Introduction
Colorectal cancer is a major cause for morbidity and
mortality and one of the most common malignant tumours of the
digestive system (~9.7% of cases), thus posing a serious threat to
human physical and mental health (1).
The most hazardous characteristics of colorectal cancer are
invasion and metastasis. Although basic and clinical cancer
research has made notable progress in recent years, the survival
rate for the majority of patients with cancer has not significantly
improved, with metastasis and relapse following treatment being the
cause for >90% of cancer-associated mortality (2). The current clinical treatment for tumour
metastasis exhibits numerous adverse effects, and there is a lack
of systematic and comprehensive knowledge regarding the biological
mechanisms underlying tumour metastasis. Therefore, prediction,
early diagnosis and effective interventions to prevent tumour
metastasis to distal tissues or organs are limited. In order to
improve the effects of therapeutics on malignant tumours, the
molecular mechanisms underlying tumour invasion and metastasis
should be investigated (3–5).
Previously, research into metastasis focused on the
actin cytoskeleton and increases in actin-related protein (ARP)
expression during metastasis. The ARP2/3 complex is an
actin-assembly nucleating agent that promotes the nucleation of
microfilaments and serves a significant function in a number of
physiological activities, including cell migration (6,7). Cell
migration is essential to normal biological processes, such as
tissue repair and regeneration; however, abnormally activated cell
movement is associated with various diseases and may eventually
lead to the development of fatal metastatic tumours. Indeed,
metastatic capacity is considered a cancer cell marker (8–10).
A previous study demonstrated that ARP subunits are
abnormally expressed in tumours, and immunohistochemical
experiments indicated that abnormally increased levels of ARPC4
were present in colorectal cancer tissues (1). Investigating the association between
ARPC4 and tumours may, therefore, provide valuable insights for the
development of strategies for early cancer diagnosis and gene
therapy. Molecular and cell biology studies previously demonstrated
abnormally increased ARPC4 expression levels in various colorectal
cancer cell lines; however, the association between the ARPC4 gene
and the occurrence and development of colorectal cancer has not yet
been fully elucidated. In the present study, further explorative
research was conducted to identify novel targets for colorectal
cancer gene therapy.
Materials and methods
Tissue samples
Colorectal carcinoma and adjacent normal colon
tissue were obtained from a female patient at the age of 67 in
August 2015 by resection at The Third People's Hospital of Chengdu
(Chengdu, China). Written informed consent was obtained from the
patient and ethical approval was granted by the Medical Ethics
Committee of The Third People's Hospital of Chengdu.
Immunohistochemical staining images were analysed with Imaris 8.01
(Bitplane AG, Zurich, Switzerland).
Immunohistochemical analysis
Detection of ARPC4 protein levels was performed as
follows: A paraffin section of the tumour sample 6–8-µm thick was
dewaxed with xylene I and II for 5 min at room temperature, then
incubated with descending ethanol series (100, 95, 90, 80 and 70%)
for 3–5 min. Slices were rinsed twice with distilled water and then
use PBS to rinse two to three times with PBS.
H2O2 solution (3%) was used to block the
endogenous peroxidase activity for 10 min at room temperature,
followed by high-pressure antigen repair. It was sealed using 10%
goat serum (cat no. ab7481; Abcam, Cambridge, UK) and the primary
monoclonal rabbit anti-ARPC4 antibody (dilution, 1:100; cat no.
ab217065; Abcam) was added, then incubated at 4°C overnight and
washed three times with PBS. The biotin-labelled mouse anti-rabbit
IgG secondary antibody (dilution, 1:100; cat no. ab6728; Abcam) was
added and incubated for 30 min at room temperature, then washed
three times with PBS, developed using DAB (93 µl ddH2O +
3 µl 0.05% DAB1 solution + 2 µl 0.05% DAB2 solution + 2 µl 0.05%
DAB3 solution) and re-dyed using 2% haematoxylin at 4°C for 10 min.
It was then sealed by neutral gum and imaged under a microscope
(BX-42; Olympus Corporation, Tokyo, Japan).
Using light microscopy at magnification, ×400, cells
from 3 fields of view were analysed and the average number of
positive cells was noted. According to the following scoring
standard, the number of positive stained cells was calculated and
divided into the ARPC4 low-expression group (<10% positive
cells) or the ARPC4 high-expression group (>10% positive cells).
This was calculated by the following formula: Number of positively
stained cells/total number of cells × 100.
Cell culture conditions and
reagents
The human colorectal cancer SW620, HT-29, HCT116 and
SW480 cell lines were obtained from the Institute of Biochemistry
and Cell Biology (Shanghai, China) and maintained by the laboratory
within the Medical Biology Department, Sichuan University (Chengdu,
China). SW620, HT-29, HCT116 and SW480 cell lines were cultured
with high-glucose Dulbecco's modified Eagle's medium (DMEM;
HyClone; GE Healthcare Life Sciences, Logan, UT, USA) containing
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and penicillin-streptomycin (10,000 U/ml; 1:100;
Gibco; Thermo Fisher Scientific, Inc.) in a Heracell™ VIOS 160i
CO2 incubator (Thermo Fisher Scientific, Inc.), and were
maintained in a 5% CO2 atmosphere at 37°C. all the cells
were replaced with new medium every 2 days and passaged when the
confluence reached ~90% by using 0.25% trypsin (Gibco; Thermo
Fisher Scientific, Inc.).
Cell culture conditions and
reagents
SW620 cells were maintained at 37°C in an atmosphere
containing 5% CO2 in Dulbecco's modified Eagle's medium
(DMEM; HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% foetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Lipofectamine® 2000 was purchased from Life Sciences
(Thermo Fisher Scientific, Inc.). Primary antibodies against
β-actin (cat no. ab8227) and ARPC4 were purchased from Abcam
(Cambridge, UK). Primary antibodies for vimentin (cat. no.
10366-1-AP), E-cadherin (cat. no. 20874-1-AP) and PCNA (cat. no.
24,036-1-AP) were supplied by ProteinTech Group, Inc. (Chicago, IL,
USA). Secondary antibodies against vimentin (cat. no. sc-2370),
E-cadherin (cat. no. sc-2030) and PCNA (cat. no. sc-2995) were
purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA).
Western blot assay
HT-29, HCT-116, SW480, SW620, SW-1116 or transfected
and null SW620 colorectal cancer cells were harvested 48 h
following transfection, during the logarithmic growth phase, and
total cellular protein was extracted. All cells were washed three
times with PBS, centrifuged at 1,000 × g for 5 min at room
temperature, and re-suspended in radioimmunoprecipitation assay
lysis (Beijing Solarbio Science & Technology, Beijing China)
and extraction buffer containing 1 mM phenylmethylsulfonyl fluoride
protease inhibitor (Beijing Solarbio Science & Technology) on
ice for 30 min to ensure completely lysis. A total volume of 5 ml
5X loading buffer [(250 mM Tris-HCL (pH 6.8), 10% (w/v) SDS, 0.5%
(w/v) BPB, 50% (v/v) glycerin and 5% (w/v) β-mercaptoethanol)] was
added and boiled at 100°C for 5–10 min. then centrifuged at 12,000
× g for 15 min at 4°C to collect the supernatant. The total protein
concentration was detected using the bicinchoninic acid method
(BCA) (Beyotime Institute of Biotechnology, Haimen, China). A total
of 40 µg protein for each sample was uploaded and separated by
SDS-PAGE, with a 5% stacking gel and 12% separating gel, and
transferred to polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were then blocked with TBS
containing 0.05% Tween-20 (TBST) and 5% non-fat milk for 1 h at
room temperature. Polyclonal rabbit anti-β actin (dilution, 1:200),
rabbit monoclonal anti-ARPC4 (dilution, 1:200), polyclonal rabbit
anti-vimentin antibody (dilution, 1:200), rabbit polyclonal
anti-E-cadherin antibody (dilution, 1:200) and PCNA rabbit
polyclonal antibody (dilution, 1:200) antibodies were added to
incubate at 4°C overnight. Following washing three times for 10 min
each with TBST, the membranes were incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit (dilution, 1:5,000;
cat no. A8275; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
peroxidase-conjugated goat anti-rabbit (dilution, 1:5,000; cat no.
A0418; Sigma-Aldrich; Merck KGaA), goat anti-rabbit IgG-HRP
secondary antibody (dilution, 1:5,000; cat no. sc-2370; Santa Cruz
Biotechnology, Inc.), goat anti-rabbit IgG-HRP secondary antibody
(dilution, 1:5,000; cat no. sc-2030; Santa Cruz Biotechnology,
Inc.) and chicken anti-rabbit IgG-HRP secondary antibody (dilution,
1:5,000; cat no. sc-2955; Santa Cruz Biotechnology, Inc.) for 1 h
at room temperature. Subsequent to washing with TBST, the membranes
were developed using an enhanced chemiluminescence western blot
detection system (Merck KGaA). Quantity One 4.62 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and Image J software
(version 2.1; National Institutes of Health, Bethesda, MD, USA) was
used analyze the results and calculate the expression of ARPC4 and
β-actin. β-actin was used as a reference protein.
ARPC4-siRNA transfection
Cells were seeded in 6-well plates
(3.5×105 cells per well). A negative control (NC) group
was transfected with non-silencing siRNA of the same length as
ARPC4-siRNA. A null group was incubated under normal conditions
(DMEM containing 10% FBS) without siRNA transfection. Transfection
was conducted using Lipofectamine® 2000 according to the
manufacturer's protocol. The GenBank database (https://www.ncbi.nlm.nih.gov/genbank/)
was used to select the RNA interference target area for the ARPC4
gene, which was used for the design and synthesis of the three
siRNA sequences and the NC (Shanghai GenePharma Co., Ltd.,
Shanghai, China). The siRNAs were designated as siRNA496, siRNA538
and siRNA679, according to the corresponding target sequence
cDNA-initiation site, and BLAST (https://blast.ncbi.nlm.nih.gov/) queries were
conducted to rule out homology with other genes (Table I).
 | Table I.Oligonucleotide sequences of the
siRNAs against actin-related protein 2/3 complex subunit 4. |
Table I.
Oligonucleotide sequences of the
siRNAs against actin-related protein 2/3 complex subunit 4.
| siRNA name | Sense | Antisense |
|---|
| Negative control |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
| siRNA 496 |
GAGCAGAGAACUUCUUUAUTT |
UAAAGAAGUUCUCUGCUCTT |
| siRNA 538 |
GGUAUGAUAUCAGCUUUCUTT |
AGAAAGCUGAUAUCAUACCTT |
| siRNA 687 |
GCUGAAGAGUUCCUUAAGATT |
UCUUAAGGAACUCUUCAGCTT |
Cell viability assays
Cells in the logarithmic growth phase immediately
following transfection were seeded in 96-well plates
(1×103 cells per well), with three wells allocated to
each experimental group. The absorbance in each well was measured
at 0, 1, 2, 3 and 4 days, using Cell Counting kit-8 (EnoGene
Biotech Co., Ltd., Nanjing, China) according to the manufacturer's
protocol. Absorbance was measured at 450 nm using a plate reader,
and growth curves were generated from the resulting data.
Flow cytometry analysis of cell cycle
distribution
A total of 48 h following transfection, cells from
each experimental group were fixed overnight at 4°C with cold 70%
ethanol prior to being incubated with RNase A at 37°C for 30 min.
The cells were stained with propidium iodide for 30 min in the dark
at room temperature, and cell cycle analysis was conducted using a
FACSCanto II flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA) and ModFit LT software 4.1 (Verity Software House, Inc.,
Topsham, ME, USA).
Cell migration assays
Cell migration assays were performed using transwell
chamber dishes (6.5 mm) with 8.0-µm pore polycarbonate membrane
inserts (EDM Millipore, Billerica, MA, USA). Serum-free cell
suspensions (200 µl, 105 cells) were seeded in the upper
compartment, while 450 µl DMEM containing 30% FBS was placed in the
lower chamber. The cells were incubated for 48 h. The upper
membrane surface was wiped to remove cells, and the migrated cells
on the lower surface were fixed with 4% methanol at room
temperature for 30 min and stained with 0.1% (g/ml) crystal violet
for 30 min at room temperature. Using a light microscope, cells in
three random fields of vision (magnification, ×400; DP-50; Olympus
Corporation, Tokyo, Japan) were counted for each sample and means
were reported.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS
16.0 statistical software (SPSS, Inc., Chicago, IL, USA). All data
are presented as the mean ± standard deviation from 3 independent
experiments. Student's t-test was used when comparing between two
groups and one-way analysis of variance with Tukey's post-hoc test
was used when comparing more than two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Immunohistochemical analysis of ARPC4
expression in human carcinoma-associated antigen-containing tissues
and adjacent non-tumour colorectal tissues
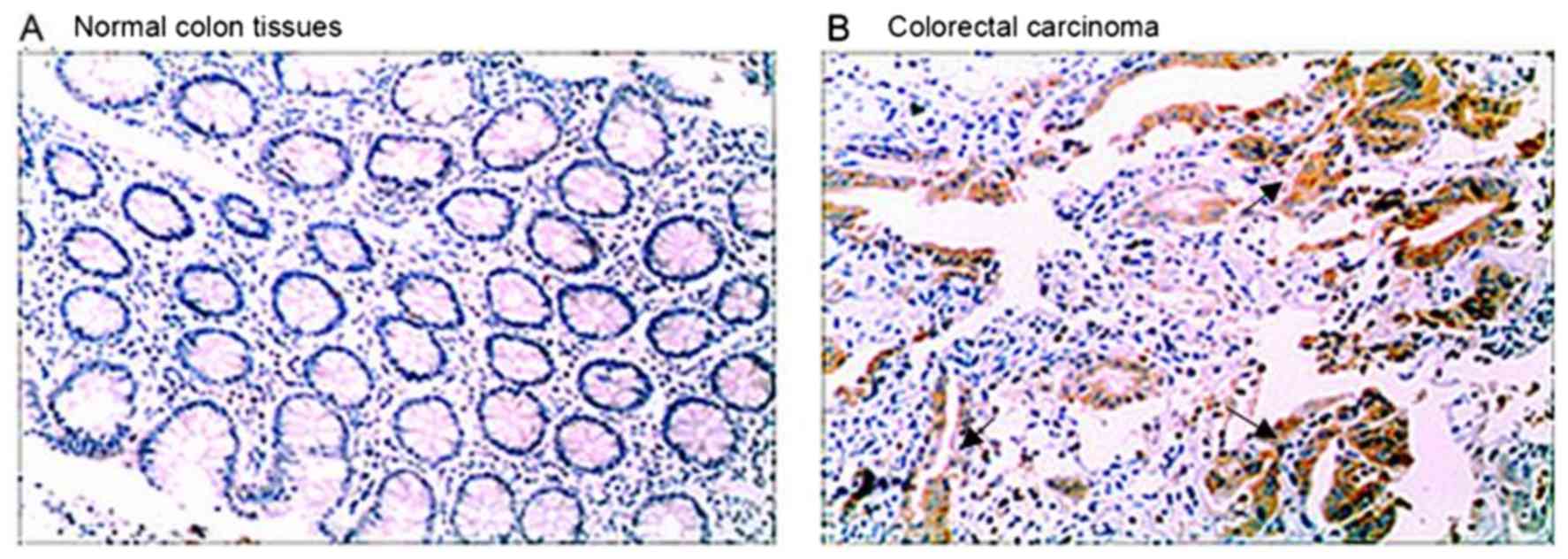
ARPC4 expression in colorectal carcinoma and normal
colon tissues was assessed by immunohistochemistry. High levels of
ARPC4 staining were observed in tumour tissues, whereas the normal
tissues were negative for ARPC4 (Fig.
1).
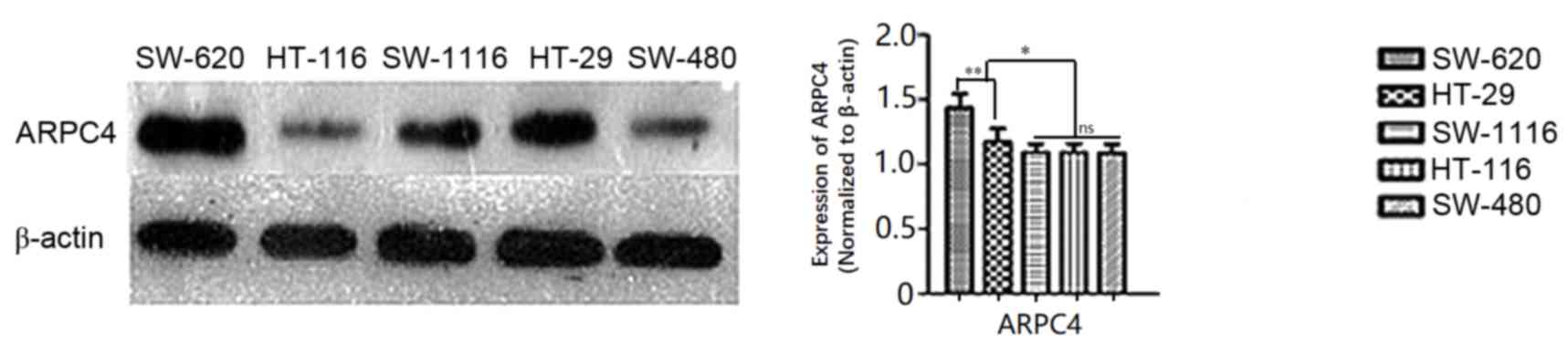
Analysis of ARPC4 expression in
colorectal cancer cells by western blot
Western blot results revealed that ARPC4 was
expressed at high levels in SW620 and HT-29 cell lines relative to
other cell lines (Fig. 2), with the
highest expression observed in SW620 cells (Fig. 2). For this reason, the SW620 cell line
was selected for subsequent experiments.
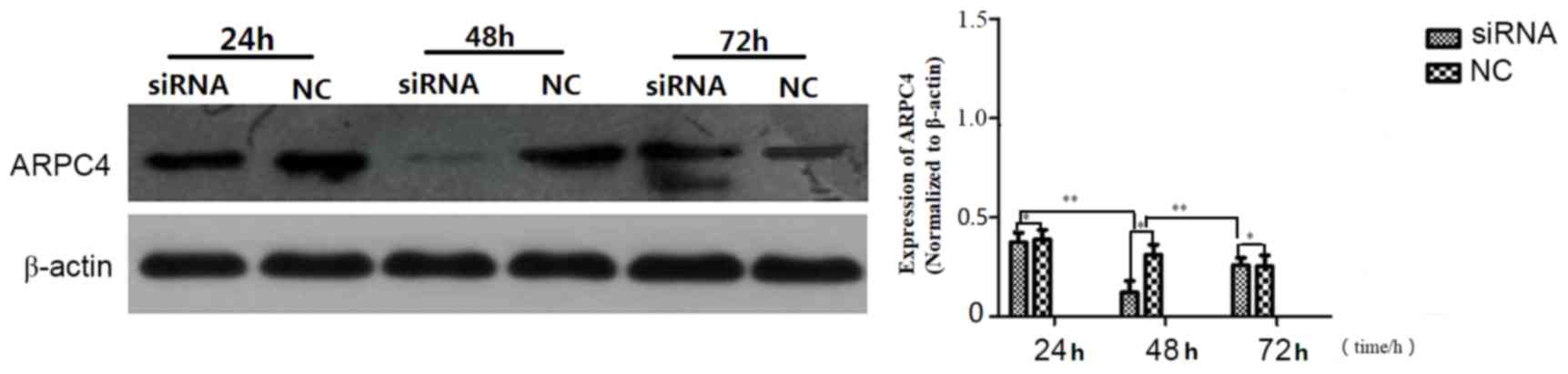
ARPC4-siRNA538 exhibited the strongest
silencing effect
Western blot analysis was carried out to determine
which of the ARPC4-siRNAs yielded the most effective silencing. As
presented in (Fig. 3), siRNA538
resulted in the highest level of ARPC4 inhibition. and was,
therefore, used in subsequent experiments. The inhibition rate was
significantly decreased at all time points, but the optimal
transfection time was 48 h. (Fig.
4).
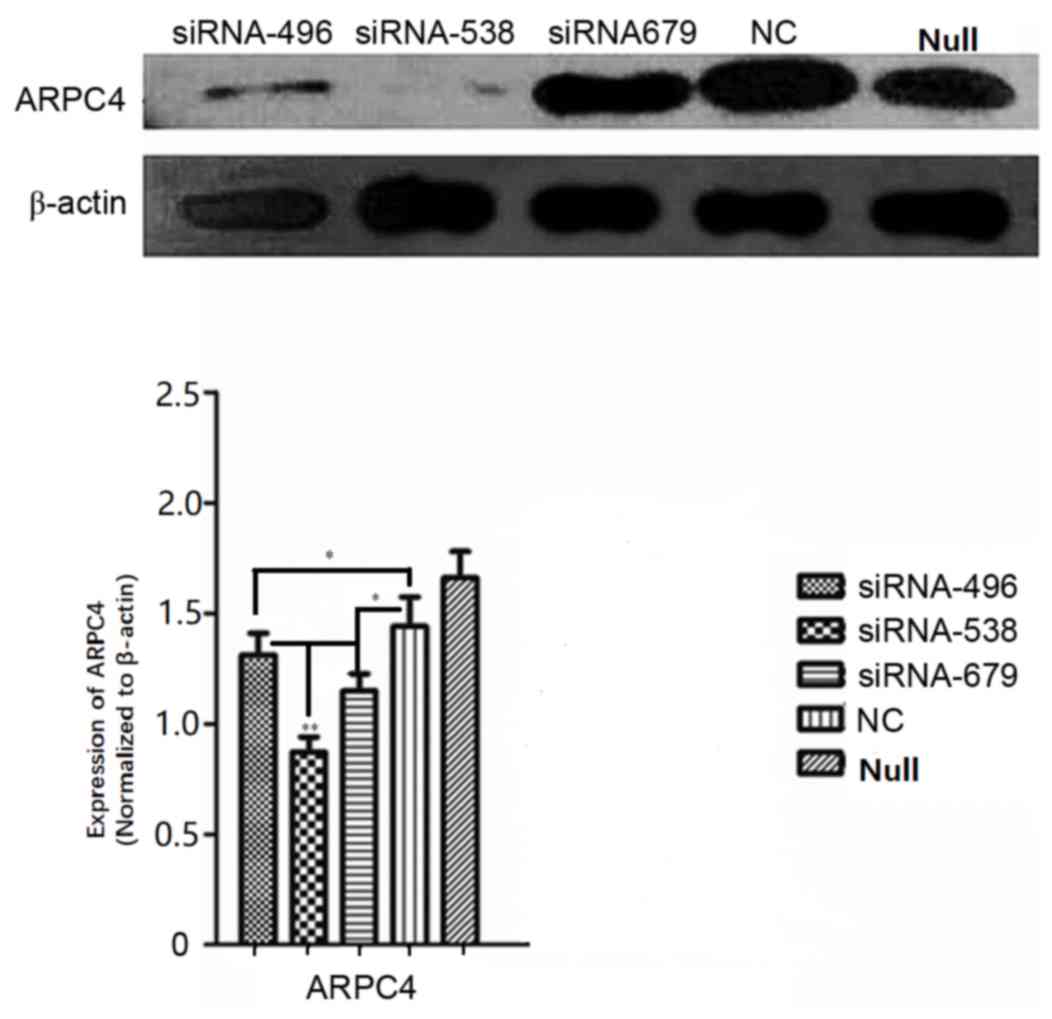 | Figure 3.Comparison of silencing efficiencies
for three ARPC4-siRNA oligonucleotide sequences. Comparison of
ARPC4 protein expression levels among siRNA, NC and Null groups in
SW620 cells after transfection with three ARPC4-siRNA
oligonucleotide sequences and NC. Following transfection with siRNA
and NC, silencing efficiencies between siRNA and NC groups were
significant (*P<0.05 vs. NC). siRNA538 resulted in the highest
level of ARPC4 inhibition when compared with the other siRNA groups
(**P<0.01 vs. siRNA496/siRNA679). ARPC4, actin-related protein
2/3 complex subunit 4; siRNA, short interfering RNA; NC,
nonspecific siRNA; siRNA496, short interfering RNA with
oligonucleotide sequences number 496; siRNA538, short interfering
RNA with oligonucleotide sequences number 538; siRNA679, short
interfering RNA with oligonucleotide sequences number 679; NC,
nonspecific siRNA; null, control group; *P<0.05;
**P<0.01. |
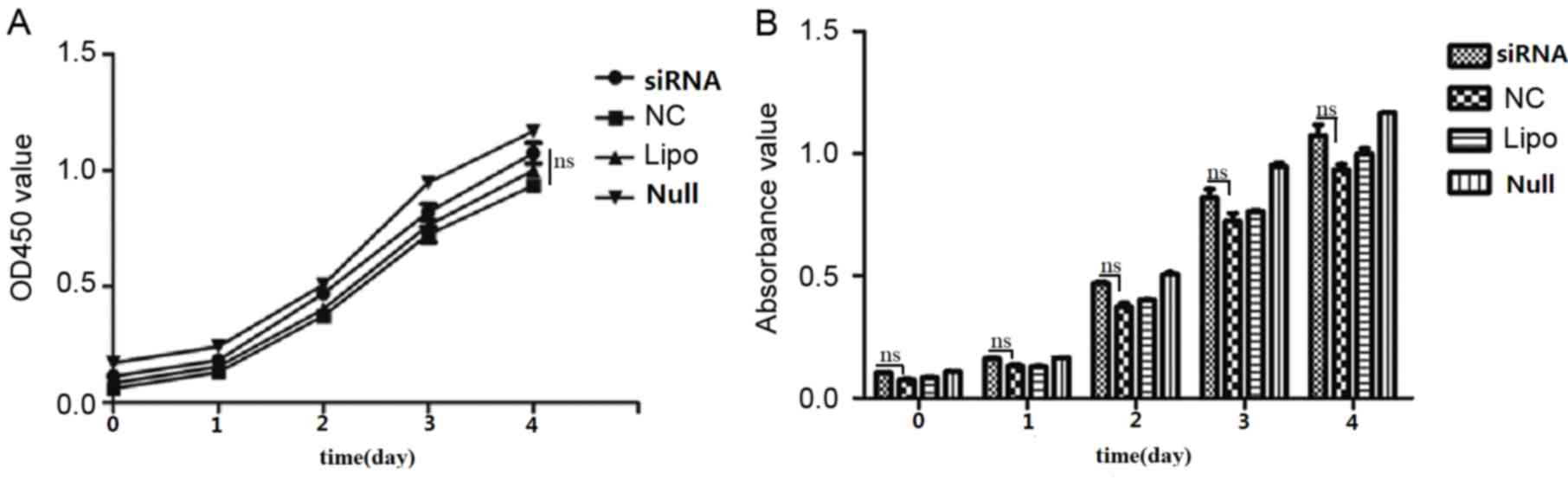
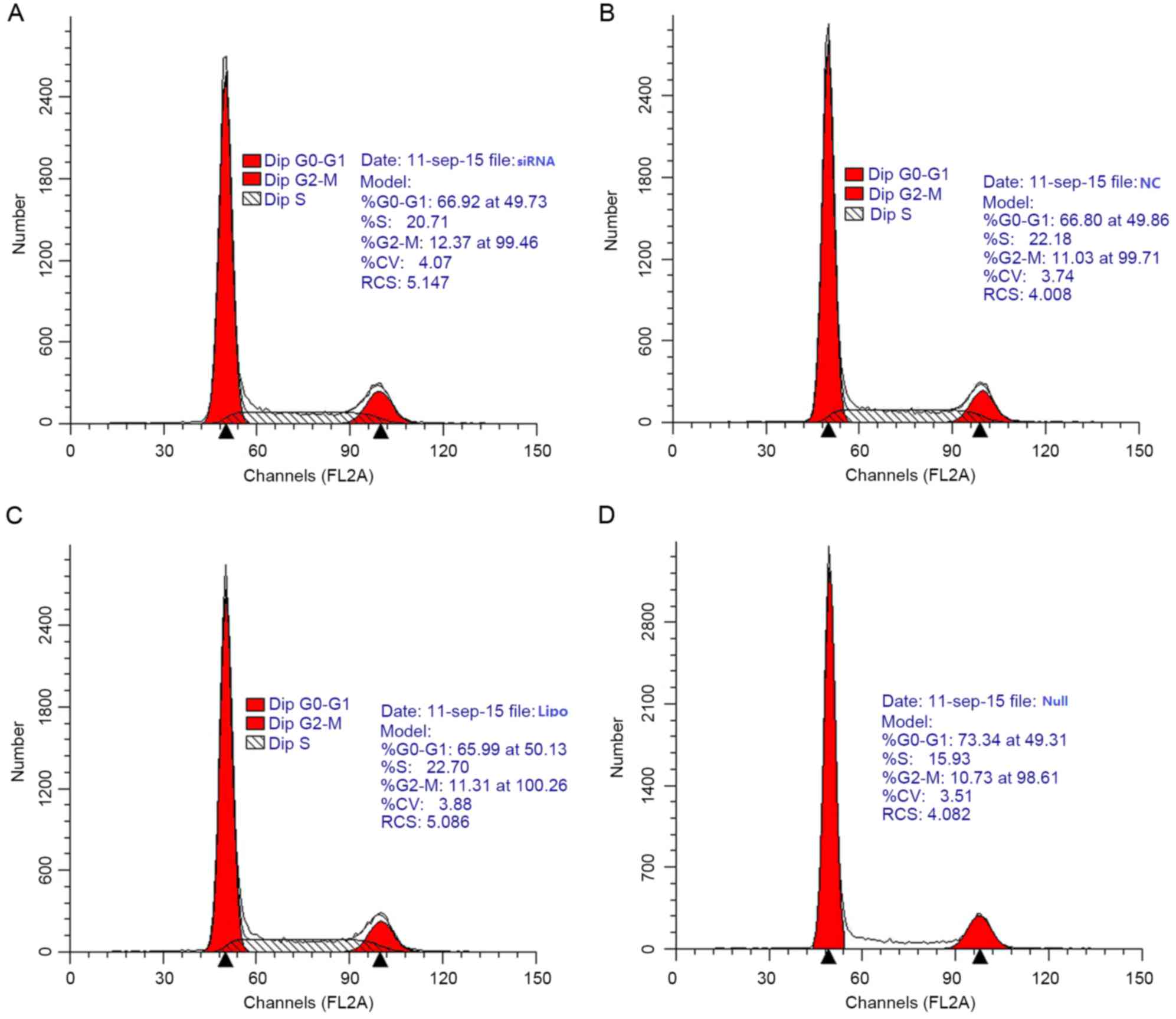
Effect of ARPC4 on viability
As determined from cell growth curves, cell
viability rates did not differ significantly among the siRNA, NC,
Lipo and Null groups (Fig. 5). Cell
cycle analysis by flow cytometry yielded the following results for
the siRNA group vs. the NC group: G0/G1
phase, 66.92 vs. 66.80%; S phase, 20.71 vs. 22.18%; and
G2/M phase, 12.37 vs. 11.03% (Fig. 6). Differences in the
G0/G1, S, and G2/M phase
distributions between the groups were not statistically
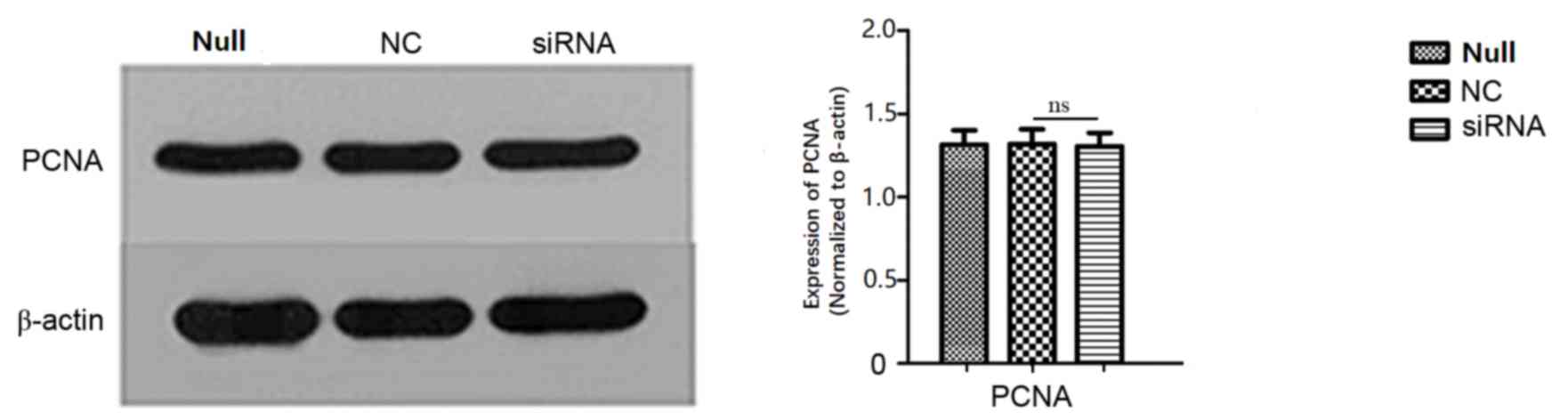
significant. Furthermore, PCNA expression, as determined by western
blot, did not differ significantly between groups (Fig. 7).
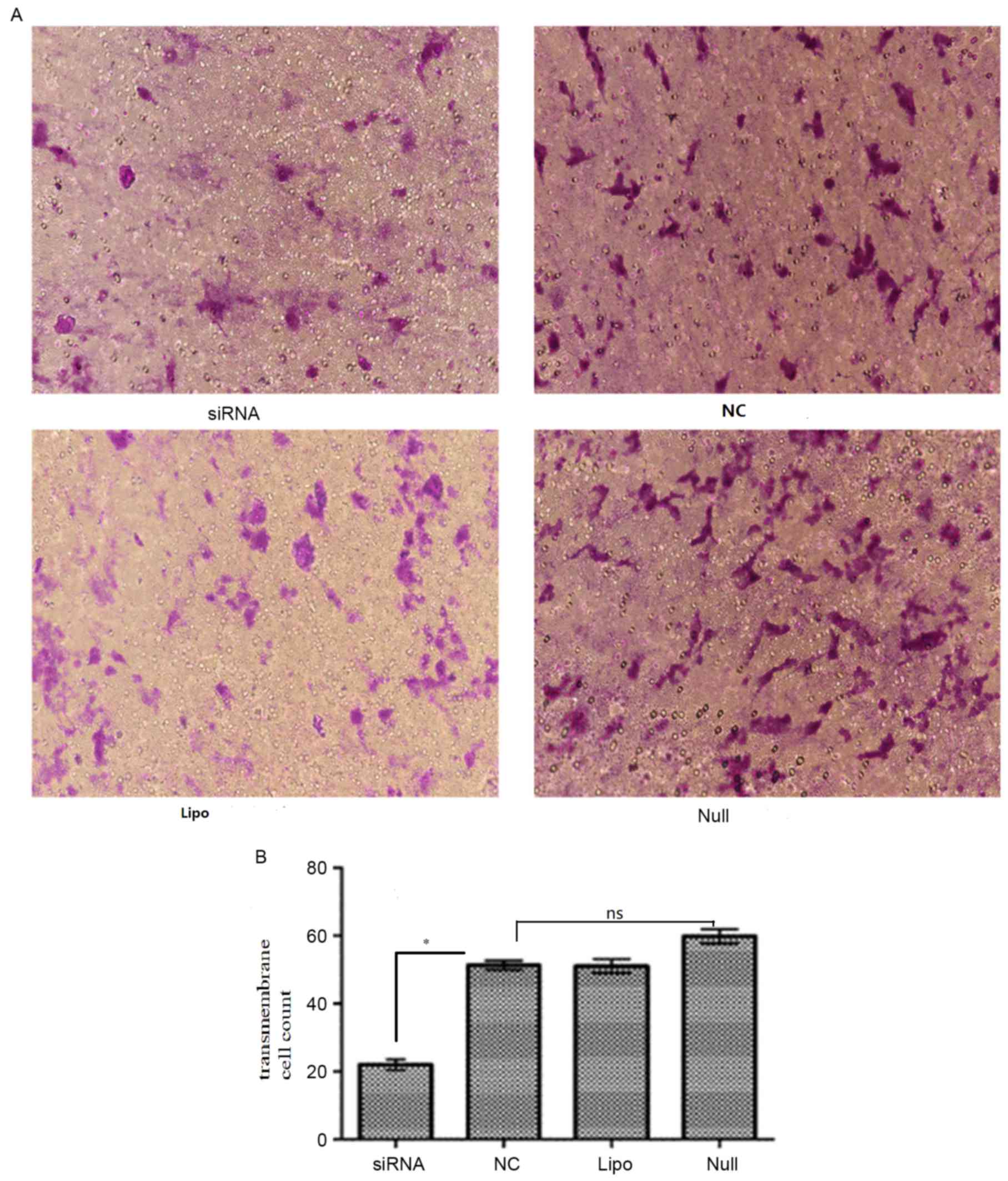
ARPC4 promotes SW620 cell
migration
To examine the effect of ARPC4 on SW620 cell
migration, Transwell and western blot analyses were conducted. In
the transwell assay, the number of migrated cells in siRNA group
were significantly decreased compared with that observed in the NC
group (transmembrane cell count, siRNA vs. NC: 20.4±1.14 vs.
60.6±2.07; P<0.05; Fig. 8), while
the differences among NC/Null/Lipo groups were not significant
(transmembrane cell count 60.6±2.07, 50.0±1.58 and 50.8±1.59;
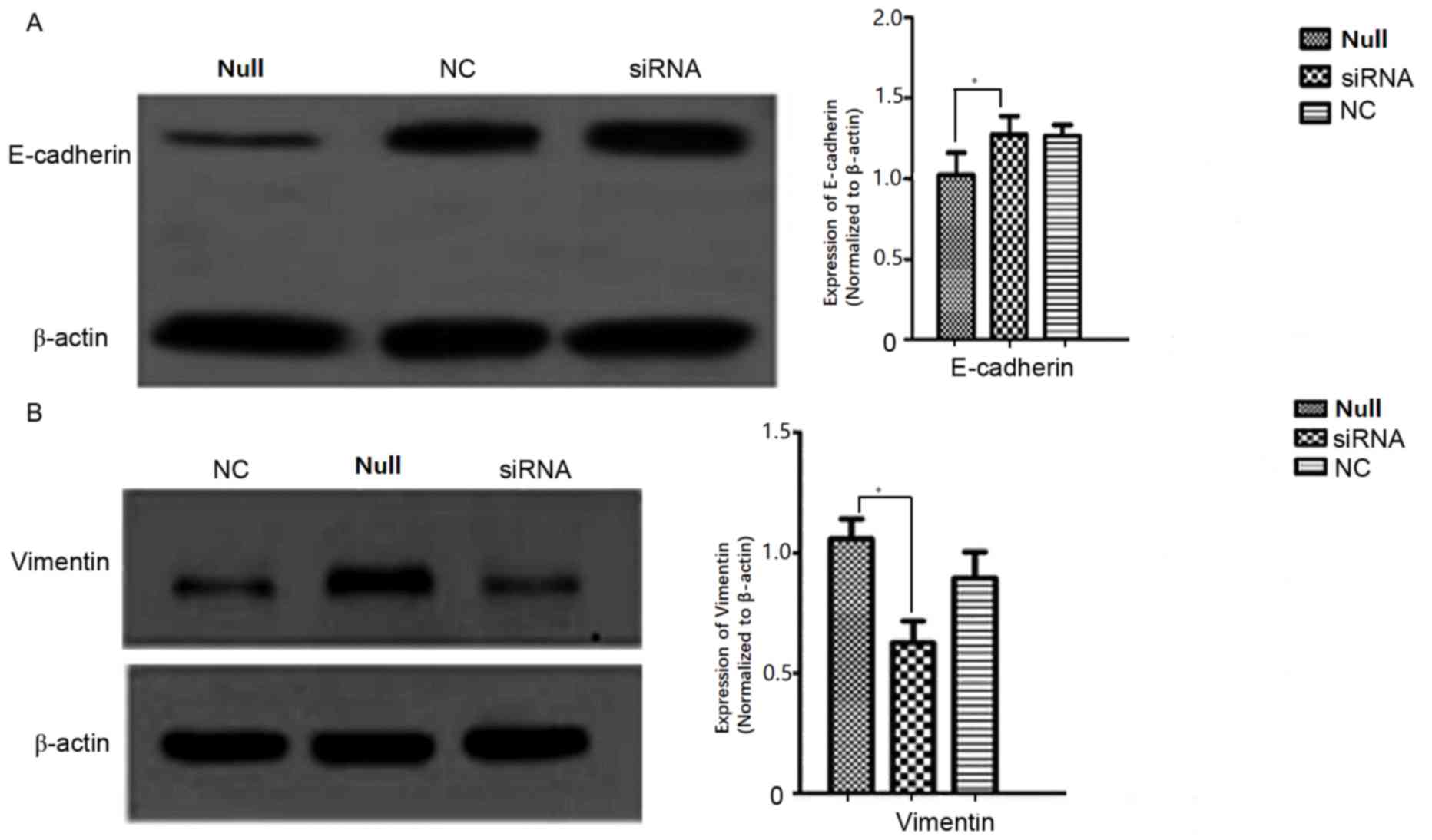
P>0.05). Western blotting results revealed that E-cadherin
(Fig. 9A) and vimentin (Fig. 9B) expression differed significantly
between the siRNA-transfected cells and the Null SW620 cells,
respectively. It was therefore concluded that cells with ARPC4
knockdown exhibited a decreased metastatic capacity compared to
Null/NC cells. NC, nonspecific siRNA; Null, control group; siRNA,
small interfering target RNA; ns; not significant.
Discussion
While high levels of ARPC4 expression have been
identified in colorectal cancer tissues, the association between
the ARPC4 gene and the occurrence and development of
colorectal cancer has not yet been elucidated (5).
Therefore, the present study explored the underlying
molecular mechanisms of the function of ARPC4 in the progression of
colorectal cancer and revealed that ARPC4 may serve a crucial
function in colorectal cancer cell migration.
The findings of the present study indicated that
although ARPC4-siRNA538 transfection did not influence cell
viability, the invasiveness of cells transfected with siRNA538 was
significantly diminished. The actin cytoskeleton formed by
monomeric globular actin serves an essential function in several
cellular processes, including division, migration, adhesion, and
endocytosis. A number of these functions involve contact with the
plasma membrane to allow the actin network outside of the cell to
respond to extracellular signals. The aforementioned processes
result from actin cytoskeleton rearrangement, which involves
numerous regulatory factors, including the ARP2/3 complex, which is
an evolutionary conserved 220-kDa complex comprised of ARP2, ARP3,
and five affiliated proteins (ARPC1-5) (6–10).
The ARP2/3 complex is an important component of the
cytoskeleton that promotes the nucleation of new microfilaments and
functions in the maintenance of cell shape, motility, and
cytokinesis. ARPC4 and ARPC2 constitute the centre of the complex,
whereas ARPC4 was previously demonstrated to serve an important
function in the biological function of ARP2/3 in pancreatic cancer
(11–14). ARPC4, the expression of which is
abnormally high in colorectal cancer cell lines, regulates the
actin nucleation process in cells, forms fusion proteins with the
products of the downstream genes and influences the migration of
pancreatic cancer cells (15,16).
PCNA is a 36 kDa protein that is only identified in
the nuclei of normal proliferative and tumour cells. PCNA is
associated with cell DNA synthesis and serves an important function
in the initiation of cell proliferation (17). Tumour cells exhibit strong
proliferative activity; PCNA may be used as an evaluation index of
the cell proliferation state. In order to further determine the
influence of ARPC4 on SW620 colorectal cancer cell proliferation in
the present study, the PCNA protein expression level was
investigated; its expression was not significantly different
between groups.
The expression of E-cadherin was markedly increased,
whereas the expression of vimentin was decreased in ARPC4-silenced
cells compared with the control cells. E-cadherin is considered to
be a tumour invasion and metastasis suppressor gene, and belongs to
the calcium-dependent cadherin family. The expression of
E-cadherin, which maintains the stability of the connection between
normal cells, is negatively correlated with the occurrence of the
epithelial-mesenchymal transition (EMT) and tumourigenesis.
E-cadherin is connected to the cytoskeleton by its interaction with
catenin to inhibit the proliferation of tumour cells and the
production of matrix metalloproteinases by the host cell (18–20).
Additionally, E-cadherin prevents the degradation of
various proteins of the matrix and basement membrane surrounding
the tumour cells, thereby inhibiting tumour cell degradation of the
matrix and basement membrane barriers (21–23).
Invasion of tumour cells is regulated by tumour-matrix
interactions. Expression of the ARP2/3 complex is associated with
stromal cells in colorectal cancer, and therefore ARP2/3 expression
enhances the motility between stromal cells and tumour cells,
thereby providing a more suitable environment for invasion by these
two cell types (24). Vimentin,
however, is considered an interstitial cell marker, the expression
of which correlates positively with the occurrence of EMT and with
tumour oncogenesis. Vimentin is the dominant central fibre in
mesenchymal cells and participates in the maintenance of cell
integrity. Decreased E-cadherin expression is associated with
elevated vimentin expression, and waveform protein expression may
interfere with cell adhesion mediated by E-cadherin (25–27).
Therefore, the results of the present study suggested that ARPC4
may enhance the expression of vimentin, whereas it may inhibit the
expression of E-cadherin, and that the expression of ARPC4 may
therefore have decreased cell adhesion to promote migration in
tumour cells, thus serving a function in tumour development.
In summary, the use of RNA interference may
effectively suppress human ARPC4 expression in colorectal cancer
SW620 cells, thereby inhibiting cell migration. These results
suggested that specific targeting of ARPC4 may represent a
potential treatment for colorectal cancer. Although previous
studies demonstrated that ARPC4 influences the migration of
pancreatic and colorectal cancer cells (15,16),
further experiments should be undertaken to define the function of
ARPC4 during the initiation of colorectal cancer. Future research
will include determination of the mechanism by which ARPC4
expression influences the biological behaviour of tumour cells.
This information will enable the elucidation of novel targets for
the treatment of colorectal cancer.
Acknowledgements
The present study was supported by the Scientific
Project of Sichuan Province (grant no. 16ZC1671) and the Program
Science and Technology Bureau of Chengdu China (grant no.
2015-HM01-00141-SF).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2839–2917. 2010. View Article : Google Scholar
|
|
2
|
Inra JA and Syngal S: Colorectal cancer in
young adults. Dig Dis Sci. 60:722–733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gross K and Brand MI: Genetic
predisposition to colorectal cancerCommon Surgical Diseases. New
York: Springer; pp. 189–191. 2014
|
|
4
|
August DA, Ottow RT and Sugarbaker PH:
Clinical perspective of human colorectal cancer metastasis. Cancer
Metastasis Rev. 3:303–324. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belvitch P, Brown ME, Brinley BN, Letsiou
E, Rizzo AN, Garcia JGN and Dudek SM: The ARP 2/3 complex mediates
endothelial barrier function and recovery. Pulm Circ. 7:200–210.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamaguchi H and Condeelis J: Regulation of
the actin cytoskeleton in cancer cell migration and invasion.
Biochim Biophys Acta. 1773:642–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le Clainche C and Carlier MF: Regulation
of actin assembly associated with protrusion and adhesion in cell
migration. Physiol Rev. 88:489–513. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Firat-Karalar EN and Welch MD: New
mechanisms and functions of actin nucleation. Curr Opin Cell Biol.
23:4–13. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goley ED and Welch MD: The ARP2/3 complex:
An actin nucleator comes of age. Nat Rev Mol Cell Biol. 7:713–726.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bailly M, Ichetovkin I, Grant W, Zebda N,
Machesky LM, Segall JE and Condeelis J: The F-actin side binding
activity of the Arp2/3 complex is essential for actin nucleation
and lamellipod extension. CurrBiol. 11:620–625. 2001. View Article : Google Scholar
|
|
11
|
Rauhala HE, Teppoi S, Niemelä S and
Kallioniemi A: Silencing of the ARP2/3 complex disturbs pancreatic
cancer cell migration. Anticancer Res. 33:45–52. 2013.PubMed/NCBI
|
|
12
|
Welch MD, DePace AH, Verma S, Iwamatsu A
and Mitchison TJ: The human Arp2/3 complex is composed of
evolutionarily conserved subunits and is localized to cellular
regions of dynamic actin filament assembly. J Cell Biol.
138:375–384. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Robinson RC, Turbedsky K, Kaiser DA,
Marchand JB, Higgs HN, Choe S and Pollard TD: Crystal structure of
Arp2/3 complex. Science. 294:1679–1684. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Higgs HN and Pollard TD: Regulation of
actin filament network formation through ARP2/3 complex: Activation
by a diverse array of proteins. Ann Rev Biochem. 70:649–676. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Otsubo T, Iwaya K, Mukai Y, Mizokami Y,
Serizawa H, Matsuoka T and Mukai K: Involvement of Arp2/3 complex
in the process of colorectal carcinogenesis. Mod Pathol.
17:461–467. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghosh A, Tousif S, Bhattacharya D,
Samuchiwal SK, Bhalla K, Tharad M, Kumar S, Prakash P, Kumar P, Das
G and Ranganathan A: Expression of the ARPC4 subunit of human
Arp2/3 severely affects Mycobacterium tuberculosis growth and
suppresses immunogenic response in murine macrophages. PLoS One.
8:e699492013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiong Y, Zhang H and Beach D: D type
cyclins associate with multiple protein kinases and the DNA
replication and repair factor PCNA. Cell. 71:505–514. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goodlad RA: Quantification of epithelial
cell proliferation, cell dynamics and cell kinetics in vivo. Wiley
Interdiscip Rev Dev Biol. 6:2017. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie Y, Li P, Gao Y, Gu L, Chen L, Fan Y,
Zhang F and Zhang X: Reduced E-cadherin expression is correlated
with poor prognosis in patients with bladder cancer: A systematic
review and meta-analysis. Oncotarget. 8:62489–62499. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao H, Xu E, Liu H, Wan L and Lai M:
Epithelial-mesenchymal transition in colorectal cancer metastasis:
A system review. Pathol Res Pract. 211:557–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berx G and van Roy F: Involvement of
members of the cadherin superfamily in cancer. Cold Spring Harb
Perspect Biol. 1:a0031292009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abu Taha A and Schnittler HJ: Dynamics
between actin and the VE-cadherin/catenin complex: Novel aspects of
the ARP2/3 complex in regulation of endothelial junctions. Cell Adh
Migr. 8:125–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen A, Beetham H, Black MA, Priya R,
Telford BJ, Guest J, Wiggins GA, Godwin TD, Yap AS and Guilford PJ:
E-cadherin loss alters cytoskeletal organization and adhesion in
non-malignant breast cells but is insufficient to induce an
epithelial-mesenchymal transition. BMC Cancer. 14:5522014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kovacs EM, Goodwin M, Ali RG, Paterson AD
and Yap AS: Cadherin-directed actin assembly: E-cadherin physically
associates with the Arp2/3 complex to direct actin assembly in
nascent adhesive contacts. Curr Biol. 12:379–382. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nijkamp MM, Span PN, Hoogsteen IJ, van der
Kogel AJ, Kaanders JH and Bussink J: Expression of E-cadherin and
vimentin correlates with metastasis formation in head and neck
squamous cell carcinoma patients. Radiother Oncol. 99:344–348.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuefer R, Hofer MD, Gschwend JE, Pienta
KJ, Sanda MG, Chinnaiyan AM, Rubin MA and Day ML: The role of an 80
kDa fragment of E-cadherin in the metastatic progression of
prostate cancer. Clin Cancer Res. 9:6447–6452. 2003.PubMed/NCBI
|