Introduction
Breast cancer is the most prevalent malignant cancer
in women in China and worldwide (1).
Outcomes are markedly improved by early breast cancer detection
(2,3).
Breast cancer screening has been employed to detect lesions at
early stages, prior to metastasis. Circulating tumor cells (CTCs)
remain a promising avenue for early breast cancer detection as it
bypasses the need for ionizing radiation (mammography) or invasive
biopsy (4).
Circulating tumor cells (CTCs) are cancer cells that
detach from primary or metastatic solid tumors into the
vasculature, where they can be sampled from the circulating
bloodstream (5). Guidelines for the
quantification of CTCs as a breast cancer biomarker have been
outlined by the American Society of Clinical Oncology (6). In metastatic breast cancer, the Food and
Drug Administration approved the CELLSEARCH® system for
routine clinical use in guiding clinical management (7). However, few studies (4,8) have been
published in CTC detection for breast cancer prior to metastasis.
Mammaglobin-positive CTC detection has previously been performed in
women suspected of breast cancer; however, it failed to detect
intraductal cancer and 50% of in situ cancers (4). CTCs also be detected in newly diagnosed
inflammatory breast cancer using CELLSEARCH system, which result in
a proportion of 54.5% non-metastatic patients with >1 CTCs
(8).
The CELLSEARCH® System detects CTCs by
detecting the expression of epithelial cell adhesion molecules
(EpCAMs) on the tumor cell surface in combination with cytokeratins
(CKs) (9). However, CTCs may variably
lose these epithelial cell markers during epithelial-mesenchymal
transition (EMT) (3,5,10), which
can result in low sensitivity and false negatives (11). To overcome these limitations,
additional detection methods for CTCs have been proposed including
manual assays such as the CytoTrack system (12), magnetophoretic techniques (13) and microfluidic chips such as
CTC-iChip™ (2).
In the present study, a strategy of
EpCAM-independent enrichment (14)
integrated with immunostaining-fluorescence in situ
hybridization (iFISH), which was previously validated in gastric
cancer, pancreatic cancer and gliomas (3,12,13), was applied to detect CTCs in patients
with newly diagnosed breast cancer. The CTCs were subtyped based on
CK expression and ploidy analysis, and were associated with tumor
size, hormone receptor status and a number of common tumor markers,
including carcinoembryonic antigen (CEA) and cancer antigen
(CA)15-3.
Patients and methods
Patients
A total of 184 female patients (age range, between
29 and 87 years) with newly diagnosed breast cancer, 26 female
patients with benign breast tumors and 10 female healthy donors
were enrolled at Changhai Hospital (Shanghai, China) between
February 2014 and June 2015. Peripheral blood samples of the
enrolled patients with no prior treatment for breast cancer and
healthy donors were collected and evaluated in the present
study.
Written consent was provided by all patients. The
present study was approved by the Ethics Committee of Changhai
Hospital and was performed according to the Declaration of Helsinki
principles.
CTC detection
Studies were performed as previously published and
according to the manufacturer's protocol of the Cytelligen CTC
enrichment kit (cat. no. SEH-003; Cytelligen, Inc., San Diego, CA,
USA) (5,15). Briefly, patient blood samples were
collected into 7.5-ml tubes containing acid-citrate-dextrose
anti-coagulant (BD Biosciences, Franklin Lakes, NJ, USA), followed
by thorough mixing by hand and addition of 3 ml of hCTC separation
matrix (Cytelligen CTC enrichment kit). The solution was
centrifuged at 450 × g for 5 min at room temperature. Supernatants
were collected and incubated with immunomagnetic particles
conjugated to anti-leukocytes monoclonal antibodies (Cytelligen CTC
enrichment kit), including anti-cluster of differentiation (CD)45,
at room temperature for 10 min with gentle agitation. The solution
was subsequently subjected to magnetic separation using a magnetic
stand (Promega Corporation, Madison, WI, USA) to remove leukocytes.
The magnetic particle-free solution was centrifuged at 500 × g for
2 min at room temperature. Cell pellets were thoroughly resuspended
in cell fixative (Cytelligen CTC enrichment kit) and immediately
applied to the coated CTC slides at room temperature for subsequent
iFISH analysis. For CK-iFISH, samples were immunostained with a
cocktail of Alexa Fluor 594-conjugated monoclonal anti-CD45 and
Alexa Fluor 488-conjugated anti-PanCK (CK4, 5, 6, 8, 10, 13 and 18)
or anti-Her2 antibodies, all supplied in the Human Tumor Cell
Identification kit (cat. no. FSH-002; Cytelligen, Inc.) for 1 h in
the dark at 37°C. Subsequently, FISH was performed with CEP 8
SpectrumOrange (Vysis; Abbott Laboratories, Abbott Park, IL, USA)
using a S500 StatSpin ThermoBrite Slide Hybridization/Denaturation
system (Abbott Laboratories), according to the manufacturer's
protocol, with the program of denaturing at 73°C for 10 min and
hybridizing at 37°C for 4 h. CTCs were identified as
DAPI+/CD45−/PanCK+ or
Her2+ with aneuploid chromosome 8.
Statistical analysis
Statistical analysis was performed with SPSS 19.0
software (IBM SPSS, Armonk, NY, USA). Differences in CTC number
between patients with cancer, patients with benign tumors and
healthy donors were compared by Mann-Whitney U test. Positive rates
of CTC with or without CK expression were compared using Fisher's
exact test. Graphical plots were generated using OriginPro 8 SR0
version 8.0 (OriginLab, Northampton, MA, USA). All the P-values
were two-sided. P<0.05 was considered to indicate a
statistically significant difference.
Results
CTCs in breast cancer
A total of 184 patients with newly diagnosed breast
cancer, 26 patients with benign breast tumors and 10 healthy
individuals were recruited at Changhai Hospital between February
2014 and June 2015. Aneuploid CTCs were detected in 167 of 184
(90.76%) patients with breast cancer (Table I), with the number of CTCs detected
ranging between 0 and 19 cells/7.5 ml blood (median, 2 cells/7.5
ml; Table II). The total number of
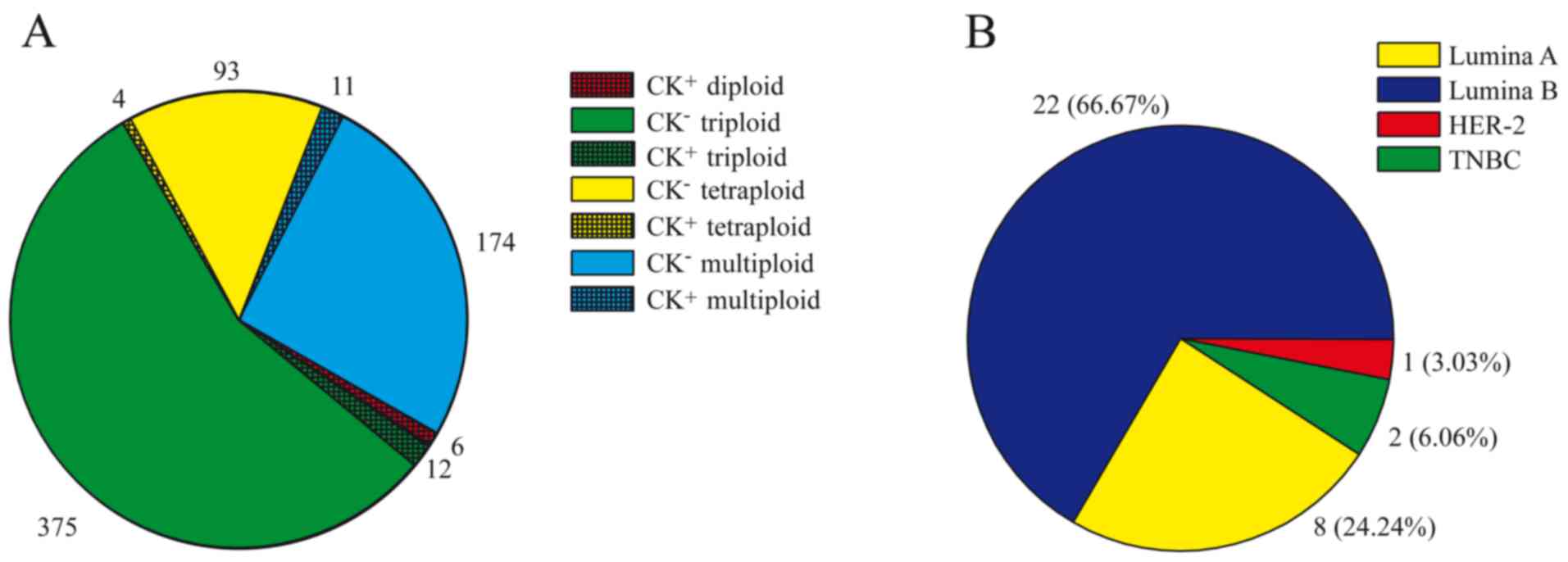
CTCs detected was 675 cells, in which 387 cells (375 cells were CK
negative and 12 cells were CK negative) were identified with
triploidy (Fig. 1A). Among the CTCs,
CK positivity was detected in only 33 (4.88%) cells from 18
patients (Fig. 1A), and CK-positive
CTCs were predominantly detected in luminal A and luminal B tumors
(Fig. 1B). Aneuploid CTCs were
detected in 8/26 benign tumors (30.77%), which was significantly
less compared with that of patients with breast cancer (P=0.007).
Furthermore, all CTC counts in benign tumors were ≤3 cells/7.5 ml.
No CTCs were identified in healthy donor blood samples (Table I).
 | Table I.Frequency of CTCs and their subtypes
in patients with breast cancera. |
Table I.
Frequency of CTCs and their subtypes
in patients with breast cancera.
|
|
| Aneuploid CTCs, n
(%) | Diploid CTCs, n
(%) | Triploid CTCs, n
(%) | Tetraploid CTCs, n
(%) | Multipoid (≥5) CTCs,
n (%) |
|---|
|
|
|
|
|
|
|
|
|---|
| Variables | Total | Total | CK+ | Total | CK+ | Total | CK+ | Total | CK+ | Total | CK+ |
|---|
| Sample type |
|
|
|
|
|
|
|
|
|
|
|
| Health
donors | 10 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Benign
tumors | 26 | 8 (30.77) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 8 (30.77) | 0 (0.00) | 2 (7.69) | 0 (0.00) | 2 (7.69) | 0 (0.00) |
| Breast
cancers | 184 | 167 (90.76) | 33 (4.88) | 5 (2.72) | 5 (2.72) | 147 (79.89) | 6 (3.26) | 68 (36.96) | 4 (2.17) | 76 (41.30) | 8 (4.35) |
| Tumor size |
|
|
|
|
|
|
|
|
|
|
|
| T1 | 80 | 73 (91.25) | 8 (10.00) | 3 (3.75) | 3 (3.75) | 66 (82.50) | 2 (2.50) | 34 (42.5) | 1 (1.25) | 33 (41.25) | 3 (3.75) |
| T2 | 68 | 63 (92.65) | 5 (7.35) | 1 (1.47) | 1 (1.47) | 52 (76.47) | 2 (2.94) | 23 (33.82) | 1 (1.47) | 24 (35.29) | 3 (4.41) |
| T3 | 10 | 9 (90.00) | 1 (10.00) | 0 (0.00) | 0 (0.00) | 9 (90.00) | 0 (0.00) | 4 (40.00) | 1 (10.00) | 8 (80.00) | 1 (10.00) |
| Molecular
typing |
|
|
|
|
|
|
|
|
|
|
|
| Luminal
A | 30 | 24 (80.00) | 5 (16.67) | 1 (3.33) | 1 (3.33) | 20 (66.67) | 2 (6.67) | 14 (46.67) | 2 (6.67) | 8 (26.67) | 2 (6.67) |
| Luminal
B | 89 | 80 (89.89) | 11 (12.36) | 4 (4.49) | 4
(4.49) | 70 (78.65) | 3 (3.37) | 28 (31.46) | 2 (2.25) | 36 (40.45) | 5 (5.62) |
|
Her2-positive | 35 | 34 (97.14) | 1 (2.86) | 0 (0.00) | 0 (0.00) | 30 (85.71) | 1 (2.86) | 16 (45.71) | 0 (0.00) | 20 (57.14) | 0 (0.00) |
|
Triple-negative | 12 | 12 (100.00) | 1 (8.33) | 0 (0.00) | 0 (0.00) | 11 (91.67) | 0 (0.00) | 4 (33.33) | 0 (0.00) | 4 (33.33) | 1 (8.33) |
| Clinical stage |
|
|
|
|
|
|
|
|
|
|
|
| I | 52 | 48 (92.31) | 6 (11.54) | 2 (3.85) | 2 (3.85) | 42 (80.77) | 1 (1.92) | 22 (42.31) | 1 (1.92) | 20 (38.46) | 3 (5.77) |
| II | 61 | 59 (96.72) | 4 (6.56) | 2 (3.28) | 2 (3.28) | 53 (86.89) | 1 (1.64) | 25 (40.98) | 1 (1.64) | 28 (45.90) | 2 (3.28) |
|
III | 39 | 34 (87.18) | 4 (10.26) | 0 (0.00) | 0 (0.00) | 28 (71.79) | 2 (5.13) | 12 (30.77) | 1 (2.56) | 16 (41.03) | 2 (5.13) |
| IV | 6 | 5 (83.33) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (50.00) | 0 (0.00) | 2 (33.33) | 0 (0.00) | 3 (50.00) | 0 (0.00) |
| Lymph node
status |
|
|
|
|
|
|
|
|
|
|
|
|
Metastasis | 80 | 73 (91.25) | 7 (8.75) | 2 (2.50) | 2 (2.50) | 64 (80.00) | 3 (3.75) | 27 (33.75) | 1 (1.25) | 36 (45.00) | 3 (3.75) |
|
Non-metastasis | 85 | 79 (92.94) | 8 (9.41) | 3 (3.53) | 3 (3.53) | 69 (81.18) | 2 (2.35) | 36 (42.35) | 2 (2.35) | 35 (41.18) | 4 (4.71) |
 | Table II.Number of CTCs and subtypes in
patients with breast cancera. |
Table II.
Number of CTCs and subtypes in
patients with breast cancera.
|
| Aneuploid CTCs,
range (median) | Diploid CTCs, range
(median) | Triploid CTCs,
range (median) | Tetraploid CTCs,
range (median) | Multipoid (≥5)
CTCs, range (median) |
|---|
|
|
|
|
|
|
|
|---|
| Variables | Counts | CK+ | Counts | CK+ | Counts | CK+ | Counts | CK+ | Counts | CK+ |
|---|
| Sample type |
|
|
|
|
|
|
|
|
|
|
| Health
donors | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Benign
tumors | 0–3 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0–3 (0.0) | 0 (0.0) | 0–1 (0.0) | 0 (0.0) | 0–2 (0.0) | 0 (0.0) |
| Breast
cancers | 0–19 (2.0) | 0–6 (0.0) | 0–2 (0.0) | 0–2 (0.0) | 0–18 (1.0) | 0–5 (0.0) | 0–4 (0.0) | 0–1 (0.0) | 0–15 (1.0) | 0–6 (1.0) |
| Tumor size |
|
|
|
|
|
|
|
|
|
|
| T1 | 0–19 (3.0) | 0–2 (0.0) | 0–2 (0.0) | 0–2 (0.0) | 0–10
(1.5)b | 0–1 (0.0) | 0–3 (0.0) | 0–1 (0.0) | 0–5 (0.0) | 0–2 (0.0) |
| T2 | 0–19 (2.0) | 0–6 (0.0) | 0–1 (0.0) | 0–1 (0.0) | 0–18
(1.0)c | 0–5 (0.0) | 0–4 (0.0) | 0–1 (0) | 0–10 (0.0) | 0–1 (0.0) |
| T3 | 0–18 (5.5) | 0–2 (0.0) | 0 (0.0) | 0 (0.0) | 0–10 (4.0) | 0 (0.0) | 0–2 (0.0) | 0–1 (0.0) | 0–6 (1.0) | 0–1 (0.0) |
| Molecular
typing |
|
|
|
|
|
|
|
|
|
|
| Luminal
A | 0–18
(2.0)d | 0–3 (0.0) | 0–1 (0.0) | 0–1 (0.0) | 0–10 (1.0) | 0–2 (0.0) | 0–3 (0.0) | 0–1 (0.0) | 0–6 (0.0) | 0–1 (0.0) |
| Luminal
B | 0–19
(2.0)e | 0–6 (0.0) | 0–2 (0.0) | 0–2 (0.0) | 0–18 (1.0) | 0–5 (0.0) | 0–4 (0.0) | 0–1 (0.0) | 0–15 (0.0) | 0–3 (0.0) |
|
Her2-positive | 0–15 (4.0) | 0–1 (0.0) | 0 (0.0) | 0 (0.0) | 0–8 (2.0) | 0–1 (0.0) | 0–4 (0.0) | 0 (0.0) | 0–10 (1.0) | 0 (0.0) |
|
Triple-negative | 1–12 (2.5) | 0–2 (0.0) | 0 (0.0) | 0 (0.0) | 0–8 (1.0) | 0 (0.0) | 0–3 (0.0) | 0 (0.0) | 0–3 (0.0) | 0–2 (0.0) |
| Clinical stage |
|
|
|
|
|
|
|
|
|
|
| I | 0–12 (3.0) | 0–2 (0.0) | 0–1 (0.0) | 0–1 (0.0) | 0–8 (1.5) | 0–1 (0.0) | 0–3 (0.0) | 0–1 (0.0) | 0–5 (0.0) | 0–2 (0.0) |
| II | 0–19 (2.0) | 0–6 (0.0) | 0–2 (0.0) | 0–2 (0.0) | 0–18 (1.0) | 0–5 (0.0) | 0–4 (0.0) | 0–1 (0.0) | 0–10 (0.0) | 0–1 (0.0) |
|
III | 0–18 (2.0) | 0–2 (0.0) | 0 (0.0) | 0 (0.0) | 0–10 (1.0) | 0–2 (0.0) | 0–2 (0.0) | 0–1 (0.0) | 0–6 (0.0) | 0–1 (0.0) |
| IV | 0–8 (3.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0–5 (0.0) | 0 (0.0) | 0–1 (0.0) | 0 (0.0) | 0–2 (0.0) | 0 (0.0) |
| Lymph node
status |
|
|
|
|
|
|
|
|
|
|
|
Metastasis | 0–19 (3.0) | 0–3 (0.0) | 0–2 (0.0) | 0–2 (0.0) | 0–10 (1.5) | 0–2 (0.0) | 0–4 (0.0) | 0–1 (0.0) | 0–6 (0.0) | 0–1 (0.0) |
|
Non-metastasis | 0–19 (2.0) | 0–6 (0.0) | 0–1 (0.0) | 0–1 (0.0) | 0–18 (1.0) | 0–5 (0.0) | 0–4 (0.0) | 0–1 (0.0) | 0–10 (0.0) | 0–2 (0.0) |
Analysis was performed by examining the association
between breast cancer CTC counts and common tumor markers,
including CEA, CA15-3, CA125, Ki-67, topoisomerase II and p53.
Overall, no significant associations were observed between CTC
counts and the tumor markers surveyed (data not shown). The
relevance of lymph node status, clinical stage and CTC of patients
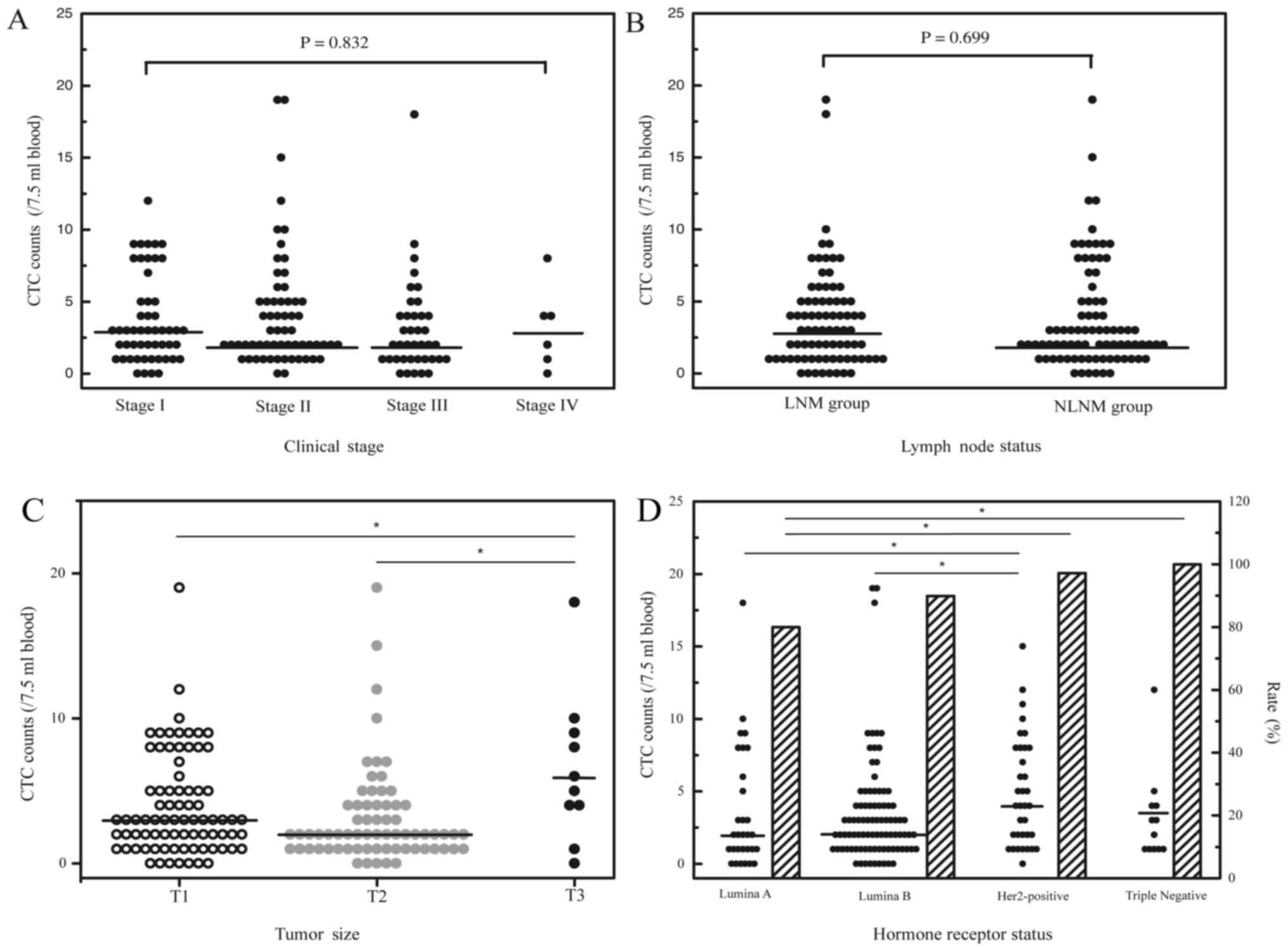
with breast cancer was also analyzed. Generally, no significant
associations were observed between CTC count and clinical stage
(Fig. 2A) or lymph node status
(Fig. 2B).
CTC and tumor size
Of the 184 breast cancer patient samples examined in
total, 158 had tumor size data available. Among this cohort, 80
were ≤2 cm (T1), 68 were between >2 and ≤5 cm (T2) and 10 were
>5 cm (T3). Despite the small sample size of T3 patients, the
highest CTC counts were detected in this group (range, 0–18
cells/7.5 ml blood; median, 5.5 cells/7.5 ml; Fig. 2C). Triploid CTC counts were notably
different between T3 and T1/T2 patients, with a median number of 4
cells/7.5 ml blood in T3 patients and a median number of 1.5
cells/7.5 ml blood and 1 cells/7.5 ml blood in T1 and T2 patients,
respectively (P=0.048 and P=0.006; Table
II). CTC subtypes based on CK expression were not significantly
different among T1, T2 and T3 patients (Table II).
CTC and molecular subtyping of breast
cancer
Of the 184 patients with breast cancer, 165 had
hormone receptor status data available, of which 30 were luminal A
(ER+/PR+, Her2− and Ki67 <14%),
89 were luminal B (ER+/PR+, Her2−
and Ki67 ≥14%; or ER+/PR+ and
Her2+), 35 were Her2-positive (ER−,
PR− and Her2+) and 12 were triple-negative
(ER−, PR− and Her2−). CTCs were
detected in 24 of the 30 luminal A patients (80.0%; range, 0–18
cells/7.5 ml; median, 2 cells/7.5 ml), 80 of 89 luminal B patients
(89.9%; range, 0–19 cells/7.5 ml; median, 2 cells/7.5 ml), 34 of 35
Her2-positive patients (97.1%; range, 0–15 cells/7.5 ml; median, 4
cells/7.5 ml) and 12 of 12 triple-negative patients (100%; range,
1–12 cells/7.5 ml; median, 2.5 cells/7.5 ml). Patients with
Her2-positive or triple-negative breast cancers had the highest
frequency and total number of CTCs compared with those in luminal A
or luminal B patients (Fig. 2D;
Tables I and II).
CTC subtypes based on karyotype, including triploid,
tetraploid and multiploid (≥5), exhibited a similar distribution in
patients with breast cancer with differing hormone receptor status
(Table I). However, CK positivity was
detected in 22 (22/363, 6.06%) cells from patients with luminal A
or luminal B tumors, which was significantly less compared with
that from patients with Her2-positive or triple-negative breast
cancers (3/199, 1.51%; P=0.017; Table
III).
 | Table III.Different CK expression rate in
breast cancer subtypes. |
Table III.
Different CK expression rate in
breast cancer subtypes.
| Molecular
typing | Total CTC
counts | CK+ CTC
counts | Expression rate,
frequency (%) |
|---|
| Luminal A | 144 | 6 | 22/363 (6.06) |
| Luminal B | 219 | 16 |
|
| Her2-positive | 166 | 1 | 3/199 (1.51) |
|
Triple-negative | 33 | 2 |
|
Discussion
In the present study, CTCs were identified in
patients with newly diagnosed breast cancer and were revealed to be
associated with aneuploidy status by the iFISH system, in addition
to tumor surface markers. Although the breast cancers examined were
not metastatic with the likely possibility of a low and difficult
to detect frequency of CTCs, the present study revealed a
relatively higher sensitivity (90.76%) in CTC detection of breast
cancer compare with the sensitivity of 54.5% in a previously study
(8), in which CTC were detected using
CELLSEARCH® system. The high sensitivity of CTC
detection in breast cancer were identical to that of other solid
tumors evaluated with the iFISH system, including gastric (90.5%)
(15), lung (92%) and esophageal
(87%) cancer (5). In a study of CTCs
detected in pancreatic cancer by the same system, 0–2 cells/3.75 ml
were detected in benign pancreatic tumors and healthy controls
(16). Similarly, 0–3 cells/7.5 ml
were detected in benign breast tumors in the present study.
In the present study, the highest rate (9/10, 90%)
and CTC counts (0–18 cells/7.5 ml, median, 5.5 cells/7.5 ml) were
observed in patients with the greatest tumor size (T3), indicating
that tumor size is associated with CTC production. Primary tumor
size is a credible predictor of breast cancer metastasis (7,9,17), as corroborated by the findings of the
present study. Patients with Her2-positive or triple-negative
tumors were also revealed to have more CTCs in rate and counts
compared with those in luminal A or luminal B patients.
Her2-positive or triple-negative breast cancers carry a worse
prognosis with an increased risk of metastasis, compared with
luminal A or luminal B patients (18–21). The
results of the present study indicated that patients with highly
aggressive disease have increased CTCs.
A large proportion (57.25% of CTCs detected, and 88%
of patients involved) of aneuploid CTCs detected were of triploid
subtype. In previous studies, triploid CTCs were detected
frequently in gastric (61.2% of patients) (15), lung (41.9% of CTCs) and esophageal
cancer (37.6% of CTCs) (17). It was
hypothesized that the ratio of triploid CTCs in patients prior to
treatment may reciprocally correlate with the chemotherapeutic
efficacy (15). In the present study,
the most significant difference between T3 and T1/T2 patients was
the frequency of triploid CTCs, indicating that triploid CTCs may
serve a role in tumor progression and treatment.
Among the 167 CTC-positive patients in the present
study, CK-positive CTCs were detected in a small cohort of
individuals (18/167, 10.78%). CK expression on the cell surface of
primary tumors may degrade or become absent in CTCs due to EMT
(3,5,10). In the
present study, the iFISH system could identify ploidy status in
CK-negative aneuploid CTCs in contrast to immunostaining alone. A
small rate of patients with CK-positive CTCs were detected in a
study evaluating pancreatic (4/22) (16), lung (8/24) and esophageal (4/13)
cancer (5). These results suggested
that CTC identification by staining of CKs alone may result in a
relatively low frequency and CTC counts.
CTCs with CK positivity were mainly detected in
patients with luminal A or luminal B tumors, indicating that almost
all CTCs with epithelial features were detected in patients with
ER/PR-positive tumors. Epithelial features were absent in CTCs
detected in Her2-positive patients, presumably due to EMT. These
results are in agreement with the conclusion of a prior study
showing that CTCs from patients with HER2+ breast
cancers were predominantly mesenchymal (22). CK expression in triple-negative tumor
CTCs requires additional study and a larger sample population.
Expression of PR indicates a functional ERα (one of the two
isoforms of ER) and ERα pathway (23), which increases E-cadherin expression
by downregulating transcriptional repressors (24,25).
Patients with Her2-positive tumors lack ER and PR expression, which
decreases the level of E-cadherin, and enhances the possibility of
EMT and tumor cell invasion.
In patients with non-metastatic breast cancer,
aneuploid CTCs independent of CK expression status can be detected
by the iFISH system. Presence of CTCs and CTC counts was associated
with tumor size and hormone receptor status in patients with breast
cancer. Triploid CTCs constituted the majority of CTCs detected in
all the patients with breast cancer evaluated regardless of hormone
receptor status and tumor size. In addition, CK-positive CTCs were
identified in a small cohort of patients and were detected at a low
rate in CTC counts. Notably, CK expression was rare in CTCs from
Her2-positive or triple-negative patients, supporting the
hypothesis that lack of ER and PR may promote EMT and enhance tumor
aggression.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81502546).
References
|
1
|
McGuire S: World Cancer Report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xue P, Wu Y, Guo J and Kang Y: Highly
efficient capture and harvest of circulating tumor cells on a
microfluidic chip integrated with herringbone and micropost arrays.
Biomed Microdevices. 17:392015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mikolajczyk SD, Millar LS, Tsinberg P,
Coutts SM, Zomorrodi M, Pham T, Bischoff FZ and Pircher TJ:
Detection of EpCAM-negative and cytokeratin-negative circulating
tumor cells in peripheral blood. J Oncol. 2011:2523612011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murray NP, Miranda R, Ruiz A and Droguett
E: Diagnostic yield of primary circulating tumor cells in women
suspected of breast cancer: The BEST (Breast Early Screening Test)
study. Asian Pac J Cancer Prev. 16:1929–1934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ge F, Zhang H, Wang DD, Li L and Lin PP:
Enhanced detection and comprehensive in situ phenotypic
characterization of circulating and disseminated heteroploid
epithelial and glioma tumor cells. Oncotarget. 6:27049–27064. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harris L, Fritsche H, Mennel R, Norton L,
Ravdin P, Taube S, Somerfield MR, Hayes DF and Bast RC Jr; American
Society of Clinical Oncology, : American Society of Clinical
Oncology 2007 update of recommendations for the use of tumor
markers in breast cancer. J Clin Oncol. 25:5287–5312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen M, Palleschi S, Khoynezhad A,
Gecelter G, Marini CP and Simms HH: Role of primary breast cancer
characteristics in predicting positive sentinel lymph node biopsy
results: A multivariate analysis. Arch Surg. 137:606–610. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mego M, Giordano A, De Giorgi U, Masuda H,
Hsu L, Giuliano M, Fouad TM, Dawood S, Ueno NT, Valero V, et al:
Circulating tumor cells in newly diagnosed inflammatory breast
cancer. Breast Cancer Res. 17:22015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yi M, Krishnamurthy S, Kuerer HM,
Meric-Bernstam F, Bedrosian I, Ross MI, Ames FC, Lucci A, Hwang RF
and Hunt KK: Role of primary tumor characteristics in predicting
positive sentinel lymph nodes in patients with ductal carcinoma in
situ or microinvasive breast cancer. Am J Surg. 196:81–87. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alix-Panabières C and Pantel K:
Circulating tumor cells: Liquid biopsy of cancer. Clin Chem.
59:110–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sheng Y, Wang T, Li H, Zhang Z, Chen J, He
C, Li Y, Lv Y, Zhang J, Xu C, et al: Comparison of analytic
performances of Cellsearch and iFISH approach in detecting
circulating tumor cells. Oncotarget. 8:8801–8806. 2017.PubMed/NCBI
|
|
12
|
Hillig T, Horn P, Nygaard AB, Haugaard AS,
Nejlund S, Brandslund I and Sölétormos G: In vitro detection of
circulating tumor cells compared by the CytoTrack and CellSearch
methods. Tumour Biol. 36:4597–4601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Min H, Jo SM and Kim HS: Efficient capture
and simple quantification of circulating tumor cells using quantum
dots and magnetic beads. Small. 11:2536–2542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu C, Hao H, Li L, Zhou X, Guo Z, Zhang L,
Zhang X, Zhong W, Guo H, Bremner RM and Lin P: Preliminary
investigation of the clinical significance of detecting circulating
tumor cells enriched from lung cancer patients. J Thorac Oncol.
4:30–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Zhang X, Ge S, Gao J, Gong J, Lu M,
Zhang Q, Cao Y, Wang DD, Lin PP and Shen L: Clinical significance
of phenotyping and karyotyping of circulating tumor cells in
patients with advanced gastric cancer. Oncotarget. 5:6594–6602.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Wang F, Ning N, Chen Q, Yang Z,
Guo Y, Xu D, Zhang D, Zhan T and Cui W: Patterns of circulating
tumor cells identified by CEP8, CK and CD45 in pancreatic cancer.
Int J Cancer. 136:1228–1233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao Y, Paner GP and Rajan PB: Sentinel
node status and tumor characteristics: A study of 234 invasive
breast carcinomas. Arch Pathol Lab Med. 129:82–84. 2005.PubMed/NCBI
|
|
18
|
Allred DC, Clark GM, Tandon AK, Molina R,
Tormey DC, Osborne CK, Gilchrist KW, Mansour EG, Abeloff M, Eudey
L, et al: HER-2/neu in node-negative breast cancer: Prognostic
significance of overexpression influenced by the presence of in
situ carcinoma. J Clin Oncol. 10:599–605. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sjögren S, Inganäs M, Lindgren A, Holmberg
L and Bergh J: Prognostic and predictive value of c-erbB-2
overexpression in primary breast cancer, alone and in combination
with other prognostic markers. J Clin Oncol. 16:462–469. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Voduc KD, Cheang MC, Tyldesley S, Gelmon
K, Nielsen TO and Kennecke H: Breast cancer subtypes and the risk
of local and regional relapse. J Clin Oncol. 28:1684–1691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui X, Schiff R, Arpino G, Osborne CK and
Lee AV: Biology of progesterone receptor loss in breast cancer and
its implications for endocrine therapy. J Clin Oncol. 23:7721–7735.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomas C and Gustafsson JÅ: The different
roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer.
11:597–608. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye Y, Xiao Y, Wang W, Yearsley K, Gao JX,
Shetuni B and Barsky SH: ERalpha signaling through slug regulates
E-cadherin and EMT. Oncogene. 29:1451–1462. 2010. View Article : Google Scholar : PubMed/NCBI
|