Introduction
Activin A, a homodimer of two inhibin βA subunits
linked by disulphide, belongs to the transforming growth factor β
(TGF-β) superfamily. It was initially isolated from porcine
follicular fluid and is also known as follicle-stimulating hormone
(FSH) releasing protein, due to its capacity to induce the release
of FSH from the pituitary gland (1).
Subsequent research has demonstrated that activin A is involved in
pleiotropic functions including inflammation, arterial pressure
regulation, embryonic development and tumourigenesis (2–5). As with
other TGF-β superfamily members, activin A functions through the
serine/threonine kinase pathway. Activin A initially binds to
activin type II receptors (ActRII), and then recruits activin type
I receptors to phosphorylate and activate SMAD family member (Smad)
2/3 in the cytoplasm, with this being the common signaling molecule
between activin and TGF-β (6). The
activated Smad2/3 forms a complex with Smad4 and then the complex
is translocated into the nucleus, resulting in downstream target
gene transcription to elicit biological effects, including cell
differentiation, proliferation and apoptosis (7,8).
Activin A is known to modulate multiple types of
cancer. It not only promotes the genesis and progression of certain
tumors, but also inhibits tumorigenesis in other instances,
depending on the cell types involved and other interacting pathways
(6). For example, activin A
suppresses the proliferation of breast cancer cells and pituitary
adenoma cells, while promoting the proliferation of ovarian
carcinoma cells. Myeloma cells induce bone marrow stromal cells to
secrete activin A, which in turn inhibits osteogenesis and induces
osteolysis and cachexia (9). However,
the effects of activin A on myeloma cell viability and apoptosis
remain unclear. Therefore, in the present study, the effects of
activin A on viability and apoptosis in the mouse myeloma cell line
NS-1 were investigated, and the mechanisms underlying activin A
action were analyzed by examining the expression of
apoptosis-associated proteins.
Materials and methods
Reagents
Activin A (cat. no. 338-AC) and
allophoycocyanin-labeled ActRIIA antibodies (cat. no. FAB340A) were
obtained from R&D Systems, Inc. (Minneapolis, MN, USA).
RPMI-1640 culture medium was bought from Gibco; Thermo Fisher
Scientfic, Inc. (Waltham, MA, USA). The Reverse
transcription-polymerase chain reaction (RT-PCR) kit was provided
by Takara Biotechnology Co., Ltd. (Dalian, China). Protein
extraction kits were bought from Thermo Fisher Scientific, Inc.
GAPDH antibodies (cat. no. KM9002) were purchased from Tianjin
Sungene Biotech Co., Ltd. (Tianjin, China). The
5-bromo-2′-deoxyuridine (BrdU) cell proliferation kit and
fluorescein isothiocyanate (FITC)-Annexin V apoptosis kit were
obtained from Roche Diagnostics (Indianapolis, IN, USA). B cell
lymphoma-2 (Bcl-2) (cat. no. 554218) antibodies were bought from BD
Biosciences (Franklin Lakes, NJ, USA). Cleaved caspase-3 antibodies
(cat. no. 9661), BCL2 associated X, apoptosis regulator (Bax) (cat.
no. 3498) and cytochrome c (Cyt c) antibodies (cat.
no. 2772) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Goat anti-rabbit immunoglobulin (Ig)
G-Peroxidase antibodies (cat. no. A0545) and anti-mouse
IgG-Peroxidase antibodies (cat. no. A3682) were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). ECL-Plus (cat. no.
RPN2232) was supplied by GE Healthcare Life Sciences (Little
Chalfont, UK).
Cell culture
The mouse myeloma NS-1 cell line was provided by the
American Type Culture Collection (Manasass, VA, USA) and cultured
in RPMI-1640 medium with 10% fetal bovine serum (cat. no.
04-001-1ACS; Biological Industries, Kibbutz Beit-Haemek, Israel) in
a 5% CO2-humidified atmosphere at 37°C.
RT-PCR
Total RNA from NS-1 cells was extracted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance
with the manufacturer's protocol). cDNA synthesis was performed
using a PrimeScript® 1st Strand cDNA Synthesis kit
(Takara Biotechnology Co., Ltd.) and the cDNA was amplified using
Premix Taq™ (cat. no. R004A; Takara Biotechnology Co.,
Ltd.) with target gene-specific primers according to the
manufacturers protocol. PCR was performed using the following
reaction conditions: 94°C for 30 sec (35 cycles), 55°C for 20 sec
(35 cycles), 72°C for 40 sec (35 cycles) and final extension was
72°C for 10 min. The primers were as follows: ActRIIA forward,
5′-ATTGGCCAGCATCCATCTCTTG-3′ and reverse,
5′-GCCACCATCATAGACTAGATTC-3′; ActRIIB forward,
5′-TGCTGAAGAGCGACCTCAC-3′ and reverse, 5′-AGCAGGTCCACATTGGTGAC-3′;
GAPDH forward, 5′-GATTGTTGCCATCAACGACC-3′ and reverse,
5′-GTGCAGGATGCATTGCTGAC-3′. GAPDH was used as a positive internal
reference, and adding no cDNA to the PCR reaction system was
regarded as a negative control. PCR products were subjected to 2%
agarose gel electrophoresis with ethidium bromide at room
temperature for 1 h. The specific bands were visualized using an
ImageMaster VDS system (GE Healthcare Life Sciences, Little
Chalfont, UK). Densitometric quantification of mRNA was normalized
to the internal control GAPDH.
BrdU cell viability assay
NS-1 cells (2×104 cells/well) were seeded
into 96-well culture plates in RPMI 1640 medium with 5% fetal
bovine serum (cat. no. 04-001-1ACS; Biological Industries, Kibbutz
Beit-Haemek, Israel) in 5% CO2 at 37°C. The cells were
then treated with 0–20 ng/ml of activin A (2 µg/ml) for 24 h,
followed by incubation with 10 µmol/l BrdU at 37°C for 2 h. The
supernatant was discarded, and 100 µl FixDenat per well was added
and incubated for a further 30 min at room temperature. The
supernatant was removed, 100 µl anti-BrdU-peroxidase working
solution was added, and cells were incubated at 37°C in the dark
for 90 min. The cells were washed three times with PBS and
substrate solution (100 µl per well) was added at room temperature
for 10 min. Finally, 1 mol/l H2SO4 25 µl was
added, and the absorbance at 450 nm was detected to evaluate cell
viability.
Hoechst 33342 staining
NS-1 cells (2×104 cells/well) were seeded
into 96-well culture plates in RPMI 1640 medium with 5% fetal
bovine serum, and treated with 10 ng/ml activin A for 24 h at 37°C.
The cells were washed three times with PBS and incubated with 50 µl
Hoechst 33342 (100 µg/ml) at room temperature for 10 min. The cells
were washed twice in PBS, and then cell morphology was observed
under a fluorescence microscope (IX71) and photographed with a
microscope digital camera system (Olympus Corporation, Tokyo,
Japan).
Flow cytometry for cells apoptosis
assay
NS-1 cells (1×106 cells/well) were seeded
in 12-well culture plates in RPMI 1640 medium with 5% fetal bovine
serum. Following treatment with 10 ng/ml of activin A at 37°C for
24 h, NS-1 cells were collected and re-suspended in 100 µl
fluorescence-activated cell sorting buffer, followed by the
addition of 1 µl FITC-Annexin V and 1 µl 7-aminoactinomycin (7-AAD)
at room temperature for 5 min in the dark. Then, the labeled cells
were analyzed using flow cytometry (BD Calibur; BD Biosciences).
Data were collected and analyzed with Cell Quest software (version
7.6; BD Biosciences) to obtain the percentage of fluorescence
cells.
Western blotting
NS-1 cells (1×106 cells/well) were seeded
into 12-well culture plates in RPMI 1640 medium with 5% fetal
bovine serum. Following treatment with activin A at 37°C for 12 h,
cells were resuspended with PBS and harvested using centrifugation
at 850 × g for 10 min at 4°C, then lysed in protein lysis buffer
(1% Nonidet P-40, 50 mmol/l Tris-HCl (pH 7.5), 150 mmol/l NaCl, 1
mmol/l NaF, 1 mmol/l phenylmethylsulfonyl fluoride, 4 µg/ml
leupeptin and 1 µg/ml aprotinin) at 37°C for 15 min, followed by
vortex at room temperature for 15 min. The lysate was removed using
centrifugation at 15,000 × g for 10 min at 4°C, and protein (30 µg)
were separated by SDS-PAGE (12% gel) and transferred onto a
polyvinylidene difluoride membrane. Then, the membrane was blocked
with 2% BSA (cat. no. B2064; Sigma-Aldrichl; Merck KGaA) for 1 h at
room temperature and probed with mouse Bcl-2 antibodies (1:1,000
dilution), rabbit Bax antibodies (1:500 dilution), rabbit cleaved
capase-3 antibodies (1:500 dilution), rabbit Cyt c
antibodies (1:500 dilution) and mouse GAPDH antibodies (1:1,000) at
4°C overnight. Following washing with 1× TBST with 0.1% Tween-20
three times, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG antibodies (1:160,000;
cat. no. A0545; Sigma-Aldrich; Merck KGaA) or anti-mouse IgG
antibodies (1:160,000; cat. no. A3682; Sigma-Aldrich; Merck KGaA)
for 1 h at room temperature. Labeled proteins were detected using
enhanced chemiluminescence reagents within the ECL-Plus with
ImageQuant LAS 4010 kit (GE Healthcare Life Sciences) and analyzed
using ImageJ software (v1.43, NIH USA). Protein levels were
normalized to the internal control GAPDH.
Statistical analysis
Experiments were repeated at least three times. All
data were presented as the mean ± standard deviation. Statistical
comparisons were performed using one-way analysis of variance,
followed by the Scheffe's test. The statistical differences between
two groups were determined using Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of ActRIIA in NS-1
cells
Activin A initially binds to ActRIIA or ActRIIB, and
then activates downstream signaling molecules (10). To confirm whether activin A exerted an
effect on NS-1 cells, ActRII mRNA expression was initially examined
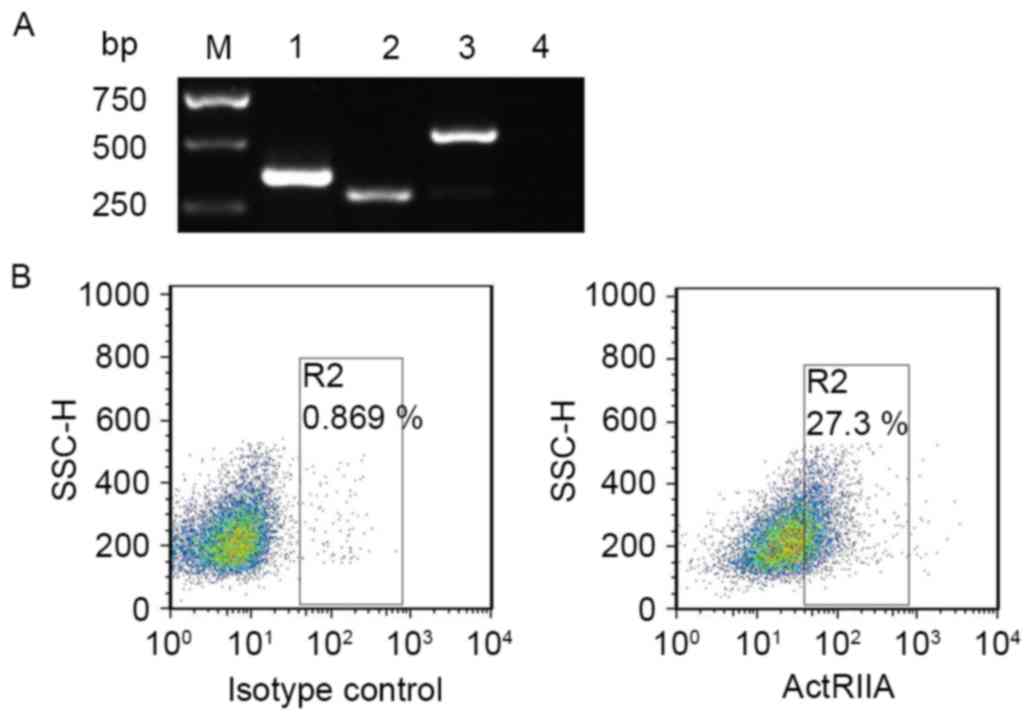
in NS-1 cells. The results of RT-PCR revealed that ActRIIA and
ActRIIB mRNA expression were detectable in NS-1 cells (Fig. 1A). Furthermore, flow cytometry results
also demonstrated that ActRIIA protein was expressed on NS-1 cells
(Fig. 1B). These data suggested that
activin A exerted an effect on NS-1 cells via binding ActRII.
Inhibitory effects of activin A on
NS-1 cell viability
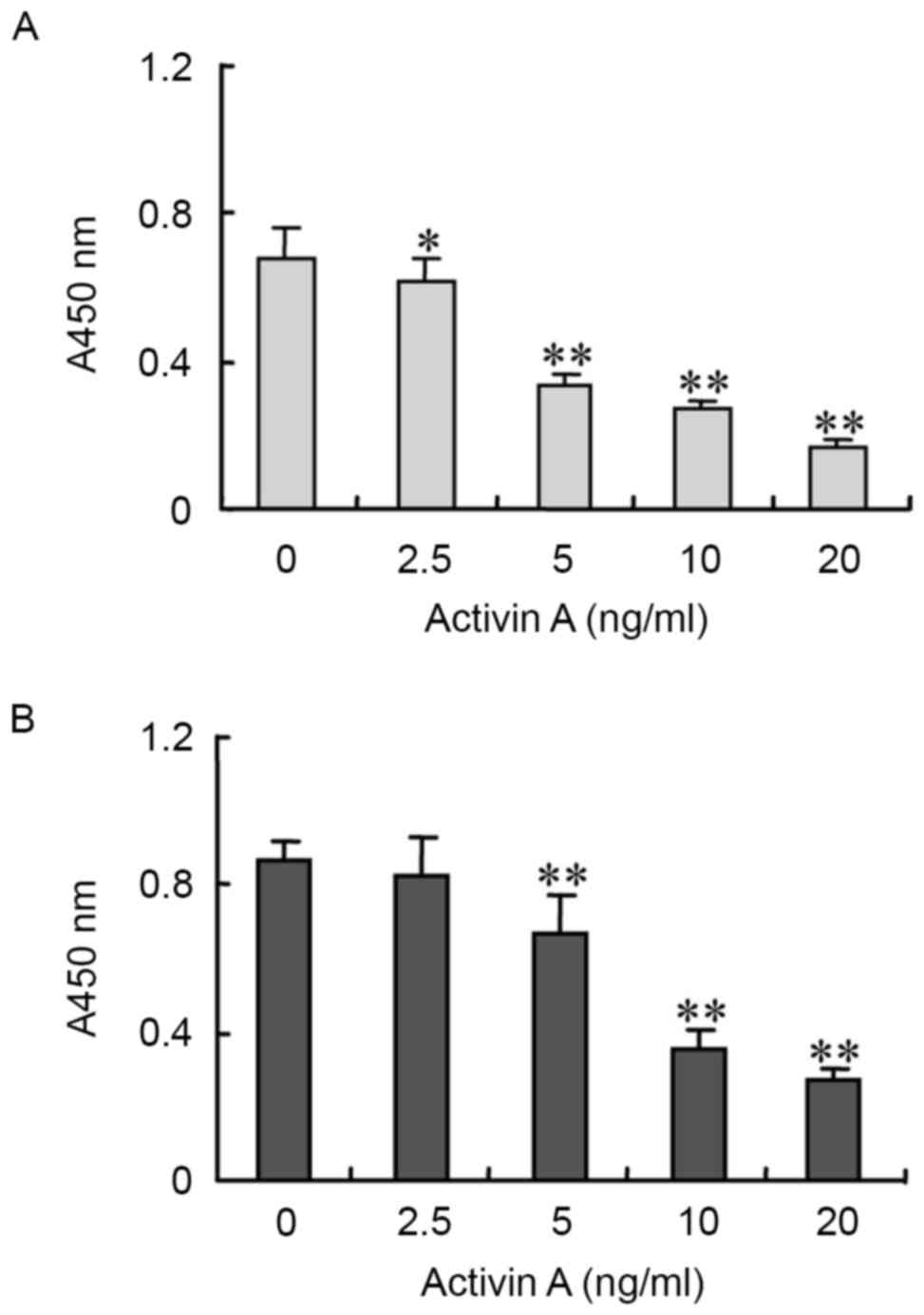
In order to evaluate the biological functions of
activin A, NS-1 cell viability was examined by BrdU assay. The
results revealed that following treatment with activin A for 24 h,
NS-1 cell viability was inhibited in a dose-dependent manner,
compared with the untreated control group (Fig. 2).
NS-1 cell apoptosis was induced by
activin A
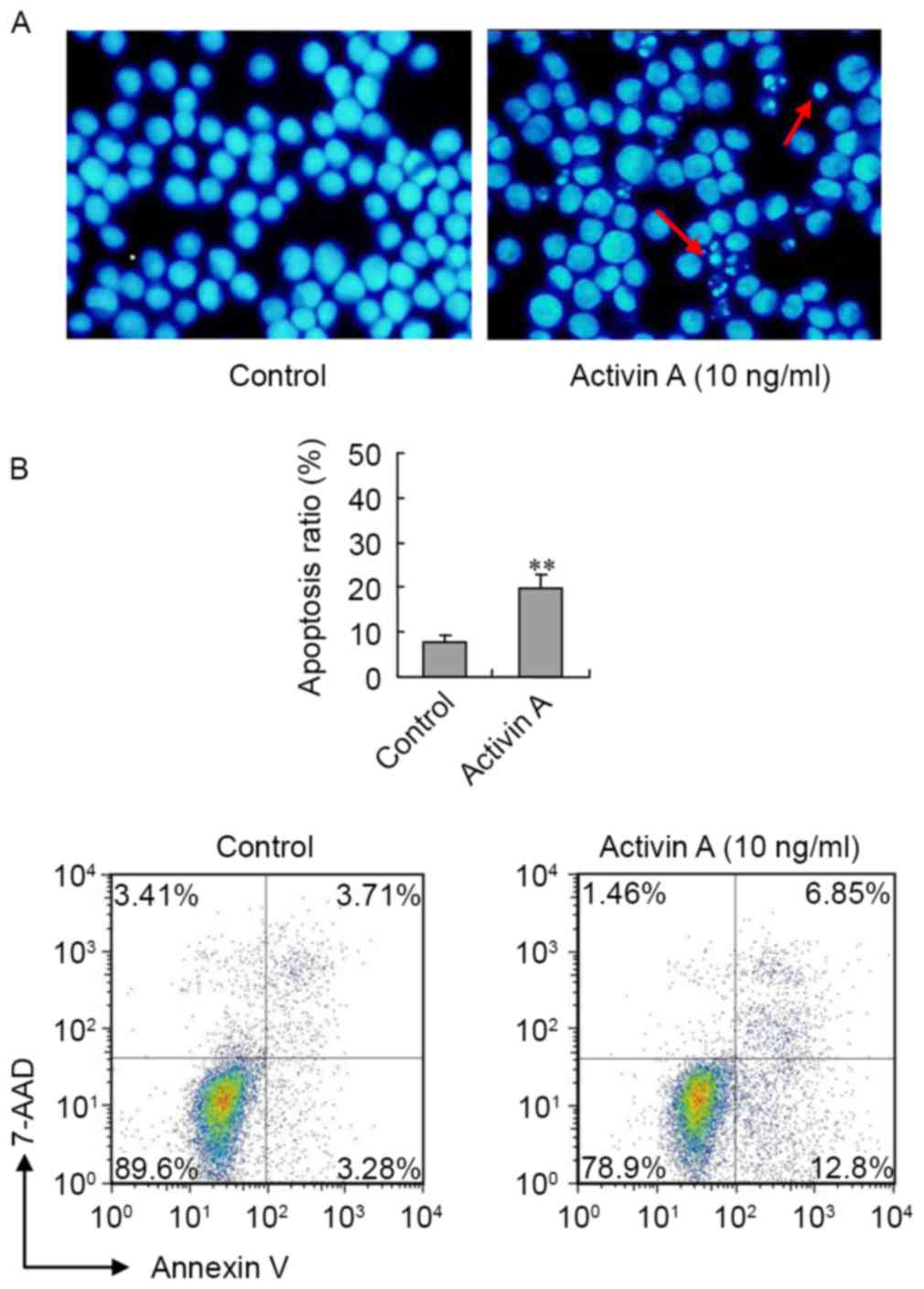
In order to assess the effect of activin A on NS-1
cell apoptosis, NS-1 cellular morphology was first examined by
Hoechst 33342 staining following treatment with activin A for 24 h.
In the control group, the cells were uniform in size and round in
shape. However, the morphology of cells treated with activin A was
notably different in terms of size and shape, and the number of
cells with nuclear condensation was visibly increased (Fig. 3A). Furthermore, the flow cytometry
results revealed that, following treatment with activin A for 24 h,
the ratio of apoptotic NS-1 cells significantly increased compared
with the control group (Fig. 3B).
These data indicated that activin A induced NS-1 cell
apoptosis.
Expression levels of
apoptosis-associated proteins in NS-1 cells treated with activin
A
Previous studies have revealed that activin A
induces B cell apoptosis through the mitochondrial apoptosis
pathway (11). In the present study,
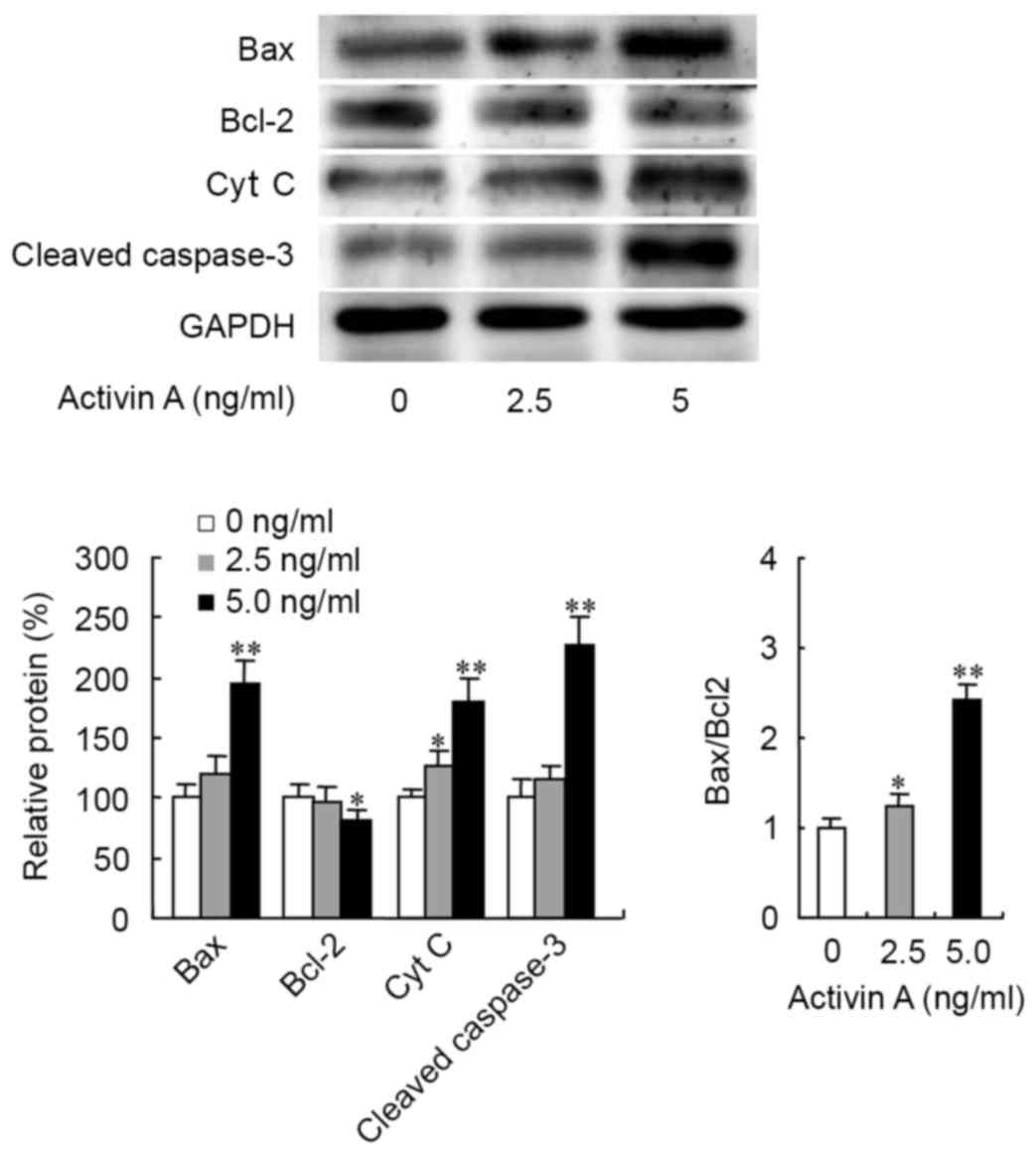
the expression levels of the apoptosis-associated proteins Bcl-2,
Bax, Cyt c and caspase-3 were examined by western blotting.
The results revealed that activin A significantly upregulated Bax
protein expression, while 5.0 ng/ml activin A treatment
downregulated Bcl-2 protein expression, which resulted in an
increased ratio of Bax/Bcl-2 (Fig.
4). Activin A also increased the expression of Cyt c (at
2.5 and 5.0 ng/ml) and cleaved caspase-3 proteins (at 5.0 ng/ml;
Fig. 4). These data suggested that
activin A may induce NS-1 cell apoptosis through the mitochondrial
apoptosis pathway.
Discussion
Activin A is a multifunctional factor of TGF-β
superfamily that is most commonly involved in embryogenesis and
gonadal hormone release (12,13). Activin A is also associated with the
maintenance of neuron survival, induction of hepatocyte apoptosis,
promotion of erythroid differentiation and dual regulation of
macrophage activation (14–17). The involvement of activin A in the
process of tumor genesis and progression has been previously
reported (18), and a significant
increase of activin A in the serum of certain patients with
colorectal adenocarcinoma has been observed (19). However, the function of activin A in
tumor progression is controversial. Activin A has been demonstrated
to exert a primarily protective function, whereby it induces cell
cycle arrest of patient-derived prostate cancer cells and
non-invasive prostate cancer LNCaP cells. In contrast, activin A
treatment resulted in an increase in proliferation of the more
aggressive prostate cancer cell line, PC3, an apparent discrepancy
(20–22). The tumor suppressive function of
activin A is also observed in breast cancer, but activing A
promotes cell proliferation and invasion in head and neck squamous
cell carcinoma, resulting in a poor prognosis (8). Multiple myeloma is characterized by the
development of osteolytic disease. Circulating activin A is
elevated in patients with advanced multiple myeloma, which
contributes to bone remodeling. Malignant plasma cells have been
reported to induce the secretion of activin A by stromal cells,
resulting in osteoblast inhibition and osteoclast stimulation in
vitro and in vivo (9,23,24). Although activin A seems to be
implicated into the pathogenesis of myeloma bone disease, the
direct effect of activin A on myeloma cell viability and apoptosis
remains unclear.
Activin A is a dimeric, multifunctional cytokine,
which is involved in a broad spectrum of functions associated with
cell viability and apoptosis, and is also associated with tumor
genesis and progression, aggressiveness and malignant degree
(3,4,19). In the
present study, the effects of activin A on myeloma NS-1 cell
viability and apoptosis were evaluated. Our data revealed that
ActRIIA and ActRIIB were expressed in NS-1 cells, indicating that
activin A exerts an effect on NS-1 cells. Moreover, the results
showed that activin A significantly inhibited the viability of NS-1
cells in a dose-dependent manner. Hoechst 33342 staining revealed
that the size of NS-1 cells was altered compared with controls, and
the shape appeared irregular following treatment with activin A. In
addition, the number of cells with nuclear condensation visibly
increased. Furthermore, the results of flow cytometry and staining
with AnnexinV-7-AAD revealed that the ratio of apoptotic NS-1 cells
increased following administration of activin A, compared with
untreated NS-1 cells. These data indicated that activin A inhibited
NS-1 cell viability and induced apoptosis in NS-1 cells.
Apoptosis is recognized as a form of programmed cell
death, characterized by nuclear fragmentation and the presence of
apoptotic bodies. It is regulated by multiple genes, and serves an
important function in the development and growth of normal adult
tissues and the maintenance of homeostasis (25). Signal transduction pathways associated
with apoptosis include the mitochondrial pathway, death receptor
pathway, and endoplasmic reticulum stress pathway, of which the
mitochondrial pathway serves a vital function in apoptosis
(26,27). The Bax/Bcl-2 ratio is the key factor
that triggers the mitochondrial pathway of apoptosis, and when the
ratio of Bax/Bcl-2 increases apoptosis is initiated (27–30). Bcl-2
and Bax proteins are located upstream of the mitochondrial
apoptosis pathway and regulate the release of Cyt c, which
is an important component of the mitochondrial electron transport
chain. The release of Cyt c from the mitochondria is a key
step in the induction of proteases downstream from caspase-3, to
regulate cell survival or death. Previous studies have demonstrated
that activin A induces B cell apoptosis through the mitochondrial
pathway (11), thus the effect of
activin A on apoptosis-associated proteins of the mitochondrial
pathway was evaluated in the present study. The results revealed
that activin A significantly upregulated Bax protein expression,
which resulted in an increase in the Bax/Bcl-2 ratio. Activin A
also increased the cleaved capase-3 and Cyt c protein
expression. These data suggest that activin A induces NS-1 cell
apoptosis via the mitochondrial pathway.
In summary, activin A serves an important function
in mouse myeloma NS-1 cell viability and apoptosis through the
mitochondrial pathway, providing a potential future target for
myeloma therapy.
Acknowledgements
The present study was supported by the Project of
Science and Technology of Jilin Province (grant no.
20140311091YY).
References
|
1
|
Woodruff TK, Besecke LM, Groome N, Draper
LB, Schwartz NB and Weiss J: Inhibin A and inhibin B are inversely
correlated to follicle stimulating hormone, yet are discordant
during the follicular phase of the rat estrous cycle and inhibin A
is expressed in a sexually dimorphic manner. Endocrinology.
137:5463–5467. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ge J, Fan Y, Lu Y, Qi Y, Wang M and Liu Z:
Activin A increases arterial pressure in the hypothalamic
paraventricular nucleus in rats by angiotension II. Neuroreport.
27:683–688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matzuk MM, Kumar TR, Vassalli A,
Bickenbach JR, Roop DR, Jaenisch R and Bradley A: Functional
analysis of activins during mammalian development. Nature.
374:354–356. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoda MA, Rozsas A, Lang E, Klikovits T,
Lohinai Z, Torok S, Berta J, Bendek M, Berger W, Hegedus B, et al:
High circulating activin A level is associated with tumor
progression and predicts poor prognosis in lung adenocarcinoma.
Oncotarget. 7:13388–13399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antsiferova M, Martin C, Huber M,
Feyerabend TB, Förster A, Hartmann K, Rodewald HR, Hohl D and
Werner S: Mast cells are dispensable for normal and
activin-promoted wound healing and skin carcinogenesis. J Immunol.
191:6147–6155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loomans HA and Andl CD: Intertwining of
activin A and TGFβ signaling: Dual roles in cancer progression and
cancer cell invasion. Cancers (Basel). 7:70–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niu L, Cui X, Qi Y, Xie D, Wu Q, Chen X,
Ge J and Liu Z: Involvement of TGF-β1/Smad3 signaling in carbon
Tetrachloride-induced acute liver injury in mice. PLoS One.
11:e01560902016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyazawa K, Shinozaki M, Hara T, Furuya T
and Miyazono K: Two major Smad pathways in TGF-beta superfamily
signaling. Genes Cells. 7:1191–1204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vallet S, Mukherjee S, Vaghela N,
Hideshima T, Fulciniti M, Pozzi S, Santo L, Cirstea D, Patel K,
Sohani AR, et al: Activin A promotes multiple myeloma-induced
osteolysis and is a promising target for myeloma bone disease. Proc
Natl Acad Sci USA. 107:pp. 5124–5129. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu HY, Wang YN, Ge JY, Li N, Cui XL and
Liu ZH: Localization and role of activin receptor-interacting
protein 1 in mouse brain. J Neuroendocrinol. 25:87–95. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamakawa N, Tsuchida K and Sugino H: The
rasGAP-binding protein, Dok-1, mediates activin signaling via
serine/threonine kinase receptors. EMBO J. 21:1684–9164. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duggal G, Heindryckx B, Warrier S, Taelman
J, Van der Jeught M, Deforce D, de Sousa Lopes S Chuva and De
Sutter P: Exogenous supplementation of Activin A enhances germ cell
differentiation of human embryonic stem cells. Mol Hum Reprod.
21:410–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okuma Y, Saito K, O'Connor AE, Phillips
DJ, de Kretser DM and Hedger MP: Reciprocal regulation of activin A
and inhibin B by interleukin-1 (IL-1) and follicle-stimulating
hormone (FSH) in rat Sertoli cells in vitro. J Endocrinol.
185:99–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang L, Wang YN, Cui XL, Fang SY, Ge JY,
Sun Y and Liu ZH: The role and mechanism of action of activin A in
neurite outgrowth of chicken embryonic dorsal root ganglia. J Cell
Sci. 125:1500–1507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walton KL, Makanji Y and Harrison CA: New
insights into the mechanisms of activin action and inhibition. Mol
Cell Endocrinol. 359:2–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ge J, Wang Y, Feng Y, Liu H, Cui X, Chen
F, Tai G and Liu Z: Direct effects of activin A on the activation
of mouse macrophage RAW264.7 cells. Cell Mol Immunol. 6:129–133.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li N, Cui X, Ge J, Li J, Niu L, Liu H, Qi
Y, Liu Z and Wang Y: Activin A inhibits activities of
lipopolysaccharide-activated macrophages via TLR4, not of TLR2.
Biochem Biophys Res Commun. 435:222–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Le Bras GF, Loomans HA, Taylor CJ, Revetta
FL and Andl CD: Activin A balance regulates epithelial invasiveness
and tumorigenesis. Lab Invest. 94:1134–1146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu S, Qi Y, Niu LM, Xie DX, Cui XL and Liu
ZH: Activin A as a novel biomarker for colorectal adenocarcinoma in
humans. Eur Rev Med Pharmacol Sci. 19:4371–437. 2015.PubMed/NCBI
|
|
20
|
Gold E and Risbridger G: Activins and
activin antagonists in the prostate and prostate cancer. Mol Cell
Endocrinol. 359:107–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujii Y, Kawakami S, Okada Y, Kageyama Y
and Kihara K: Regulation of prostate-specific antigen by activin A
in prostate cancer LNCaP cells. Am J Physiol Endocrinol Metab.
286:E927–E931. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balanathan P, Williams ED, Wang H,
Pedersen JS, Horvath LG, Achen MG, Stacker SA and Risbridger GP:
Elevated level of inhibin-alpha subunit is pro-tumourigenic and
pro-metastatic and associated with extracapsular spread in advanced
prostate cancer. Br J Cancer. 100:1784–1793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garcia-Gomez A, Sanchez-Guijo F, Del
Cañizo MC, San Miguel JF and Garayoa M: Multiple myeloma
mesenchymal stromal cells: Contribution to myeloma bone disease and
therapeutics. World J Stem Cells. 6:322–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terpos E, Kastritis E, Christoulas D,
Gkotzamanidou M, Eleutherakis-Papaiakovou E, Kanellias N,
Papatheodorou A and Dimopoulos MA: Circulating activin-A is
elevated in patients with advanced multiple myeloma and correlates
with extensive bone involvement and inferior survival; no
alterations post-lenalidomide and dexamethasone therapy. Ann Oncol.
23:2681–2686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prokhorova EA, Zamaraev AV, Kopeina GS,
Zhivotovsky B and Lavrik IN: Role of the nucleus in apoptosis:
Signaling and execution. Cell Mol Life Sci. 72:4593–4612. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delgado Y, Morales-Cruz M, Hernández-Román
J, Martínez Y and Griebenow K: Chemical glycosylation of cytochrome
c improves physical and chemical protein stability. BMC Biochem.
15:162014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng X, Yu Z, Liang N, Chi X, Li X, Jiang
M, Fang J, Cui H, Lai W, Zhou Y and Zhou S: The mitochondrial and
death receptor pathways involved in the thymocytes apoptosis
induced by aflatoxin B1. Oncotarget. 7:12222–12234. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roset R, Ortet L and Gil-Gomez G: Role of
Bcl-2 family members on apoptosis: What we have learned from
knock-out mice. Front Biosci. 12:4722–4730. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mignard V, Lalier L, Paris F and Vallette
FM: Bioactive lipids and the control of Bax pro-apoptotic activity.
Cell Death Dis. 5:e12662014. View Article : Google Scholar : PubMed/NCBI
|