Introduction
Alzheimer's disease (AD) is a degenerative disorder
characterized histopathologically by amyloid β (Aβ) aggregations,
neurofibrillary tangles and neuronal impairment finally leading to
progressive loss of recent and episodic memory and other cognitive
functions (1). Compared with males,
females are more susceptible to suffer from AD (2). At present, therapeutic method for
treating AD mainly include increasing the concentration of
acetylcholine and other neurotransmitters such as glutamate, while
also combining other pharmacological and non-pharmacological
strategies for treatment of the AD associated comorbidities to
alleviate symptom burden and improve life quality, but still have
no practical significance for the progression of AD (3).
Recent studies have shown that mesenchymal stem
cells (MSCs) as a promising therapeutic strategy for AD therapy
(4,5).
MSCs are multi-potent progenitor cells with ability to
differentiate into multiple lineages that are mainly derived from
the three sources including bone marrow, adipose tissue, and the
umbilical cord. Bone marrow MSCs (BM-MSCs) have properties of
self-renewal, rapid proliferation, immunosuppression, low
immunogenicity, pluripotency and tissue regeneration and repair
ability, which can also increase the number of positive cells for
choline acetyltransferase and survivin expression (4,6).
Furthermore, human MSCs were able to improve memory by reducing the
level of Aβ in the hippocampus and Aβ deposition through the
activation of M2-like microglia and modulation neuroinflammation in
an AβPP/PS1 transgenic AD mouse model (7). Moreover, human MSCs enhanced Aβ
immunoreactivity and autophagosome induction, decreased
intracellular Aβ levels, promote Aβ clearance in AD models, which
may lead to increased neuronal survival against Aβ toxicity
(8). Recent work had confirmed that
the potential therapeutic role of BMMSCs against AD through their
anti-apoptotic, neurogenic and immunomodulatory properties in the
experimental AD model, however, the molecular mechanisms are
largely unclear.
Selective Alzheimer's disease indicator-1
(Seladin-1) is a specific AD marker and is an important
neuroprotective effector. A large number of studies have proven
that Seladin-1 is closely linked to neuronal degeneration in AD,
which is downregulated in brain regions affected by AD (9,10). Nestin
is usually used as a marker of neural stem and progenitor cells. It
is expressed in astrocytes and neurons in AD patient and other
neurodegenerative disease (11). In
addition, nestin transgenic was used to establish AD animal models,
and the number of nestin cells showed statistically significant
decrease in the dentate gyrus of the hippocampus within the AD
groups when compared with the controls (12). Collectively, these data strongly
support the idea that Seladin-1 and nestin play pivotal roles in AD
pathogenesis, although the exact molecular mechanisms still need to
be further elucidated to facilitate better treatment for AD.
The purpose of the current work was to determine the
roles of BM-MSCs transplantation in AD model and explore the
underlying molecular mechanism. The expression of Seladin-1 and
nestin was detected using quantitative polymerase chain reaction
(qPCR) and western blot analysis, respectively. We also used the
phosphoinositide 3-kinase (PI3K) inhibitor and extracellular
signal-regulated kinase (ERK)1/2 inhibitor (LY294002, PD98059) to
explore the molecular mechanism. We sought to test the hypothesis
that BM-MSCs transplantation regulates Seladin-1 and nestin
expression through a mechanism associated with activating the
PI3K/protein kinase B (Akt) and ERK1/2 signaling pathways.
Materials and methods
Animals and experimental groups
All experiments were performed out accordance with
the guidelines of the National Institutes of Health for Care and
Use of Laboratory Animals and approved by the Animal Welfare
Committee of Tangdu Hospital, Fourth Military Medical University.
Sprague-Dawley female rats (body weight 130–150 g) were acquired
from the Experimental Animal Center of the Fourth Military Medical
University (Xi'an, China). The animals were housed at constant
temperature at 20–22°C and humidity at 40–60%, with a 12-h day and
dark illumination cycle and free access to food and water. All
animals were randomized into six groups (n=8 per group) as follow:
Group 1, rats as a healthy control group; Group 2, rats as
Aluminium chloride induced AD (17 mg/kg b. wt daily for 75 days
according to previous report (13);
Group 3, induced Alzheimer rats that received BM-MSCs only; Group
4, induced Alzheimer rats that received BM-MSCs and LY294002; Group
5, induced Alzheimer rats that received BM-MSCs and PD98059; and
Group 6, induced Alzheimer rats that received BM-MSCs, LY294002 and
PD98059 treatment group.
Rats in the control group received a lateral
ventricular injection of sterile saline (1 ml). As previously
described (14,15), 1 µg LY294002 (Selleck Chemicals in
China, Shanghai, China) and/or 2 µg PD98059 (Selleck Chemicals,
Shanghai, China) or phosphate-buffered saline (PBS) 10 min were
administered a lateral ventricular injection in the rats after
treatment with aluminum chloride. The volume of 2 µl was injected
in each rat. A single dose of BM-MSCs (3×106 cells/rat)
(16) was slowly injected into rats
by the tail vein in 5 min with a 27G needle.
Primary culture of BM-MSCs
The femoral bones were harvested from 4 donor male
rats, and the medullary cavity was bathed with the Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Bone marrow cells were cultured in DMEM
low glucose (21885-108; Gibco; Thermo Fisher Scientific, Inc.) with
10% fetal calf serum (SV30160.03; HyClone; GE Healthcare Life
Sciences, Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin (15070-063; Gibco; Thermo Fisher Scientific, Inc.)
(37°C, 5% CO2 for 48 h), and this culture medium were
changed every 3 days. When the adherent cells reached 70–80%
confluence, the cells were passaged at a ratio of 1:2 with 0.25%
trypsin (contained 0.02% EDTA). Identity was confirmed by flow
cytometry for MSC-specific markers (the expression of CD105, CD73
and CD90 was more than 95%, whereas the expression of CD34, CD45,
CD31 and CD146 was 2% or less. The fourth generation was used for
transplantations in the experiments.
RT-PCR
Total RNA was extracted from hippocampus tissue of
female rats using TRIzol (Invitrogen, Thermo Fisher Scientific,
Inc.). cDNA was synthesized from 1 µg of total RNA from each sample
using RevertAidTM First Strand cDNA Synthesis kit (no. K1621;
Fermentas, Thermo Fisher Scientific, Inc.). The primers used for
Seladin-1 were forward, 5′-GGGTGTTTGTGTGCCTCTTCC-3′ and reverse,
5′-GCTCCTTCCACTCCCGTACC-3′; for nestin were forward,
5′-TGGAGCGGGAGTTAGAGGCT-3′, and reverse,
5′-ACCTCTAAGCGACACTCCCGA-3′; and for β-actin were forward,
5′-CTGTCTGGCGGCACCACCAT-3′, and reverse, 5′-GCAACTAAGTCATAGTCCGC-3
(17). RT-PCR was performed in a
Bio-Rad IQ™ system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
using SYBR-Green qPCR analysis. A total of 40 PCR cycles were used
for amplification of Seladin-1, nestin, and β-actin. We used ΔCq
method to estimate the relative quantification of Seladin-1 and
nestin mRNA. The β-actin amplification levels were used as
reference to normalize the relative quantification of the target
gene.
Western blot analysis
Hippocampus tissue were homogenized and lysed with
tissue protein lysis solution. Protein concentrations were
determined using BCA kit. Standard samples were fractionated on a
10% SDS-PAGE gel, and then transferred onto polyvinylidene
difluoride (PVDF) membranes and blocked with 5% bovine serum
albumin (BSA) at room temperature for 2 h. Followed by incubation
with Seladin-1 (2033S; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), nestin (4760S; Santa Cruz Biotechnology, Inc.), Akt (1085S;
Santa Cruz Biotechnology, Inc.), p-Akt (10001S; Santa Cruz
Biotechnology, Inc.), ERK1/2 (1240S; Santa Cruz Biotechnology,
Inc.), p-ERK1/2 (1150S; Santa Cruz Biotechnology, Inc.), and
β-actin ((13E5) Rabbit mAb (Alexa Fluor®594 Conjugate)
9470S; Santa Cruz Biotechnology, Inc.) (all antibody were 1:1,000
diluted) overnight at 4°C followed by incubation with secondary
antibody (1:5,000 diluted; Cell Signaling Technology, Inc.,
Danvers, MA, USA). Signal was visualized with enhanced
chemiluminescence (ECL) reagent in the Tanon 4500 imaging system
and the band intensities were quantified using ImageJ software.
Normalization was based on the protein level of β-actin.
Statistical analysis
All data are presented as the mean ± standard
deviation. Data were tested for normality used Kolmogorov-Smirnov
test. The equality of variances between the groups was assessed
used Levene's test. For statistical analysis, significant
differences were analyzed using a one-way ANOVA analysis followed
by the Tukey post-hoc comparisons (SPSS 17.0 software; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
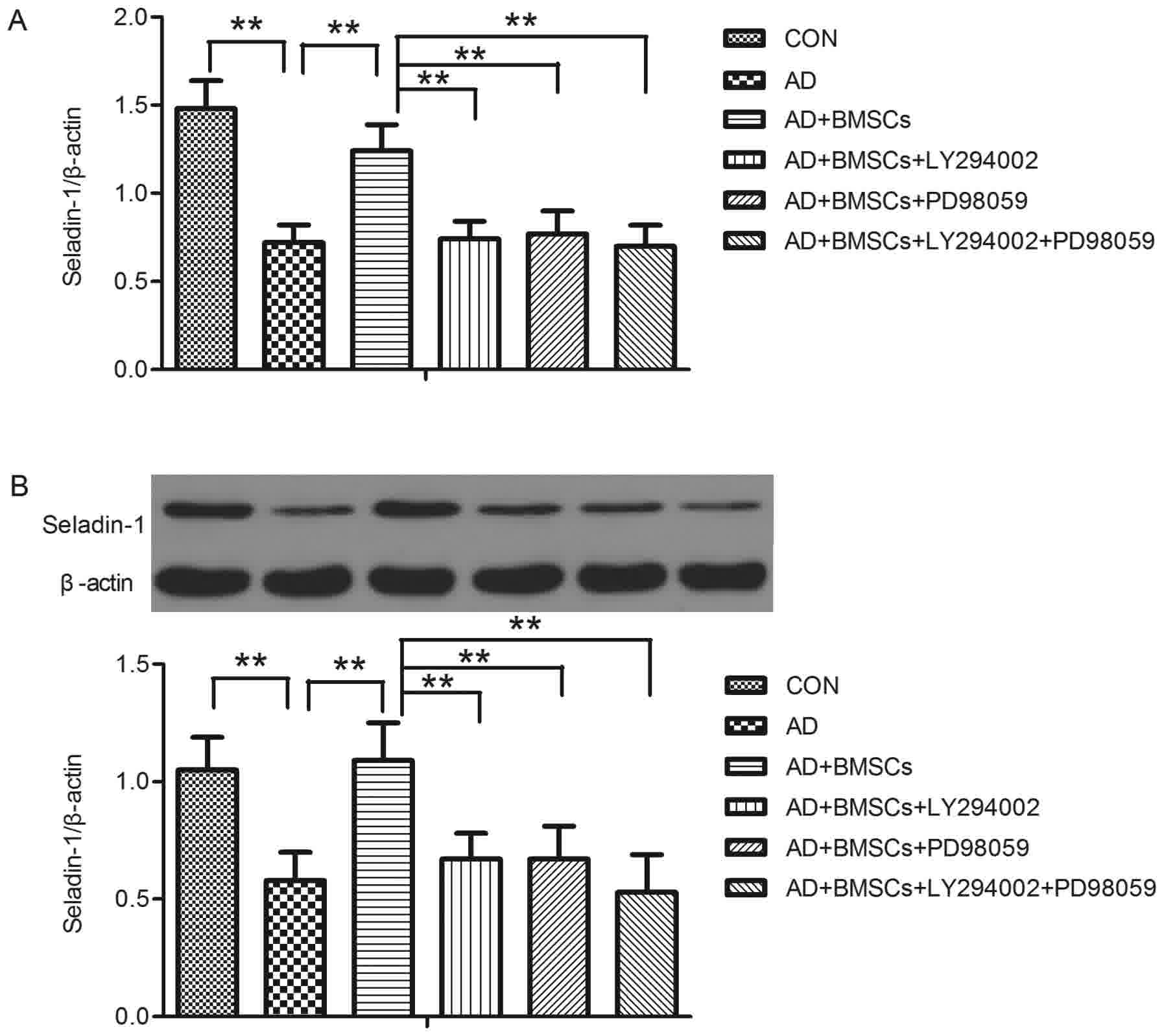
Molecular study of Seladin-1 gene
expression and protein expressions in AD model
The results of RT-PCR showed that Seladin-1 gene
level showed significant difference between groups [F(5,42)=55.046,
P<0.001] (Fig. 1A). Tukey post hoc
test revealed that Seladin-1 mRNA level was lower in the AD group
compared to that in the control group (P<0.001, AD vs. CON
group). After BM-MSCs transplantation, the Seladin-1 mRNA level was
significantly increased (P<0.001, BM-MSCs vs. AD group). When
treated with a PI3K and p-ERK1/2 inhibitor, LY294002, PD98059 or
combination the two inhibitors, Seladin-1 mRNA level was
significantly decreased (all P<0.001, LY294002, PD98059 or
LY294002 + PD98059 vs. BM-MSCs group, respectively). Furthermore,
we used western blot analysis to detect the change of Seladin-1
protein expression, and the results showed as that of Seladin-1
mRNA. There were significant differences of Seladin-1 protein
expression between groups [F=24.508 (5,42), P<0.001] (Fig. 1B). After Tukey post-hoc test, the AD
group showed a significant decrease in brain Seladin-1 protein
expression in comparison with the control group (P<0.001, AD vs.
CON group), BM-MSCs transplantation significantly increased
Seladin-1 protein expression in comparison with the AD group
(P<0.001, BM-MSCs vs. AD group), but treatment of the AD group
with LY294002, PD98059 or LY294002+ PD98059 significantly decreased
brain Seladin-1 protein expression respectively in comparison with
the BM-MSCs group (all P<0.001, LY294002, PD98059 or LY294002 +
PD98059 vs. BM-MSCs group, respectively).
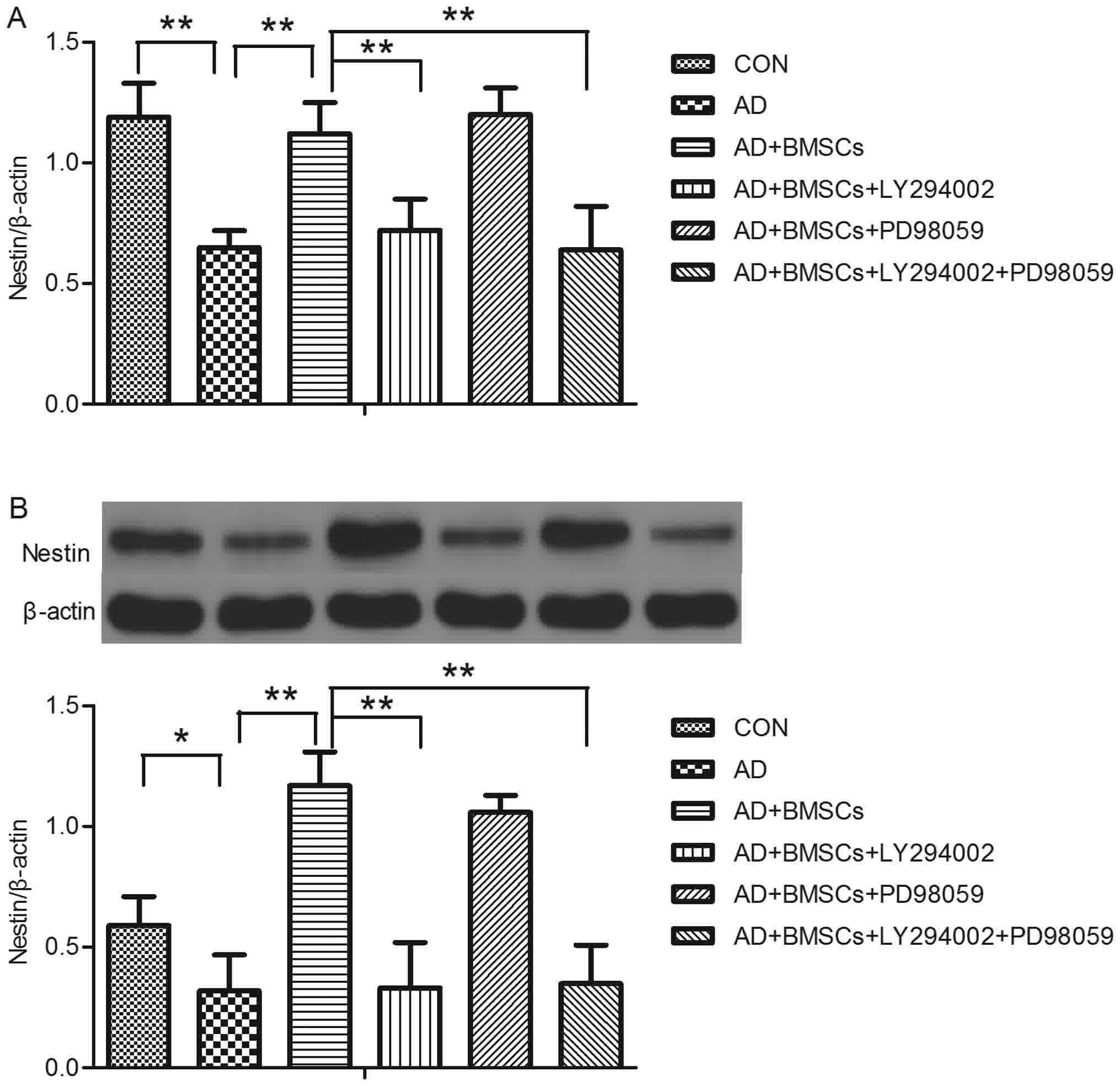
Molecular study of nestin gene and
protein expressions in AD model
Fig. 2 represents the
effect of treatment with LY294002, PD98059, or BM-MSCs on brain
nestin gene and protein expression in AD model. There was
significant difference on the nestin gene level between groups
[F(5,42)=35.852, P<0.001]. Tukey post hoc test showed that
nestin mRNA level was lower in the AD group compared to that in the
control group (P<0.001, AD vs. CON group). After BM-MSCs
transplantation, the nestin mRNA level was significantly increased
(P<0.001, BM-MSCs vs. AD group). When treated with a PI3K
inhibitor, LY294002, nestin mRNA level was significantly decreased
(all P<0.001, LY294002, or LY294002 + PD98059 group vs. BM-MSCs,
respectively), while there was no effect of PD98059 on the nestin
mRNA level (P>0.05, PD98059 vs. BM-MSCs group). The expression
of nestin was analyzed using western blot analysis, and the result
showed that there was significant difference of nestin protein
expression between groups [F=57.439 (5,42), P<0.001]. After
Tukey post-hoc test, the AD group showed a significant decrease in
the nestin protein expression in comparison with the control group
(P=0.007, AD vs. CON group), BM-MSCs transplantation significantly
increased nestin protein expression in comparison with the AD group
(P<0.001, BM-MSCs vs. AD group), treatment of the AD group with
LY294002 or LY294002 + PD98059 significantly decreased in the
nestin protein expression respectively in comparison with the
BM-MSCs group (all P<0.001, LY294002 or LY294002 + PD98059 vs.
BM-MSCs group, respectively), but there was no effect of PD98059 on
the nestin protein expression (P>0.05, PD98059 vs. BM-MSCs
group).
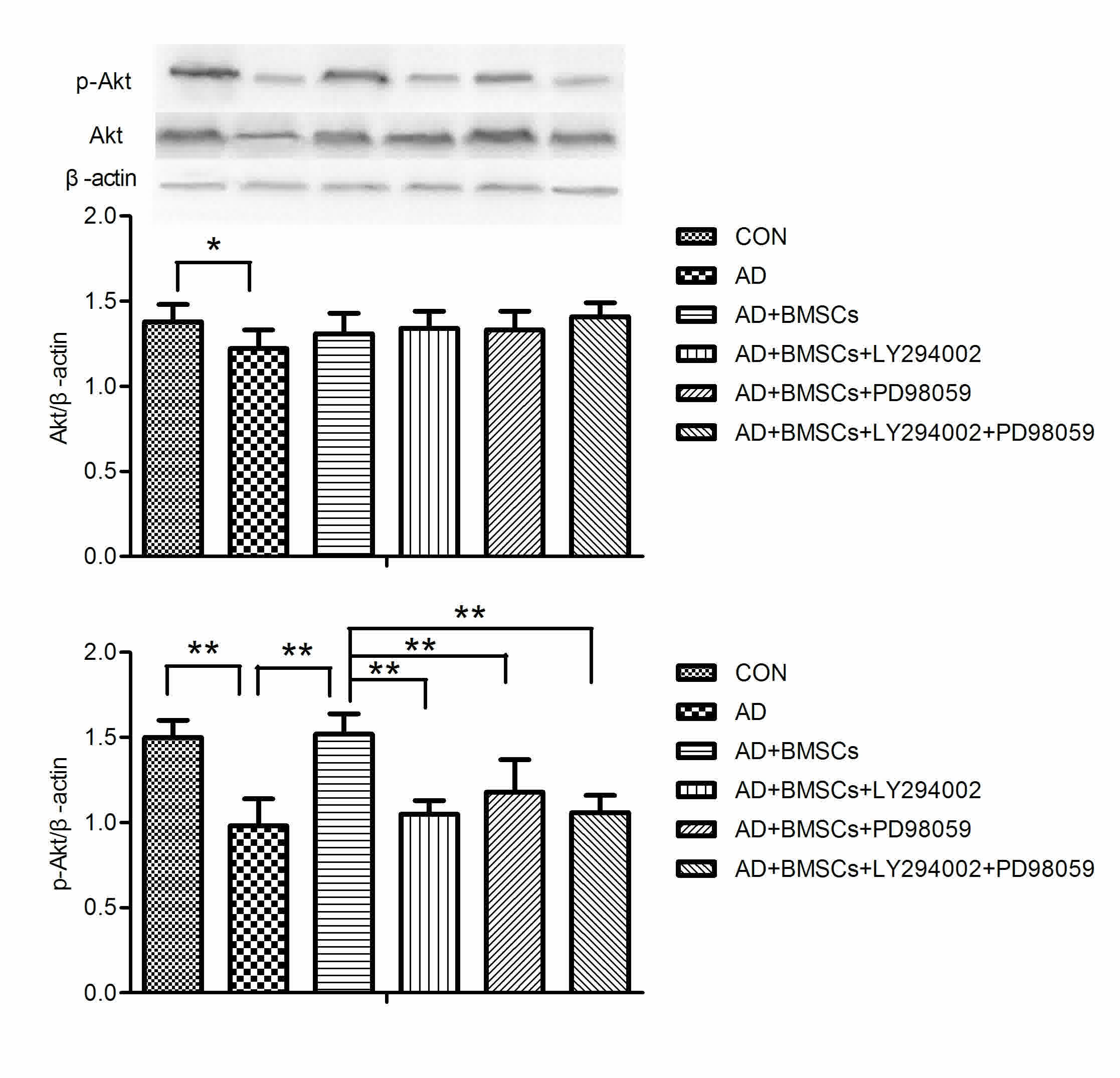
Activation of PI3K/Akt pathway after
BM-MSCs transplantation in AD model
As shown in Fig. 3,
there was significant difference on the Akt and p-Akt protein
expression between groups [F(5,42)=2.949, P=2.949; F(5,42)=26.237,
P<0.001]. Tukey post-hoc test showed that Akt and p-Akt protein
expression was lower in the AD group in comparison with the control
group (P=0.004, P<0.001 respectively, AD vs. CON group). BM-MSCs
transplantation significantly enhanced p-Akt protein expression in
comparison with the AD group (P<0.001, BM-MSCs vs. AD group).
PD98059 or/and LY294002 treatment dramatically inhibited p-Akt
expression (all P<0.001, LY294002, PD98059 or LY294002 + PD98059
vs. BM-MSCs group).
Activation of ERK1/2 pathway after
BM-MSCs transplantation in AD model
Fig. 4 showed that
there was significant difference on the p-ERK1/2 protein expression
between groups [F(5,42)=18.812, P<0.001]. Tukey post-hoc test
showed that p-ERK1/2 protein expression was lower in the AD group
in comparison with the control group (P<0.001, AD vs. CON
group). BM-MSCs transplantation significantly increased p-ERK1/2
protein expression in comparison with the AD group (P<0.001,
BM-MSCs vs. AD group). LY294002 treatment significantly attenuated
p-ERK1/2 expression (P<0.001, LY294002 vs. BM-MSCs group). There
was no significant difference of p-ERK1/2 expression between
PD98059 or LY294002 + PD98059 and BM-MSCs group (all P>005,
PD98059 or LY294002 + PD98059 vs. BM-MSCs group).
Discussion
Aluminum chloride was widely used as an inductor for
AD model (18,19). Aluminum exposure significantly lowered
the acetylcholinesterase activity in the crude synaptosomal
fraction of cortex, hippocampus, thalamus and nucleus caudatus in
Mongolian gerbils comparing to the control group (20). The in vitro experiment also
indicated that aluminum chloride was potent enough to significantly
inhibit acetylcholinesterase (21).
When the cultured human lymphocytes were treated with 5, 10, 15 and
25 µM concentrations of aluminum, all phases of the cell cycle
resulted in polyploidy and endoreduplication and DNA damage
(22). These data indicate that AlCl3
is a valid inductor for AD model.
In the present study we demonstrated that the
expression of Seladin-1 gene and protein was decreased in the
AlCl3-induced AD model compared with that in the control rats.
After a single dose of BM-MSCs (3×106 cells/rat) into
the hippocampus, the expression of Seladin-1 gene and protein was
found to be enhanced, supporting the data of Abdel Aziza et
al (23) who reported that
BM-MSCs exert beneficial effects in AD rats possibly through
decreasing the brain cholesterol level and increasing Seladin-1
gene expression. Moreover, the results of the therapeutic potential
of comparing BM-MSCs with 2 conventional therapies (Rivastigmine
and Cerebrolysin) of AD also indicated that BM-MSCs exerted the
potential therapeutic role via enhancing the Seladin-1 and
nestin gene expression (19). Nestin
is an intermediate filament protein expressed in the neural
progenitor cells and it's also a marker of proliferating and
migrating cells. The decreased expression in nestin gene and
protein in the AD rats in the present study was detected, but
BM-MSCs transplantation significantly reversed this reduction,
which showed that BM-MSCs exerted the potential therapeutic role
for AD possibly via enhancing the expression of Seladin-1
and nestin gene or protein. To further elucidate the underlying
molecular mechanisms, we employed two indictors (LY294002, PD98059)
of PI3K and p-ERK1/2 signaling pathway and found that treatment
with LY294002 effectively blocked the therapeutic effect of BM-MSCs
as indicated from the decreasing expression levels of Seladin-1,
nestin, p-Akt and p-ERK1/2. Treatment with PD98059 decreased the
expression of Seladin-1, p-Akt, but no action on the expression of
nestin and p-ERK1/2. Treatment with LY294002 and PD98059 also
decreased the expression of Seladin-1, nestin, p-Akt, but no impact
on the expression of p-ERK1/2. These observations indicated that
PI3K and ERK1/2 signaling pathway were closely linked the mechanism
of the therapeutic role of BM-MSCs transplantation for AD.
The aim of the present work is to present a
preliminary study of the effect BM-MSCs transplantation on the
expression of Seladin-1 and nestin and the underlying molecular
mechanisms in Alzheimer model. There are some limitations to our
study. First, although aluminum exposure had been confirmed as a
valid inductor for constructing AD model, and aluminum exposure
rats showed significant increase in escape latency and decrease in
the percentage of time in residence in the original platform
quadrant in Morris water maze, we should also use some methods such
as morris water maze to detect the valid of our present AD model.
Second, cells composition in the hippocampus tissue is complex, we
detected the whole RNA and protein, but did not used
immunohistochemistry and immunofluorescence test to confirm which
cell type from of the RNA and protein. Third, due to the design of
the experiment is preliminary, the study only offer preliminary
evidence that treatment with BM-MSCs may represent the potential
therapeutic approach against the brain lesion in AD. Our future
direction of work will be proceed based on all above
limitations.
In summary, the results from this study indicated
that BM-MSCs transplantation enhanced the Seladin-1 and nestin
expression may through a mechanism associated with activating the
PI3K/Akt and ERK1/2 pathways. Our study offers preliminary evidence
that treatment with BM-MSCs may represent the potential therapeutic
approach against the neurodegeneration characterized AD.
References
|
1
|
Bekris LM, Yu CE, Bird TD and Tsuang DW:
Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol.
23:213–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Podcasy JL and Epperson CN: Considering
sex and gender in Alzheimer disease and other dementias. Dialogues
Clin Neurosci. 18:437–446. 2016.PubMed/NCBI
|
|
3
|
Jicha GA and Carr SA: Conceptual evolution
in Alzheimer's disease: Implications for understanding the clinical
phenotype of progressive neurodegenerative disease. J Alzheimers
Dis. 19:253–272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li M, Guo K and Ikehara S: Stem cell
treatment for Alzheimer's disease. Int J Mol Sci. 15:19226–19238.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naaldijk Y, Jäger C, Fabian C, Leovsky C,
Blüher A, Rudolph L, Hinze A and Stolzing A: Effect of systemic
transplantation of bone marrow-derived mesenchymal stem cells on
neuropathology markers in APP/PS1 Alzheimer mice. Neuropathol Appl
Neurobiol. 43:299–314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tirino V, Paino F, D'Aquino R, Desiderio
V, De Rosa A and Papaccio G: Methods for the identification,
characterization and banking of human DPSCs: Current strategies and
perspectives. Stem Cell Rev. 7:608–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang H, Xie Z, Wei L, Yang H, Yang S, Zhu
Z, Wang P, Zhao C and Bi J: Human umbilical cord mesenchymal stem
cell-derived neuron-like cells rescue memory deficits and reduce
amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem
Cell Res Ther. 4:762013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin JY, Park HJ, Kim HN, Oh SH, Bae JS,
Ha HJ and Lee PH: Mesenchymal stem cells enhance autophagy and
increase β-amyloid clearance in Alzheimer disease models.
Autophagy. 10:32–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iivonen S, Hiltunen M, Alafuzoff I,
Mannermaa A, Kerokoski P, Puoliväli J, Salminen A, Helisalmi S and
Soininen H: Seladin-1 transcription is linked to neuronal
degeneration in Alzheimer's disease. Neuroscience. 113:301–310.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benvenuti S, Saccardi R, Luciani P, Urbani
S, Deledda C, Cellai I, Francini F, Squecco R, Rosati F, Danza G,
et al: Neuronal differentiation of human mesenchymal stem cells:
Changes in the expression of the Alzheimer's disease-related gene
seladin-1. Exp Cell Res. 312:2592–2604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu G, Kwong WH, Li Q, Wang X, Feng Z and
Yew DT: Bcl2, bax, and nestin in the brains of patients with
neurodegeneration and those of normal aging. J Mol Neurosci.
27:167–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng Q, Zheng M, Zhang T and He G:
Hippocampal neurogenesis in the APP/PS1/nestin-GFP triple
transgenic mouse model of Alzheimer's disease. Neuroscience.
314:64–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krasovskiĭ GN, Vasukovich LY and Chariev
OG: Experimental study of biological effects of leads and aluminum
following oral administration. Environ Health Perspect. 30:47–51.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barros DM, Mello e Souza T, de Souza MM,
Choi H, DeDavid e Silva T, Lenz G, Medina JH and Izquierdo I:
LY294002, an inhibitor of phosphoinositide 3-kinase given into rat
hippocampus impairs acquisition, consolidation and retrieval of
memory for one-trial step-down inhibitory avoidance. Behav
Pharmacol. 12:629–634. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zamin LL, Dillenburg-Pilla P,
Argenta-Comiran R, Horn AP, Simão F, Nassif M, Gerhardt D, Frozza
RL and Salbego C: Protective effect of resveratrol against
oxygen-glucose deprivation in organotypic hippocampal slice
cultures: Involvement of PI3-K pathway. Neurobiol Dis. 24:170–182.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM,
Li SN and Xiang P: Bone marrow-derived mesenchymal stem cells
protect against experimental liver fibrosis in rats. World J
Gastroenterol. 11:3431–3440. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gaytan F, Barreiro ML, Caminos JE, Chopin
LK, Herington AC, Morales C, Pinilla L, Paniagua R, Nistal M,
Casanueva FF, et al: Expression of ghrelin and its functional
receptor, the type 1a growth hormone secretagogue receptor, in
normal human testis and testicular tumors. J Clin Endocrinol Metab.
89:400–409. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang QL, Jia L, Jiao X, Guo WL, Ji JW,
Yang HL and Niu Q: APP/PS1 transgenic mice treated with aluminum:
An update of Alzheimer's disease model. Int J Immunopathol
Pharmacol. 25:49–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salem AM, Ahmed HH, Atta HM, Ghazy MA and
Aglan HA: Potential of bone marrow mesenchymal stem cells in
management of Alzheimer's disease in female rats. Cell Biol Int.
38:1367–1383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vučetić-Arsić S, Radonjić NV, Jovanović M,
Selaković V, Nikolić T, Velimirović M, Stojković T, Milovanović A,
Milovanović J and Petronijević ND: Oxidative stress precedes
mitochondrial dysfunction in gerbil brain after aluminum ingestion.
Environ Toxicol Pharmacol. 36:1242–1252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pohanka M: Copper, aluminum, iron and
calcium inhibit human acetylcholinesterase in vitro. Environ
Toxicol Pharmacol. 37:455–459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lima PD, Leite DS, Vasconcellos MC,
Cavalcanti BC, Santos RA, Costa-Lotufo LV, Pessoa C, Moraes MO and
Burbano RR: Genotoxic effects of aluminum chloride in cultured
human lymphocytes treated in different phases of cell cycle. Food
Chem Toxicol. 45:1154–1159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aziza Abdel MT, Atta HM, Samer H, Ahmed
HH, Rashed LA, Sabry D, Raouf Abdel ER and Alkaffas MA: Heme
oxygenase effect on mesenchymal stem cells action on experimental
Alzheimer's disease. EXCLI J. 12:778–792. 2013.PubMed/NCBI
|