Introduction
Worldwide, colorectal cancer (CRC) is a leading
cause of cancer-associated mortality (1). Despite significant advances in medical
and surgical treatment options, the mortality rate for CRC remains
high (2), with a reported
812,000–855,000 mortalities resulting from colon and rectal cancer
in 2015 (3). In order to address this
issue and improve the prognosis of patients with CRC, a more
thorough understanding of the underlying pathological processes of
CRC carcinogenesis is required.
The adenoma-carcinoma sequence represents the
process by which the majority of CRC arise (4,5). In 70–80%
of colorectal tumors, this sequence is initiated by the mutation
and inactivation of the adenomatous polyposis coli (APC) gene,
which acts as the gatekeeper of colorectal tumorigenesis (6). The APC protein combines with glycogen
synthase kinase 3β, axin and casein kinase 1α to form a
‘destruction complex’ that degrades β-catenin (7). As a consequence of APC protein
inactivation, the Wnt/β-catenin signaling pathway is often
aberrantly activated in CRC (6). The
Wnt/β-catenin signaling pathway is important as it serves a key
function in the regulation of intestinal stem cells (ISCs)
(8). These are multipotent cells with
the capacity to self-renew and thus sustain the proliferative
capacity of the intestinal epithelium (9). Cancer stem cells (CSCs) act in a manner
similar to normal ISCs to promote tumor expansion and progression
in adenomas and adenocarcinomas (10,11).
In addition to Wnt/β-catenin, two other key
signaling pathways regulate colorectal ISCs; the Notch signaling
pathway, which is associated with generating cells with ISC-like
properties (12,13), and the bone morphogenetic protein
(BMP) signaling pathway, which counteracts the effects of the
Wnt/β-catenin pathway (14,15). Cellular activation of the
Wnt/β-catenin signaling pathway is characterized by the nuclear
localization of β-catenin; these cells act as CSCs and are capable
of tumorigenesis (6,12,16).
Additionally, membrane-bound β-catenin is associated with the
presence of epithelial cadherin, which maintains epithelial
cell-cell adhesion; loss of membrane-bound β-catenin may contribute
to the activation of the Wnt/β-catenin signaling pathway (17,18).
Leucine-rich repeat-containing G protein-coupled
receptor 5 (LGR5) acts downstream of the Wnt gene and has been
identified as a marker of ISCs and CSCs in CRC (19–23). It
has been reported that increased expression of LGR5 occurs in colon
cancer cell lines as well as samples of colorectal adenoma and
adenocarcinoma, compared with noncancerous controls (24). In addition, LGR5 silencing in
colorectal cell lines has been reported to result in downregulated
Notch signaling (22). Specific
activation of the Wnt/β-catenin signaling pathway via the loss of
APC in ISCs expressing LGR5 is sufficient to promote the formation
of adenomas in the mouse intestine (21).
GATA6, a member of the zinc-finger DNA binding
transcription factor family, has been identified as a regulator of
the Wnt/β-catenin signaling pathway, the BMP pathway and LGR5
expression. GATA6 deficiency suppresses colonic tumorigenesis by
antagonizing the β-catenin/transcription factor 4 complex by
binding to the BMP4 regulatory region in APC null mice (25). Furthermore, GATA6 is able to bind to
the LGR5 promoter region and, as such, knockdown of GATA6 decreases
LGR5 mRNA levels to a similar extent as β-catenin knockdown
(25). Decreasing the expression of
LGR5 via suppressing GATA6 therefore inhibits the tumorigenic
properties of CRC cells (26). Based
on the available evidence, it was hypothesized in the present study
that the nuclear translocation of β-catenin and increased
expression of LGR5 and GATA6 may serve a cooperative role in human
colorectal tumorigenesis. The aim of the present study was to
investigate this cooperative role by observing the varied
expression of β-catenin, LGR5 and GATA6 in a normal
mucosa-adenoma-adenocarcinoma sequence in colorectal tissue
samples.
Materials and methods
Subjects
A total of 65 patients with a pathological diagnosis
of colorectal adenocarcinoma and a history of adenoma that
underwent surgical resections at The First Affiliated Hospital of
Jinzhou Medical University, Department of General Surgery
(Liaoning, China) between June 2012 and September 2014 were
retrospectively included in the present study. The
Tumor-Node-Metastasis (TNM) stage of patients was determined
according to the 7th edition of the American Joint Committee on
Cancer staging manual (27,28). The baseline characteristics of the
patients are presented in Table
I.
 | Table I.Characteristics of patients with
colorectal adenoma and adenocarcinoma. |
Table I.
Characteristics of patients with
colorectal adenoma and adenocarcinoma.
| Characteristic | n (%) |
|---|
| Age, years |
|
|
<65 | 31 (47.69) |
|
≥65 | 34 (52.31) |
| Sex |
|
|
Male | 47 (72.31) |
|
Female | 18 (27.69) |
| Location of
adenoma |
|
|
Colon | 25 (38.46) |
|
Rectum | 40 (61.54) |
| Histological grade
of adenomatous dysplasia |
|
|
Mild | 29 (44.62) |
|
Moderate/severe | 31 (47.69) |
| Number of
adenoma |
|
|
Single | 56 (86.15) |
|
Multiple | 9 (13.85) |
| Location of
adenocarcinoma |
|
|
Colon | 26 (40.00) |
|
Rectum | 39 (60.00) |
| TNM staging of
adenocarcinoma |
|
| Stage
I/II | 46 (70.77) |
| Stage
III/IV | 19 (29.23) |
| pTNM T stage |
|
|
T1/T2 | 18 (27.69) |
|
T3/T4 | 47 (72.31) |
| pTNM N stage |
|
| N0 | 47 (72.31) |
|
N1/N2 | 18 (27.69) |
Adenoma, adenocarcinoma and adjacent normal tissue
samples (3-µm thick) were fixed in 4% formalin overnight at 4°C and
embedded in paraffin. The majority of adenomas (89.25%; n=58/65)
were in the same location as adenocarcinomas diagnosed in the same
patient. The present study was approved by The Ethics Committee of
The First Affiliated Hospital of Jinzhou Medical University.
Immunohistochemistry (IHC)
The tissue sections were initially deparaffinized in
an oven at 65°C for 2 h, immersed in xylene 3 times and
subsequently heated to 100°C in 10 mM citrate buffer (Origene
Technologies, Inc., Beijing, China), pH 6.0, for 30 min. Sections
were blocked with 1% bovine serum albumin (A1933; Sigma-Aldrich,
Merck KGaA, Darmstadt, Germany) and 5% normal goat serum (S-1000;
Vector Laboratories, Inc., Burlingame, CA, USA) in PBS plus 0.04%
tween 20 at room temperature for 1 h. Sections were subsequently
incubated with primary anti-LGR5 (1:100; ab75850; Abcam, Cambridge,
UK), anti-GATA6 (1:150; sc-9055; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and anti-β-catenin antibodies (1:200; 51067-2-AP;
ProteinTech Group, Inc., Chicago, IL, USA) at 4°C overnight.
Samples were treated with a 2-step plus Poly-HRP anti-mouse/rabbit
IgG detection system (cat. no. PV-9000; Origene Technologies, Inc.)
and 3,3′-diaminobenzidine tetrahydrochloride (cat. no. ZLI-9032;
Origene Technologies, Inc.), according to manufacturer's protocols.
Finally, samples were counterstained with Mayer's hematoxylin (cat.
no. MHS16; Sigma-Aldrich; Merck KGaA) at room temperature for 5 min
and mounted with Histomount mounting medium (HS-103; National
Diagnostics, Atlanta, GA, USA).
Evaluation of IHC staining
IHC results were evaluated as previously described
(29). Images were evaluated in at
least three fields of view at ×200 magnification using an Olympus
BX53 light microscope (Olympus Corporation, Tokyo, Japan) which
included either a whole section or whole layer of colorectal wall
(mucosa-submucosa-muscularis-serosa) were evaluated and selected
unanimously by two experienced pathologists. The pathologists also
took into consideration the staining intensity and the proportion
of positive staining. The intensity score refers to four grades of
staining intensity: 0, negative staining; 1, weak/light yellow
staining; 2, moderate/yellow staining; and 3, strong/dark yellow
staining. The quantity score refers to the proportion of positively
stained cells in the tissue sections: 0, 0–4% positively stained
cells; 1, 5–24% positively stained cells; 2, 25–49% positively
stained cells; 3, 50–74% positively stained cells; and 4, 75–100%
positively stained cells. The final overall score ranged from 0 to
12 and was obtained by multiplying the intensity score by the
quantity score. The mean of the overall scores of the selected
fields was analyzed as the evaluative scores of staining. Tissue
sections with unavailable staining were excluded from the final
analysis.
Statistical analysis
SAS-JMP software (version 11; SAS Institute, Inc.,
Cary, NC, USA) was used for statistical analysis. For comparisons
between groups, a Kruskal-Wallis test followed by Steel-Dwass
method was performed for abnormally distributed data comprising
more than two groups. A Wilcoxon rank-sum was used when there were
two groups. Data are presented as the median [interquartile range,
(IQR)]. Spearman rank correlation was used to analyze the
correlation between β-catenin, LGR5 and GATA6 expression. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of β-catenin, LGR5 and
GATA6 in a normal mucosa-adenoma-adenocarcinoma sequence of
colorectal tissue
To simultaneously observe the effect of
Wnt/β-catenin signaling pathway activation, LGR5 and GATA6 on
colorectal tumorigenesis, the expression of β-catenin, LGR5 and
GATA6 in adenocarcinoma, adjacent adenoma and normal tissue samples
were evaluated. It was identified that β-catenin, LGR5 and GATA6
were expressed in distinct locations within intestinal epithelial
cells: β-catenin was expressed on the cell membrane, in the
cytoplasm and in the nucleus; LGR5 was predominantly expressed in
the cytoplasm; and GATA6 was expressed in the cell nucleus and
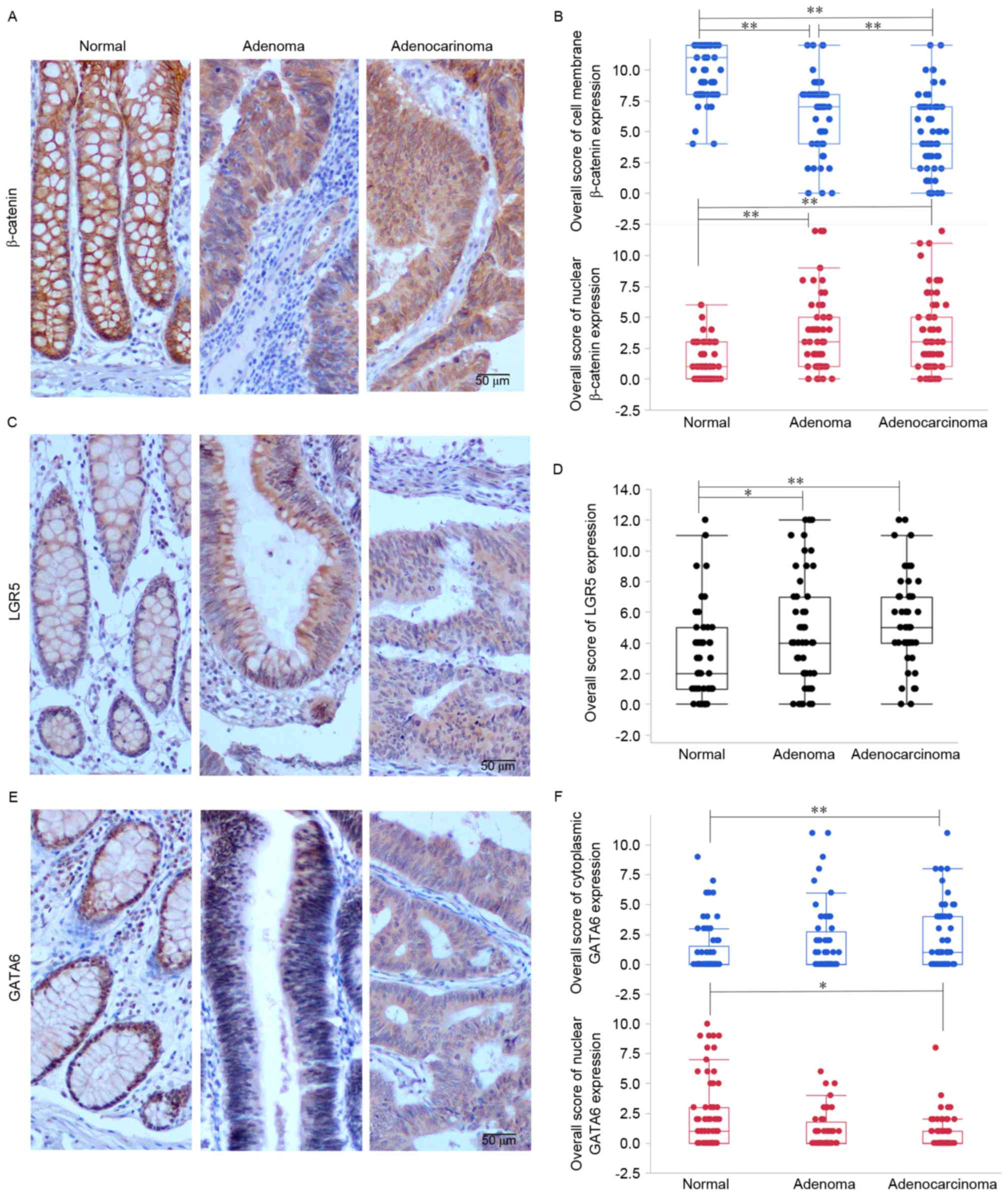
cytoplasm (Fig. 1).
Nuclear expression of β-catenin increased in samples
as they progressed from normal mucosa to adenoma to adenocarcinoma
(P<0.0001), whereas membrane-bound β-catenin expression
decreased (P<0.0001; Fig. 1A and
B). A statistically significant difference was identified
between all pairwise comparisons, with the exception of nuclear
β-catenin expression in adenoma and adenocarcinoma samples. This
suggests that increased nuclear β-catenin expression and decreased
cell-membrane β-catenin expression may serve a function in the
transformation from normal colorectal mucosa to adenoma.
To investigate the expression of LGR5 and its
function in the normal mucosa-adenoma-adenocarcinoma
transformation, IHC staining of LGR5 in tissue sections were
evaluated. It was revealed that, similar to nuclear β-catenin
expression, there was an increase in LGR5 expression as the tissue
progressed from normal mucosa to adenocarcinoma (P=0.0003; Fig. 1C and D).
Similarly, cytoplasmic GATA6 was overexpressed in
adenocarcinomas compared with normal mucosal samples (P=0.015;
Fig. 1E and F). In contrast, nuclear
GATA6 was downregulated in adenocarcinomas compared with normal
mucosa (P=0.001; Fig. 1E and F).
These data demonstrate that nuclear β-catenin and
cytoplasmic LGR5 expression is higher in adenoma and adenocarcinoma
samples compared with normal mucosal samples, whereas cytoplasmic
GATA6 expression is higher in adenocarcinoma samples compared with
normal mucosal samples. These findings suggest that nuclear
β-catenin and LGR5 may serve a function in the transition from
normal mucosa to adenoma and increased expression of cytoplasmic
GATA6 may promote the formation of adenocarcinoma.
Correlation between the expression of
β-catenin, LGR5 and GATA6 in normal mucosa, adenoma and
adenocarcinoma
Using normal mucosa, adenoma and adenocarcinoma
tissue sections obtained from the same patient, the association
between disease stage and β-catenin, LGR5 and GATA6 expression in
distinct intracellular locations was investigated (Tables II–IV). In adenomas and adenocarcinomas, a
significant positive correlation was observed between cytoplasmic
GATA6 expression and LGR5 expression (P=0.001 rs=0.481,
Table III; P=0.007,
rs=0.377, Table IV). In
contrast, there was a negative association between nuclear GATA6
expression and LGR5 expression in adenomas (P=0.022,
rs=−0.329; Table V). No
significant associations were observed in normal mucosa (Table II). These results suggest that there
is an interaction between GATA6 and LGR5 expression in colorectal
tumors but not in normal mucosa. No significant association between
nuclear β-catenin and GATA6 expression was observed in any tissues
(Tables II–IV).
 | Table II.Correlation between β-catenin, LGR5
and GATA6 in colorectal normal mucosa. |
Table II.
Correlation between β-catenin, LGR5
and GATA6 in colorectal normal mucosa.
| Protein | Cell membrane
β-catenin | Nuclear
β-catenin | LGR5 | Cytoplasmic
GATA6 | Nuclear GATA6 |
|---|
| Cell membrane
β-catenin |
rs=1.000 |
rs=0.085 |
rs=0.146 |
rs=−0.176 |
rs=−0.048 |
|
| – | P=0.516 | P=0.308 | P=0.176 | P=0.715 |
| Nuclear
β-catenin |
rs=0.085 |
rs=1.000 |
rs=0.134 |
rs=−0.001 |
rs=0.056 |
|
| P=0.516 | – | P=0.353 | P=0.993 | P=0.674 |
| LGR5 |
rs=0.146 |
rs=0.134 |
rs=1.000 |
rs=0.122 |
rs=0.012 |
|
| P=0.308 | P=0.353 | – | P=0.399 | P=0.934 |
| Cytoplasmic
GATA6 |
rs=−0.176 |
rs=−0.001 |
rs=0.122 |
rs=1.000 |
rs=0.074 |
|
| P=0.176 | P=0.993 | P=0.399 | – | P=0.573 |
| Nuclear GATA6 |
rs=−0.048 |
rs=0.056 |
rs=0.012 |
rs=0.074 |
rs=1.000 |
|
| P=0.715 | P=0.674 | P=0.934 | P=0.573 | – |
 | Table IV.Correlation between β-catenin, LGR5
and GATA6 in colorectal adenocarcinoma. |
Table IV.
Correlation between β-catenin, LGR5
and GATA6 in colorectal adenocarcinoma.
|
| Cell membrane
β-catenin | Nuclear
β-catenin | LGR5 | Cytoplasmic
GATA6 | Nuclear GATA6 |
|---|
| Cell membrane
β-catenin |
rs=1.000 |
rs=−0.141 |
rs=−0.086 |
rs=−0.056 |
rs=−0.002 |
|
| – | P=0.270 | P=0.554 | P=0.665 | P=0.985 |
| Nuclear
β-catenin |
rs=−0.141 |
rs=1 |
rs=−0.233 |
rs=−0.212 |
rs=0.251 |
|
| P=0.270 | – | P=0.103 | P=0.098 |
P=0.049a |
| LGR5 |
rs=−0.086 |
rs=−0.233 |
rs=1 |
rs=0.377 |
rs=−0.270 |
|
| P=0.554 | P=0.103 | – |
P=0.007b | P=0.058 |
| Cytoplasmic
GATA6 |
rs=−0.056 |
rs=−0.212 |
rs=0.377 |
rs=1 |
rs=0.123 |
|
| P=0.665 | P=0.098 |
P=0.007b | – | P=0.343 |
| Nuclear GATA6 |
rs=−0.002 |
rs=0.251 |
rs=−0.270 |
rs=0.122 |
rs=1.000 |
|
| P=0.985 |
P=0.049a | P=0.058 | P=0.343 | – |
 | Table III.Correlation between β-catenin, LGR5
and GATA6 in colorectal adenoma. |
Table III.
Correlation between β-catenin, LGR5
and GATA6 in colorectal adenoma.
| Protein | Cell membrane
β-catenin | Nuclear
β-catenin | LGR5 | Cytoplasmic
GATA6 | Nuclear GATA6 |
|---|
| Cell membrane
β-catenin |
rs=1.000 |
rs=0.022 |
rs=−0.135 |
rs=−0.168 |
rs=0.305 |
|
| – | P=0.866 | P=0.367 | P=0.209 |
P=0.020a |
| Nuclear
β-catenin |
rs=0.022 |
rs=1.000 |
rs=−0.087 |
rs=−0.170 |
rs=0.148 |
|
| P=0.866 | – | P=0.560 | P=0.202 | P=0.269 |
| LGR5 |
rs=−0.135 |
rs=−0.087 |
rs=1.000 |
rs=0.481 |
rs=−0.329 |
|
| P=0.367 | P=0.560 | – |
P=0.001b |
P=0.022a |
| Cytoplasmic
GATA6 |
rs=−0.168 |
rs=−0.170 |
rs=0.481 |
rs=1.000 |
rs=−0.235 |
|
| P=0.209 | P=0.202 |
P=0.001b | – | P=0.071 |
| Nuclear GATA6 |
rs=0.305 |
rs=0.148 |
rs=−0.329 |
rs=−0.235 |
rs=1.000 |
|
|
P=0.020a | P=0.269 |
P=0.022a | P=0.071 | – |
 | Table V.β-catenin, LGR5 and GATA6 expression
in adenoma stratified by histological grade of dysplasia. |
Table V.
β-catenin, LGR5 and GATA6 expression
in adenoma stratified by histological grade of dysplasia.
|
| Cell membrane
β-catenin | Nuclear
β-catenin | LGR5 | Cytoplasmic
GATA6 | Nuclear GATA6 |
|---|
|
|
|
|
|
|
|
|---|
| Dysplasia | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 |
|---|
| Mild | 27 | 8 (2) | 2.17 | 27 | 4 (3) | 4.33a | 24 | 4 (7) | 0.46 | 27 | 0 (4) | 0.02 | 27 | 0 (3) | 0.57 |
|
Moderate/severe | 29 | 7 (6) |
| 29 | 2 (3) |
| 22 | 4 (4) |
| 29 | 1 (3) |
| 29 | 0 (1) |
|
Analysis of β-catenin, LGR5 and GATA6
expression in colorectal adenoma and adenocarcinoma stratified by
pathological parameters
β-catenin, LGR5 and GATA6 expression was
investigated in samples of adenomas and adenocarcinomas stratified
by pathological parameters (Tables V
and VI). Adenomas with mild-grade
dysplasia exhibited increased levels of nuclear β-catenin
expression compared with adenomas with moderate and severe-grade
dysplasia (P=0.038; Table V). This
suggests that upregulation of nuclear β-catenin may be an early
event in the pathogenesis of colorectal tumors. When protein
expression was evaluated in adenocarcinomas with distinct
pathological TNM stages, nuclear GATA6 was observed to be
significantly downregulated in stage T3 and T4 adenocarcinomas
compared with stage T1 and T2 adenocarcinomas (P=0.024; Table VI).
 | Table VI.β-catenin, LGR5 and GATA6 expression
in adenocarcinoma stratified by pathological parameters. |
Table VI.
β-catenin, LGR5 and GATA6 expression
in adenocarcinoma stratified by pathological parameters.
|
| Cell membrane
β-catenin | Nuclear
β-catenin | LGR5 | Cytoplasmic
GATA6 | Nuclear GATA6 |
|---|
|
|
|
|
|
|
|
|---|
| Parameters | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 |
|---|
| TNM staging |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
I/II | 44 | 5 (5) | 0.09 | 44 | 3 (5) | 0.04 | 38 | 5 (3) | 1.74 | 44 | 1 (4) | 0.97 | 44 | 1 (1) | 0.69 |
|
III/IV | 19 | 4 (4) |
| 19 | 3 (4) |
| 12 | 6 (4) |
| 18 | 1 (4) |
| 18 | 0 (1) |
|
| pTNM T stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
T1/T2 | 17 | 4 (5) | 0.04 | 17 | 4 (7) | 0.23 | 15 | 6 (3) | 0.10 | 17 | 1 (3) | 0.07 | 17 | 1 (3) | 5.10a |
|
T3/T4 | 46 | 4 (5) |
| 46 | 3 (4) |
| 35 | 5 (3) |
| 45 | 1 (4) |
| 45 | 0 (1) |
|
| pTNM N stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| N0 | 45 | 4 (5) | 0.64 | 45 | 2 (5) | 0.08 | 39 | 5 (3) | 0.09 | 45 | 1 (4) | 0.43 | 45 | 0 (2) | 0.36 |
|
N1&N2 | 18 | 4 (5) |
| 18 | 3 (5) |
| 11 | 6 (3) |
| 17 | 1 (5) |
| 17 | 0 (1) |
|
Nuclear β-catenin expression is
positively correlated with the depth of invasion of colorectal
adenocarcinomas, however LGR5 and GATA6 expression is not
To explore the association between β-catenin, LGR5
and GATA6 expression and the depth of tumor invasion, protein
expression was evaluated at distinct invasive depths (mucosa,
submucosa, muscularis and serosa) in the same sample of
adenocarcinoma. Nuclear β-catenin expression was significantly
increased in the serosal layer compared with all other layers
(P=0.042; Table VII). No
significant difference in the cell-membrane expression of
β-catenin, or the cytoplasmic expression of LGR5 or GATA6, was
observed among layers (Table VII).
These results suggest that nuclear β-catenin expression may
contribute to the invasive capacity of colorectal cancer. In
addition, these results are consistent with those of previous
reports, which demonstrated increased expression of nuclear
β-catenin in the invasive front (30)
and that the Wnt/β-catenin pathway regulates the epithelial to
mesenchymal transition and increases the invasive capabilities of
tumor cells (31,32).
 | Table VII.β-catenin, LGR5 and GATA6 expression
in different invasive depth of colorectal adenocarcinoma. |
Table VII.
β-catenin, LGR5 and GATA6 expression
in different invasive depth of colorectal adenocarcinoma.
|
| Cell membrane
β-catenin | Nuclear
β-catenin | LGR5 | Cytoplasmic
GATA6 | Nuclear GATA6 |
|---|
|
|
|
|
|
|
|
|---|
| Parameters | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 |
|---|
| Mucosa | 28 | 4 (7) | 1.63 | 28 | 2 (7) | 8.23a | 14 | 7 (4) | 1.28 | 8 | 1 (4) | 3.79 | 8 | 2 (4) | 6.41 |
| Submucosa | 45 | 4 (4) |
| 45 | 2 (4) |
| 29 | 4 (3) |
| 29 | 1 (2) |
| 29 | 0 (1) |
|
| Muscularis | 53 | 4 (6) |
| 53 | 3 (5) |
| 36 | 6 (4) |
| 42 | 2 (4) |
| 42 | 0 (1) |
|
| Serosa | 10 | 2 (7) |
| 10 | 6 (9) |
| 7 | 4 (3) |
| 4 | 4 (6) |
| 4 | 0 (0) |
|
Discussion
Activation of the Wnt/β-catenin signaling pathway
results in β-catenin translocation to the nucleus and transcription
of Wnt target genes; this process has been implicated in the
initiation of colorectal tumorigenesis (33,34). LGR5
is a target of the Wnt/β-catenin signaling pathway (19) and has been identified in colorectal
CSCs with an expansive phenotype (35,36);
furthermore, this protein may be regulated by GATA6 during CRC
formation (26).
To evaluate the contribution and interactions of
β-catenin, LGR5 and GATA6 in the adenoma-carcinoma sequence of
human intestinal samples, matched samples of normal mucosa, adenoma
and adenocarcinoma were used. The expression of these proteins in
distinct intracellular locations and the associations between them
were assessed. Compared with normal mucosal samples, adenoma and
adenocarcinoma samples exhibited increased nuclear β-catenin and
cytoplasmic LGR5 expression. These results are consistent with
previous studies that have identified increased levels of nuclear
β-catenin expression in colorectal tumors (16,30).
Furthermore, suppression of the Wnt/β-catenin signaling pathway in
CRC cells has been demonstrated to inhibit tumor formation
(37), whereas silencing LGR5 in CRC
cell lines decreases proliferation, migration and colony formation
in vitro, as well as tumorigenic capacity in vivo
(22). Furthermore, ISCs that are
LGR5+ and in which the Wnt/β-catenin signaling pathway
is activated tend to progress more efficiently towards intestinal
adenoma (21). These results of this
aforementioned study, together with the results of the present
study, suggest that activation of the Wnt/β-catenin pathway and
increased expression of LGR5 may occur in the early stages of
colorectal tumorigenesis, and are sustained at high levels during
the malignant transformation.
The present study also demonstrated that the
expression of nuclear β-catenin in adenomas characterized by
mild-grade dysplasia was significantly increased (P=0.038) compared
with those characterized by moderate and severe-grade dysplasia,
whereas there was no significant difference in the expression of
LGR5 among adenomas of different stages. Taken together, these
results suggest that the activated Wnt/β-catenin signaling pathway
may serve an important function in the initiation of adenoma
formation, whereas subsequent transcription of the LGR5 gene
sustains self-renewing ISCs in order to propagate CRC development
(20,22,38).
With respect to GATA6 expression, the results of the
present study revealed that higher levels of cytoplasmic GATA6
expression were exhibited in adenocarcinomas compared with adenomas
and normal mucosal samples. This suggests that there may be a
cumulative effect of cytoplasmic GATA6 expression on colorectal
tumorigenesis. A previous study also demonstrated strong
cytoplasmic GATA6 staining in adenocarcinomas (39) and GATA6 was identified as a key
regulator that sustains the tumorigenic capability of CRC cells
(26). Taken together, these results
suggest that overexpressed cytoplasmic GATA6 serves a role in the
formation of colorectal adenocarcinomas.
In the present study, a positive correlation was
identified between cytoplasmic GATA6 and LGR5 expression in
colorectal adenomas and adenocarcinomas, but not in normal mucosa.
This suggests that the expression of cytoplasmic GATA6 is
associated with the expression of LGR5 in colorectal tumors.
Indeed, it has previously been demonstrated that GATA6 is able to
regulate LGR5 expression and is required for tumorigenesis in colon
cancer cells with APC mutations, as characterized by
aberrant activation of the Wnt/β-catenin signaling pathway
(6,26). In summary, the results of these
previous studies, and those of the present study, implicate LGR5 as
an important regulator of colorectal tumorigenesis.
The expression of nuclear GATA6 was demonstrated to
decrease in the normal mucosa-adenoma-adenocarcinoma sequence in
the present study, which is inconsistent with the results of our
previous study (39). Furthermore,
the correlation between LGR5 and nuclear GATA6 expression was
negative in adenomas. It has previously been reported that GATA6
may regulate the expression of LGR5 by binding to the lgr5
promoter (25). The reason for this
difference may be due in part to the interplay between the
expression of GATA6 in the cytoplasm and the nucleus during
colorectal tumorigenesis, or due to distinct modes of regulation of
LGR5. However, the mechanism of cytoplasmic GATA6 regulation of
LGR5 during colorectal tumorigenesis is unclear. Future studies are
warranted to investigate the expression of nuclear and cytoplasmic
GATA6 in human CRC tissues.
In conclusion, the results of the present study
suggest that aberrantly activated Wnt/β-catenin signaling and
increased expression of LGR5 may act together to drive the
transition from colorectal normal mucosa to adenoma and to sustain
the expansive proliferation of tumor cells. Simultaneously, the
cumulative increase in cytoplasmic GATA6 expression, which is
correlated with LGR5 expression, may promote the progression to
colorectal adenocarcinoma. It has been reported that the activated
Wnt/β-catenin pathway, LGR5 and GATA6 exhibit pro-tumorigenic
effects on colonic and rectal epithelial cells. The results of the
present study provide additional evidence for the cooperative role
of these proteins during colorectal tumorigenesis, and suggest that
LGR5 may be a novel and useful target for CRC prevention and
treatment. Nevertheless, additional studies are required to further
investigate the interaction between these proteins during the
transformation from normal colorectal mucosa to adenomas and
adenocarcinomas.
Acknowledgements
The present study was supported by Aohongboze
Graduate Sci-tech Innovation Foundation (the President Fund of
Jinzhou Medical University; grant no. AH2015001).
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
ISCs
|
intestinal stem cells
|
|
CSCs
|
cancer stem cells
|
|
LGR5
|
leucine-rich repeat-containing G
protein-coupled receptor 5
|
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The Global Burden of Cancer, 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al:
Global, regional and national cancer incidence, mortality, years of
life lost, years lived with disability and disability-adjusted
life-years for 32 cancer groups, 1990 to 2015: A systematic
analysis for the global burden of disease study. JAMA Oncol.
3:524–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fearon ER: Molecular genetics of
colorectal cancer. Annu Rev Pathol. 6:479–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Novellasdemunt L, Antas P and Li VS:
Targeting Wnt signaling in colorectal cancer. A review in the
theme: Cell signaling: Proteins, pathways and mechanisms. Am J
Physiol Cell Physiol. 309:C511–C521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Basu S, Haase G and Ben-Ze'ev A: Wnt
signaling in cancer stem cells and colon cancer metastasis.
F1000Res. 5:F10002016. View Article : Google Scholar
|
|
9
|
Wright NA: Epithelial stem cell repertoire
in the gut: Clues to the origin of cell lineages, proliferative
units and cancer. Int J Exp Pathol. 81:117–143. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vermeulen L, Todaro M, de Sousa Mello F,
Sprick MR, Kemper K, Alea M Perez, Richel DJ, Stassi G and Medema
JP: Single-cell cloning of colon cancer stem cells reveals a
multi-lineage differentiation capacity. Proc Natl Acad Sci USA.
105:pp. 13427–13432. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeuner A, Todaro M, Stassi G and De Maria
R: Colorectal cancer stem cells: From the crypt to the clinic. Cell
stem cell. 15:692–705. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vermeulen L, De Sousa E, Melo F, van der
Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M,
Merz C, Rodermond H, et al: Wnt activity defines colon cancer stem
cells and is regulated by the microenvironment. Nat Cell Biol.
12:468–476. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sikandar SS, Pate KT, Anderson S, Dizon D,
Edwards RA, Waterman ML and Lipkin SM: NOTCH signaling is required
for formation and self-renewal of tumor-initiating cells and for
repression of secretory cell differentiation in colon cancer.
Cancer Res. 70:1469–1478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kosinski C, Li VS, Chan AS, Zhang J, Ho C,
Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, et al: Gene
expression patterns of human colon tops and basal crypts and BMP
antagonists as intestinal stem cell niche factors. Proc Natl Acad
Sci USA. 104:pp. 15418–15423. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He XC, Zhang J, Tong WG, Tawfik O, Ross J,
Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, et al: BMP
signaling inhibits intestinal stem cell self-renewal through
suppression of Wnt-beta-catenin signaling. Nature genetics.
36:1117–1121. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Serafino A, Moroni N, Zonfrillo M,
Andreola F, Mercuri L, Nicotera G, Nunziata J, Ricci R, Antinori A,
Rasi G and Pierimarchi P: WNT-pathway components as predictive
markers useful for diagnosis, prevention and therapy in
inflammatory bowel disease and sporadic colorectal cancer.
Oncotarget. 5:978–992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hinck L, Näthke IS, Papkoff J and Nelson
WJ: Dynamics of cadherin/catenin complex formation: Novel protein
interactions and pathways of complex assembly. J Cell Biol.
125:1327–1340. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heuberger J and Birchmeier W: Interplay of
cadherin-mediated cell adhesion and canonical Wnt signaling. Cold
Spring Harb Perspect Biol. 2:a0029152010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Van der Flier LG, Sabates-Bellver J, Oving
I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S,
Van de Wetering M, Marra G and Clevers H: The intestinal Wnt/TCF
signature. Gastroenterology. 132:628–632. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barker N, van Es JH, Kuipers J, Kujala P,
van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H,
Peters PJ and Clevers H: Identification of stem cells in small
intestine and colon by marker gene Lgr5. Nature. 449:1003–1007.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barker N, Ridgway RA, van Es JH, van de
Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR,
Sansom OJ and Clevers H: Crypt stem cells as the cells-of-origin of
intestinal cancer. Nature. 457:608–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hirsch D, Barker N, McNeil N, Hu Y, Camps
J, McKinnon K, Clevers H, Ried T and Gaiser T: LGR5 positivity
defines stem-like cells in colorectal cancer. Carcinogenesis.
35:849–858. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kemper K, Prasetyanti PR, De Lau W,
Rodermond H, Clevers H and Medema JP: Monoclonal antibodies against
Lgr5 identify human colorectal cancer stem cells. Stem cells.
30:2378–2386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uchida H, Yamazaki K, Fukuma M, Yamada T,
Hayashida T, Hasegawa H, Kitajima M, Kitagawa Y and Sakamoto M:
Overexpression of leucine-rich repeat-containing G protein-coupled
receptor 5 in colorectal cancer. Cancer Sci. 101:1731–1737. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Whissell G, Montagni E, Martinelli P,
Hernando-Momblona X, Sevillano M, Jung P, Cortina C, Calon A, Abuli
A, Castells A, et al: The transcription factor GATA6 enables
self-renewal of colon adenoma stem cells by repressing BMP gene
expression. Nat Cell Biol. 16:695–707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsuji S, Kawasaki Y, Furukawa S, Taniue K,
Hayashi T, Okuno M, Hiyoshi M, Kitayama J and Akiyama T: The
miR-363-GATA6-Lgr5 pathway is critical for colorectal
tumourigenesis. Nat Commun. 5:31502014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Edge SB and Byrd DR: Compton CC:AJCC
cancer staging manual. 7th. New York, NY: Springer; 2010
|
|
28
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Q, Cao HZ and Zheng PS: LGR5 promotes
the proliferation and tumor formation of cervical cancer cells
through the Wnt/beta-catenin signaling pathway. Oncotarget.
5:9092–9105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suzuki H, Masuda N, Shimura T, Araki K,
Kobayashi T, Tsutsumi S, Asao T and Kuwano H: Nuclear beta-catenin
expression at the invasive front and in the vessels predicts liver
metastasis in colorectal carcinoma. Anticancer Res. 28:1821–1830.
2008.PubMed/NCBI
|
|
31
|
Hu TH, Yao Y, Yu S, Han LL, Wang WJ, Guo
H, Tian T, Ruan ZP, Kang XM, Wang J, et al: SDF-1/CXCR4 promotes
epithelial-mesenchymal transition and progression of colorectal
cancer by activation of the Wnt/β-catenin signaling pathway. Cancer
Lett. 354:417–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jones S, Chen WD, Parmigiani G, Diehl F,
Beerenwinkel N, Antal T, Traulsen A, Nowak MA, Siegel C, Velculescu
VE, et al: Comparative lesion sequencing provides insights into
tumor evolution. Proc Natl Acad Sci USA. 105:pp. 4283–4288. 2008;
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van de Wetering M, Sancho E, Verweij C, de
Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D,
Haramis AP, et al: The beta-catenin/TCF-4 complex imposes a crypt
progenitor phenotype on colorectal cancer cells. Cell. 111:241–250.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vermeulen L and Snippert HJ: Stem cell
dynamics in homeostasis and cancer of the intestine. Nat Rev
Cancer. 14:468–480. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin YU, Wu T, Yao Q, Zi S, Cui L, Yang M
and Li J: LGR5 promotes the proliferation of colorectal cancer
cells via the Wnt/β-catenin signaling pathway. Oncology Lett.
9:2859–2863. 2015. View Article : Google Scholar
|
|
37
|
Kanwar SS, Yu Y, Nautiyal J, Patel BB and
Majumdar AP: The Wnt/beta-catenin pathway regulates growth and
maintenance of colonospheres. Mol Cancer. 9:2122010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schepers AG, Snippert HJ, Stange DE, van
den Born M, van Es JH, van de Wetering M and Clevers H: Lineage
tracing reveals Lgr5+ stem cell activity in mouse intestinal
adenomas. Science. 337:730–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Belaguli NS, Aftab M, Rigi M, Zhang M,
Albo D and Berger DH: GATA6 promotes colon cancer cell invasion by
regulating urokinase plasminogen activator gene expression.
Neoplasia. 12:856–865. 2010. View Article : Google Scholar : PubMed/NCBI
|