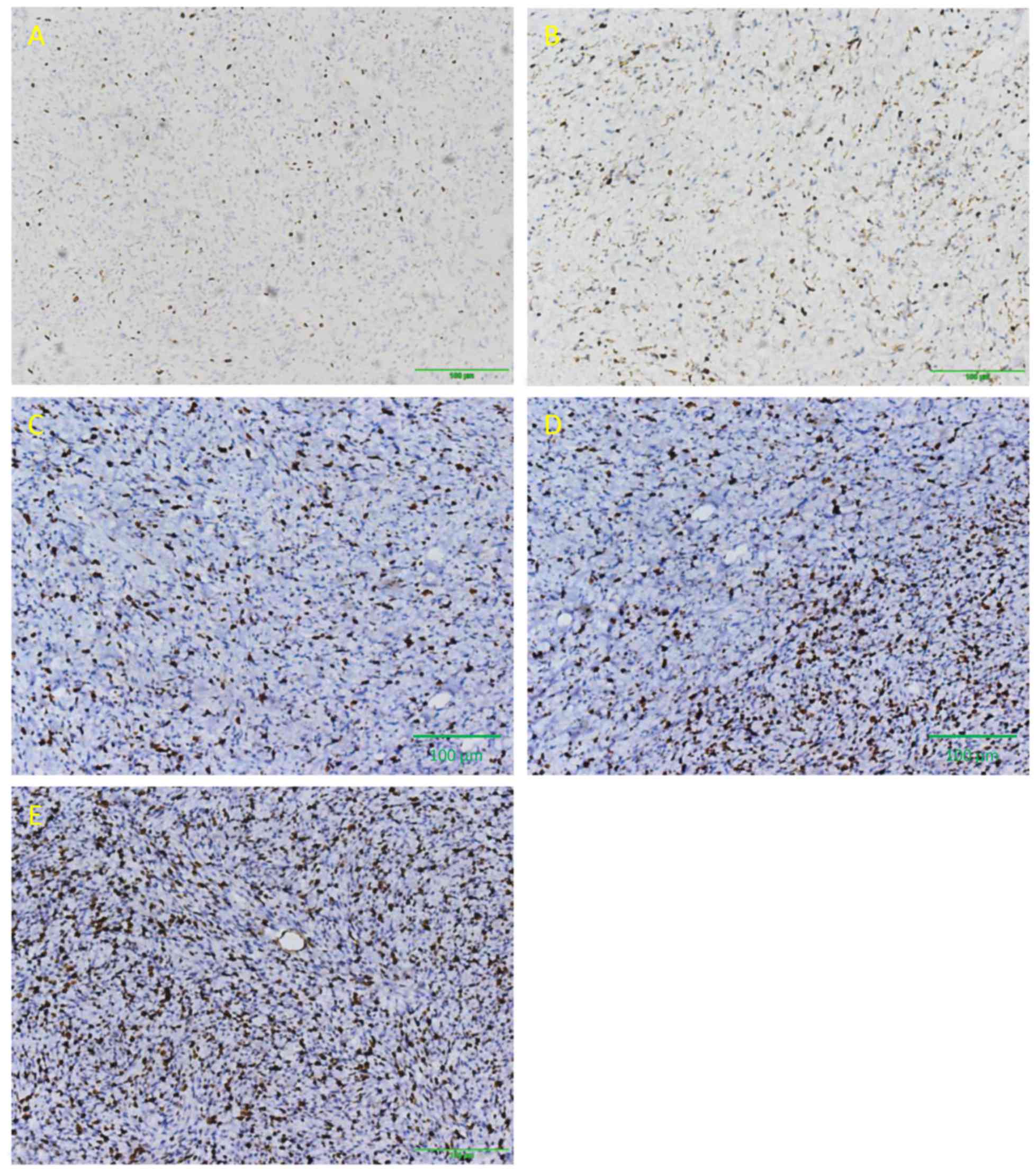
Immunohistochemical analysis of PRPLS tumors stained
for S-100, vimentin and Ki-67 is presented in Figs. 1–3.
Tumors from 23 patients were positive for S-100, whereas vimentin
and Ki-67 were expressed in 62 and 64 patients, respectively. S-100
expression was predominantly observed in well-differentiated PRPLS.
The rates of vimentin- and Ki-67-positive staining were
significantly higher compared with that of S-100 (P<0.0001,
P<0.0001 and P=0.157 respectively; Table III). Ki-67 was highly expressed in
high-grade PRPLS, with the most pronounced expression observed in
de-differentiated, pleomorphic and mixed-type tumors. Furthermore,
a negative correlation was observed between Ki-67 expression index
and DSS time (Table IV).
 | Table III.Analysis on the specificity of S-100,
vimentin and Ki-67 staining. |
Table III.
Analysis on the specificity of S-100,
vimentin and Ki-67 staining.
| Groups | Positive, n | Negative, n | Positive rate,
% | Z-value |
P-valuea |
|---|
| Group 1 |
|
|
| −6.245 | <0.0001 |
|
S-100 | 23 | 42 | 35.4 |
|
|
|
Vimentin | 62 | 3 | 95.4 |
|
|
| Group 2 |
|
|
| −6.403 | <0.0001 |
|
S-100 | 23 | 42 | 35.4 |
|
|
|
Ki-67 | 64 | 1 | 98.5 |
|
|
| Group 3 |
|
|
| −1.414 | 0.157 |
|
Vimentin | 62 | 3 | 95.4 |
|
|
|
Ki-67 | 64 | 1 | 98.5 |
|
|
 | Table IV.Correlation between the intension of
Ki-67 expression and survival time. |
Table IV.
Correlation between the intension of
Ki-67 expression and survival time.
| Ki-67 staining
intensity |
|
|
|
|---|
|
|
|
|
|---|
| Threshold (%) | Symbol | Patients, n | R-value |
P-valuea |
|---|
| ≤0 and 10 | + | 29 | −0.542 | 0.0001 |
| ≤10 and 20 | ++ | 13 |
|
|
| ≤20 and <30 | +++ | 8 |
|
|
| ≤30 and <40 | ++++ | 8 |
|
|
| >40 | +++++ | 6 |
|
|
Association between pathological
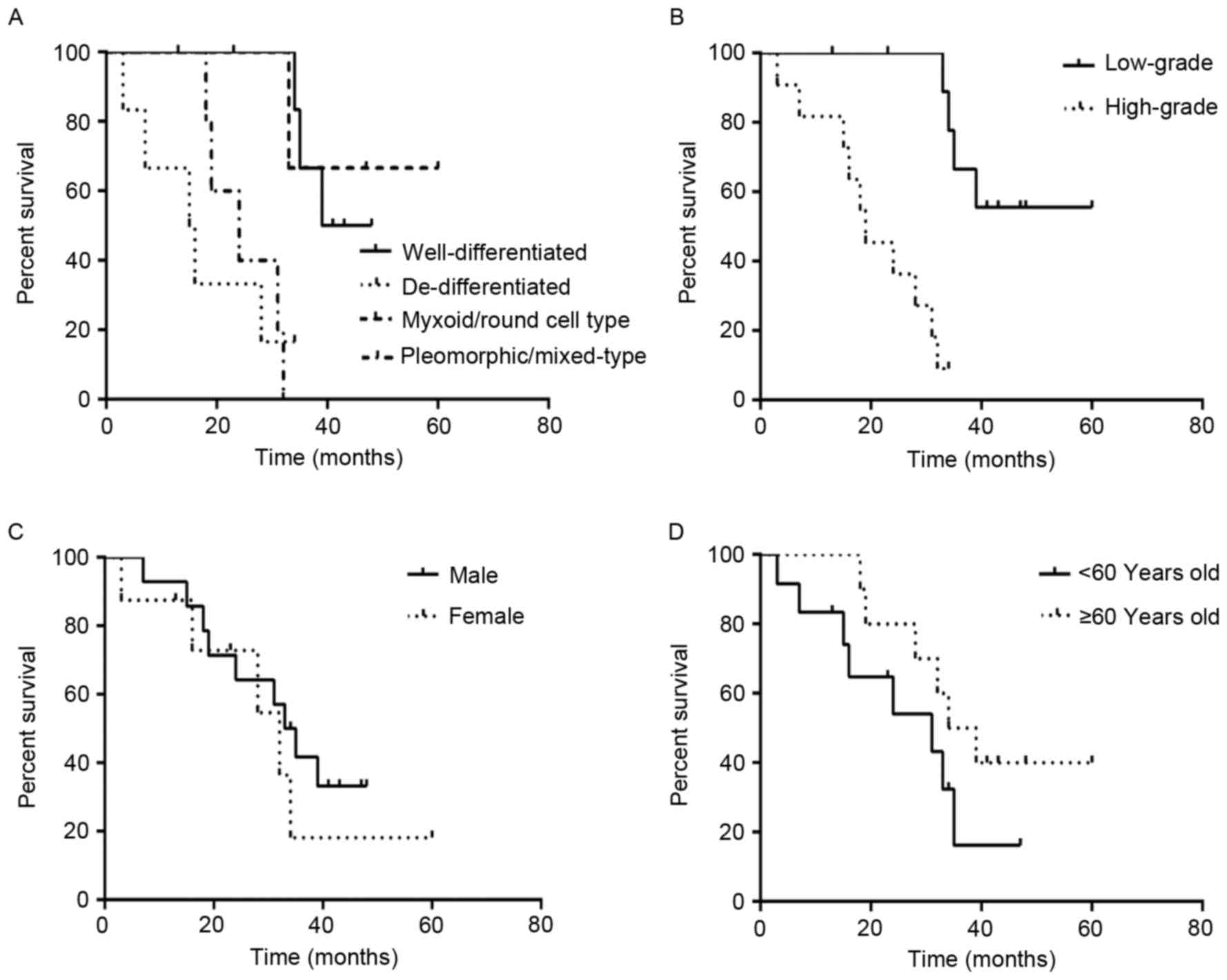
subtype and DSS time
The duration of follow-up period of patients ranged
between 3 and 114 months. The median survival time of the 51
surgical patients was 43.3 months. The median survival time of the
25 patients with well-differentiated PRPLS was 51.2 months, and the
rate of survival was 84.0%, compared with 20.7 months and 50.0% for
the 10 patients with de-differentiated PRPLS. Additionally, the 8
patients with the myxoid/round cell subtype had a median survival
time of 59.9 months and a survival rate of 62.5%, compared with
30.1 months and 25.0% for the 8 patients with
pleomorphic/mixed-type tumors (Table
II). Statistical analysis indicated that DSS time was
significantly increased in patients with well-differentiated PRPLS,
compared with those with de-differentiated PRPLS
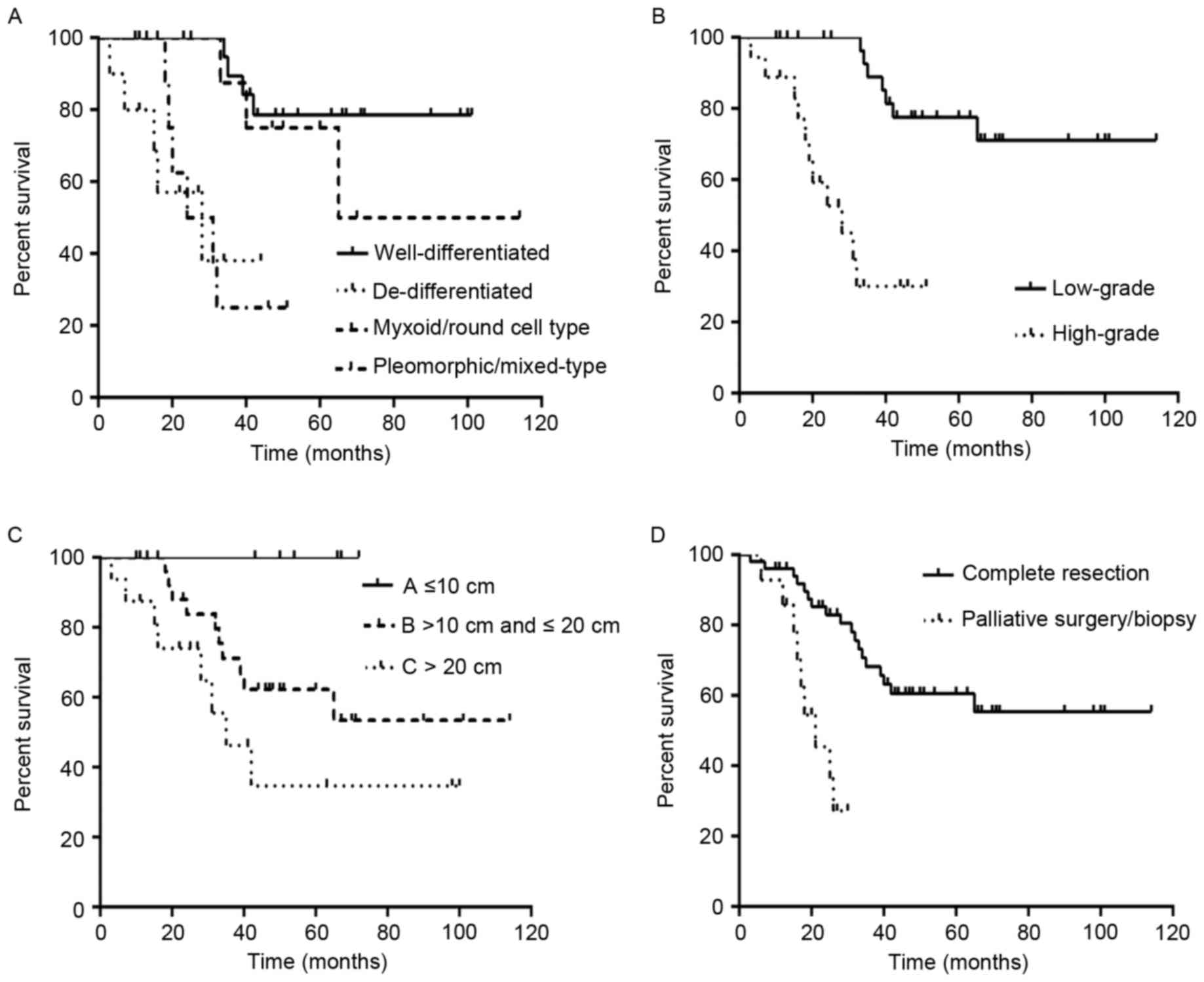
(χ2=19.467, P<0.0001; Fig.
4).
Association between histological grade
and DSS time
Analysis of tumor grade and DSS was performed using
the follow-up data of the 51 patients with PRPLS who received
complete primary resection. Histological subtype was used to define
the histological grade, which included de-differentiated and
pleomorphic/mixed-type subtypes and low-grade included
well-differentiated and myxoid/round cell subtypes. The median
survival time of the 18 patients with high-grade PRPLS was 24.9
months and the overall survival rate was 38.9%, compared with 53.3
months and 78.8% for the 33 patients with low-grade PRPLS (Fig. 4). DSS was significantly increased in
the patients with low-grade PRPLS compared with patients with
high-grade PRPLS (χ2=19.053, P<0.001).
Association between tumor burden and
DSS time
The present study investigated the association of
tumor burden with postoperative survival time. In the 51 patients
that received complete primary resection, the total median tumor
burden was 18.0 cm (range, 3–45 cm). There were 7 patients in group
A (tumor size, ≤10 cm), with a median survival time of 52.1 months.
There were 28 patients in group B (tumor size, >10 cm and ≤20
cm), and the median survival time was 45.6 months, compared with
35.3 months for the 16 patients in group C (tumor size, >20 cm).
Statistical analysis demonstrated that tumor burden was
significantly associated with DSS (χ2=6.826, P=0.033;
Fig. 4).
Association between the DSS time of
patients and complete resection or palliative surgery/biopsy
The median survival time of the 51 patients that
received complete resection was 43.3 months and the survival rate
was 64.7%, compared with 19.4 months and 35.7% for the 14 patients
that underwent palliative surgery or biopsy. Thus, complete tumor
resection was demonstrated to be significantly associated with
increased DSS time (χ2=15.471, P<0.0001), and also
observably improved the survival rate and survival time (Fig. 4).
Association between DSS time and
contiguous organ resection or palliative surgery/biopsy
To achieve complete tumor resection, contiguous
resection was performed where necessary. The median survival time
following contiguous organ resection (32 patients) was 42.7 months
and the rate of survival was 53.1%, compared with 19.4 months and
35.8% following palliative surgery or biopsy (14 patients).
Analysis demonstrated that DSS time was significantly increased in
patients who received contiguous organ resection, compared with
those who underwent only palliative surgery or biopsy
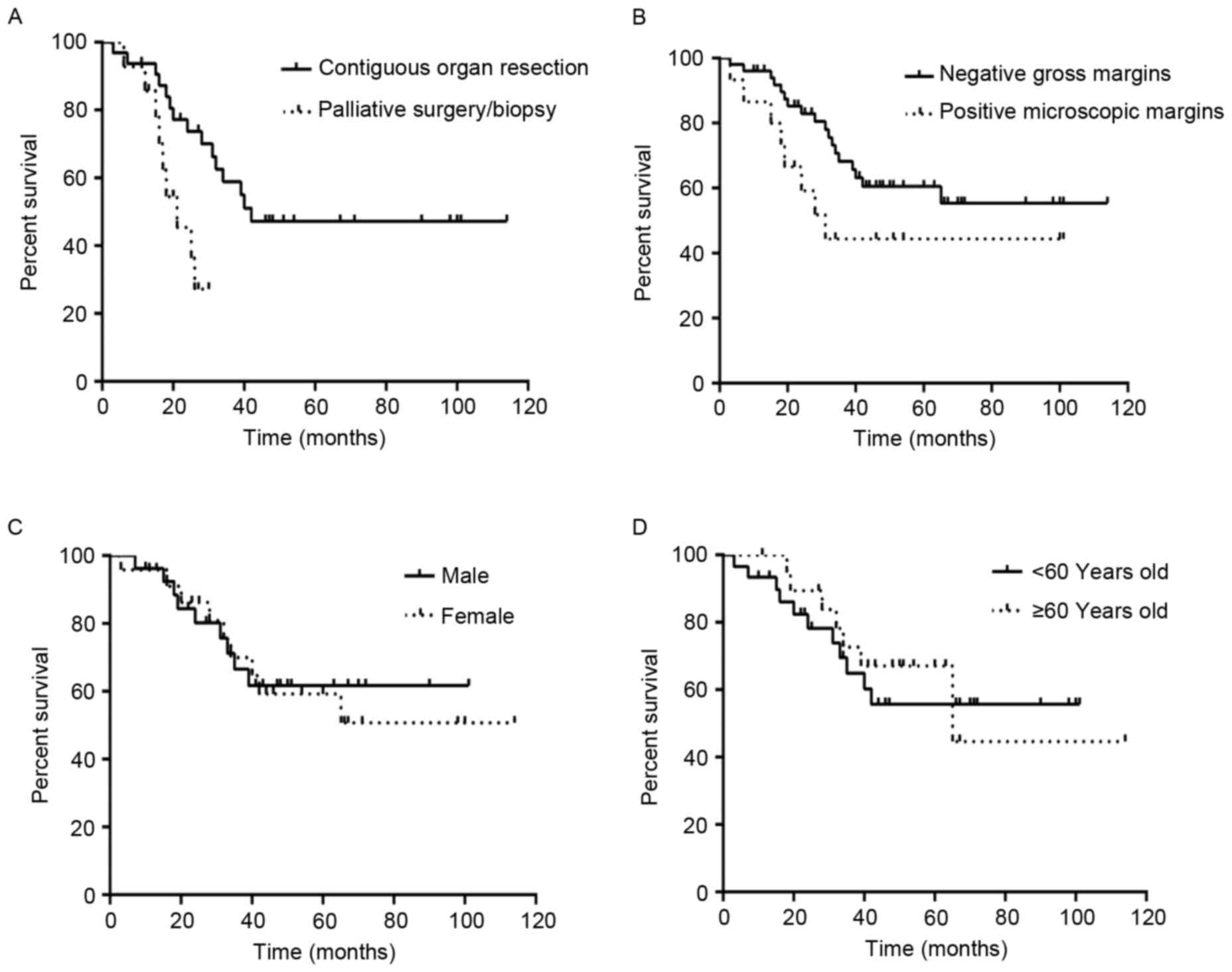
(χ2=7.130, P=0.008; Fig.
5).
Margin of resection
Of the 51 patients who underwent complete resection,
all had negative gross margins. The median survival time for these
patients was 43.3 months and the rate of survival was 64.7%,
compared with 19.4 months and 35.7% in the 14 patients who received
palliative surgery or biopsy with tumor-positive gross margins. Of
the 32 patients who underwent contiguous organ resection, 15
patients had tumor-positive microscopic margins. The median
survival time for these patients was 36.9 months and the survival
rate was 46.7% in these 15 patients. There was no statistically
significant difference between the DSS of patients with
tumor-negative gross margins and positive microscopic margins
(χ2=2.240, P=0.134; Fig.
6). However, the status of the gross margins was associated
with prognosis based on a comparison of the DSS of the 51 patients
with negative gross margins and the 14 patients with positive gross
margins who received palliative surgery or biopsy
(χ2=15.471, P<0.0001; Fig.
5).
Association between DSS time, gender
and age at diagnosis
Among the 65 patients included in the present study,
the 35 males had a median survival time of 36.1 months and a 60.0%
survival rate, compared with a 40.5-month median survival time and
56.7% survival rate in the 30 female patients. Analysis
demonstrated that gender did not affect DSS time
(χ2=0.005, P=0.821; Fig.
5). For patients with age <60 years (n=40), the median
survival time was 37.1 months, and the survival rate was 57.5%,
compared with 38.9 months and a 60.0% survival rate for patients
with age ≥60 years (n=25). The age at diagnosis did not affect DSS
(χ2=0.005, P=0.671; Fig.
5).
Multivariate analysis of factors
affecting prognosis
The pathological subtype, histological grade, tumor
burden, contiguous organ resection, local recurrence, tumor
margins, gender and age at presentation were analyzed using the Cox
proportional hazards regression model. The results revealed that
pathological subtype, histological grade and contiguous organ
resection were independent factors that affected prognosis, whereas
the remaining variables were not (Table
V).
 | Table V.Multivariate analysis of patient
prognosisa. |
Table V.
Multivariate analysis of patient
prognosisa.
| Variables | B | SE | Wald | df | P-value | Exp (B) | 95% CI |
|---|
| Histological
subtype | −0.491 | 0.247 | 3.952 | 1 | 0.047 | 0.612 | 0.377–0.993 |
| Histological
grade | 2.262 | 0.385 | 12.172 | 1 | <0.0001 | 9.602 | 2.695–34.219 |
| Tumor burden | 0.141 | 0.283 | 0.249 | 1 | 0.618 | 1.151 | 0.662–2.003 |
| Contiguous organ
resection | −1.115 | 0.451 | 6.117 | 1 | 0.013 | 0.328 | 0.135–0.793 |
| Primary local
recurrence | 0.944 | 0.541 | 3.046 | 1 | 0.081 | 2.570 | 0.890–7.419 |
| Margin | −0.549 | 0.578 | 0.903 | 1 | 0.342 | 0.577 | 0.186–1.793 |
| Gender | −0.044 | 0.385 | 0.013 | 1 | 0.908 | 0.957 | 0.450–2.036 |
| Age | 0.305 | 0.419 | 0.529 | 1 | 0.467 | 1.357 | 0.596–3.087 |
Association between pathological
subtype and DSS time in patients with primary local recurrence
Of the 51 patients who underwent complete resection,
22 experienced primary local recurrence. The median time to
recurrence of the 8 patients with well-differentiated PRPLS was
34.5 months and the survival rate was 62.5%, compared with 17.2
months and 16.7% for the 6 patients with de-differentiated PRPLS.
Additionally, the 3 patients with myxoid/round cell tumors
exhibited a median time-to-recurrence of 46.7 months and survival
rate of 66.7%, compared with 24.8 months and 0% survival rate in
the 5 patients with pleomorphic/mixed-type PRPLS. Statistical
analysis demonstrated that, in patients with primary local
recurrence, the pathological subtype was associated with DSS
(χ2=14.995, P=0.002; Fig.
6).
Association between histological grade
and DSS in patients with primary local recurrence
Of the 22 patients with primary local recurrence,
the median time to recurrence of the 11 patients with high-grade
PRPLS was 20.6 months and the rate of survival was 9.1%, compared
with 37.8 months and 63.6% in the 11 patients with low-grade PRPLS.
Analysis demonstrated that the DSS time was significantly higher
among patients with low-grade PRPLS, compared with those with
high-grade PRPLS (χ2=14.810, P<0.0001; Fig. 6).
Multivariate analysis of factors
affecting local recurrence
The pathological subtype, histological grade, tumor
burden, gender and age at presentation were examined in the
patients who exhibited local recurrence following resection (n=22)
using the Cox proportional hazards regression model. The analysis
demonstrated that histological grade was an independent factor
affecting local recurrence, while the remaining variables were not
independently associated with recurrence (Table VI).
 | Table VI.Multivariate analysis of local
recurrencea. |
Table VI.
Multivariate analysis of local
recurrencea.
| Variables | B | SE | Wald | df | P-value | Exp (B) | 95% CI |
|---|
| Histological
subtype | 0.064 | 0.306 | 0.044 | 1 | 0.834 | 1.066 | 0.586–1.941 |
| Histological
grade | 2.506 | 0.963 | 6.772 | 1 | 0.009 | 12.260 | 1.857–80.958 |
| Tumor burden | 1.006 | 0.582 | 2.992 | 1 | 0.084 | 2.736 | 0.875–8.557 |
| Gender | −0.155 | 0.752 | 0.042 | 1 | 0.837 | 0.857 | 0.196–3.740 |
| Age | −0.006 | 0.723 | 0.007 | 1 | 0.993 | 0.994 | 0.241–4.099 |
Correlation between local recurrence
and DSS time
Of the 51 patients who received complete resection,
22 developed primary local recurrence, with a survival rate of
36.4%. By comparison, the survival rate was 86.2% for the 29
patients without local recurrence. This finding demonstrated that
local recurrence strongly affected DSS, and statistical analysis
indicated that there was a negative association between local
recurrence and DSS (R=0.517, P=0.000; Table VII). Thus, local tumor recurrence
following complete resection was the predominant cause of mortality
in patients with PRPLS.
 | Table VII.Correlation between disease-dependent
survival and local recurrence. |
Table VII.
Correlation between disease-dependent
survival and local recurrence.
| Parameter | Patients, n | Median survival
rate, % | R-value |
P-valuea |
|---|
| Primary local
recurrence |
|
| 0.517 | <0.001 |
|
Yes | 22 | 36.4 |
|
|
| No | 29 | 86.2 |
|
|
Correlation between tumor burden and
histological grade
Of the 51 patients who underwent complete tumor
resection, the median tumor burden was 18.0 cm. The median tumor
burden of the 33 patients with low-grade PRPLS was 17.2 cm, and
value was 19.4 cm in the patients with high-grade PRPLS.
Statistical analysis revealed that histological grade was not
correlated with tumor burden (R=0.222, P=0.117; Table VIII).
 | Table VIII.Correlation between tumor burden and
histological grade, tumor invasion of adjacent organs or local
recurrence. |
Table VIII.
Correlation between tumor burden and
histological grade, tumor invasion of adjacent organs or local
recurrence.
| Parameters | Patients, n | Median tumor
burden, cm | R-value |
P-valuea |
|---|
| Histological
grade |
|
| 0.222 | 0.117 |
|
High | 18 | 19.4±5.1 |
|
|
|
Low | 33 | 17.2±8.4 |
|
|
| Adjacent organ
invasion |
|
| 0.225 | 0.112 |
|
Yes | 32 | 19.3±7.2 |
|
|
| No | 19 | 15.9±7.6 |
|
|
| Primary local
recurrence |
|
| 0.159 | 0.265 |
|
Yes | 22 | 18.6±5.7 |
|
|
| No | 29 | 17.5±8.6 |
|
|
Correlation between tumor burden and
tumor invasion of adjacent organs
Of the 51 PRPLS patients who underwent complete
resection, 32 underwent resection of adjacent organs as a result of
tumor invasion. The median tumor burden in patients who received
adjacent organ resection was 19.3 cm, compared with 15.9 cm in the
19 patients without tumor invasion of adjacent organs. Statistical
analysis demonstrated that there was no significant correlation
between tumor burden and tumor invasion of adjacent organs
(R=0.225; P=0.112; Table
VIII).
Correlation between tumor burden and
local recurrence
Following complete primary surgical resection, 22
patients developed primary local recurrence and the median tumor
burden was 18.6 cm, compared with 17.5 cm in the 29 patients that
did not exhibit local recurrence. Statistical analysis indicated
that tumor burden was not correlated with the development of local
recurrence (R=0.159, P=0.265; Table
VIII).
Correlation between tumor invasion of
adjacent organs and histological grade
Of all 65 patients with PRPLS, a median of 1 organ
was invaded in each of the 38 patients with low-grade PRPLS,
compared with 2 in the 27 patients with high-grade PRPLS.
Statistical analysis demonstrated that there was a positive
correlation between tumor invasion of adjacent organs and tumor
histological grade (R=0.666, P<0.001; Table IX). Thus, the histological grade was
strongly associated with tumor invasion of adjacent organs.
 | Table IX.Correlation between adjacent organs
invasion and histological grade. |
Table IX.
Correlation between adjacent organs
invasion and histological grade.
| Parameter | Patients, n | Median integral
number of organs invadeda | R-value |
P-valueb |
|---|
| Histological
grade |
|
| 0.666 | <0.001 |
|
High | 27 | 2±1 |
|
|
|
Low | 38 | 1±1 |
|
Discussion
RPLS is the most common soft tissue malignancy of
the retroperitoneum and accounts for ~40% of cases of primary soft
tissue sarcoma in the retroperitoneum (1–3). PRPLS
typically occurs in patients of 40–60 years, with a 1:1 ratio
between male and female patients (1,19). Primary
liposarcoma most commonly develops in the arms, legs,
retroperitoneum (20) or the bottom
of pelvic cavity (21). PRPLS lacks
typical clinical symptoms, meaning it is difficult to diagnose. The
majority of patients with PRPLS are diagnosed at an advanced
disease stage, therefore PRPLS tumors often grow to a large size
and have invaded adjacent organs upon diagnosis (22).
The benefits of using adjuvant chemotherapy and
radiation therapy to treat PRPLS remains controversial (23,24). A
number of studies have suggested that chemotherapy may worsen
patient prognosis (25,26). Other studies have reported that
chemotherapy has limited effectiveness for PRPLS, but is beneficial
for liposarcomas originating from the lower or upper extremities
(23,27). Radiation can result in clinical
complications, including nerve lesions, hydronephrosis, ureteral
fistula and ileus (28). For PRPLS,
due to the deep location in the enterocoelia and close proximity to
important visceral organs, damage to the viscera by radiation must
be considered when deciding upon treatment modalities.
According to the criteria of the WHO Classification
of Tumors of Soft Tissue and Bone (29), the histological tumor subtype defines
the histological grade: High-grade includes de-differentiated,
pleomorphic and mixed cell subtypes, whereas low-grade comprises
well-differentiated, myxoid and round cell subtypes, with
histological subtype predicting DSS (17,26). In
previous reports, well-differentiated and myxoid PRPLS cases
exhibited low rates of local recurrence, and long intervals between
treatment and recurrence compared with other subtypes of PRPLS
(17,30). The present study, which was based on a
Chinese population from a single medical center, comprised 49.0%
cases of well-differentiated, 19.6% cases of de-differentiated and
15.7% cases of myxoid/round cell PRPLS. The percentage of PRPLS
histological subtypes was approximately consistent with previous
reports (14,17). The biological behavior of these tumors
indicated that well-differentiated tumors grew slowly. By contrast,
de-differentiated tumors grew faster and centrifugally. It is more
likely for de-differentiated tumors to lead to increased invasion
of adjacent organs. In the present study, the histological tumor
grade was significantly associated with tumor burden, as the
majority of PRPLS cases exhibited expansive growth and formed a
pseudocapsule. However, histological grade was associated with
tumor invasion of adjacent organs. This may be due to the highly
invasive nature of high-grade tumors and the ability of some of the
tumor cells to pass through the tumor pseudocapsule to invade
adjacent organs.
Pathological diagnosis currently remains the gold
standard for the diagnosis of PRPL. Common markers for
investigating the clinicopathological features and biological
behavior in immunohistochemical analysis of PRPL tumors include
S-100, vimentin and Ki-67 (31–34). S-100
is an acidic calcium-binding protein that is predominantly present
in the cytosol of astroglial cells of the central nervous system.
Vimentin is an intermediate filament protein that is expressed in
mesenchymal cells and is closely associated with the occurrence and
metastasis of tumors (35). Ki-67 is
a proliferation-associated nuclear antigen and may be used to
measure the proliferative activity of tumor cells (36). Ki-67 is specifically expressed in the
cytoplasm (membrane) of adipocytes and in the nuclei of other tumor
cells. The results of the present study demonstrated that vimentin
and Ki-67 were more sensitive markers for PRPLS diagnosis compared
with S-100. S-100 protein was predominantly expressed in
well-differentiated PRPLS. Furthermore, vimentin and Ki-67 were
expressed in the majority of the PRPLS cases, and there was a
higher expression of Ki-67 in high-grade PRPLS, including
de-differentiated, pleomorphic and mixed-type PRPLS. By contrast,
in low-grade tumors, ~20.0% of the cell population was
Ki-67-positive. However, the Ki-67-positive cell population ranged
from 20.0 to 60.0% in high-grade PRPLS. These results demonstrated
that Ki-67 expression reflected the proliferative activity of the
tumor cells, and its positive expression was associated with
DSS.
In recent years, certain reports have indicated that
de-differentiated liposarcoma is associated with poor prognosis,
whereas the sclerotic subtype has the best prognosis in
well-differentiated PRPLS cases (30). Tseng et al (37) reported that the 5-year survival rate
of patients with differentiated retroperitoneal liposarcoma was
20.0%, compared with 83.0% for those with well-differentiated
PRPLS. In the present study, the survival times for patients with
well-differentiated and myxoid/round cell PRPLS were improved
compared with those with de-differentiated and
pleomorphic/mixed-type tumors. Additionally, the prognosis for
patients with low-grade PRPLS was improved compared with those with
high-grade. Furthermore, survival analysis demonstrated that the
pathological subtype and histological grade were important
prognostic factors for DSS, and that there was a positive
association between tumor invasion of adjacent organs and
histological grade.
Serio et al (38) proposed that the complete resection of
PRPLS (according to the gross margins) was an effective surgical
treatment for PRPLS. Previous studies reported that ~80.0% of
patients with PRPLS that required aggressive treatment were
suitable for complete surgical resection, and this treatment
strategy resulted in a median survival time of 83 months and a
5-year DSS of 60.0% (39–41). Singer et al (17) reported 3- and 5-year survival rates of
73.0 and 60.0%, respectively, when complete resection was
performed. Additionally, Milone et al (42) reported a 5-year survival rate of
85.7%. The rate of complete primary tumor resection was 78.5% in
the present study, compared with 81.0% in a previous report
(17). This discrepancy may be due to
the fact that 46 patients in the present study presented with tumor
invasion of adjacent organs. However, there was no significant
difference in the prognosis of patients with negative gross margins
during operation compared with that of patients with positive
microscopic margins postoperatively. It is hypothesized that this
may be because these PRPLS tumors had complete pseudocapsules and
exhibited local expansive growth. However, tumor-positive gross
margins strongly affected DSS.
In the present study, the tumor burden was
associated with DSS. However, tumor burden did not directly affect
tumor invasion of adjacent organs and local recurrence. This is
potentially due to ~80.0% of patients exhibiting a tumor burden of
>10 cm, indicating that the tumor grew expansively and the
majority of the tumor pseudocapsules were complete. A previous
study has reported that the 5-year survival rate of patients who
underwent complete tumor resection was 75.0%, compared with 34.0%
for patients who underwent palliative surgery or biopsy (43). This effect on survival rate was also
observed in the present study. However, the follow-up period of
certain patients was short, and long-term survival must also be
evaluated. Biopsy is not generally recommended for patients without
the ability to undergo complete resection due to the likelihood of
tumor seeding (44). Shibata et
al (45) observed that, for
patients with PRPLS that were not able to undergo complete
resection, palliative surgery is able to increase the survival time
compared with simple biopsy and reduce 75.0% of the clinical
symptoms. Neuhaus et al (25)
reported similar results.
To achieve a complete removal of the tumor,
57.0–83.0% of patients with PRPLS required contiguous organ
resection (25,46), and the resected organs included
kidney, adrenals, ureter, colon, small intestine, omentum, spleen
and other celiac organs (47).
Patients appear to benefit from this aggressive approach.
Furthermore, complete resection that includes resection of
tumor-invaded adjacent organs has been shown to be beneficial for
the prevention of local recurrence (17). In the present study, 32 patients
received contiguous organ resection. The prognosis of these
patients was improved compared with the 14 patients who received
palliative surgery or biopsy.
Multivariate analysis demonstrated that the
pathological subtype and histological grade of the tumors were
independent markers of prognosis, therefore, the greater the
differentiation of the tumor, the better the prognosis of the
patient. Additionally, contiguous organ resection was an
independent prognostic factor; if patients exhibited tumor invasion
of the adjacent organs, the prognosis of patients who received
contiguous organ resection was improved compared with patients who
received palliative surgery. However, although tumor burden
affected DSS, it was not an independent prognostic marker. The
likely reason for this is that larger tumors are able to infiltrate
adjacent organs more easily. Additionally, the rate of complete
resection was lower in patients with a larger tumor burden. In the
present study, gender and age were not independent prognostic
markers for DSS.
Clinical practice demonstrates that PRPLS has a very
high local recurrence rate following surgery (5). The majority of patients succumb to
disease as a result of local recurrence. Thus, the majority of
patients with local recurrence require reoperation (25). It is generally considered that the
cause of recurrence is the presence of pseudocapsules containing
malignant cells, which can be observed in the majority of cases of
PRPLS, and the rapid growth of the tumor to oppress the surrounding
normal structures. PRPLS frequently recurs in situ (48). The probability of recurrence doubles
over time (48). Certain studies have
reported that first, second and third complete resections were 57,
22 and 10.0%, respectively, in primary local recurrence of PRPLS
following the first resection (39,49). For
patients with recurrence who cannot undergo complete resection, the
aim of treatment is to attenuate symptoms, remove oppression of the
surrounding organs and obstruction of the tumor, maintain visceral
function, extend survival time and improve the quality of life. A
previous study indicated that if the speed of growth of a recurrent
tumor is >0.9 cm per month, multiple surgeries do not improve
the survival rate (50).
Research has demonstrated that local recurrence of
liposarcomas is closely associated with tumor grade. In the present
study, of the 22 patients with local recurrence, half of original
primary tumors were high grade and almost all cases exhibited tumor
invasion of adjacent organs, which is in accordance with the
literature (17,44). Tumor burden was not associated with
local recurrence, and it was observed that almost all the tumors
had whole pseudocapsules and grew expansively. Of the patients with
local recurrence, 30 underwent further surgery and only 8 survived
to the end of the study, with a survival rate of 36.4%, compared
with 86.2% in patients without recurrence. According to the present
study, local recurrence was negatively correlated with DSS time.
Thus, postoperative local recurrence is considered to be the most
common cause of mortality in patients with PRPLS. Multivariate
analysis demonstrated that histological grade was an independent
factor that affected local recurrence, whereas tumor burden was not
an independent prognostic factor, potentially as the majority of
patients exhibited recurrence had primary tumors >10 cm and over
half of these patients exhibited tumor invasion of adjacent organs.
Furthermore, half of the patients that developed recurrence had
high-grade primary tumors. Gender and age at presentation were not
independent factors.
In conclusion, despite the high rate of recurrence
and mortality of PRPLS, surgical resection is the most effective
treatment. Local recurrence is the predominant cause of mortality
in PRPLS. Staining for vimentin and Ki-67 demonstrated higher
sensitivity for PRPLS diagnosis compared with S-100 protein. S-100
protein was predominantly expressed in well-differentiated PRPLS,
whereas vimentin and Ki-67 exhibited positive expression in almost
all PRPLS samples. Additionally, Ki-67 was highly expressed in
high-grade PRPLS. Notably, there was a negative association between
the Ki-67 expression index and DSS. DSS was strongly associated
with pathological subtype, histological grade, tumor burden,
complete primary tumor resection and contiguous organ resection.
Positive microscopic margins did not affect DSS, whereas the status
of gross margins was strongly associated with DSS. Pathological
subtype, histological grade and contiguous organ resection were
independent markers of prognosis. Pathological subtype and
histological grade were associated with local recurrence, with
histological grade demonstrated to be an independent marker for
local recurrence. Patient age at presentation and gender were not
useful markers of prognosis or local recurrence.
References
|
1
|
Mack TM: Sarcomas and other malignancies
of soft tissue, retroperitoneum, peritoneum, pleura, heart,
mediastinum, and spleen. Cancer. 75 Suppl 1:S211–S244. 1995.
View Article : Google Scholar
|
|
2
|
Nathan H, Raut CP, Thornton K, Herman JM,
Ahuja N, Schulick RD, Choti MA and Pawlik TM: Predictors of
survival after resection of retroperitoneal sarcoma: A
population-based analysis and critical appraisal of the AJCC
staging system. Ann Surg. 250:970–976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Porter GA, Baxter NN and Pisters PW:
Retroperitoneal sarcoma: A population-based analysis of
epidemiology, surgery, and radiotherapy. Cancer. 106:1610–1616.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dalai KM, Antonescu CR and Singer S:
Diagnosis and management of lipomatous tumors. J Surg Oncol.
97:298–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SY, Goh BK, Teo MC, Chew MH, Chow PK,
Wong WK, Ooi LL and Soo KC: Retroperitoneal liposarcomas: The
experience of a tertiary Asian center. World J Surg Oncol.
9:122011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fuks D, Verhaeghe JL, Marchal F, Guillemin
F, Beckendorf V, Peiffert D, Leroux A, Rios M, Troufléau P and
Marchal C: Surgery and postoperative radiation therapy in primary
retroperitoneal sarcomas: Experience of the cancer centre
Alexis-Vautrin. Cancer Radiother. 16:194–200. 2012.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
EI-Bared N, Taussky D, Mehiri S, Patocskai
E, Roberge D and Donath D: Preoperative intensity modulated
radiation therapy for retroperitoneal sarcoma. Technol Cancer Res
Treat. 13:211–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alldinger I, Yang Q, Pilarsky C, Saeger
HD, Knoefel WT and Peiper M: Retroperitoneal soft tissue sarcomas:
Prognosis and treatment of primary and recurrent disease in 117
patients. Anticancer Res. 26:1577–1581. 2006.PubMed/NCBI
|
|
9
|
Rubin BP and Fletcher CD: The
cytologenetics of lipomatous tumours. Histopathology. 30:507–511.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma YL, Peng JY, Zhang P, Liu WJ, Huang L
and Qin HL: Immunohistochemical analysis revealed CD34 and Ki67
protein expression as significant prognostic factors in coloreetal
cancer. Med Oncol. 27:304–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors-definition, clinical, histological,
immunohistochemical, and molecular genetic features and
differential diagnosis. Virchows Arch. 438:1–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Czyzewska J, Guzińska-Ustymowicz K, Lebelt
A, Zalewski B and Kemona A: Evaluation of proliferating markers
Ki-67, PCNA in gastric cancers. Rocz Akad Med Bialymst. 49 Suppl
1:S64–S66. 2004.
|
|
13
|
Na JC, Choi KH, Yang SC and Han WK:
Surgical experience with retroperitoneal liposarcoma in a single
korean tertiary medical center. Korean J Urol. 53:310–316. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tseng WW, Madewell JE, Wei W, Somaiah N,
Lazar AJ, Ghadimi MP, Hoffman A, Pisters PW, Lev DC and Pollock RE:
Locoregional disease patterns in well-differentiated and
de-differentiated retroperitoneal liposarcoma: Implications for the
extent of resection? Ann Surg Oncol. 21:2136–2143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guillou L and Aurias A: Soft tissue
sarcomas with complex genomic profiles. Virchows Arch. 456:201–217.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stout AP: Liposarcoma-the malignant tumor
of lipoblasts. Ann Surg. 119:86–107. 1944. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singer S, Antonescu CR, Riedel E and
Brennan MF: Histologic subtype and margin of resection predict
pattern of recurrence and survival for retroperitoneal liposarcoma.
Ann Surg. 238:358–370. 2003.PubMed/NCBI
|
|
18
|
Liu JJ, Liu JY, Chen J, Wu YX, Yan P, Ji
CD, Wang YX, Xiang DF, Zhang X, Zhang P, et al: Scinderin promotes
the invasion and metastasis of gastric cancer cells and predicts
the outcome of patients. Cancer Lett. 376:110–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mendenhall WM, Zlotecki RA, Hochwald SN,
Hemming AW, Grobmyer SR and Cance WG: Retroperitoneal soft tissue
sarcoma. Cancer. 104:669–675. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Herrera-Gómez A, Ortega-Gutiérrez C,
Betancourt AM and Luna-Ortiz K: Giant retroperitoneal liposarcoma.
World J Surg Oncol. 6:1152008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshida Y, Inoue K, Ohsako T, Nagamoto N,
Tanaka E and Tsuruzoe S: Weekly paclitaxel therapy is curative for
patients with retroperitoneal liposarcoma. Gan to Kagaku Ryoho.
34:465–467. 2007.(In Japanese). PubMed/NCBI
|
|
22
|
Windham TC and Pisters PW: Retroperitoneal
sarcomas. Cancer Control. 12:36–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paryani NN, Zlotecki RA, Swanson EL,
Morris CG, Grobmyer SR, Hochwald SN, Marcus RB Jr and Indelicato
DJ: Multimodality local therapy for retroperitoneal sarcoma. Int J
Radiat Oncol Biol Phys. 82:1128–1134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tseng WH, Martinez SR, Do L, Tamurian RM,
Borys D and Canter RJ: Lack of survival benefit following adjuvant
radiation in patients with retroperitoneal sarcoma: A SEER
analysis. J Surg Res. 168:e173–e180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neuhaus SJ, Barry P, Clark MA, Hayes AJ,
Fisher C and Thomas JM: Surgical management of primary and
recurrent retroperitoneal liposarcoma. Br J Surg. 92:246–252. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dalal KM, Kattan MW, Antonescu CR, Brennan
MF and Singer S: Subtype specific prognostic nomogram for patients
with primary liposarcoma of the retroperitoneum, extremity, or
trunk. Ann Surg. 244:381–391. 2006.PubMed/NCBI
|
|
27
|
Eilber FC, Eilber FR, Eckardt J, Rosen G,
Riedel E, Maki RG, Brennan MF and Singer S: The impact of
chemotherapy on the survival of patients with high-grade primary
extremity liposarcoma. Ann Surg. 240:686–695. 2004.PubMed/NCBI
|
|
28
|
Stilidi IS, Nikulin MP, Nered SN, Davydov
MM, Bolotskiĭ VI and Gubina GI: Combined operations by
retroperitoneal liposarcoma. Khirurgiia (Mosk). 1–25. 2013.
|
|
29
|
Weiss SW, Sobin LH and Enzinger FM:
Histologic typing of soft tissue tumors. World Health Organization
International Histological Classification of Tumours. 2th.
Springer-Verlag; Berlin, Germany: pp. 23–26. 1994
|
|
30
|
Fabre-Guillevin E, Coindre JM, Nde S
Somerhausen, Bonichon F, Stoeckle E and Bui NB: Retroperitoneal
liposarcomas: Follow-up analysis of dedifferentiation after
clinicopathologic reexamination of 86 liposarcomas and malignant
fibrous histiocytomas. Cancer. 106:2725–2733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang L, Ren W, Zhou X, Sheng W and Wang J:
Pleomorphic liposarcoma: A clinicopathological, immunohistochemical
and molecular cytogenetic study of 32 additional cases. Pathol Int.
63:523–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng J, Yu H, Wang L, Wang X and Shen G:
Primary oral and maxillofacial liposarcoma: A clinicopathological
and immunohistochemical study of eleven cases. Arch Med Sci.
8:316–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dziegiel P, Salwa-Zurawska W, Zurawski J,
Wojnar A and Zabel M: Prognostic significance of augmented
metallothionein (MT) expression correlated with Ki-67 antigen
expression in selected soft tissue sarcomas. Histol Histopathol.
20:83–89. 2005.PubMed/NCBI
|
|
34
|
Wang HJ, Li CY and Wang YK: Diagnostic
implications of Ki-67 expression in adipocytes and lipoblasts: An
immunohistochemical study. Int J Clin Exp Pathol. 7:8899–8904.
2014.PubMed/NCBI
|
|
35
|
Yu JQ, Zhou Q, Zheng YF and Bao Y:
Expression of Vimentin and Ki-67 proteins in cervical squamous cell
carcinoma and their relationships with clinicopathological
features. Asian Pac J Cancer Prev. 16:4271–4275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wintzer HO, Zipfel I, Schulte-Mönting J,
Hellerich U and von Kleist S: Ki-67 immunostaining in human breast
tumors and its relationship to prognosis. Cancer. 67:421–428. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tseng W, Martinez SR, Tamurian RM, Borys D
and Canter RJ: Histologic type predicts survival in patients with
rrtroperitoneal soft tissue sarcoma. J Surg Res. 172:123–130. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Serio G, Tenehini P, Nifosi F and Iacono
C: Surgical strategy in primary retroperitoneal tumours. Br J Surg.
76:385–389. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singer S, Corson JM, Demetri GD, Healey
EA, Marcus K and Eberlein TJ: Prognostic factors predictive of
survival for truncal and retroperitoneal soft-tissue sarcoma. Ann
Surg. 221:185–195. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vijay A and Ram L: Retroperitoneal
liposarcoma: A comprehensive review. Am J Clin Oncol. 38:213–219.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lewis JJ, Leung D, Woodruff JM and Brennan
MF: Retroperitoneal soft-tissue sarcoma: Analysis of 500 patients
treated and followed at a single institution. Ann Surg.
228:355–365. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Milone M, Pezzullo LS, Salvatore G,
Pezzullo MG, Leongito M, Esposito I and Milone F: Management of
high-grade retroperitoneal liposarcomas: Personal experience.
Updates Surg. 63:119–124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bonvalot S, Miceli R, Berselli M, Causeret
S, Colombo C, Mariani L, Bouzaiene H, Le Péchoux C, Casali PG, Le
Cesne A, et al: Aggressive surgery in retroperitoneal soft tissue
sarcoma carried out at high-volume centers is safe and is
associated with improved local control. Ann Surg Oncol.
17:1507–1514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Clark MA and Thomas JM: Portsite
recurrence after laparoscopy for staging of retroperitoneal
sarcoma. Surg Laparosc Endosc Percutan Tech. 13:290–291. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shibata D, Lewis JJ, Leung DH and Brennan
MF: Is there a role for incomplete resection in the management of
retroperitoneal liposarcomas? J Am Coll Surg. 193:373–379. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sato T, Yamaguchi T, Azekura K, Ueno M,
Ohyama S, Ohya M, Yamamoto J, Muto T, Ishikawa Y and Kanda H:
Repeated resection for intra-abdominal and retroperitoneal
liposarcoma: Long-term experience in a single cancer in Japan. Int
Surg. 91:267–271. 2006.PubMed/NCBI
|
|
47
|
Chikatani K, Baba H, Sobajima J, Ishiguro
T, Kumamoto K, Kumagai Y, Ishibashi K, Haga N, Tsuji Y, Iwama T and
Ishida H: Clinicopathological characteristics and treatment outcome
of retroperitoneal liposarcoma. Gan To Kagaku Ryoho. 39:2426–2428.
2012.(In Japanese). PubMed/NCBI
|
|
48
|
Huang XH, Li PY, Zhao XD and Liu N: The
therapeutic strategy of primary retroperitoneal liposarcoma. Chin J
Pract Surg. 2:156–158. 2013.(In Chinese).
|
|
49
|
Theodosopoulos T, Psychogiou V, Yiallourou
AI, Polymeneas G, Kondi-Pafiti A, Papaconstantinou I and Voros D:
Management of retroperitoneal sarcomas: Main prognostic factors for
local recurrence and survival. J BUON. 17:138–142. 2012.PubMed/NCBI
|
|
50
|
Park JO, Qin LX, Prete FP, Antonescu C,
Brennan MF and Singer S: Predicting outcome by growth rate of
locally recurrent retroperitoneal liposarcoma: The one centimeter
per month rule. Ann Surg. 250:977–982. 2009. View Article : Google Scholar : PubMed/NCBI
|