Introduction
Furin is a member of the pro-protein convertase (PC)
family that activates precursor proteins by cleaving a specific
recognition sequence, and has served an important function in the
activation of bacterial toxins and viral glycoproteins, in addition
to the metastatic progression of certain types of tumor (1,2). It was
revealed that furin may be a target for the development of potent
and selective antiproteolytic agents, owing to the notable function
of furin in the proteolytic activation of numerous pathogenic
precursor proteins, including the pro-toxins of bacteria and
viruses, such as influenza A, Ebola virus and anthrax infection
(3–5).
Furthermore, furin may process molecules associated with tumor
aggression and metastatic potential, including transforming growth
factor-β (TGF-β), membrane type 1 matrix metalloproteinase (MMP)
and vascular endothelial growth factor (6–8). Furin is
required for the activation of numerous pathogenic precursor
proteins and therefore, furin inhibition is a logical approach to
inhibiting the activation of those proteins.
In previous studies, a variety of putative
inhibitors of furin have been identified, the most attractive among
these being small molecule compounds, including
decanoyl-RVKR-chloromethylketone (CMK) (9), α1-antitrypsin Portland (α1-PDX)
(10), CCG 8294 (11) and hexa-D-arginine (D6R) (12). D6R, a type of small synthetic
inhibitor, is less toxic and more effective compared with other
small molecule compounds in vitro, including α1-PDX, furin
propeptide and proteinase inhibitor-8 (12), with inhibitory constant values for
furin, PACE4 and PC1 being 0.106, 0.580 and 13.200 µM,
respectively. It has been reported that D6R may be a treatment for
bacterial and viral infections (4,13,14). For example, D6R appeared to block the
cleavage of pseudomonas aeruginosa exotoxin A in
vitro and in vivo (15),
to reduce hepatitis B e-antigen secretion in patients with chronic
hepatitis B viral infection and to facilitate the decrease of
immune tolerance (9). However, little
is known regarding the function of D6R in the progression of a
tumor.
Pancreatic cancer is a highly fatal disease with a
high mortality rate and a 5-year survival rate of ~5% (16). Pancreatic cancer lacks noticeable
symptoms, progresses rapidly, and is characterized by early
dissemination and poor prognosis (17). The present study revealed that D6R is
able to suppress the proliferation and epithelial-mesenchymal
transition (EMT) of pancreatic cancer cells. The results of the
present study indicated that D6R may function as an ideal compound
for anti-pancreatic cancer treatment.
Materials and methods
Cell culture
Pancreatic cancer SW1990 and PaTu8988 cell lines
were obtained from the Second Military Medical University
(Shanghai, China). Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) with 10% fetal bovine serum (FBS; Invitrogen; Thermo
Fisher Scientific, Inc, Waltham, MA, USA) at 37°C in a humidified
incubator with a 5% CO2 supply. Cells were treated with
or without D6R (Fumeisi Biotechnology Co., Ltd., Nanjing, China) (1
µg/ml) for 48–72 h.
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8, Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) was used to
analyze cell viability. Briefly, cells were seeded onto a 96-well
plate at 2,000 cells/well. A CCK-8 assay was used to assess the
cells viability at the first, second, third, fourth and fifth day.
Briefly, 10 µl CCK-8 was added to each well respectively and cells
were incubated in the dark at 37°C for 2 h, and absorbance measured
at 490 nm with a iMark™ microplate absorbance reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Colony formation assay
A colony formation assay was then used to detect the
anchorage-independent growth of SW1990 and PaTu8988 cells. Cells
were incubated in 6-well culture plates at 1,000 cells/well. The
cell colonies were formed following incubation for 1–2 weeks, and
then they were fixed with 4% paraformaldehyde (http://www.aladdin-e.com, Aladdin Shanghai Biochemical
Technology Co., Ltd., Shanghai, China) for 20 min and stained with
crystal violet (Aladdin Shanghai Biochemical Technology Co., Ltd.)
for 30 min at room temperature. The numbers of colonies were
counted and a graph was constructed.
Scratch wound healing assay
A scratch wound healing assay was used to detect the
migration ability of SW1990 and PaTu8988 cells. Cells were seeded
in a 24-well plate at 2×105 cells/well and incubated for
6 h, then a 10-µl pipette tip was used to disrupt the confluent
monolayer and the cell layer was washed with PBS three times. The
width of scratch was visualized using a light microscope
(magnification, ×4; Olympus Corporation, Tokyo, Japan). Cells were
then cultured in DMEM with or without 1 µg/ml D6R for 24 h. The
wounded monolayer was visualized using a light microscope
(magnification, ×4; Olympus Corporation, Tokyo, Japan).
Cell invasion assay
Cell invasion assays were performed using Transwell
chambers (Corning, NY, USA) according to the manufacturer's
protocol. A total of 4 µl BD Matrigel Basement Membrane matrix (BD
Biosciences, Franklin Lakes, NJ, USA) was placed in each chamber.
Cells were seeded at a density of 1×105 cells/well in
Matrigel chambers in DMEM, and 10% FBS was added to the lower
chambers. Following incubation for 24 h, with or without 1 µg/ml
D6R, cells that remained on top of the filter were wiped off, and
cells that had invaded to the lower chamber were stained and
counted. Cells were fixed with 4% paraformaldehyde for 20 min and
stained with 1% crystal violet for 30 min at room temperature. The
numbers of invaded cells were counted and a graph was
constructed.
Western blotting
Cells were rinsed with PBS 3 times on ice prior to
treatment with radioimmunoprecipitation assay lysis buffer
(Shanghai BioSun Sci&Tech Co., Ltd., Shanghai, China) at 100°C
for 10 min. The mixture was then centrifuged at 4°C at 9,000 ×
g/min (Heal Force Development Ltd, Hong Kong, SAR, China) for 10
min. The supernatant was removed and the total cellular protein
concentration was measured using the BCA method. Approximately 30
µg of protein was loaded in each lane and separated using SDS-PAGE
(10% gel) and transferred onto polyvinylidene fluoride membranes.
Membranes were blocked with 5% non-fat milk at room temperature for
1 h, then incubated with the primary antibodies at 4°C for 8 h and
then secondary horseradish peroxidase (HRP)-conjugated antibodies
at room temperature for 1 h. Bands were detected using an enhanced
chemiluminescence system (Minichemill, SageCreation, Beijing,
China). Primary antibodies included; rabbit anti-Furin (1:500; cat
no. 18413-1-AP; Proteintech Group, Inc., Chicago, IL, USA) and
mouse anti-β-Tubulin (1:10,000, cat no. 6181), rabbit
anti-N-Cadherin (1:1,000; cat no. 13116), rabbit anti-E-Cadherin
(1:1,000; cat no. 3195), rabbit anti-Vimentin (1:1,000; cat no.
5741), rabbit anti-DBF2 kinase activator protein MOB1 (Mob1;
1:1,000, cat no. 13730), rabbit anti-p-Mob1 (1:1,000, cat no.
8699), rabbit anti-yes-associated protein (YAP; 1:1,000; cat no.
8418), rabbit anti-p-YAP (1:1,000, cat no. 13619) (all purchased
from Cell Signaling Technology, Inc., Danvers, MA, USA). The
secondary HRP-conjugated antibodies were goat anti-rabbit IgG (H+L)
secondary antibodies (1:10,000; cat. no. 31460; Invitrogen; Thermo
Fisher Scientific) and goat anti-mouse IgG (H+L) (1:5,000; cat. no.
31430; Invitrogen; Thermo Fisher Scientific).
Statistical analysis
All data were presented as the mean ± the standard
deviation. All statistical analyses were carried out using SPSS
Statistical software (version 19; IBM Corp., Armonk, NY, USA). An
unpaired Student's t-test was used to compare the significance
between the experimental group and the control. P<0.05 was
considered to indicate a statistically significant difference.
Results
D6R inhibits the proliferation of
SW1990 and PaTu8988 cells
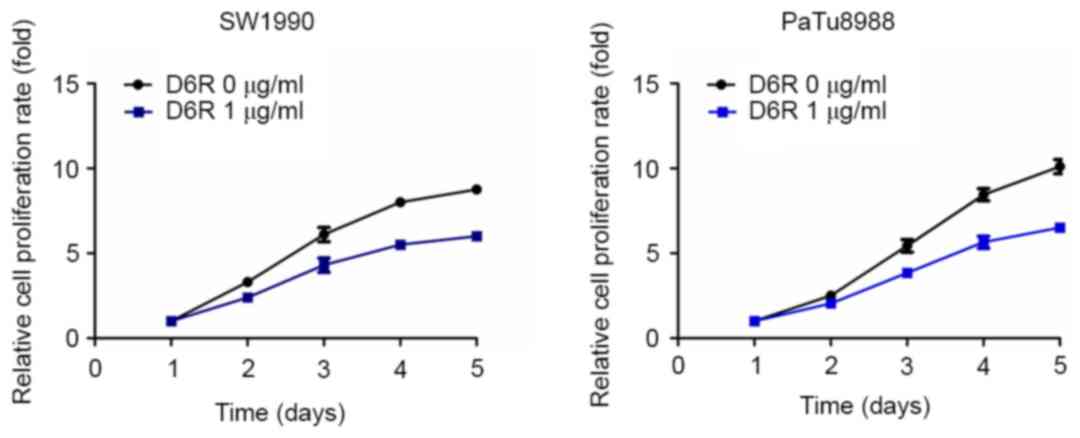
CCK-8 assays were used to examine the relative
proliferation rates in SW1990 and PaTu8988 cells. As presented in
Fig. 1, D6R treatment resulted in
decreased relative rates of proliferation in SW1990 and PaTu8988
cells, indicating that D6R inhibited the proliferation of SW1990
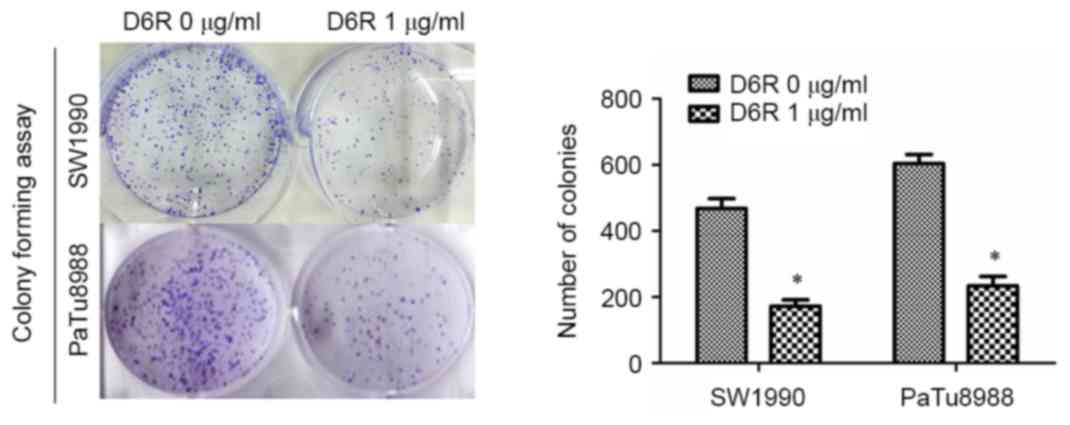
and PaTu8988 cells. Furthermore, a colony-forming assay revealed
that the number of colonies were 468±21 and 173±14 in the D6R-free
and D6R treated groups of SW1990 cells, respectively, and 603±21
and 234±19 in D6R-free and D6R-treated groups of PaTu8988 cells,
respectively (Fig. 2). D6R treated
cells demonstrated a significantly decreased number of colonies
(P<0.05) for SW1990 and PaTu8988 cell lines compared with the
untreated groups, suggesting that D6R suppresses
anchorage-independent growth. This data suggests that D6R inhibited
the proliferation ability of SW1990 and PaTu8988 cells.
D6R reduces cell invasiveness in
SW1990 and PaTu8988 cells
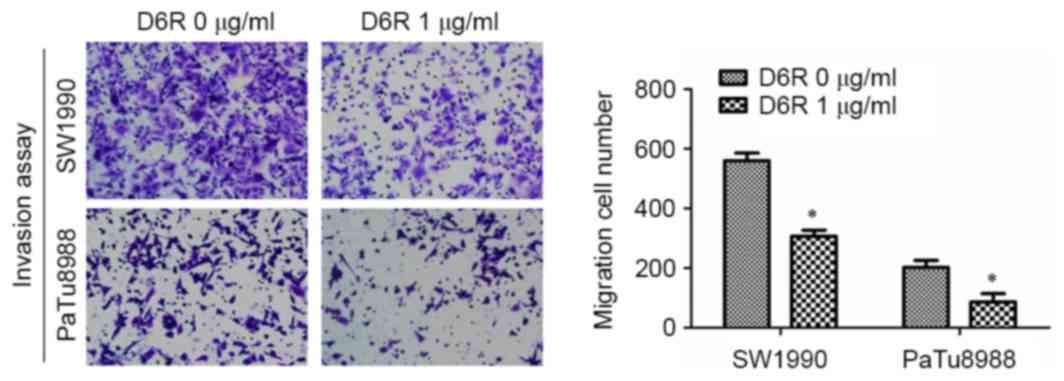
A Transwell invasion assay was used to examine the
effect of D6R on the invasive abilities of SW1990 and PaTu8988
cells. As presented in Fig. 3, the
numbers of invaded cells were 610±27 and 306±15 in D6R-free and D6R
treated groups of SW1990 cells, respectively, and 203±16 and 87±20
in D6R-free and D6R treated groups of PaTu8988 cells, respectively.
The number of invaded cells was significantly lower in the D6R
treated groups for SW1990 and PaTu8988 cell lines (P<0.05)
compared with the untreated groups. This data suggests that D6R
inhibited the invasion ability of SW1990 and PaTu8988 cells.
D6R inhibits the migration ability of
SW1990 and PaTu8988 cells
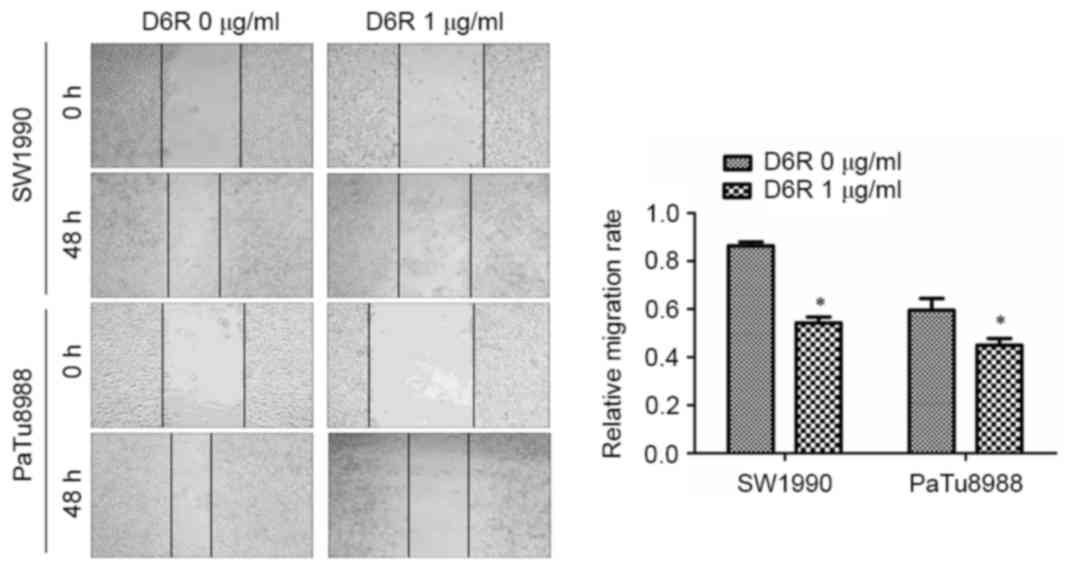
To determine the function of D6R in cell migration,
a wound-healing assay was used to detect the migration ability of
SW1990 and PaTu8988 cells. The width of the scratch at the
beginning was 0.890±0.106 and 1.043±0.210 in D6R-free and D6R
treated groups of SW1990 cells, respectively, and 0.893±0.182 and
1.228±0.201 in D6R-free and D6R treated groups of PaTu8988 cells.
The migration rates were 0.864±0.011 and 0.543±0.017 in D6R-free
and D6R treated groups of SW1990 cells, respectively, and
0.595±0.035 and 0.450±0.020 in D6R-free and D6R treated groups of
PaTu8988 cells, respectively. The relative migration rate was
significantly lower in the D6R treated groups of cells for SW1990
and PaTu8988 cell lines (P<0.05) compared with untreated groups.
These results suggest that D6R inhibited the migration ability of
SW1990 and PaTu8988 cells (Fig.
4).
D6R inhibits EMT in SW1990 and
PaTu8988 cells
EMT serves a key function in allowing primary tumor
cells to be capable of metastasizing. To determine whether D6R
affects EMT, the expression of EMT relative proteins were examined
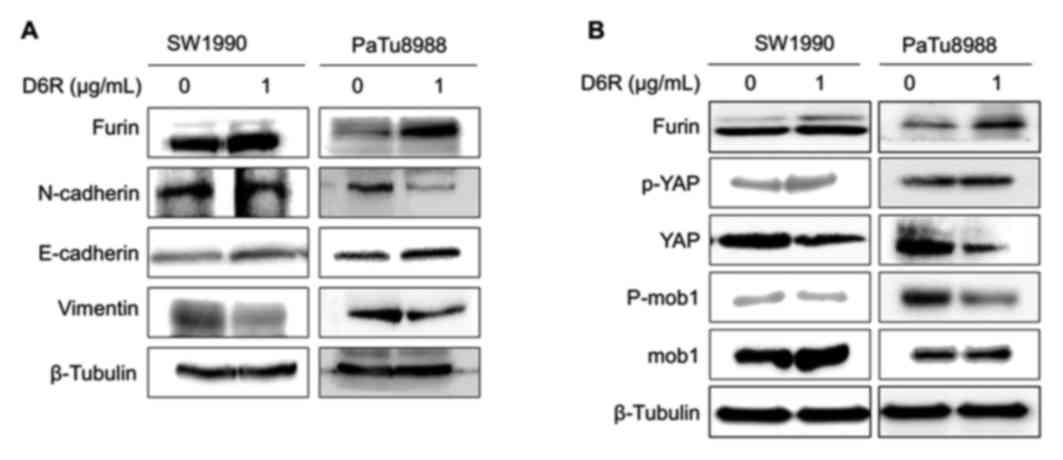
with or without D6R treatment. Fig.
5A demonstrates that D6R resulted in the downregulation of
N-cadherin and vimentin, and the upregulation of E-cadherin in
SW1990 and PaTu8988 cells. Mature furin expression was slightly
increased in cells treated with D6R, thus suggesting that D6R
suppressed the activity of furin, and may have caused to some
extent an accumulation of the enzyme. This data confirms that D6R
suppressed EMT in SW1990 and PaTu8988 cells.
D6R affects the Hippo-YAP pathway in
SW1990 and PaTu8988 cells
It has been reported that Hippo-YAP signaling is
associated with EMT (2,6,7). To
explore whether the Hippo-YAP pathway reacted to D6R inhibiting EMT
in SW1990 and PaTu8988 cells, the expression of the relevant
proteins in the Hippo-YAP pathway was examined with or without D6R
treatment in SW1990 and PaTu8988 cells. The results of the present
study revealed that D6R resulted in the downregulation of total YAP
and p-Mob1, and the upregulation of p-YAP and Mob1 protein levels.
This data suggests that D6R affected the Hippo-YAP signaling
pathway in SW1990 and PaTu8988 cells (Fig. 5B).
Discussion
In the present study, it was identified that D6R,
functioning as a furin inhibitor, suppressed the proliferation,
migration and invasion of SW1990 and PaTu8988 cells, and
characterized furin as an oncogene. Notably, D6R inhibited EMT
potentially via the Hippo-YAP signaling pathway.
Differing types of small molecule components,
including α1-PDX, Furin propeptide and proteinase inhibitor-8,
which function as competitive furin inhibitors, have been well
characterized. A number of them demonstrated the potential to be
used for the treatment of certain infections, including bacterial
and viral infections, such as Pseudomonas aeruginosa exotoxin A,
Bacillus anthraci and Hepatitis B (4,13,14). Additionally, a number of them have
been reported to reduce the growth and invasiveness of numerous
types of tumor cells (10,11,18,19).
α1-PDX resulted in the reduction of the growth and invasive
ability, and malignant phenotypes of HT-29 human colon carcinoma
cells (18), glioma tumor cells
(20) and head and neck squamous cell
carcinoma cells (10). A
small-molecule inhibitor of furin named CCG 8294 and decRVKR-CMK
inhibited the maturation of MMPs and the invasiveness of human
fibrosarcoma cells (11,21). However, collective studies have
demonstrated the limitations of these inhibitors. It has been
revealed that polyarginines were characterized by high potency,
specificity and low toxicity (12,22),
compared with other small molecules. Previous studies have
demonstrated the potency of D6R treatment on viral and bacterium
infections, but little is known about the anti-metastatic potential
of D6R. Therefore, the migration and invasion potential were
measured using treatments of D6R at a concentration of 1 µg/ml in
SW1990 and PaTu8988 cells. It was demonstrated that D6R inhibited
the migration and invasion ability and EMT in SW1990 and PaTu8988
cells.
EMT, a crucial cellular mechanism in tumor
metastasis, is a developmental process in which cells lose their
epithelial features and develop a mesenchymal phenotype allowing
cells to escape and spread to distant sites (23). Accumulating evidence indicates that
substrate molecules processed by furin, including MMPs and TGF-β,
are critical for the promotion of EMT, which contributes to cancer
metastasis (6,7,24–26). From the results of the present study,
it may be concluded that D6R suppressed EMT and served a crucial
function in pancreatic cancer metastasis. In the present study, it
was confirmed that D6R treatment resulted in the downregulation of
N-cadherin and vimentin, and the upregulation of E-cadherin,
consistent with the reduction in the invasion and migration ability
of SW1990 and PaTu8988 cells. Furthermore, it was conclusively
demonstrated that D6R inhibited EMT in line with alterations in YAP
phosphorylation levels and the total YAP protein level, suggesting
that YAP was involved in the regulation of EMT suppressed by D6R in
SW1990 and PaTu8988 cells. Despite these notable findings, further
investigations are required to elucidate the mechanism of D6R in
the regulation of EMT via the Hippo-YAP pathway.
In summary, the present study indicates that D6R
reduced the proliferation, migration and invasive ability of SW1990
and PaTu8988 cells, and that D6R-suppressed EMT may be regulated
via the Hippo-YAP signaling pathway. Altogether, D6R has the
potential to be used as a drug candidate to ameliorate a malignant
phenotype in SW1990 and PaTu8988 cells.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81672402, 81472333
and 81372718) and the Natural Science Foundation of Jiangsu
Province (grant no. BK20131247).
References
|
1
|
Bassi DE, Mahloogi H and Klein-Szanto AJ:
The proprotein convertases furin and PACE4 play a significant role
in tumor progression. Mol Carcinog. 28:63–69. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pei D and Weiss SJ: Furin-dependent
intracellular activation of the human stromelysin-3 zymogen.
Nature. 375:244–247. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shiryaev SA, Remacle AG, Ratnikov BI,
Nelson NA, Savinov AY, Wei G, Bottini M, Rega MF, Parent A,
Desjardins R, et al: Targeting host cell furin proprotein
convertases as a therapeutic strategy against bacterial toxins and
viral pathogens. J Biol Chem. 282:20847–20853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Becker GL, Lu Y, Hardes K, Strehlow B,
Levesque C, Lindberg I, Sandvig K, Bakowsky U, Day R, Garten W and
Steinmetzer T: Highly potent inhibitors of proprotein convertase
furin as potential drugs for treatment of infectious diseases. J
Biol Chem. 287:21992–22003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thomas G: Furin at the cutting edge: From
protein traffic to embryogenesis and disease. Nat Rev Mol Cell
Biol. 3:753–766. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dubois CM, Blanchette F, Laprise MH, Leduc
R, Grondin F and Seidah NG: Evidence that furin is an authentic
transforming growth factor-beta1-converting enzyme. Am J Pathol.
158:305–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yana I and Weiss SJ: Regulation of
membrane type-1 matrix metalloproteinase activation by proprotein
convertases. Mol Biol Cell. 11:2387–2401. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
López de Cicco R, Watson JC, Bassi DE,
Litwin S and Klein-Szanto AJ: Simultaneous expression of furin and
vascular endothelial growth factor in human oral tongue squamous
cell carcinoma progression. Clin Cancer Res. 10:4480–4488. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pang YJ, Tan XJ, Li DM, Zheng ZH, Lei RX
and Peng XM: Therapeutic potential of furin inhibitors for the
chronic infection of hepatitis B virus. Liver Int. 33:1230–1238.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bassi DE, Lopez De Cicco R, Mahloogi H,
Zucker S, Thomas G and Klein-Szanto AJ: Furin inhibition results in
absent or decreased invasiveness and tumorigenicity of human cancer
cells. Proc Natl Acad Sci USA. 98:pp. 10326–10331. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coppola JM, Bhojani MS, Ross BD and
Rehemtulla A: A small-molecule furin inhibitor inhibits cancer cell
motility and invasiveness. Neoplasia. 10:363–370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cameron A, Appel J, Houghten RA and
Lindberg I: Polyarginines are potent furin inhibitors. J Biol Chem.
275:36741–36749. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Komiyama T, Swanson JA and Fuller RS:
Protection from anthrax toxin-mediated killing of macrophages by
the combined effects of furin inhibitors and chloroquine.
Antimicrob Agents Chemother. 49:3875–3882. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ozden S, Lucas-Hourani M, Ceccaldi PE,
Basak A, Valentine M, Benjannet S, Hamelin J, Jacob Y, Mamchaoui K,
Mouly V, et al: Inhibition of Chikungunya virus infection in
cultured human muscle cells by furin inhibitors: Impairment of the
maturation of the E2 surface glycoprotein. J Biol Chem.
283:21899–21908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sarac MS, Cameron A and Lindberg I: The
furin inhibitor hexa-D-arginine blocks the activation of
Pseudomonas aeruginosa exotoxin A in vivo. Infect Immun.
70:7136–7139. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reubi JC: Peptide receptors as molecular
targets for cancer diagnosis and therapy. Endocr Rev. 24:389–427.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khatib AM, Siegfried G, Prat A, Luis J,
Chrétien M, Metrakos P and Seidah NG: Inhibition of proprotein
convertases is associated with loss of growth and tumorigenicity of
HT-29 human colon carcinoma cells: Importance of insulin-like
growth factor-1 (IGF-1) receptor processing in IGF-1-mediated
functions. J Biol Chem. 276:30686–30693. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Longuespée R, Couture F, Levesque C,
Kwiatkowska A, Desjardins R, Gagnon S, Vergara D, Maffia M,
Fournier I, Salzet M and Day R: Implications of Proprotein
Convertases in Ovarian Cancer Cell Proliferation and Tumor
Progression: Insights for PACE4 as a Therapeutic Target. Transl
Oncol. pii:S1936–5233. 2014.
|
|
20
|
Mercapide J, Lopez De Cicco R, Bassi DE,
Castresana JS, Thomas G and Klein-Szanto AJ: Inhibition of
furin-mediated processing results in suppression of astrocytoma
cell growth and invasiveness. Clin Cancer Res. 8:1740–1746.
2002.PubMed/NCBI
|
|
21
|
Maquoi E, Noël A, Frankenne F, Angliker H,
Murphy G and Foidart JM: Inhibition of matrix metalloproteinase 2
maturation and HT1080 invasiveness by a synthetic furin inhibitor.
FEBS Lett. 424:262–266. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Angliker H: Synthesis of tight binding
inhibitors and their action on the proprotein-processing enzyme
furin. J Med Chem. 38:4014–4018. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bartholin L: Pancreatic cancer and the
tumor microenvironment: Mesenchyme's role in pancreatic
carcinogenesisPancreatic Cancer and Tumor Microenvironment. Grippo
PJ and Munshi HG: Trivandrum (India): 2012
|
|
24
|
Rasheed ZA, Yang J, Wang Q, Kowalski J,
Freed I, Murter C, Hong SM, Koorstra JB, Rajeshkumar NV, He X, et
al: Prognostic significance of tumorigenic cells with mesenchymal
features in pancreatic adenocarcinoma. J Natl Cancer Inst.
102:340–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hotz B, Arndt M, Dullat S, Bhargava S,
Buhr HJ and Hotz HG: Epithelial to mesenchymal transition:
Expression of the regulators snail, slug, and twist in pancreatic
cancer. Clin Cancer Res. 13:4769–4776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|