Introduction
Ewing's sarcoma (EWS) is a highly aggressive tumor
that occurs mostly in children and young adults and rapidly
disseminates to bones, bone marrow, and lungs (1). Despite advances in primary EWS
management, the improvement of survival rates for patients with
metastases or recurrence has remained modest over the last decades
(2,3).
Consequently, there is a persistent pursuit for new approaches for
its treatment.
On this regard, a recent article by Pinca et
al (4) published in Oncology
Reports portrayed the effects of ROCKs (Rho-associated
coiled-coil containing protein kinases) specific inhibition on the
growth, migration and differentiation of two EWS cell lines. The
authors showed that exposure of cells to Y27632 (ROCK
pan-inhibitor) or SR3677 (ROCK2 inhibitor) significantly reduced
migration and growth, while favoring morphology changes and neural
differentiation. As a result, the authors embrace the possible use
of ROCK2 as a molecular target for the treatment of EWS.
The role of ROCK1 and ROCK2 in cancer cell
dissemination through their contribution in actin cytoskeleton
organization, cell adhesion and motility has been extensively
studied in many tumors of different origins (5–10).
However, a deeper appreciation of the role of the dysregulation of
these kinases in EWS and their possible associations with patient's
prognosis is still indispensable.
Materials and methods
Clinical samples
Twenty-three consecutive primary EWS tumor samples
were obtained by surgeons from the Department of Biomechanics,
Medicine and Rehabilitation of the Locomotor System of the Clinics
University Hospital (Ribeirão Preto School of Medicine-University
of São Paulo) between May 2005 and September 2015. The survival
analysis was followed until June 2016. No local or systemic
treatment had been conducted in these patients before the surgery.
All samples were obtained with informed consent and the research
approved by the Ethics Committee of the University of São Paulo
(no. 43619215.9.0000.5407). Tissues were included in paraffin by
the Pathology department of the Clinics University Hospital
(Ribeirão Preto School of Medicine, University of São Paulo,
Ribeirão Preto, SP, Brazil).
Cell lines and reagents
The EWS cell lines SK-ES-1 and RD-ES were acquired
from the Rio de Janeiro Cell Bank (Rio de Janeiro, Brazil). Before
the experiments each cell line authentication was conducted in the
laboratory of Biochemical Genetics-FMRP/USP, by examining the
CSF1PO, D13S317, D16S539, D5S818, D7S820, THO1, TPOX, vWA, and AMEL
polymorphic loci for Short tandem repeat profiling (STR) under the
supervision of Professor Dr Aguinaldo Luiz Simões. Cells were grown
in McCoy's or RPMI medium (Gibco; Grand Island, NY, USA)
supplemented with 10% of fetal bovine serum and an antibiotic
mixture (100 units/ml penicillin, and 100 µg/ml streptomycin) and
maintained in an incubator at 37°C with 5% CO2 in a
humidified atmosphere.
Drug and treatments
The drugs, hydroxifasudil (pan-ROCK inhibitor),
GSK429286 and SR3677 (ROCK1 and ROCK2 inhibitor respectively) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). For all
experiments, the drugs were added on the culture medium immediately
before applied to cells. Corresponding control cultures received
equal volumes of solvent dimethyl sulfoxide (DMSO).
RNA isolation, reverse transcription
and quantitative real-time PCR of mRNA
Total RNA from cell lines was extracted using TRIzol
Reagent (Invitrogen, Karlsruche, Germany) following the
manufacturer's protocol. RNA samples from 9 osteoblast primary
cultures were kindly provided by Professor Adalberto Luiz Rosado
from the School of Dentistry of Ribeirão Preto, University of São
Paulo, and used as controls. The concentration and quality of the
RNA was accessed using a ND-1000 NanoDrop spectrophotometer
(NanoDrop Technologies; Thermo Fisher Scientific, Wilmington, DE,
USA). cDNA was synthetized using the High Capacity kit (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's instructions. The qRT-PCR was
performed using Taqman® gene assays [ROCK1
(Hs01127699-m1), ROCK2 (Hs00178154-m1)], according to the
manufacturer's protocol on the 7500 Real Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). As reference genes,
GUS was used to normalize expression levels. The MRC5 (normal
fibroblast) cell line were used as calibrator. Relative expression
was calculated by 2−ΔΔCT analysis method (11).
Western blot
Total protein was extracted from EWS cell lines with
RIPA buffer (Thermo Scientific, Inc.) according to manufacture
instructions. Equal amounts of heat-denatured protein samples (50
mg per lane) were separated on 10% SDS-polyacrylamide gel
electrophoresis and transferred to nitrocellulose membranes
(Amersham Pharmacia Biotech, Piscataway, NJ, USA). The antibodies
included primary rabbit monoclonal anti-ROCK1 (Ab45171; dilution,
1:500; Abcam, Cambridge, MA, USA), primary rabbit monoclonal
anti-ROCK2 (Ab125025; dilution, 1:10,000; Abcam) and rabbit
monoclonal anti-GAPDH antibody (AbEPR6256; dilution, 1:10,000;
Abcam). The immunoblots were developed using goat anti-rabbit
secondary antibody (Ab6721; dilution, 1:5,000; Abcam) followed by
detection with the ECL Western Blotting Substrate kit (Abcam,
Cambridge, UK) and visualized in a ChemiDoc Bioimaging System
(Bio-Rad, Hercules, CA, USA). Expression levels were quantified
using ImageJ® software and normalized to loading
controls.
Immunohistochemistry
Immunohistochemistry for ROCK1 and ROCK2 was
performed in 23 and 22 available tissue samples, respectively
(Anti-ROCK1; Ab45171, dilution, 1:250; Anti-ROCK2; Ab125025;
dilution, 1:200; Abcam). The reactions were performed with the
EXPOSE Mouse and Rabbit Specific HRP/DAB Detection IHC kit
(ab80436; Abcam) according to the manufacturer's recommendations.
Briefly, histological sections were submitted to xylol and alcohol
baths for complete dewaxing and subsequent hydration. Antigen
retrieval was performed in steam cooker for 40 min in Tris-EDTA pH
9.0 buffer. Blocking of endogenous peroxidase and nonspecific
binding was performed, respectively, with hydrogen peroxide (15
min) and blocking solution of the kit for 10 min. The primary
antibodies were diluted as recommended by the manufacturer and
incubated at room temperature for ~2 h. After washing, the sections
were incubated with the kit complement solution for 10 min and then
with the HRP conjugate for 15 min. Finally, the slides were
incubated with diaminobenzidine solution (DAB) for a standard time
for each antibody and counterstained with Harris Hematoxylin. A
colon adenocarcinoma biopsy was used as a positive control for the
anti-ROCK1 antibody, whereas a non-neoplastic kidney sample was
used as a control for ROCK2. For negative control of the reactions
the primary antibody was replaced with PBS buffer. For evaluation
of the immunostaining, the slides were scanned with an Olympus
BX61VS Slide Scanner system (Olympus Optical do Brasil Ltda, São
Paulo, Brasil) and at least five regions representative of the
tumor were analyzed using the IHC Profiler plugin, according to
Varghese et al (12). For
subsequent statistical analysis, the samples were classified into
two groups: Negative or positive (low positive, positive or high
positive).
Survival assay
Cell survival assays were performed through the XTT
kit (XTT II; Roche Molecular Biochemicals, Mannheim, Germany). In
summary, 2,000 cells were seeded in 96-well plates and allowed to
attach overnight. Subsequently, cells were treated with different
concentrations of each drug and incubated for 48, 72 and 96 h (h).
After treatment, the culture medium was replaced with medium
containing 10 µl of XTT dye (3 mg/ml) in each well. The plates were
incubated for 4 h at 37°C and the formazan product was measured at
455 and 650 nm by using an iMarkmicroplate reader (Bio-Rad). As a
control, cells treated with the same concentration of drug
vehicule, DMSO (Sigma-Aldrich) were used. Each experiment was
performed at least in triplicate wells and repeated in three sets
of tests.
Colony formation assay
Colony formation assays were performed according to
Franken et al (13). Briefly,
single cell suspensions of 1,000 cells were seeded in 6-well plates
and treated with several concentration of each drug for 48 h. After
this period, the culture medium were replaced with a drug-free
medium and cells incubated at 37°C for 7 to 15 days. Then, the
colonies were fixed with methanol and stained with Giemsa 3%. Only
colonies containing more than 50 cells were scored. Assays were
performed in duplicate in three independent sets of tests.
Cell cycle assay
Cells treated with each drug at different
concentrations for 24 h were detached by trypsin, fixed in 100%
ethanol and stained with propidium iodide for analysis on a Guava
Personal Cell Analysis system (Guava Technologies, Hayward, CA,
USA) according to the standard protocol provided by the
manufacturer. The percentages of cells in G0/G1, S and G2/M phase
were analyzed using the GUAVA Cytosoft software version 4.2.1
(Guava Technologies). All cell cycle assays were performed in
triplicate in three independent sets of tests.
Migration assay
Wound healing assays were performed according to
Liang et al (14). with minor
modifications. Succinctly, cells were grown to confluence on
12-well plates, and scratch wounds were created using a pipet tip
(200 µl) and photographed at time zero. Then, cells were treated
with different concentrations of each drug and cultured for 24 h in
medium with only 1% of fetal bovine serum. After that period, cells
were photographed. The cell-free area was measured with the Motic
Images Plus v2.0 software (Motic China Group Co., Ltd., Xiamen,
China). Cell migration rates were calculated as the distance
travelled by the cells in this area over time. Assays were
performed in duplicate in three independent sets of tests.
Invasion assay
Cell invasion was measured by migration of cells
through gel-coated Transwell inserts. EWS cells were harvested,
re-suspended in serum-free medium, treated with different
concentrations of each drug and seeded on the top of
Matrigel-coated invasion 8 µm pore size chambers (Becton Dickinson
& Co., Franklin Lakes, NJ, USA; 5×105 cell/insert).
Bellow the insert, the well was filled with medium containing 10%
fetal bovine serum. Cells were then allowed to migrate for 24 h in
an incubator and after that period non-invasive cells were removed
from the membrane upper surface with swabs. The ones attached to
the lower side of the membrane were fixed with 100% methanol and
stained with Giemsa 3%. The membranes were removed from the
inserts, placed on microscope slides with Entelan (Merk, NY, USA)
and counted with ImageJ® software in ten random fields
at magnification, ×20. Assay was performed in three sets of
independent experiments.
Statistical analysis
Associations between ROCK1 and ROCK2 protein
expression and the clinical variables [age (<14 years vs. >14
years old); sex (male vs. female); EWS/FLI1 status (positive vs.
negative); Huvos grade (<90% of necrotic areas-Huvos levels 1
and 2- vs. >90% necrotic areas-huvos levels 3 and 4; tumor
volume (<200 cm3 vs. >200 cm3); tumor
skeletal location (axial vs. appendicular); remission (incomplete
vs. complete); metastasis (presence vs. absence); relapse (presence
vs. absence); death (alive vs. deceased] were determined by
two-tailed Fisher's exact test. Survival analysis was carried out
based on Log-Rank tests represented on Kaplan-Meier curves. The
functional assays data was statistically analyzed by Student's
two-tailed t-test or One-Way Repeated Measures Analysis of Variance
(ANOVA) followed by the Bonferroni Pairwise Multiple Comparison.
All tests were carried out for α=0.05. All analyses were performed
using the SPSS 21.0 software (IBM SPSS, Armonk, NY, USA) and
expressed as the mean ± standard deviation.
Results
ROCK1 and ROCK2 expression in
pediatric EWS
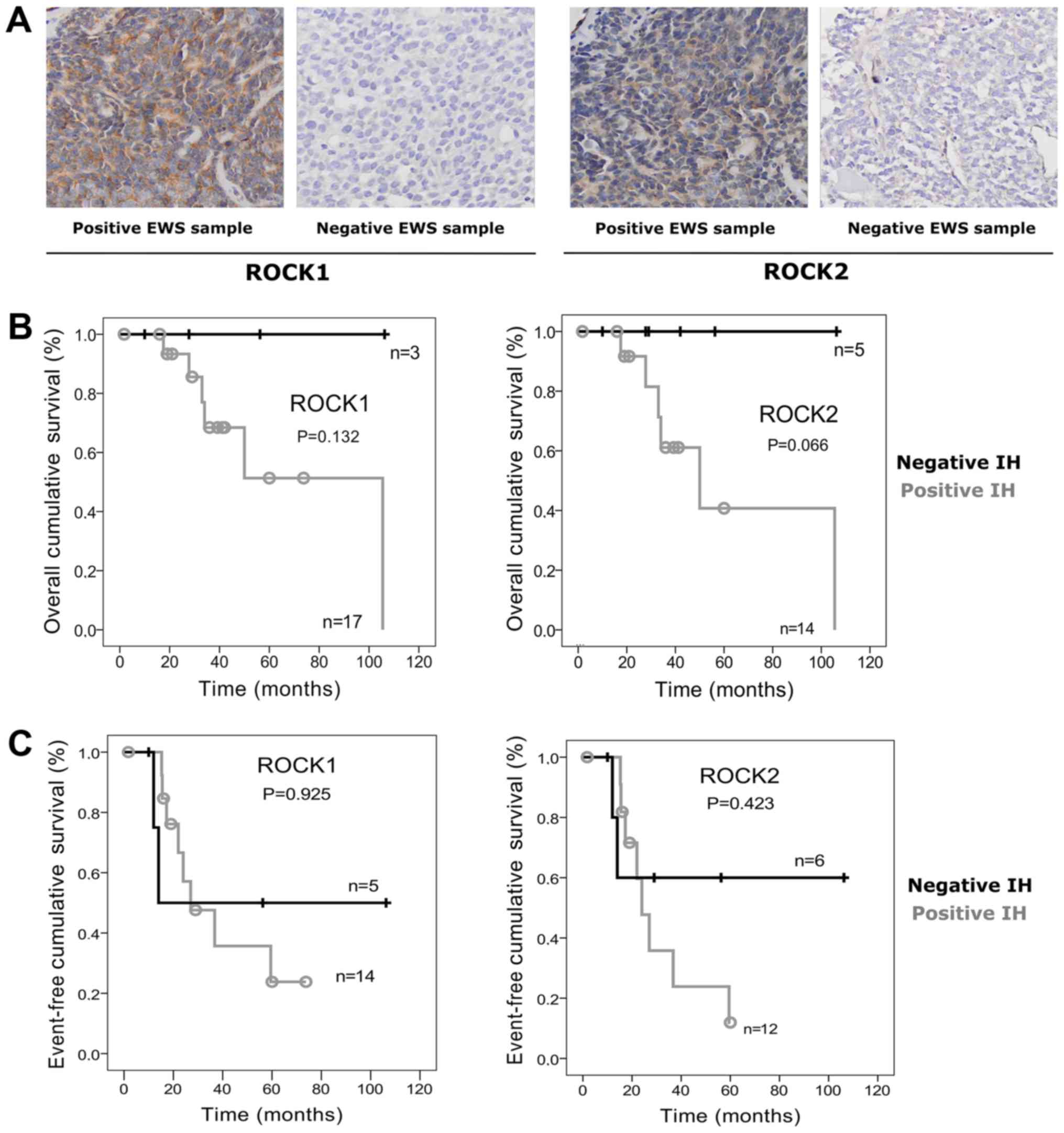
The majority of tumor samples presented positive
immunostaining for ROCK1 (17/23; 75%) and ROCK2 (14/23; 65%)
proteins (Fig. 1A). However, no
significant associations were observed between their expression and
any of the relevant clinical features such as Huvos classification,
FLI1/EWS status, relapse, metastasis, or death. Nonetheless,
we observed a trend for poorer outcome in patients with positive
samples, and significant higher risk of incomplete remission in
patients with ROCK2 positive tumors (OR=35.0, 95% CI, 1.74–702.9;
P=0.026). In addition, positivity for ROCK2 seems to indicate
increased risk of larger tumor volume (OR=2.33, 95% CI, 0.22–25.24;
P=0.58) (Table I). Moreover, ROCK1
and ROCK2 positivity was also suggestive of lower patient's
survival, even though no significant differences were found
(Fig. 1B). Event-free survival (EFS)
for ROCK1 was estimated at 23.8±14.1% for positive samples vs.
50±25% for negative ones (P=0.925). EFS of ROCK2 positive patients
was 11.9±11.1% vs. 60±21.9% (P=0.423) (Fig. 1C).
 | Table I.Clinical and pathological features of
patients with EWS and corresponding ROCK1 and ROCK2 immunostaining
profiles. |
Table I.
Clinical and pathological features of
patients with EWS and corresponding ROCK1 and ROCK2 immunostaining
profiles.
|
| ROCK1 (n=23) | ROCK2 (n=22) |
|---|
|
|
|
|
|---|
| Characteristic | (+) n=17 | (−) n=6 | Odds ratio (95%
CI) | P-value | (+) n=14 | (−) n=8 | Odds ratio (95%
CI) | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
Male | 8 | 5 | 5.6
(0.52–58.91) | 0.179 | 7 | 5 | 1.7
(0.28–9.82) | 0.675 |
|
Female | 9 | 1 |
|
| 7 | 3 |
|
|
| Age |
|
|
|
|
|
|
|
|
| <14
years | 7 | 2 | 1.4
(0.20–9.87) | 1.000 | 6 | 3 | 1.2
(0.21–7.41) | 1.000 |
| >14
years | 10 | 4 |
|
| 8 | 5 |
|
|
| Tumor
volumea |
|
|
|
|
|
|
|
|
| >200
cm3 | 7 | 2 | 1.2
(0.07–18.35) | 1.000 | 7 | 2 | 3.5
(0.28–43.2) | 0.530 |
| <200
cm3 | 3 | 1 |
|
| 2 | 2 |
|
|
| Huvos
gradea |
|
|
|
|
|
|
|
|
|
1–2 | 5 | 1 | 2.5
(0.19–32.19) | 0.604 | 4 | 2 | 1.2
(0.13–11.0) | 1.000 |
|
3–4 | 6 | 3 |
|
| 5 | 3 |
|
|
| Skeletal
locationa |
|
|
|
|
|
|
|
|
|
Axial | 8 | 2 | 2.0
(0.28–14.2) | 0.646 | 8 | 2 | 4.8
(0.68–33.8) | 0.183 |
|
Appendicular | 8 | 4 |
|
| 5 | 6 |
|
|
|
Remissiona |
|
|
|
|
|
|
|
|
|
Incomplete | 7 | 1 | 5.2
(0.40–68.95) | 0.282 | 7 | 1 | 35.0
(1.7–702.9) | 0.026b |
|
Complete | 4 | 3 |
|
| 1 | 5 |
|
|
|
EWS/FL1a |
|
|
|
|
|
|
|
|
|
Positive | 9 | 3 | Not calculated |
| 8 | 3 | Not calculated |
|
|
Negative | 1 | 0 |
|
| 1 | 0 |
|
|
| Events |
|
|
|
|
|
|
|
|
|
Metastasis |
|
|
|
|
|
|
|
|
|
Yes | 7 | 3 | 0.7
(0.11–4.54) | 1.000 | 7 | 3 | 1.7
(0.28–9.82) | 0.675 |
| No | 10 | 3 |
|
| 7 | 5 |
|
|
|
Relapsea |
|
|
|
|
|
|
|
|
|
Yes | 7 | 1 | 2.3
(0.20–27.6) | 0.619 | 7 | 1 | 5.8
(0.52–64.8) | 0.177 |
| No | 9 | 3 |
|
| 6 | 5 |
|
|
| Deatha |
|
|
|
|
|
|
|
|
|
Yes | 6 | 0 | Not calculated |
| 6 | 0 | Not calculated |
|
| No | 11 | 4 |
|
| 8 | 6 |
|
|
ROCK2, but not ROCK1 is overexpressed
in EWS cell lines
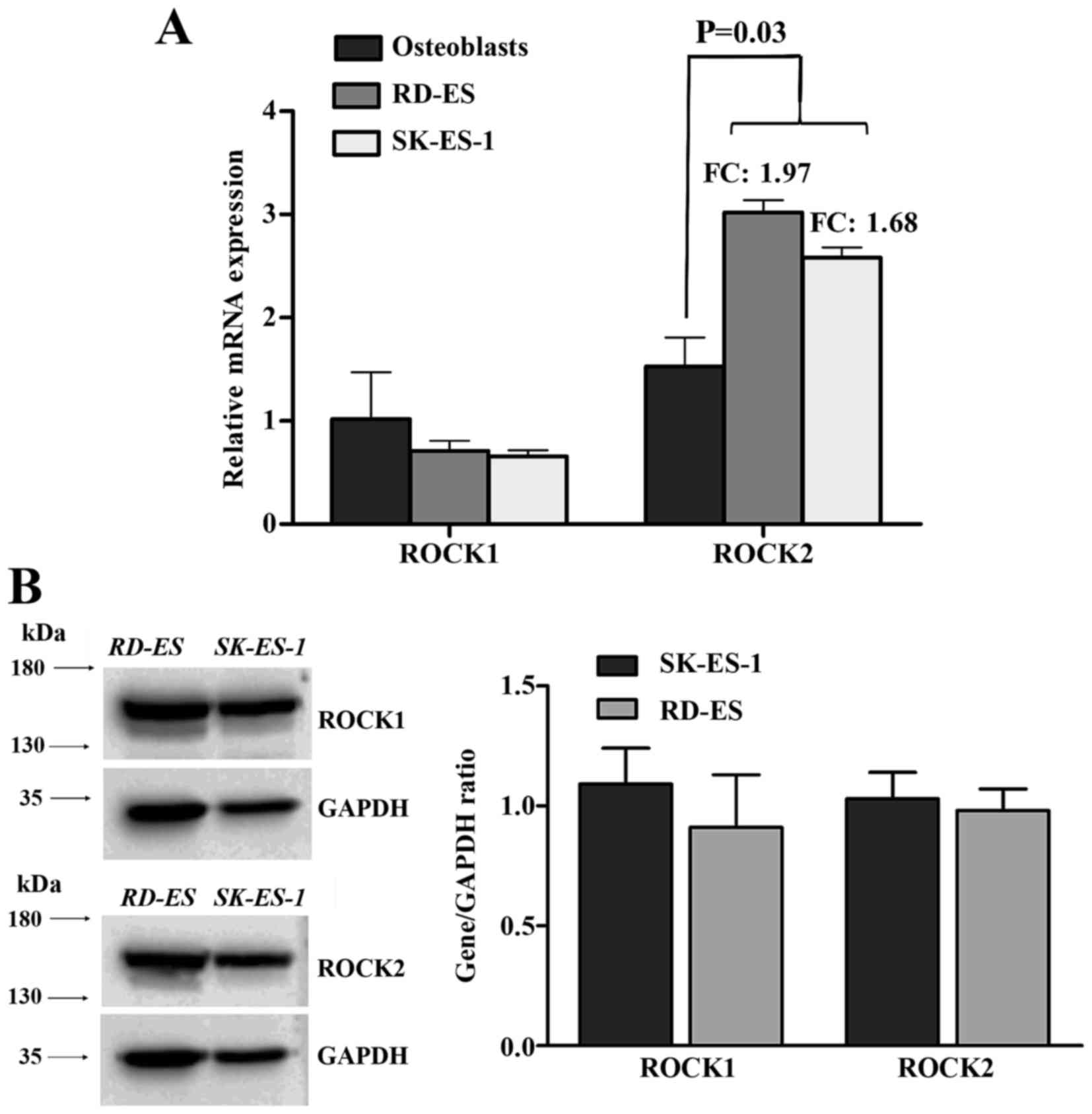
mRNA expression levels of ROCK1 and ROCK2 were
evaluated in two EWS cell lines, SK-ES-1 and RD-ES through
quantitative real-time PCR. As seen in Fig. 2A, ROCK1 did not show any significant
difference in expression when compared to the control (nine primary
osteoblast cell lines). Conversely, ROCK2 was found with
significant higher expression (P=0.03) compared to controls
(fold-change 1.97 for RD-ES and 1.68 for SK-ES-1). Protein
expression levels of both kinases were found comparable (Fig. 2B).
Inhibition of ROCK1 or ROCK2 does not
show antitumor effects
To investigate the prospect of targeting ROCK1 and
ROCK2 in EWS we evaluated the in vitro effects of three
specific inhibitors on cell viability, clonogenicity and cell cycle
in the SK-ES-1 and RD-ES EWS cell lines. For each experiment the
drugs GSK924286, a specific ROCK1 inhibitor, SR3677, a specific
ROCK2 inhibitor and hydroxyfasudil, a pan-ROCK inhibitor were used
at different doses according with manufacturer's instructions.
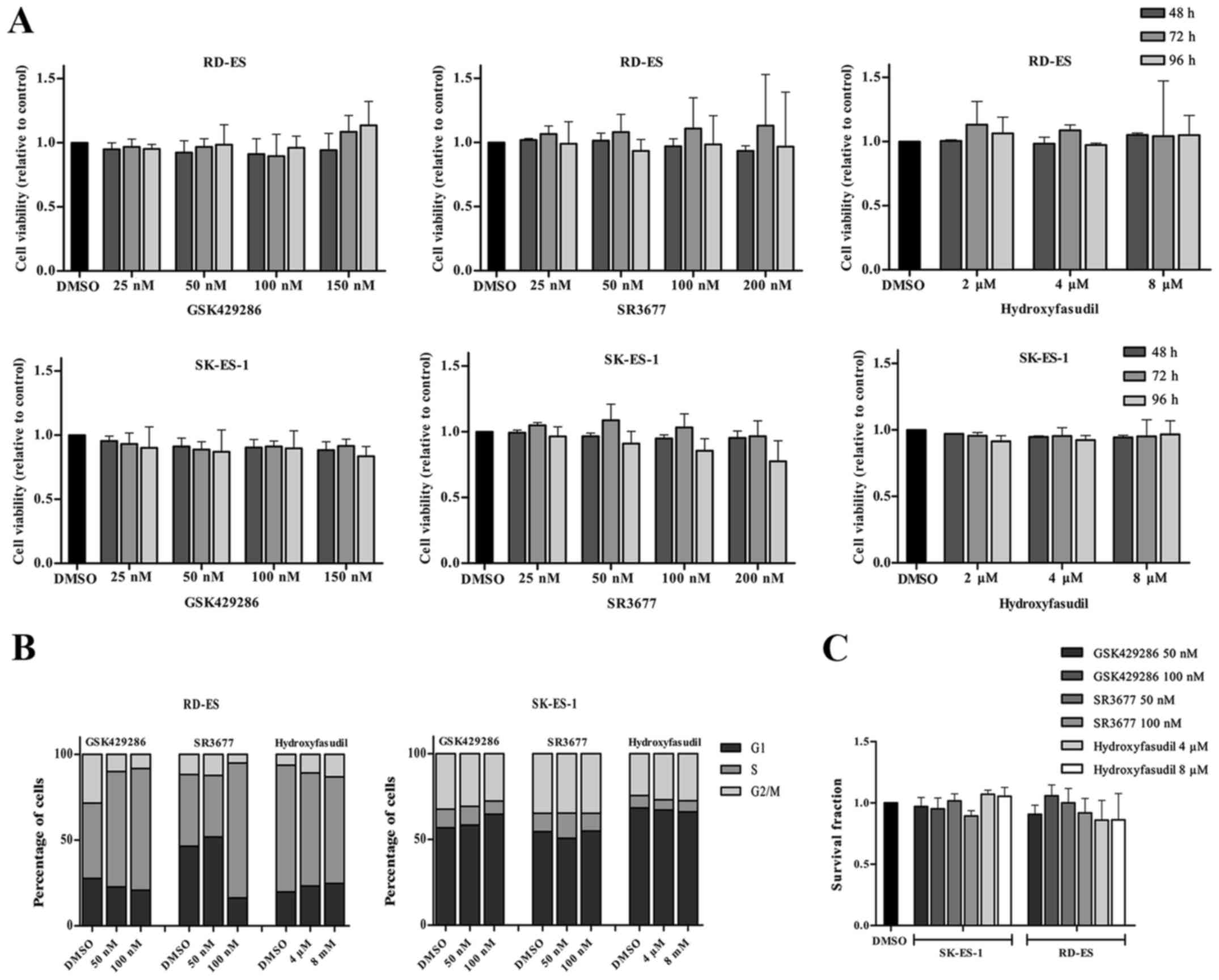
Firstly, doses comprising the IC50 reported values were
used, being 3.5, 7, 14 and 21 nM for GSK924286, 1.25 nM, 2.5, 5 and
10 nM for SR3677 and 0.3, 0.6, 1.2 and 2.4 µM for hydroxyfasudil.
Cells were treated for 24, 48, 72 and 96 h though none of these
treatments affected cell viability at any time (data not shown).
Then, doses were increased to 25, 50, 100 and 150 nM for GSK924286,
25, 50, 100 and 200 nM for SR3677 and 2, 4 and 8 µM for
hydroxyfasudil and cells treated for the same periods. Nonetheless,
cell viability was not affected again (Fig. 3A). For the other functional assays,
doses of 50 nM e 100 nM were chosen for GSK924286 and SR3677 and of
4 and 8 µM for hydroxyfasudil. Likewise, and cell cycle dynamics
and the clonogenic capacity were not significantly affected by
inhibition of ROCKs (Fig. 3B and
C).
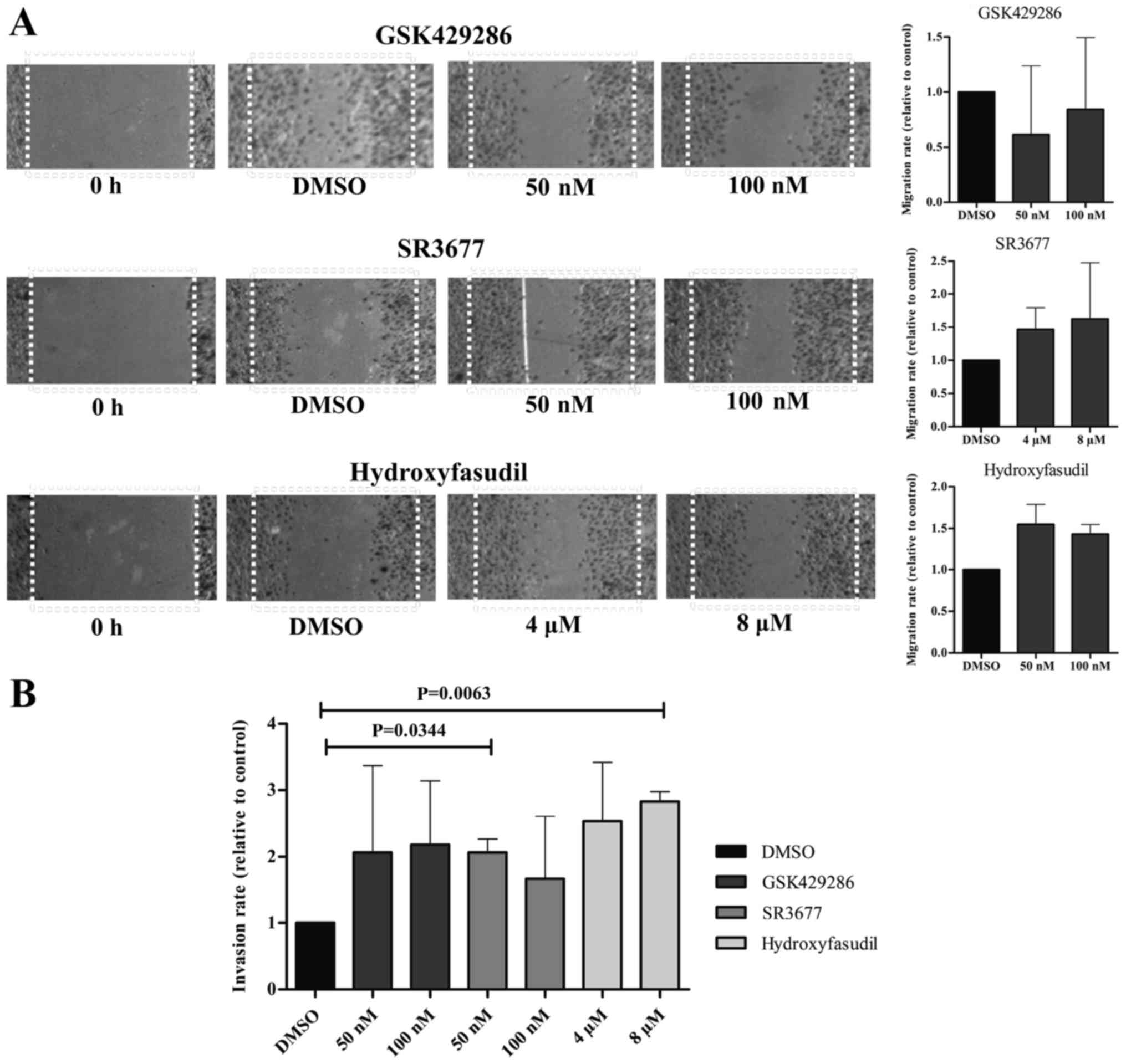
Moreover, the migration capacity of SK-ES-1 cell
line was not significantly altered after treatment with any of the
drugs as seen through the wound healing assay. Nonetheless, gap
closure under treatment with the ROCK2-inhibitor and the
pan-inhibitor was increased in ~50% (Fig.
4A). This effect on the migratory capacity of the cells was
evinced by the invasion assays, where treatment with SR377 50 nM
and hidroyfasudil 8 µM induced higher penetrance of cells through
the Matrigel layer (P=0.0063 and P=0.0344, respectively) (Fig. 4B).
Discussion
The Rho-associated kinases ROCK1 and ROCK2 are key
regulators of cellular shape and motility by acting on the
cytoskeleton (15,16). Over the last decade, their
dysregulation has been frequently associated with several
carcinogenic and metastasis-related processes such as cell
adhesion, migration and invasion (5,6,10,17).
Nevertheless, their roles in EWS tumorigenesis/progression and
their clinical significance have not been clearly elucidated.
In most tumors studied so far, ROCK1 and
ROCK2 are described as oncogenes (7,18–22). Moreover, strong associations between
ROCK1 and ROCK2 upregulation and poor prognosis have been described
in osteosarcoma, gastric and laryngeal squamous cell carcinoma
(7,20,22).
In agreement with these studies, our results showed
positive immunostaining for ROCK1 and ROCK2 in the majority of
pediatric EWS tumor samples. Furthermore, even though the
correlation between patient's survival and ROCK1 or ROCK2
positivity was only suggestive and there were no associations with
clinical features such as HUVOS classification, FLI1/EWS
status, relapse, metastasis or death, we found a significantly
increased risk of incomplete remission in patients with positive
immunostaining for ROCK2. Nonetheless, these results need to be
viewed with caution because of the small number of samples
evaluated.
Higher levels of ROCK2 gene expression were
also found in EWS cell lines (SK-ES-1 and RD-ES) with conspicuous
protein expression of both kinases. In view of these, we tested the
prospect of using the pharmacological inhibition of either ROCK
through several functional assays in vitro. Three ROCK
inhibitors were used: One specific for ROCK1 (GSK429286), one
specific for ROCK2 (SR3677) and a pan-inhibitor
(hydroxyfasudil).
Initially, we used the doses ranging within the
IC50 indicated by the manufacturers, but there were no
changes on cell viability after treatment with any of the drugs at
such doses. Treatment was also ineffective even after increasing
the doses ~10 times (proliferation and cell cycle). The clonogenic
capacity assay, which not only predicts long-term cell viability,
but also evaluates the sum of all forms of cell death, also failed
to demonstrate any cellular influence irrespective of inhibition of
either ROCK1, ROCK2 or both kinases.
Several authors have demonstrated that inhibition of
ROCK1 and ROCK2 using other strategies (such as siRNA or microRNAs)
causes a decrease in cell invasion and migration in various types
of neoplasia (6,8,9,23,24).
Similar results have also been reported after treating cells with
other inhibitory compounds such as HA-1077 (fasudil), WF-536,
Y-27632 and RKI-1447 (8,23,25–28).
The potentiality and selectivity of GSK429286,
SR3677, hydroxyfasudil have been repeatedly confirmed in tumors of
different origins even using comparable or lower doses than those
used in the present study (29–36).
Most recently, Pinca et al (4) performed and in vitro study were
they demonstrated ROCK1 and ROCK2 expression in a panel (n=9) of
EWS cell lines, and showed that inhibition of these kinases with
Y27632 or SR3677 resulted in diminished growth and migration
capacity in two EWS cell lines (SK-ES-1 and 6647). However, the
inhibitory effects were independent of the protein levels (ROCK1
was ~3× less expressed in SK-ES-1, for instance). Moreover,
individually, the authors showed that ROCK1 expression was higher
in the RD-ES cell line whereas ROCK2 expression was higher in the
SK-ES-1 cell line, what was not reflected in our study. Similarly,
even after treating the same cell line (SK-ES-1) with the same drug
(SR3677) functional assays were not reproducible, though Pinca
et al (4) used a ×100 higher
concentration, which is more than ×3,000 higher than the
IC50 reported by the manufacturers. Consequently, their
in vitro data might point towards a certain resistance to
ROCK inhibition in EWS cells. Of note, it is well established that
>1 µM SR3677 acts on several off-target kinases (33) including PKA [which promotes tumor
growth and metastasis in EWS (37),
MRCK [another mediator of cell contractibility (38) and AKT1 that plays important roles in
EWS survival (39).
Moreover, our experiments also showed disparate
results on cell migration and invasion which were increased after
treatment of the SK-ES-1 cell line with SR3677 and Hydroxyfasudil,
suggesting a stimulating effect after ROCK2 inhibition. Abe et
al (28) previously reported
similar results after treating urothelial carcinoma cells with
HA-1077. Likewise, Mertsch and Thanos (40) demonstrated that knockdown of ROCK2
significantly increases the invasive potential of cells in a
substrate independent manner.
In this way, even though the majority of EWS samples
included in our study showed positivity for ROCK1 and ROCK2, the
lack of conspicuous associations with prognosis and absence of
effective responses to their inhibition in vitro, do not
support their prospect use as therapeutic targets for the treatment
of this highly metastatic tumor.
In the future, larger cohort studies might provide
more evidence on whether there is a specific role of ROCK kinases
in EWS physiopathology.
Acknowledgements
The authors would like to acknowledge FAPESP for
financial support and to thank Mrs. Monica Azevedo de Abreu for
technical assistance. FAPESP (Fundação de Amparo à Pesquisa do
Estado de São Paulo; grant 2014/03877-3) and VGM fellowship
2014/07118-0.
References
|
1
|
West DC: Ewing sarcoma family of tumors.
Curr Opin Oncol. 12:323–329. 2017. View Article : Google Scholar
|
|
2
|
Ladenstein R, Pötschger U, Le Deley MC,
Whelan J, Paulussen M, Oberlin O, van den Berg H, Dirksen U, Hjorth
L, Michon J, et al: Primary disseminated multifocal Ewing sarcoma:
Results of the Euro-EWING 99 trial. J Clin Oncol. 28:3284–3291.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bernstein M, Kovar H, Paulussen M, Randall
RL, Schuck A, Teot LA and Juergens H: Ewing's sarcoma family of
tumors: Current management. Oncologist. 11:503–519. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pinca RS, Manara MC, Chiadini V, Picci P,
Zucchini C and Scotlandi K: Targeting ROCK2 rather than ROCK1
inhibits Ewing sarcoma malignancy. Oncol Rep. 37:1387–1393. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu CY, Chang ZF and Lee HH:
Immunohistochemical evaluation of ROCK activation in invasive
breast cancer. BMC Cancer. 15:9432015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vigil D, Kim TY, Plachco A, Garton AJ,
Castaldo L, Pachter JA, Dong H, Chen X, Tokar B, Campbell SL and
Der CJ: ROCK1 and ROCK2 are required for non-small cell lung cancer
anchorage-independent growth and invasion. Cancer Res.
72:5338–5347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, He X, Ma Y, Liu Y, Shi H, Guo W
and Liu L: Overexpression of ROCK1 and ROCK2 inhibits human
laryngeal squamous cell carcinoma. Int J Clin Exp Pathol.
8:244–251. 2015.PubMed/NCBI
|
|
8
|
Zhang P, Lu Y, Liu XY and Zhou YH:
Knockdown of Rho-associated protein kinase 1 suppresses
proliferation and invasion of glioma cells. Tumour Biol.
36:421–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kroiss A, Vincent S, Decaussin-Petrucci M,
Meugnier E, Viallet J, Ruffion A, Chalmel F, Samarut J and Allioli
N: Androgen-regulated microRNA-135a decreases prostate cancer cell
migration and invasion through downregulating ROCK1 and ROCK2.
Oncogene. 34:2846–2855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun K, Duan X, Cai H, Liu X, Yang Y, Li M,
Zhang X and Wang J: Curcumin inhibits LPA-induced invasion by
attenuating RhoA/ROCK/MMPs pathway in MCF7 breast cancer cells.
Clin Exp Med. 16:37–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Varghese F, Bukhari AB, Malhotra R and De
A: IHC Profiler: An open source plugin for the quantitative
evaluation and automated scoring of immunohistochemistry images of
human tissue samples. PLoS One. 9:e968012014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsumura F: Regulation of myosin II
during cytokinesis in higher eukaryotes. Trends Cell Biol.
15:371–377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Izawa I and Inagaki M: Regulatory
mechanisms and functions of intermediate filaments: A study using
site- and phosphorylation state-specific antibodies. Cancer Sci.
97:167–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi J, Surma M, Zhang L and Wei L:
Dissecting the roles of ROCK isoforms in stress-induced cell
detachment. Cell Cycle. 12:1492–1500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–2641. 2003.PubMed/NCBI
|
|
19
|
Lane J, Martin TA, Watkins G, Mansel RE
and Jiang WG: The expression and prognostic value of ROCK I and
ROCK II and their role in human breast cancer. Int J Oncol.
33:585–593. 2008.PubMed/NCBI
|
|
20
|
Liu X, Choy E, Hornicek FJ, Yang S, Yang
C, Harmon D, Mankin H and Duan Z: ROCK1 as a potential therapeutic
target in osteosarcoma. J Orthop Res. 29:1259–1266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Babeto E, Conceição AL, Valsechi MC,
Junior P Peitl, de Campos Zuccari DA, de Lima LG, Bonilha JL, de
Freitas Calmon M, Cordeiro JA and Rahal P: Differentially expressed
genes in giant cell tumor of bone. Virchows Arch. 458:467–476.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu YJ, Tang Y, Li ZF, Li Z, Zhao Y, Wu ZJ
and Su Q: Expression and significance of Rac1, Pak1 and Rock1 in
gastric carcinoma. Asia Pac J Clin Oncol. 10:e33–e39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Itoh K, Yoshioka K, Akedo H, Uehata M,
Ishizaki T and Narumiya S: An essential part for Rho-associated
kinase in the transcellular invasion of tumor cells. Nat Med.
5:221–225. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Genda T, Sakamoto M, Ichida T, Asakura H,
Kojiro M, Narumiya S and Hirohashi S: Cell motility mediated by rho
and Rho-associated protein kinase plays a critical role in
intrahepatic metastasis of human hepatocellular carcinoma.
Hepatology. 30:1027–1036. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takamura M, Sakamoto M, Genda T, Ichida T,
Asakura H and Hirohashi S: Inhibition of intrahepatic metastasis of
human hepatocellular carcinoma by Rho-associated protein kinase
inhibitor Y-27632. Hepatology. 33:577–581. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakajima M, Katayama K, Tamechika I,
Hayashi K, Amano Y, Uehata M, Goto N and Kondo T: WF-536 inhibits
metastatic invasion by enhancing the host cell barrier and
inhibiting tumour cell motility. Clin Exp Pharmacol Physiol.
30:457–463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patel RA, Forinash KD, Pireddu R, Sun Y,
Sun N, Martin MP, Schönbrunn E, Lawrence NJ and Sebti SM: RKI-1447
is a potent inhibitor of the Rho-associated ROCK kinases with
anti-invasive and antitumor activities in breast cancer. Cancer
Res. 72:5025–5034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abe H, Kamai T, Hayashi K, Anzai N,
Shirataki H, Mizuno T, Yamaguchi Y, Masuda A, Yuki H, Betsunoh H,
et al: The Rho-kinase inhibitor HA-1077 suppresses
proliferation/migration and induces apoptosis of urothelial cancer
cells. BMC Cancer. 14:4122014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohnaka K, Shimoda S, Nawata H, Shimokawa
H, Kaibuchi K, Iwamoto Y and Takayanagi R: Pitavastatin enhanced
BMP-2 and osteocalcin expression by inhibition of Rho-associated
kinase in human osteoblasts. Biochem Biophys Res Commun.
287:337–342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimokawa H: Rho-kinase as a novel
therapeutic target in treatment of cardiovascular diseases. J
Cardiovasc Pharmacol. 39:319–327. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hinderling PH, Karara AH, Tao B, Pawula M,
Wilding I and Lu M: Systemic availability of the active metabolite
hydroxy-fasudil after administration of fasudil to different sites
of the human gastrointestinal tract. J Clin Pharmacol. 47:19–25.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goodman KB, Cui H, Dowdell SE,
Gaitanopoulos DE, Ivy RL, Sehon CA, Stavenger RA, Wang GZ, Viet AQ,
Xu W, et al: Development of dihydropyridone indazole amides as
selective Rho-kinase inhibitors. J Med Chem. 50:6–9. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng Y, Yin Y, Weiser A, Griffin E,
Cameron MD, Lin L, Ruiz C, Schürer SC, Inoue T, Rao PV, et al:
Discovery of substituted
4-(pyrazol-4-yl)-phenylbenzodioxane-2-carboxamides as potent and
highly selective Rho kinase (ROCK-II) inhibitors. J Med Chem.
51:6642–6645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang HL, Hu SH, Chou AH, Wang SS, Weng YH
and Yeh TH: H1152 promotes the degradation of
polyglutamine-expanded ataxin-3 or ataxin-7 independently of its
ROCK-inhibiting effect and ameliorates mutant ataxin-3-induced
neurodegeneration in the SCA3 transgenic mouse. Neuropharmacology.
70:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nichols RJ, Dzamko N, Hutti JE, Cantley
LC, Deak M, Moran J, Bamborough P, Reith AD and Alessi DR:
Substrate specificity and inhibitors of LRRK2, a protein kinase
mutated in Parkinson's disease. Biochem J. 424:47–60. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chapman S, McDermott DH, Shen K, Jang MK
and McBride AA: The effect of Rho kinase inhibition on long-term
keratinocyte proliferation is rapid and conditional. Stem Cell Res
Ther. 5:602014. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo W, Xu C, Ayello J, et al: Protein
phosphatase 1 regulatory subunit 1A (PPP1R1A) promotes tumor growth
and metastasis via inhibition of protein phosphatase 1 in Ewing
sarcoma. AACR 107th Annual Meeting 2016 American Association for
Cancer Research. New Orleans, LA. 2016;
|
|
38
|
Bouin AP, Kyumurkov A, Régent-Kloeckner M,
Ribba AS, Faurobert E, Fournier HN, Bourrin-Reynard I, Manet-Dupé
S, Oddou C, Balland M, et al: ICAP-1 monoubiquitylation coordinates
matrix density and rigidity sensing for cell migration through
ROCK2-MRCKα balance. J Cell Sci. 130:626–636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hotfilder M, Sondermann P, Senss A, van
Valen F, Jürgens H and Vormoor J: PI3K/AKT is involved in mediating
survival signals that rescue Ewing tumour cells from fibroblast
growth factor 2-induced cell death. Br J Cancer. 92:705–710. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mertsch S and Thanos S: Opposing signaling
of ROCK1 and ROCK2 determines the switching of substrate
specificity and the mode os migration of glioblastoma cells. Mol
Neurobiol. 49:900–915. 2014. View Article : Google Scholar : PubMed/NCBI
|