Introduction
Cervical cancer is the second most common type of
female malignancy, subsequent to breast cancer. There were an
estimated 527,600 new cervical cancer cases and 265,700 deaths
worldwide in 2012 (1). Although
cervical cytology screening has enabled early detection and early
treatment of cervical cancer, the incidence and mortality rates
continue to increase each year (2,3). The
majority of patients with cervical cancer are treated with standard
radiation therapy and chemotherapy, but therapeutic response
varies. Thus, studies are required to determine the pathogenesis of
cervical cancer in order to identify a more effective treatment for
cervical cancer.
MicroRNAs (miRNA/miR) are endogenous non-coding RNAs
containing between 19 and 21 nucleotides. miRNA may regulate
proteins at the post-transcriptional level, resulting in the
degradation or inhibition of proteins. miRNAs are involved in the
regulation of a number of biological functions, including cell
cycle, proliferation, differentiation and apoptosis. Previous
studies have revealed abnormal expression of a number of miRNAs in
cervical cancer, including miR-214, miR-21, miR-143 and miR-145
(4–7).
In cervical cancer, miR-214 expression is decreased, which has been
reported to inhibit cancer progression through targeting
mitochondrial transcription factor A (8). miR-21 overexpression may inhibit the
expression of phosphatase and tensin homolog (PTEN), and promote
cancer cell proliferation and migration (9). In addition, miR-143 expression was
identified to be significantly decreased in cervical cancer, and
associated with tumor size, positive lymph node metastasis and
HPV16 infection (10). A previous
study demonstrated that the expression level of miR-145 was
significantly decreased in human cervical cancer tissues when
compared with corresponding adjacent normal tissues and associated
with tumor progression, and poor prognosis (11). Furthermore, miR-494 serves as a tumor
suppressor miRNA and abnormal expression of miR-494 has been
identified in a number of tumor tissue types (8,9). However,
whether the expression level of miR-494 is altered in cervical
cancer remains unknown. In addition, there are few detailed reports
focusing on the role of miR-494 in cervical cancer.
The present study analyzed the expression of miR-494
in cervical tissue using reverse transcription-quantitative
polymerase chain reaction (RT-qPCR), its association with
clinicopathological characteristics of cervical cancer, and its
effects on HeLa cells through transfection with anti-miR-494 and
miR-494 mimics. The present study aimed to identify the effect of
miR-494 on HeLa cell viability and invasion. Additionally, the
present study aimed at exploring the association between miR-494
and cervical cancer development, metastasis and invasion, in order
to identify novel therapeutic targets.
Materials and methods
Patients and tissue samples
Cervical cancer tissues were selected from 40
patients with cervical cancer who were admitted to the Qi Lu
Hospital of Shandong University (Qingdao, China) between December
2011 and December 2014. No patients previously received
preoperative chemotherapy or radiotherapy. Patients age range was
between 25–72 years; mean age 52.23±20.13 years. All cervical
cancer tissue samples were validated by independent pathologists.
Clinical and pathological classification and staging were performed
according to the International Federation of Gynecology and
Obstetrics criteria (10). The
present study additionally included 40 cervical intraepithelial
lesions and 40 matched normal cervical tissues as the control
group. All specimens were stored in liquid nitrogen within 30 min
of resection. All protocols performed in the present study were
approved by the Ethics Committee of the Qi Lu Hospital of Shandong
University. Written informed consent was obtained from all
patients.
Reagents and equipment
The following equipment and reagents were utilized
in the present study: CO2 incubator (Thermo Fisher
Scientific, Inc., Waltham, MA, USA); pipette (Eppendorf, Hamburg,
Germany); weighing balance; inverted microscope; Milli-Q plus
ultra-pure water system (EMD Millipore, Billerica, MA, USA);
cervical cancer cell line HeLa (Institute of Basic Medical Chinese
Academy of Medical Sciences, Beijing, China); miR-494 mimic and
anti-miR-494 (Invitrogen; Thermo Fisher Scientific, Inc.), RPMI
1640 medium (Gibco; Thermo Fisher Scientific, Inc.); fetal bovine
serum (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA),
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.); ready-to-use
PCR kit (BBI solutions, Cardiff, UK); primers (Sangon Biotech Co.,
Ltd., Shanghai, China). Cells were cultured in RPMI-1640 medium
containing 10% fetal bovine serum at 37°C in a 5% CO2
incubator.
RT-qPCR
Total RNA was extracted from tissues using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. cDNAs were synthesized with the
Prime-Script RT reagent kit (Takara Bio, Inc., Otsu, Japan). The
qPCR reaction conditions were as follows: 95°C for 5 min, 95°C for
10 sec and 60°C for 20 sec, for 40 cycles. The primer sequences
(11) were as follows: miR-494
forward, 5′-TGACCTGAAACATACACGGGA-3′ and miR-494 reverse,
5′-TATCGTTGTACTCCACTCCTTGAC-3′. GADPH was used as the internal
reference gene and the primers used were as follows: Forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′; and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. Data was subjected to quantitative
analysis by comparing the Cq values and using the 2−ΔΔCq
method (12).
Cell transfection methods
HeLa cells were seeded in a 24-well plate at a
density of 4×104 cells/well. Prior to transfection, all
transfection reagents were at room temperature and medium was
placed into incubator for 30 min. Following 48 h transfection at
37°C, cells were washed with PBS containing 1% bovine serum albumin
(Sigma Aldrich; Merck KGaA, Darmstadt, Germany) twice and
resuspended with 100 µl Nucleofector® electroporation
(Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, the
corresponding RNA was added (300 pmol/sample). The cell suspension
was placed into an electroporation cuvette, tapped to ensure that
there were no air bubbles at the bottom of the electrical rotor,
and the electroporation program was selected and run according to
the manufacturer's protocol. Cells were immediately taken out of
the electrical rotor, transferred into 12-well plates and cultured
in the incubator.
MTT viability assay
The transfected cells (0.5×104
cells/well) were seeded in 96-well culture plates and incubated
with 20 µl MTT at 37°C for 4 h. Subsequently, the supernatant was
aspirated and 15 ul dimethyl sulfoxide solution was added. After 10
min of low agitation, the optical density was determined at a
wavelength of 490 nm.
Invasion assay
Following 48 h transfection, 0.25% trypsin
(containing EDTA) was used to digest cells. Cells were stained with
0.16% trypan blue dye solution for 3 min at room temperature. Cells
(5×105) were suspended in serum-free RPMI 1640 medium
and the lower layer of the Transwell chamber was plated with RPMI
1640 medium, supplemented with 10% fetal bovine serum. Cells were
plated in the upper chamber and incubated at 37°C in an atmosphere
containing 5% CO2 for 24 h. The membrane was coated with
Matrigel (BD Bioscience, San Jose, CA, USA). Subsequently, the
number of invaded cells were counted in 16 fields of view. Images
were captured at a wavelength of 490 nm on a Leica DC 300F camera
(Leica Microsystems GmbH, Wetzlar, Germany).
Bioinformatics prediction of miR-494
target genes
TargetScan (http://www.targetscan.org/vert_71/) target gene
prediction software selected suppressor of cytokine signaling 6
(SOCS6) as a target gene of miR-494. TargetScan target gene
prediction software identified the 3726–3733 site at the 3′ end of
the untranslated region (3′UTR) of SOCS6 mRNA as a possible site of
action of miR-494.
Dual-luciferase reporter assay
The 3′UTR of SOCS6, containing putative
miR-99a-binding sites was amplified and cloned into pMIR (Ambion;
Thermo Fisher Scientific, Inc.). In vitro synthesized DNA
fragment containing the position and DNA fragment containing the
mutant site, cloned into dual luciferase start after a child
carrier pMIR. PMIR carrier (Ambion, Austin, TX, USA) and miR494
were co-transfected into HeLa cells using Dharmafect Duo
transfection reagent (Thermo Fisher Scientific, Inc.); the cells
were collected 48 h post-transfection. The PMIR carrier containing
wild-type and mutated full-length 3′UTR from human SOCS6 (RefSeq
NM_004232.3), was purchased from Labomics (GeneCopoeia, Inc.,
Rockville, MD, USA). The sense siRNA sequence was GCU GCG AUA UCA
ACG GUG Att; the antisense siRNA sequence was UCA CCG UUG AUA UCG
CAG Ctg. Luciferase activity was measured using the Dual-Luciferase
Reporter Assay system (Promega Corporation) according to the
manufacturer's recommendations. The luciferase activity was
detected on a GLOMAX20/20 luminometer (Promega Corporation) and
normalized to the Renilla luciferase activity.
Western blot analysis
Total protein was extracted from cells using the
protein extraction reagent (Novagen; Merck KGaA) accordingly to the
manufacturer's protocol and total protein concentration was
determined using a BCA Protein Assay kit (Thermo Fisher Scientific,
Inc.). A total of 50 µg extracted protein per lane was separated
using SDS-PAGE (8–10% gel) and 5% stacking gel isolated, and
transferred to nitrocellulose membranes. Subsequently, membranes
were blocked at room temperature with Tris-Buffered Saline-Tween-20
(TBST) containing 5% bovine serum albumin for 1 h. Membranes were
incubated with anti-β-actin (1:5,000; cat. no. A5441;
Sigma-Aldrich; Merck KGaA) or SOCS6 antibody (1:1,000; cat. no.
ab53181; Abcam, Cambridge, UK) overnight at 4°C. The following day,
membranes were washed with 0.1% TBST 3 times (5 min each), the
horseradish peroxidase-labeled-anti-rat serum (1:1,000; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) secondary
antibody was added and incubated at room temperature for 1 h.
Subsequently, membranes were washed with 0.1% TBST and Supersignal
West Femto HRP sensitive chemiluminescent substrate (Pierce; Thermo
Fisher Scientific, Inc.) was added to visualize the bands. β-actin
was used as the internal control. All experiments are
representative of a minimum of three independent repeats.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS software (version 16.0; SPSS, Inc., Chicago, IL, USA) was used
for statistical data analysis. P<0.05 was considered to indicate
a statistically significant difference. Differences between miR-494
expression and clinicopathological features of cervical cancer were
assessed using Student's t-test (unpaired). The differences in
expression between three groups were examined by one-way analysis
of variance followed by a Dunnett's post hoc test.
Results
miR-494 expression is decreased in
cervical cancer
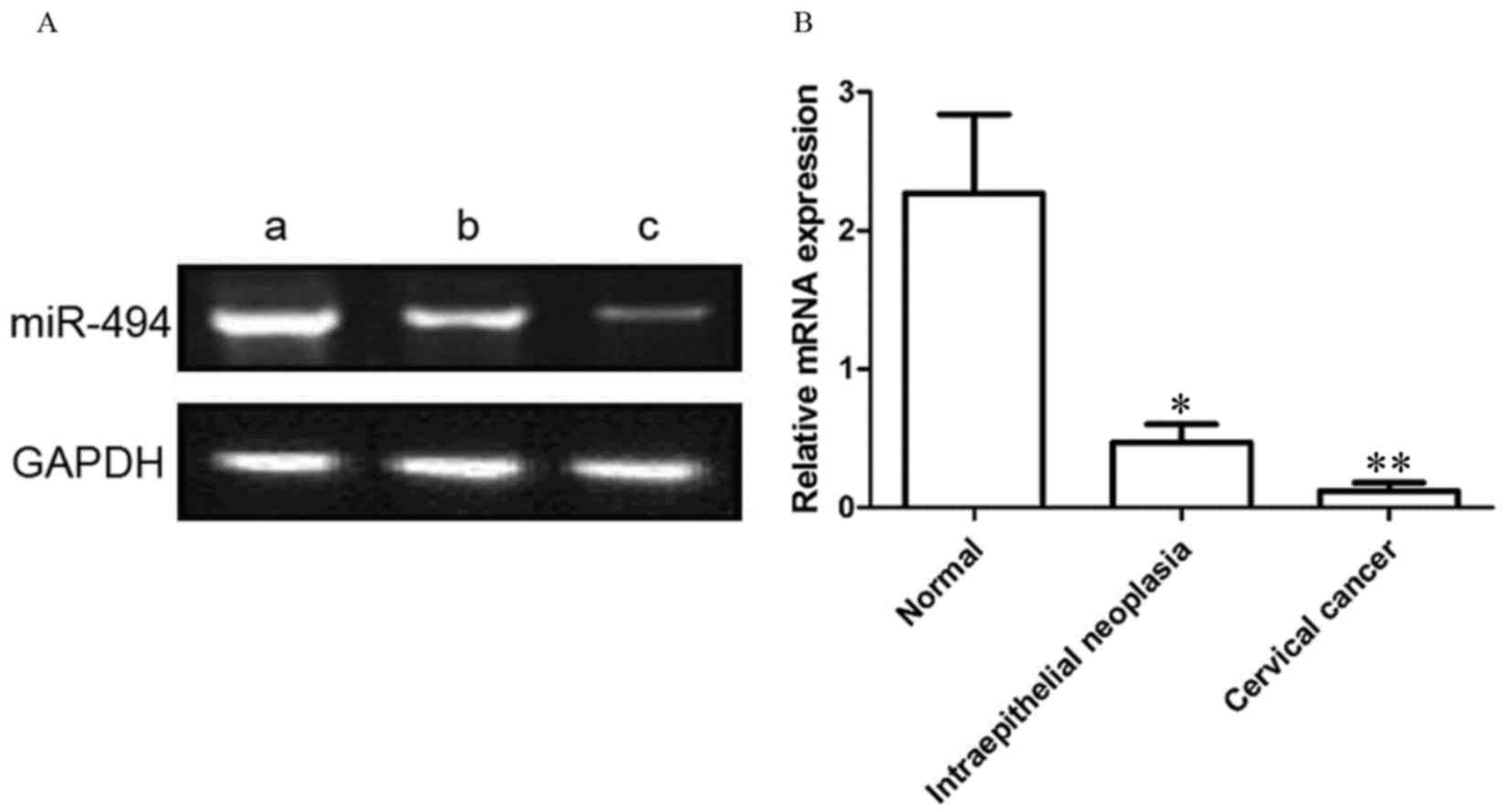
As presented in Fig.
1, the relative expression level of miR-494 was 0.12±0.06,
0.47±0.13 and 2.27±0.57 in cervical cancer, cervical
intraepithelial neoplasia and normal cervical tissues,
respectively. Compared with the cervical intraepithelial neoplasia,
the miR-494 expression level in cervical cancer samples was
significantly decreased (P<0.01). In addition, compared with
normal cervical tissue, miR-494 expression level was significantly
decreased in cervical intraepithelial lesions (P<0.05).
miR-494 expression is associated with
clinicopathological features of cervical cancer
As presented in Table
I, the expression level of miR-494 in patients with cervical
cancer was associated with clinical stage, depth of stromal
invasion and lymph node metastasis (P<0.01), but not with age,
tumor diameter, and menopause (P>0.05).
 | Table I.miR494 expression level and
clinicopathological features of cervical cancer. |
Table I.
miR494 expression level and
clinicopathological features of cervical cancer.
| Parameter | n | miR-494 | t-value | P-value |
|---|
| Age, years |
|
| 0.356 | 0.736 |
|
<40 | 21 |
2.28±0.79 |
|
|
| ≥40 | 19 |
2.19±0.71 |
|
|
| Tumor diameter,
cm |
|
| 0.177 | 0.866 |
|
<2.5 | 20 |
2.17±0.72 |
|
|
| ≥2.5 | 20 |
2.22±0.67 |
|
|
| Menopause |
|
| 0.106 |
|
| Yes | 18 |
2.25±0.52 |
| 0.919 |
| No | 22 |
2.41±0.50 |
|
|
| Clinical stage |
|
| 5.556 | 0.001 |
| I–II | 19 |
2.93±0.44 |
|
|
|
III–IV | 21 |
1.11±0.61 |
|
|
| Interstitial
infiltration |
|
| 5.444 | 0.002 |
|
<2/5 | 24 |
2.65±0.48 |
|
|
| ≥2/5 | 16 |
1.29±0.72 |
|
|
| Lymph node
metastasis |
|
| 5.261 | 0.002 |
| No | 22 |
2.79±0.57 |
|
|
| Yes | 18 |
1.44±0.62 |
|
|
miR-494 mimic transfection increases
the expression level of miR-494
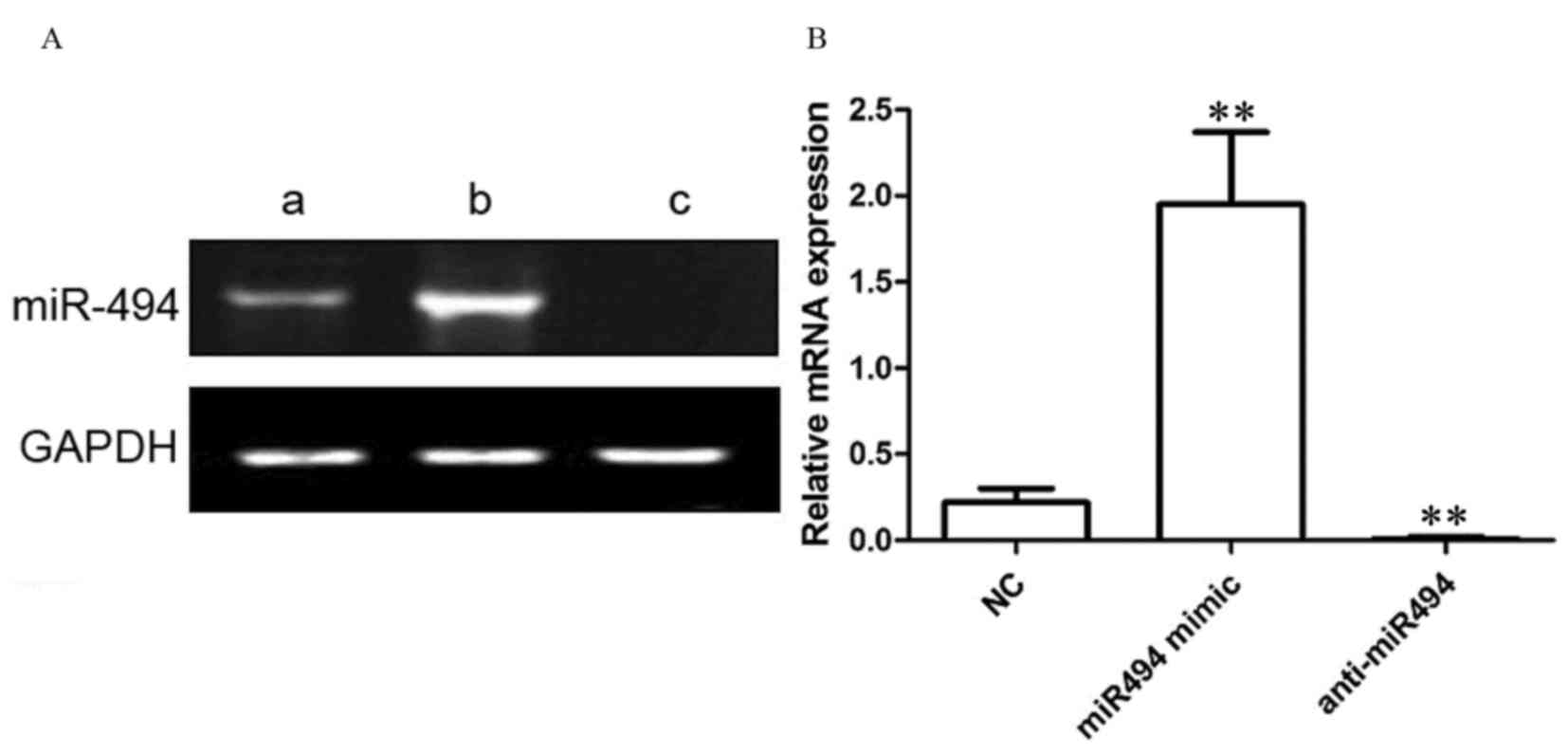
As presented in Fig.
2, in NC-transfected HeLa cells, the relative expression level
of miR-494 was 0.22±0.08. Following transfection with the miR-494
mimic, the relative expression level of miR-494 in HeLa cells was
1.95±0.42. Following transfection with the anti-miR-494 in HeLa
cells, the relative expression level of miR-494 was 0.01±0.01.
Compared with control group, miR-494 mimic transfection
significantly increased the expression level of miR-494
(P<0.01), and anti-miR494 transfection significantly inhibited
the expression level of miR-494 (P<0.01).
Transfection with the miR-494 mimic
inhibits the viability of HeLa cells
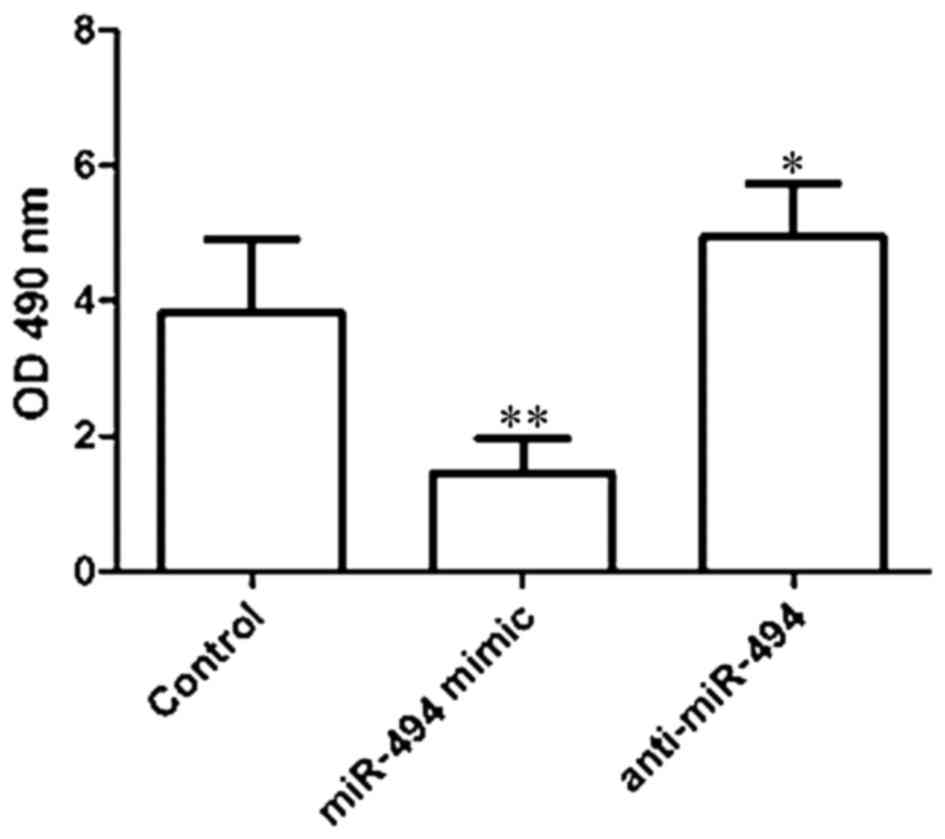
As presented in Fig.
3, the viability of HeLa cells was determined to be 3.83±1.08,
1.44±0.52 and 4.94±0.79 in the control, miR-494 mimic, and
anti-miR494 groups, respectively. Compared with the control group,
transfection with the miR-494 mimic significantly inhibited the
viability of HeLa cells (P<0.01), and transfection with the
anti-miR-494 significantly increased the viability of HeLa cells
(P<0.05).
Transfection with miR-494 mimic
inhibits the invasive ability of HeLa cells
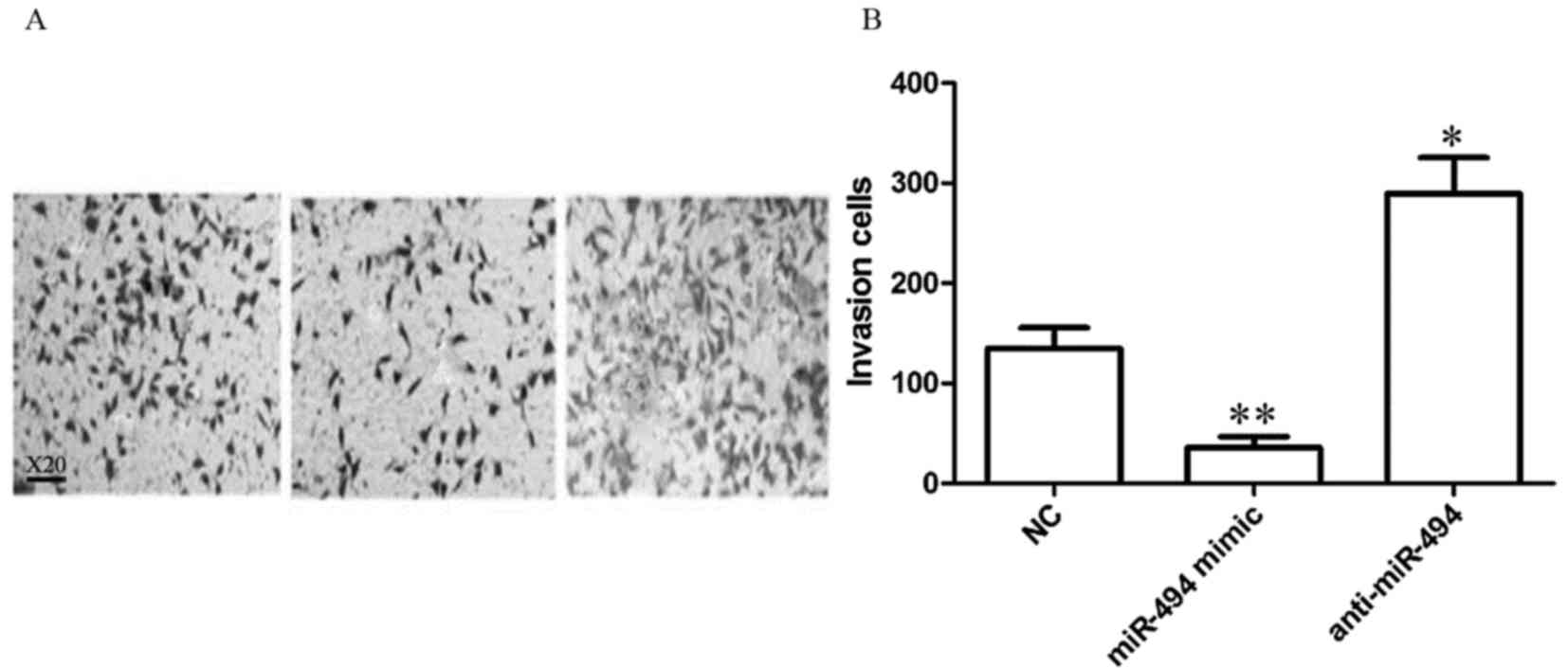
As presented in Fig.
4, the number of invasive HeLa cells was 134.84±20.75,
35.75±11.04 and 289.45±35.94 in the control, miR-494 mimic, and
anti-miR-494 groups, respectively. Compared with the control group,
miR-494 mimic transfection significantly inhibited HeLa cells
invasiveness (P<0.01), and transfection with anti-miR-494
significantly increased the ability of HeLa cell invasion
(P<0.05).
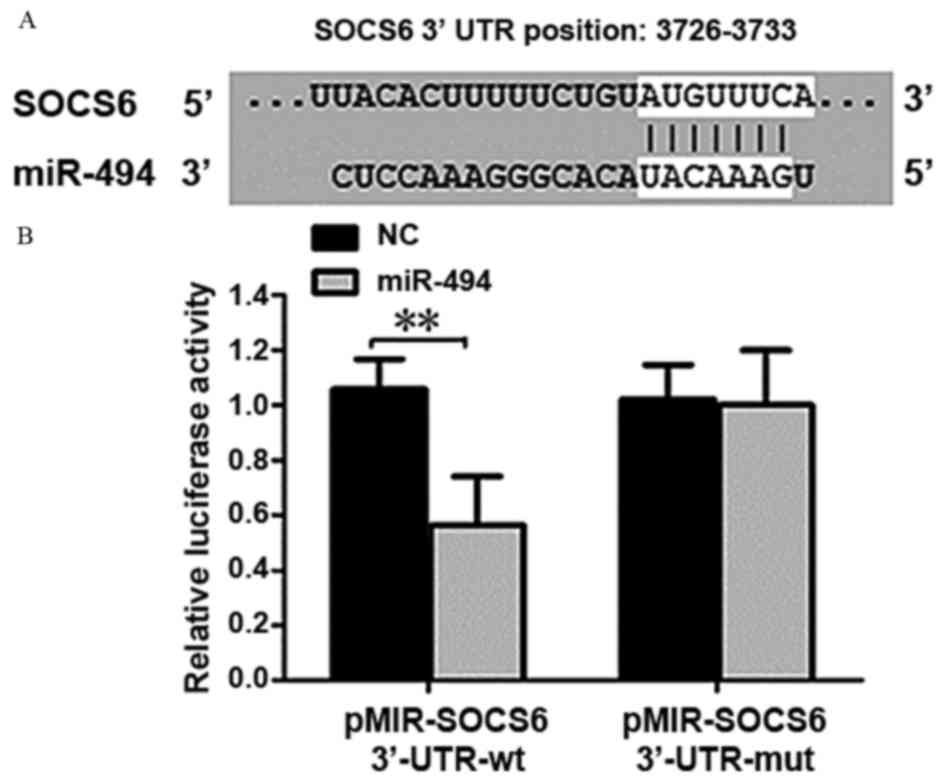
miR-494 target gene prediction
TargetScan prediction software indicated that SOCS6
may be a target gene of miR-494. The forecasting SOCS6 3′-UTR
target sites are presented in Fig.
5A. The luciferase reporter gene assay demonstrated that
luciferase activity was significantly decreased when co-transfected
with wild-type pMIR-SOCS6 3′-UTR carrier and miR-494 (P<0.01;
Fig. 5B). Following co-transfection
of mutant pMIR-SOCS6 3′-UTR carrier and miR-494, there was no
significant difference was identified, suggesting an interaction
between miR-494 and SOCS6.
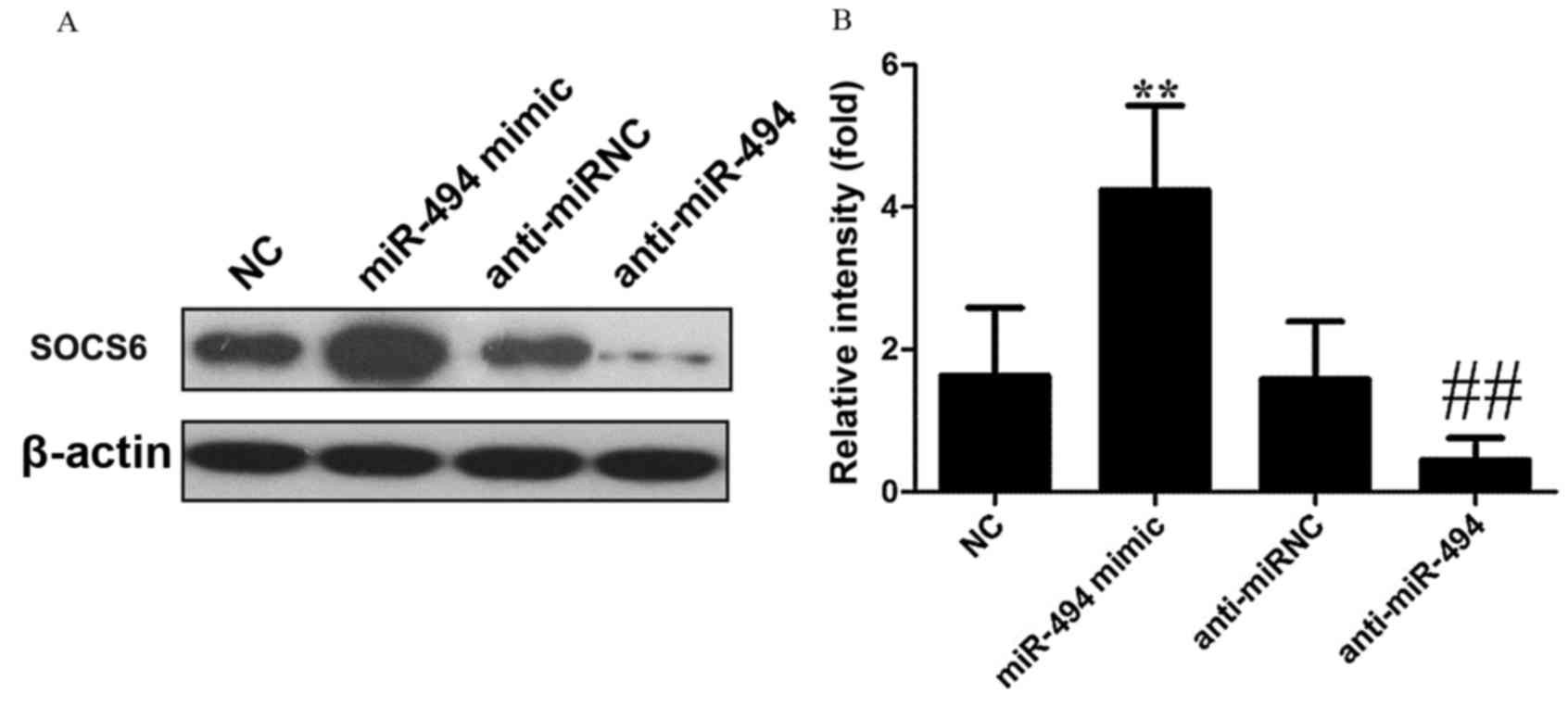
miR-494 mimic transfection increases
the expression of SOCS6
As presented in Fig.
6, when compared with NC, miR-494 mimic transfection
significantly increased SOCS6 expression in HeLa cells (P<0.01),
while anti-miR-494 transfection significantly inhibited SOCS6
(P<0.01).
Discussion
miRNAs are endogenous non-coding RNAs that contain
between 19 and 21 nucleotides. miRNAs are involved in regulating a
number of biological functions including cell cycle, proliferation,
differentiation and apoptosis. Previous studies have demonstrated
that a variety of miRNAs exhibit abnormal expression levels in
tumor tissue. Yanaihara et al (13) explored miRNA expression in >100
cases of primary malignant tumor and adjacent normal tissues. A
total of 43 miRNAs exhibited altered expression levels in primary
malignant tumor and cancerous tissues, whereby 28 miRNAs were
downregulated, and 15 were upregulated (13). Cervical cancer is one of the most
common types of gynecologic cancer, and is the fourth leading cause
of cancer-associated mortality among females worldwide (14). Previous studies have demonstrated that
miRNAs may be abnormally expressed in cervical cancer and this is
associated with the prognosis of cervical cancer (15,16). Zhao
et al (17) validated that the
expression level of miR-491-5p was significantly decreased in
cervical cancer and that miR-491-5p inhibited cancer cell
proliferation, primarily by regulating human telomerase reverse
transcriptase. Furthermore, Deng et al (18) revealed that the expression level of
miR-142-3p was significantly decreased in cervical cancer, and that
miR142-3p inhibited cancer cell proliferation and invasion,
primarily by regulating Frizzled-7.
Previous studies have identified that miR-494 is
associated with the development of tumors (8,9). However,
there are a limited number of studies on the expression and the
function of miR-494 in cervical cancer. The present study was
performed on 40 cases of cervical cancer, 40 cases of cervical
intraepithelial lesions and 40 cases of normal cervical tissue. The
results of the present study determined the expression of miR-494,
which demonstrated that miR-494 was significantly decreased in
cervical cancer, compared with the other two types of tissue
(P<0.01). In addition, the expression level of miR-494 in
cervical cancer tissue was associated with clinical stage, depth of
stromal invasion and lymph node metastasis (P<0.01); however, no
associated was determined between miR-494 expression level and the
patient's age, tumor diameter and menopause (P>0.05). The
results of the present study suggested that decreased expression of
miR-494 is associated with the development of cervical cancer.
Furthermore, transfection was performed in the present study which
demonstrated that miR-494 mimic transfection significantly
inhibited HeLa cell viability and invasion (P<0.01).
Transfection with anti-miR-494 significantly increased the HeLa
cell viability and invasion (P<0.05). These results suggest
that, miR-494 may serve a function in the development of cervical
cancer by regulating the viability and invasiveness of cancer
cells.
miRNAs exhibit biological effects primarily by
adjusting the downstream target genes; therefore, the role of
downstream target genes determine the function of miRNAs. A
previous study has demonstrated that miR-494 regulates the
PTEN/protein kinase B signaling pathway, which results in the
regulation of glioma cell proliferation, invasion and migration
(19). Sun et al (20) identified that miR-494 may effect
colorectal cancer cell migration and invasion by directly targeting
PTEN. Additionally, in breast cancer, miR-494 exerts effects on the
Wnt/β-catenin signaling pathway, which inhibits breast cancer
(21). Accordingly, in the present
study, target genes of miR-494 were selected using TargetScan
software. Previous studies (22,23) have
identified SOCS6 to exhibit functions in the progression of human
cancers, which prompted the focus on this gene.
A previous study demonstrated that miR-17-5p
regulated the proliferation of gastric cancer cells by modulating
SOCS6 expression (24). Therefore,
the present study investigated the expression of SOCS6 in HeLa
cells, which demonstrated that miR-494 mimic transfection increased
SOCS6 expression (P<0.05) and anti-miR-494 transfection
significantly inhibited SOCS6 expression (P<0.05). The results
of the present study identified SOCS6 as a downstream target gene
of miR-494.
The results of the present study demonstrated that
the expression of miR-494 was significantly decreased in cervical
cancer, and miR-494 inhibited cancer cell proliferation and
invasion by regulating the expression of SOCS6. The present study
may have provided a novel cancer therapeutic target; however, the
molecular mechanism underlying miR-494-induced inhibition of cancer
cell proliferation and invasion remains unknown.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao S, Yao D, Chen J, Ding N and Ren F:
MiR-20a promotes cervical cancer proliferation and metastasis in
vitro and in vivo. PLoS One. 10:e01209052015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Handler AS, Henderson VA, Rosenfeld A,
Rankin K, Jones B and Issel LM: Illinois breast and cervical cancer
program: Implementing effective public-private partnerships to
assure population health. J Public Health Manag Pract. 21:459–466.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen Z, Lei Z, Jin-An M, Xue-Zhen L,
Xing-Nan Z and Xiu-Wen D: The inhibitory role of miR-214 in
cervical cancer cells through directly targeting mitochondrial
transcription factor A (TFAM). Eur J Gynaecol Oncol. 35:676–682.
2014.PubMed/NCBI
|
|
5
|
Xu J, Zhang W, Lv Q and Zhu D:
Overexpression of miR-21 promotes the proliferation and migration
of cervical cancer cells via the inhibition of PTEN. Oncol Rep.
33:3108–3116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Y, Ma C, Zhang W, Chen Z and Ma L:
Down regulation of miR-143 is related with tumor size, lymph node
metastasis and HPV16 infection in cervical squamous cancer. Diagn
Pathol. 9:882014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q, Qin J, Chen A, Zhou J, Liu J,
Cheng J, Qiu J and Zhang J: Downregulation of microRNA-145 is
associated with aggressive progression and poor prognosis in human
cervical cancer. Tumour Biol. 36:3703–3708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang YK, Xi WY, Xi RX, Li JY, Li Q and Gao
YE: MicroRNA-494 promotes cervical cancer proliferation through the
regulation of PTEN. Oncol Rep. 33:2393–2401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan J, Wang K and Xi M: MiR-494 inhibits
epithelial ovarian cancer growth by targeting c-Myc. Med Sci Monit.
22:617–624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
FIGO staging for carcinoma of the vulva,
cervix and corpus uteri, . International journal of gynaecology and
obstetrics: The official organ of the International Federation of
Gynaecology and Obstetrics. 125:1–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou RP, Chen G, Shen ZL and Pan LQ:
Cinobufacin suppresses cell proliferation via miR-494 in BGC-823
gastric cancer cells. Asian Pac J Cancer Prev. 15:1241–1245. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zou Ruanmin, Hu Zhi, Chen Hao, et al:
MiR199a in cervical cancer and cervical intraepithelial lesions
Expression and significance. J Med Res. 40:55–59. 2011.
|
|
13
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma G, Dua P and Agarwal SM: A
comprehensive review of dysregulated miRNAs involved in cervical
cancer. Curr Genomics. 15:310–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Villegas-Ruiz V, Juárez-Méndez S,
Pérez-González OA, Arreola H, Paniagua-García L, Parra-Melquiadez
M, Peralta-Rodríguez R, López-Romero R, Monroy-García A,
Mantilla-Morales A, et al: Heterogeneity of microRNAs expression in
cervical cancer cells: Over-expression of miR-196a. Int J Clin Exp
Pathol. 7:1389–1401. 2014.PubMed/NCBI
|
|
17
|
Zhao Q, Zhai YX, Liu HQ, Shi YA and Li XB:
MicroRNA-491-5p suppresses cervical cancer cell growth by targeting
hTERT. Oncol Rep. 34:979–986. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng B, Zhang Y, Zhang S, Wen F, Miao Y
and Guo K: MicroRNA-142-3p inhibits cell proliferation and invasion
of cervical cancer cells by targeting FZD7. Tumour Biol.
36:8065–8073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li XT, Wang HZ, Wu ZW, Yang TQ, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Huang YL, et al: miR-494-3p
regulates cellular proliferation, invasion, migration, and
apoptosis by PTEN/AKT signaling in human glioblastoma cells. Cell
Mol Neurobiol. 35:679–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun HB, Chen X, Ji H, Wu T, Lu HW, Zhang
Y, Li H and Li YM: miR-494 is an independent prognostic factor and
promotes cell migration and invasion in colorectal cancer by
directly targeting PTEN. Int J Oncol. 45:2486–2494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song L, Liu D, Wang B, He J, Zhang S, Dai
Z, Ma X and Wang X: miR-494 suppresses the progression of breast
cancer in vitro by targeting CXCR4 through the Wnt/β-catenin
signaling pathway. Oncol Rep. 34:525–531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang Y, Sun B, Xiang J and Chen Z:
MiR-301a promotes colorectal cancer cell growth and invasion by
directly targeting SOCS6. Cell Physiol Biochem. 35:227–236. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tanaka T, Arai M, Jiang X, Sugaya S, Kanda
T, Fujii K, Kita K, Sugita K, Imazeki F, Miyashita T, et al:
Downregulation of microRNA-431 by human interferon-β inhibits
viability of medulloblastoma and glioblastoma cells via
upregulation of SOCS6. Int J Oncol. 44:1685–1690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Q, Luo G, Yang Z, Zhu F, An Y, Shi Y
and Fan D: miR-17-5p promotes proliferation by targeting SOCS6 in
gastric cancer cells. FEBS Lett. 588:2055–2062. 2014. View Article : Google Scholar : PubMed/NCBI
|