Introduction
Recently, it was reported that cessation of
abiraterone acetate (AA) is associated with an AA withdrawal
syndrome (AAWS) in metastatic castration-resistant prostate cancer
(mCRPC) which was first reported in 2012 (1). AAWS is characterized by a transient
prostate-specific antigen (PSA) decrease after AA discontinuation,
mimicking anti-androgen withdrawal syndrome (AWS) (2). The use of AA in patients with mCRPC has
been increasing since its registration. With the widespread use of
AA, AAWS has become gradually recognized. So far, limited evidence
is available on this phenomenon and the mechanisms leading to AAWS
is still difficult to explain. In this manuscript, we report the
case of a patient who had a drastic PSA decrease after stopping AA.
We also provide a review of recent literature and describe
potential explanation of the mechanism underlying this phenomenon.
Particularly, activation of mutant androgen receptor (mAR) by
probable alternative ligands such as prednisolone, progesterone, AA
itself and its steroidal metabolites are mainly discussed in this
manuscript.
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images. No ethics approval sought as this was a case report with no
direct impact on patient outcome.
Case report
A 72-year-old man complaining of lumbar and right
chest pain was referred to our hospital in June 2011 for further
evaluation of serum PSA elevation that had reached 2,000.0 ng/ml.
Prostate needle biopsy was performed and pathological examination
showed prostatic adenocarcinoma and the Gleason score of 4+4.
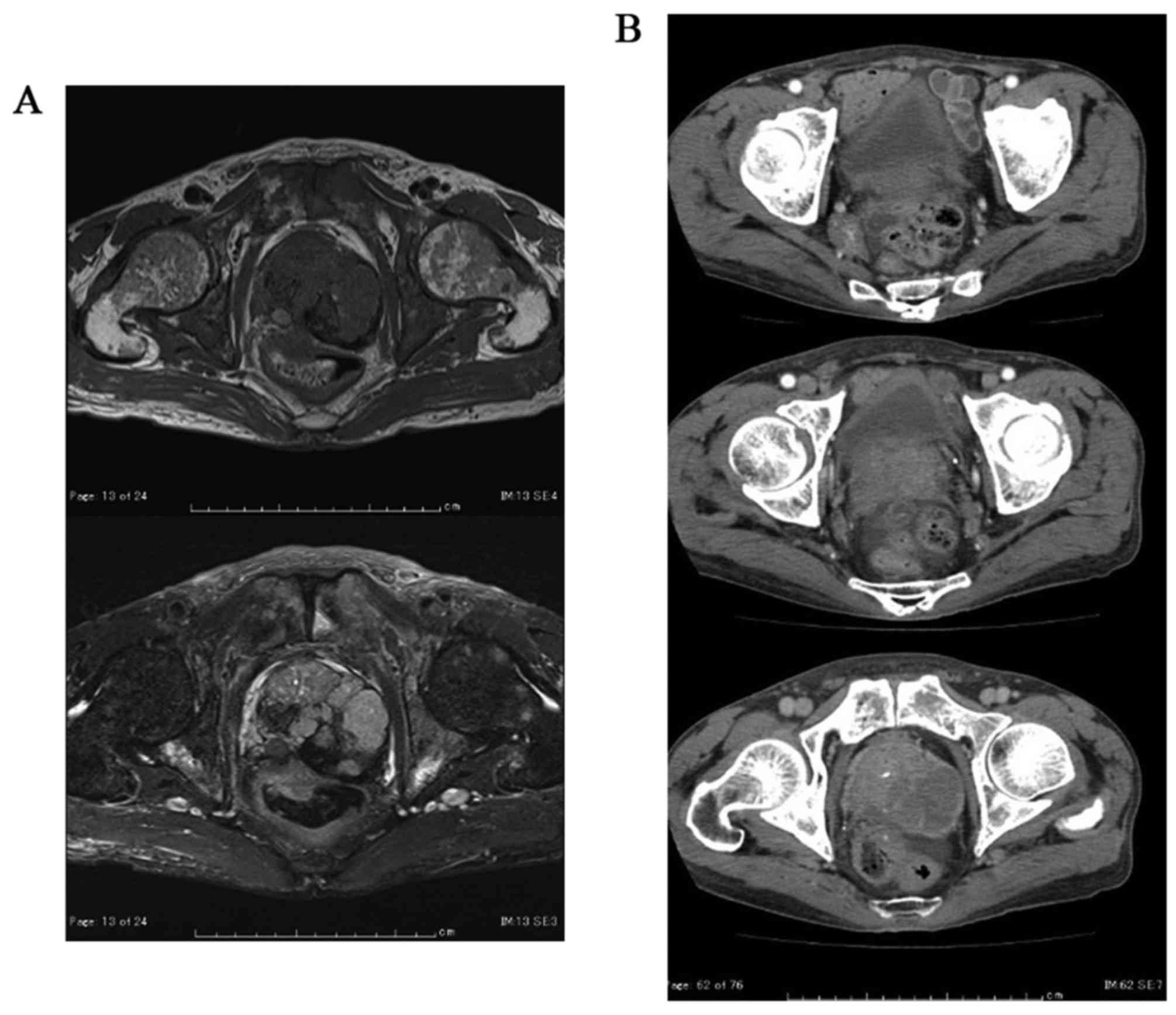
Magnetic resonance imaging (MRI) showed massive prostate tumor that
invaded the rectum and the left seminal vesicle directly (Fig. 1A). Computed tomography (CT) revealed
metastasis of the pelvic lymph nodes and bone scintigraphy revealed
multiple bone metastasis (Fig. 1B and
C). He was diagnosed with prostate cancer, cT4N1M1b.
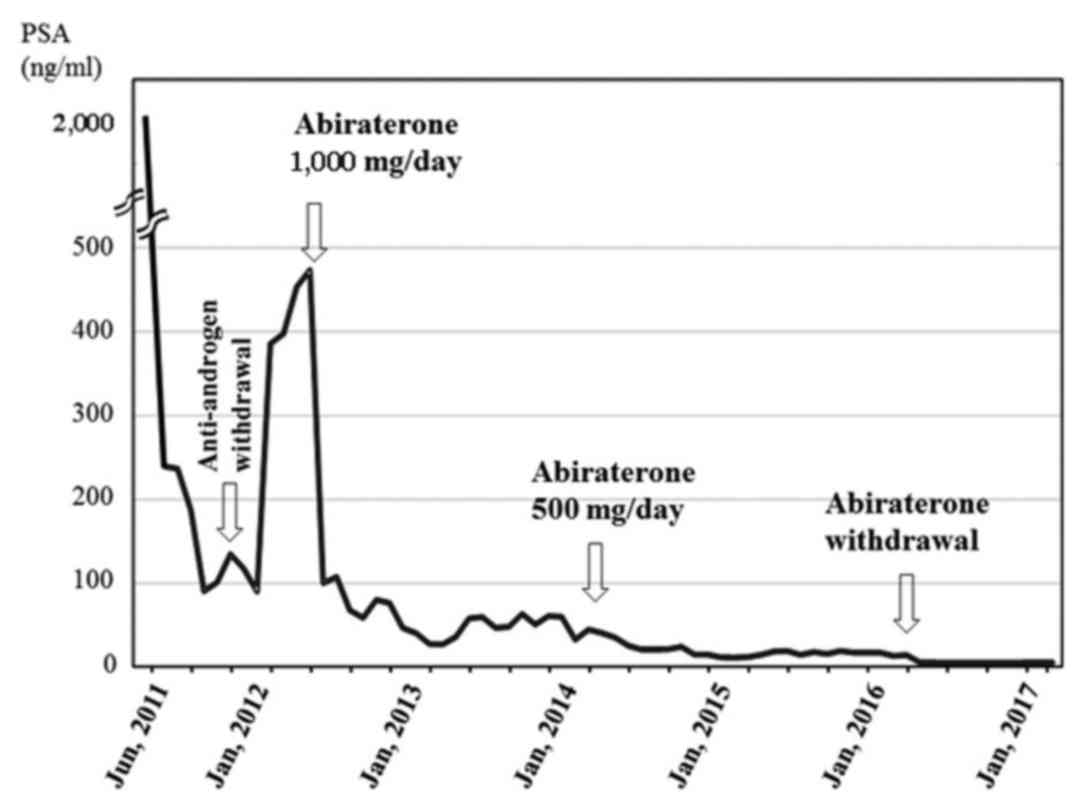
The PSA kinetics during treatment are shown in
Fig. 2. Initially, combined androgen
blockade (CAB) with leuprolide acetate and bicalutamide had been
started. The serum PSA level had decreased to 85.1 ng/ml in
November 2011, but then gradually increased again to 113.3 ng/ml in
February 2012. Then, bicalutamide was discontinued to determine
AWS.
Subsequently the serum PSA level had temporarily
decreased however, then gradually increased again and serum PSA
increased to 517.0 ng/ml in July 2012. Consequently, AA was
initiated at the standard dose of 1,000 mg daily in combination
with prednisolone (10 mg daily). PSA rapidly decreased to a nadir
of 20.1 ng/ml, thereafter, his PSA had remained stable between
20–50 ng/ml during this period.
In July 2014, he confessed that he had felt nauseous
after taking AA for a long time. Thereafter, arbitrarily on his
own, he reduced taking AA to half of the standard dose (500 mg
daily). However, contrary to our expectations, his serum PSA level
promptly decreased to a nadir of 8.1 ng/ml. At that time, he still
continued taking prednisolone at the standard dose of 10 mg.
Thereafter, his PSA has become a lot lower between 8–16 ng/ml than
before. Although the association between AA and his nausea is
unclear, his nausea resolved spontaneously thereafter.
Subsequently, in April 2016, he complained of
steroid purpura on bilateral forearms, which may not require
particular treatment. We recommended him to continue taking AA.
However, he self-indulgently stopped taking AA. He had also quit
taking prednisolone at the same time. Surprisingly, his serum PSA
levels decreased to <1.0 ng/ml after AA discontinuation without
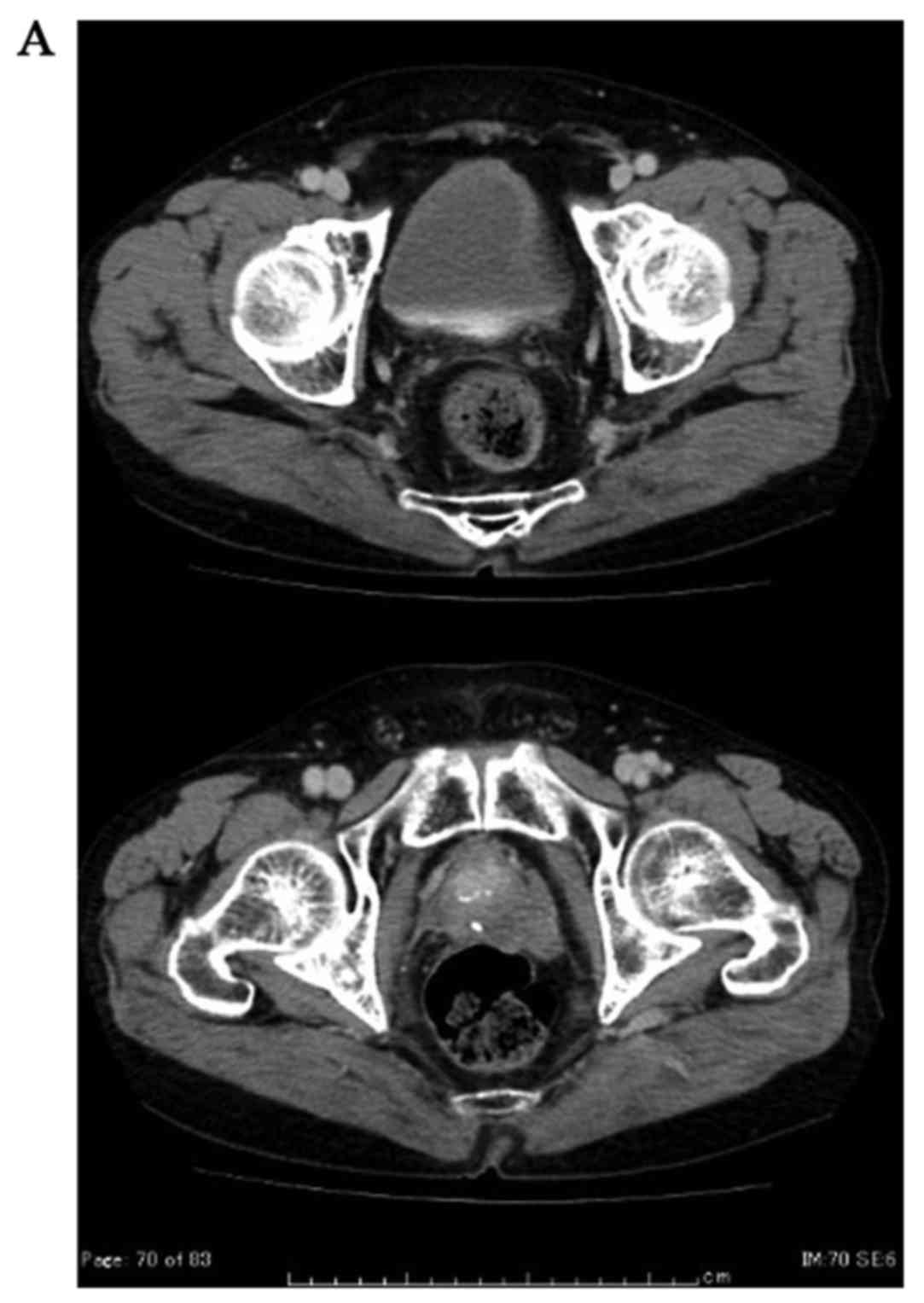
radiological progression. The patient is still well and alive 1
year after AA cessation without a biological, clinical or
radiological progression under leuprolide acetate (Fig. 3A and B).
Discussion
Similar to AWS, a PSA decrease after AA
discontinuation, namely AAWS, is recently recognized and reported
in mCRPC (2). Initially, AAWS had
been thought to be minor short-term event (3). AAWS with a biochemical response >50%
were retrospectively observed in 6% of the patients who stopped AA
because of disease progression (3).
However, in the subsequent manuscript, AAWS has proved to be not so
uncommon event. PSA response >50% was retrospectively observed
in 15% (3 of 26) of mCRPC patients after stopping AA (4). It was also demonstrated that AAWS was
observed in 16% (3 of 19) mCRPC patients and 2 of them associated
with clinical and radiological response (4). It has gradually become known that AAWS
can occasionally induce a long-lasting biochemical response
accompanied by a clinical and radiological improvement (4). More recently, it was also suggested that
patients who experienced AAWS may have better survival outcomes
(5).
Currently proposed leading mechanisms of resistance
to AA includes activation of mutant AR by alternative ligands,
ligandless AR activation by constitutively active AR variants
lacking the ligand-binding domain and/or cross-talk with relative
signaling pathways (6). Among these
mechanisms, AR mutations in the hormone-binding domain which allow
activation by alternative ligands other than testosterone is
supposed to be the main cause of AAWS.
Among withdrawal syndromes, AWS associated with
anti-androgens has been mainly investigated. AWS is the phenomenon
characterized by tumor regression and decline in serum PSA levels
on discontinuation of the anti-androgen (7). AR mutations in the ligand-binding domain
(LBD) and/or hinge region, such as T877A or H874Y, are speculated
to be responsible for stimulating effects of anti-androgens on
prostate cancer (8). Recently, F876 L
AR mutation, which converted enzalutamide into an AR agonist, is
also reported (6). Various mARs can
reduce ligand binding specificity, and induce mAR activation (mARA)
by several ligands other than testosterone. These alternative
ligands are not restricted only to anti-androgen. Various mARs are
demonstrated to be activated by various alternative ligands, such
as progesterone, estradiol, adrenal androgens, hydrocortisone or
anti-androgens (6). Several
withdrawal syndromes associated with hormonal agents other than
anti-androgens are also reported (6).
With regard to AAWS, the following ligands has been
supposed to bind to LBD of mAR: A) prednisolone taken with AA, B)
slightly elevated progesterone induced by AA administration and C)
AA itself or steroidal metabolites of AA.
AA is generally administered in association with
prednisolone, at a daily dose of 10 mg, to prevent the
mineralocorticoid excess caused by the loss of a negative feedback
of the adrenocorticotropic hormone (ACTH) (9). In most cases, both AA and prednisolone
were stopped at the same time. Therefore, it had been hypothesized
that prednisolone taken with AA is responsible for the withdrawal
response (4). Prednisolone is also
known to act as alternative ligand for certain mAR (6). It is probable that AAWS might partly
depend on prednisolone discontinuation rather than AA
discontinuation. However, it is difficult to explain this
phenomenon only by means of prednisolone. As a matter of fact, AAWS
is documented even in those who continued steroids after AA
cessation (10). The underlying
mechanisms other than prednisolone are speculated from our case
too. Our case initially reduced AA to half dose with continuing
prednisolone at the standard dose of 10 mg, which results in more
decreased PSA level.
AA is a potent CYP17 inhibitor that blocks androgen
synthesis which drastically reduces circulating and tissue androgen
levels which otherwise increases steroids upstream of CYP17
including progesterone. Serum and tissue progesterone level
slightly increases through AA administration (11). Progesterone can also activate several
mARs (11). Among them, T878A mAR is
suggested to be upregulated through AA treatment, which is one of
the possible mechanisms of AAWS (12). Due to the agonistic effect on mAR,
even a small amount of progesterone might be responsible for
AAWS.
Participation of AA itself and/or steroidal
metabolites of AA are also suggested as possible mechanism for
AAWS. AA itself could have direct AR antagonistic properties and
AAWS might possibly be linked to direct abiraterone-AR interactions
(13). Moreover, multiple steroidal
metabolites of AA such as D-4-abiraterone (D4A) and 5a-abiraterone
which are previously unappreciated, has recently been identified.
AA is metabolized to D4A, which act as an AR antagonist (14). D4A is converted to 5a-abiraterone,
which act as an AR agonist (15).
Furthermore, various steroidal metabolites of AA had been
successively detected and these metabolites are speculated to
interact directly with wildtype or mutant AR (16).
Conventionally, AWS is defined as a disease response
and clinical improvement after discontinuation of the anti-androgen
in patients developing progressive prostate cancer during CAB
(7), however, we emphasize that the
definition of withdrawal syndrome should be reconsidered. As
mentioned above, withdrawal syndromes could be derived by various
ligands other than anti-androgen including progesterone, estradiol,
corticosteroids, AA and other steroidal derivatives. Taking account
of these phenomenon, these should all be widely categorized an
‘alternative-ligand withdrawal syndromes’. Besides, withdrawal
syndromes are not the phenomenon restricted to disease progression
periods. In our patient, AAWS with additional PSA decreases were
observed when his PSA levels once reached his PSA nadir. From
another point of view, his nadir PSA had never reached an
undetectable level during AA treatment which indicated that drug
resistance for AA potentially existed. Recently, there have been
several reports regarding the relationships between nadir PSA level
and clinical outcome. In the population-based database includes
26,272 Japanese men with prostate cancer, it is demonstrated that
patients with nadir PSA levels <0.2 ng/ml following primary
androgen deprivation therapy had better clinical outcomes than
patients with nadir PSA levels ≥0.2 ng/ml which indicates
underlying drug resistance (17).
Even while on novel AR targeting therapies, drug resistance may
concealed if PSA did not reach undetectable levels.
Although the mechanisms underlying AAWS requires
further study, clinicians should pay attention to this phenomenon.
Considering the frequency of AAWS, it might not be proper to
routinely anticipate AAWS. However, when patient's conditions
allow, it would be acceptable to wait for a brief period before
starting further therapy after AA progression because it would be a
good opportunity to presume existing drug resistance including
mARA. Through evaluation of PSA kinetics on daily medical
examination, useful information on managing mCRPC can sometimes be
obtained.
Glossary
Abbreviations
Abbreviations:
|
AA
|
abiraterone acetate
|
|
D4A
|
D-4-abiraterone
|
|
AAWS
|
abiraterone acetate withdrawal
syndrome
|
|
AWS
|
anti-androgen withdrawal syndrome
|
|
PSA
|
prostate-specific antigen
|
|
ACTH
|
adrenocorticotropic hormone
|
|
mCRPC
|
metastatic castration-resistant
prostate cancer
|
|
CAB
|
combined androgen blockade
|
|
mAR
|
mutant androgen receptor
|
|
mARA
|
mutant androgen receptor
activation
|
|
LBD
|
ligand-binding domain
|
|
MRI
|
magnetic resonance imaging
|
|
CT
|
computed tomography
|
References
|
1
|
Gauthier H, Bousquet G, Pouessel D and
Culine S: Abiraterone acetate withdrawal syndrome: Does it exist?
Case Rep Oncol. 5:385–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Witjes JA: A case of abiraterone acetate
withdrawal. Eur Urol. 64:517–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Albiges L, Auclin E, Rousseau B, Boughalem
E, Levy A, Loriot Y, Palma MD, Massard C and Fizazi K: Is there a
withdrawal syndrome with abiraterone acetate (AA)? J Clin Oncol.
31:892013. View Article : Google Scholar
|
|
4
|
Caffo O, Palermo A, Veccia A, Maines F,
Chierichetti F, Berruti A and Galligioni E: Biochemical and
objective response to abiraterone acetate withdrawal: Incidence and
clinical relevance of a new scenario for castration-resistant
prostate cancer. Urology. 82:1090–1093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caffo O, Maines F, Trentin C, Veccia A and
Galligioni E: Long-term outcomes and predictive factors in patients
(pts) with metastatic castration-resistant prostate cancer (mCRPC)
showing abiraterone withdrawal syndrome (AWS) after docetaxel (DOC)
treatment. J Clin Oncol. 34 2 Suppl:S3242016. View Article : Google Scholar
|
|
6
|
Lorente D, Mateo J, Zafeiriou Z, Smith AD,
Sandhu S, Ferraldeschi R and de Bono JS: Switching and withdrawing
hormonal agents for castration-resistant prostate cancer. Nat Rev
Urol. 12:37–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paul R and Breul J: Anti-androgen
withdrawal syndrome associated with prostate cancer therapies:
Incidence and clinical significance. Drug Saf. 23:381–390. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taplin ME, Bubley GJ, Shuster TD, Frantz
ME, Spooner AE, Ogata GK, Keer HN and Balk SP: Mutation of the
androgen-receptor gene in metastatic androgen-independent prostate
cancer. N Engl J Med. 332:1393–1398. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: Abiraterone and increased survival in metastatic prostate
cancer. N Engl J Med. 364:1995–2005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Azad AA and Eigl BJ: Evaluation of
prostate-specific antigen response following cessation of
abiraterone acetate: Is there evidence for a withdrawal syndrome?
Eur Urol. 65:504–505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taplin ME, Montgomery B, Logothetis CJ,
Bubley GJ, Richie JP, Dalkin BL, Sanda MG, Davis JW, Loda M, True
LD, et al: Intense androgen-deprivation therapy with abiraterone
acetate plus leuprolide acetate in patients with localized
high-risk prostate cancer: Results of a randomized phase II
neoadjuvant study. J Clin Oncol. 32:3705–3715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen EJ, Sowalsky AG, Gao S, Cai C,
Voznesensky O, Schaefer R, Loda M, True LD, Ye H, Troncoso P, et
al: Abiraterone treatment in castration-resistant prostate cancer
selects for progesterone responsive mutant androgen receptors. Clin
Cancer Res. 21:1273–1280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Richards J, Lim AC, Hay CW, Taylor AE,
Wingate A, Nowakowska K, Pezaro C, Carreira S, Goodall J, Arlt W,
et al: Interactions of abiraterone, eplerenone, and prednisolone
with wild-type and mutant androgen receptor: A rationale for
increasing abiraterone exposure or combining with MDV3100. Cancer
Res. 72:2176–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Bishop AC, Alyamani M, Garcia JA,
Dreicer R, Bunch D, Liu J, Upadhyay SK, Auchus RJ and Sharifi N:
Conversion of abiraterone to D4A drives anti-tumour activity in
prostate cancer. Nature. 523:347–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Z, Alyamani M, Li J, Rogacki K, Abazeed
M, Upadhyay SK, Balk SP, Taplin ME, Auchus RJ and Sharifi N:
Redirecting abiraterone metabolism to fine-tune prostate cancer
anti-androgen therapy. Nature. 533:547–551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caffo O and Sharifi N: Could steroidal
abiraterone metabolites possibly explain abiraterone withdrawal
syndrome? Eur Urol. 70:898–899. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kitagawa Y, Ueno S, Izumi K, Mizokami A,
Hinotsu S, Akaza H and Namiki M: Nadir prostate-specific antigen
(PSA) level and tome to PSA nadir following primary androgen
deprivation therapy as independent prognostic factors in a Japanese
large-scale prospective cohort study (J-CaP). J Cancer Res Clin
Oncol. 140:673–679. 2014. View Article : Google Scholar : PubMed/NCBI
|