Introduction
Lung cancer, the leading cause of cancer-related
deaths worldwide, is commonly divided into two categories, small
cell lung cancer (SCLC) and non-SCLC (NSCLC), depending on its
degree of differentiation and morphological characteristics
(1,2).
NSCLC accounts for ~80% of primary lung cancers, including squamous
cell carcinoma, adenocarcinoma, and large cell carcinoma (3). The 5-year survival rate of NSCLC is only
7% (4). Moreover, lymph nodes and
distant organ metastasis are the main reasons leading to treatment
failure in NSCLC patients with radical resection (5,6).
Epithelial to mesenchymal transition (EMT), a
reversible biological process, is characterized by the loss of
epithelial cell junction proteins (E-cadherin, Zo-1) (7,8), the gain
of mesenchymal markers (vimentin, N-cadherin) (9,10), and the
activation of transcription factors (Snail1, Slug, ZEB1, Twist)
(11–15). Accumulating evidence indicates that
EMT enhances tumor invasion, distant metastasis, and chemotherapy
resistance in NSCLC, underscoring the need for a comprehensive
understanding of the EMT function in NSCLC progression (16–19).
Monoamine oxidase A (MAOA), a mitochondria-bound
enzyme, catalyzes the oxidative deamination of dietary amines and
monoamine neurotransmitters, such as serotonin, norepinephrine, and
dopamine (20,21). The functions of MAOA have been
extensively studied in the context of neurological disorders,
including mental depression, aggressive behaviors, and Parkinson's
disease (22,23). Recent studies have indicated the role
of MAOA in the progression of prostate cancer (24–30),
hepatocellular carcinoma (HCC)(31),
and cholangiocarcinoma (32). High
Gleason grade or poorly differentiated prostate cancer exhibited
increased MAOA expression (24), and
the increased MAOA promoted prostate cancer metastasis (25,26).
Furthermore, overexpression of MAOA was found to dramatically
downregulate the expression of E-cadherin and upregulate the
expression of vimentin and Twist at both mRNA and protein levels in
prostate cancer (25). These studies
suggested that MAOA might promote the progression of prostate
cancer by mediating EMT. However, conflicting results were reported
for HCC (31) and cholangiocarcinoma
(32). Therefore, the role of MAOA
may vary across cancer types, and therefore, it is essential to
further understand the function of MAOA in other cancers.
Little is known about the function of MAOA in NSCLC.
Accordingly, in this study, we investigated the expression of MAOA
in NSCLC tissues and analyzed the association between the
expression of MAOA and EMT or the development of
clinicopathological features. We found for the first time, to the
best of our knowledge, that MAOA protein and mRNA expressions in
NSCLC tissues were significantly higher than those observed in the
matched non-tumor adjacent lung tissues, and the increased MAOA
expression was related to EMT, clinical stages, and lymph node
metastases in NSCLC, suggesting that MAOA may be involved in
mediating the progression of NSCLC.
Materials and methods
Reagents
Rabbit anti-human MAOA monoclonal antibody was
obtained from Abcam (ab126751; Cambridge, UK). Mouse anti-human
E-cadherin monoclonal antibody, rabbit anti-human vimentin,
N-cadherin, Snail1, Slug, Zo-1, ZEB1 and Twist monoclonal
antibodies, and horseradish peroxidase (HRP)-conjugated secondary
antibodies were purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA). The RNA extraction kit (RNAprep Pure FFPE kit)
was purchased from Tiangen Biotech Co., Ltd. (Beijing, China). The
reverse transcription (RT) kit (PrimeScript™ RT reagent
kit) and qPCR analysis kit (SYBR Premix Ex Taq™ II) were obtained
from Takara Biotechnology Co., Ltd. (Dalian, China).
NSCLC patients and control cases
NSCLC tissue specimens were obtained from 45
patients who were definitively diagnosed with NSCLC and had
undergone curative surgery between 2007 and 2010 at the Affiliated
Hospital of Guangdong Medical University (Guangdong, China). The
matched non-tumor adjacent lung tissues (1 cm from the tumor) were
also collected from the same patients, as the controls. Among these
patients, the complete clinicopathological and histopathological
data were collected from 30 cases. The patients that met the
following criteria were enrolled. First, the patients were
definitively diagnosed with NSCLC based on histological
examinations. Second, the patients had not received chemotherapy,
immunotherapy, or radiotherapy before pulmonary lobectomy. Third,
the patients showed normal hepatic and renal functions and no
abnormality of the endocrine system.
Ethics approval
Either the patients or their close relatives
provided informed written consents. Our investigation received the
ethic approval from the local Committee of the Affiliated Hospital
of Guangdong Medical University. All clinical investigations were
performed according to the principles defined by the Declaration of
Helsinki.
Immunohistochemistry
Immunohistochemical staining was performed on
paraffin-embedded tissue specimens, including NSCLC and matched
non-tumor adjacent lung tissues, from 45 cases. Briefly,
paraffin-embedded tissue specimens were cut into 4-µm sections,
transferred onto Superfrost Ultra Plus slides, and placed in a 60°C
oven overnight. The slides were deparaffinized with xylene,
rehydrated in a descending alcohol series (100, 95, 90, 80 and
70%), and then rinsed with sterile distilled water for 5 min.
Antigen retrieval was performed by boiling the tissue sections in
citrate buffer (10 mM trisodium citrate, 0.05% Tween-20, pH=6) for
10 min. The slides were further treated with 3% hydrogen peroxide
for 15 min to inactivate any endogenous peroxidase activity. After
rinsing in phosphate-buffered saline (PBS), non-specific sites were
blocked with normal goat serum for 15 min. The slides were
subsequently incubated with primary antibodies (1:100) overnight at
4°C. One slide was incubated with PBS, as the negative control.
After washing with precooled PBS, the slides were incubated with a
secondary biotinylated antibody for 15 min at room temperature. The
slides were washed with precooled PBS three times, and then treated
with a streptavidin-biotin complex for 15 min at room temperature.
The slides were then stained with diaminobenzidine (DAB), and
counterstained with hematoxylin, before being observed and analyzed
in a double-blind manner, under light microscopy, by two senior
pathologists. Ten randomly selected fields were examined at ×400
magnification and 100 cancer cells were counted in each field
(total 10,000 cells) to determine the proportion of positive cells.
A semi-quantitative analysis was performed to evaluate the protein
expression levels as described previously (33,34). In
brief, staining intensity was scored on a scale of 0 to 3, 0 for no
intensity, 1 for low intensity (light yellow), 2 for moderate
intensity (claybank), and 3 for high intensity (sepia). The cell
positivity was scored on a scale of 0 to 4: 0, <5% cells
stain-positive; 1, 5 to 25% cells stain-positive; 2, 26 to 50%
cells stain-positive; 3, 51 to 75% cells stain-positive; and 4,
>75% cells stain-positive. The scores obtained relative staining
intensity and proportion of positive cells were multiplied together
to generate a final score ranging from 0 to 12, interpreted as
follows: 0, negative (−); 1 to 4, weakly positive (+); 5 to 8,
moderately positive (++); 9 to 12, strongly positive (+++). The
scores were evaluated by two pathologists.
RT-qPCR
Total RNA was extracted from paraffin-embedded
tissue specimens using the TIANGEN RNAprep Pure FFPE kit and then
converted to cDNA using the PrimeScript™ RT reagent kit
(both from Tiangen Biotech Co., Ltd.). qPCR analysis was performed
using SYBR Premix Ex Taq™ II (Takara Biotechnology Co., Ltd.),
according to the manufacturer's instructions. All the primers were
synthesized by Takara Biotechnology Co., Ltd., and are listed in
Table I. The housekeeping gene
β-actin was used as an internal control to normalize mRNA
levels. The optimum reaction conditions for qPCR were as follows:
Pre-treatment at 42°C for 5 min, initial denaturation at 95°C for
10 sec, followed by 40 cycles at 95°C for 5 sec, and 60°C for 31
sec. The experiment was carried out in triplicate.
 | Table I.Primers for real-time quantitative
PCR. |
Table I.
Primers for real-time quantitative
PCR.
| GenBank no. | Genes | Primer sequence
(5′-3′) | Length (bp) |
|---|
| NM_000240.3 | MAOA | Forward primer
AGTGAGCGAACGGATAATGG | 114 |
|
|
| Reverse primer
TGTTCATGGTTCAGCGTCTC |
|
| NM_004360.4 |
E-cadherin | Forward primer
TTGCTACTGGAACAGGGACAC | 179 |
|
|
| Reverse primer
CCCGTGTGTTAGTTCTGCTGT |
|
| NM_003257.4 | Zo-1 | Forward primer
GGATGTTTATCGTCGCATTGTA | 158 |
|
|
| Reverse primer
AAGAGCCCAGTTTTCCATTGTA |
|
| NM_001792.4 |
N-cadherin | Forward primer
TTATCCTTGTGCTGATGTTTGTG | 139 |
|
|
| Reverse primer
TCTTCTTCTCCTCCACCTTCTTC |
|
| NM_003380.3 |
Vimentin | Forward primer
TGGCACGTCTTGACCTTGAA | 176 |
|
|
| Reverse primer
GGTCATCGTGATGCTGAGAA |
|
| NM_005985.3 | Snail1 | Forward primer
TCCTTCGTCCTTCTCCTCTACTT | 155 |
|
|
| Reverse primer
TGTTGCAGTATTTGCAGTTGAAG |
|
| NM_003068.4 | Slug | Forward primer
GCCTTTTTCTTGCCCTCAC | 115 |
|
|
| Reverse primer
GGTTTTGGAGCAGTTTTTGC |
|
| NM_030751.5 | ZEB1 | Forward primer
TCCCCATCACCTCTAAACCTT | 122 |
|
|
| Reverse primer
CCCTGTTGCTTTGGTAGTGAA |
|
| NM_000474.3 | Twist | Forward primer
AGTCCGCAGTCATACGAGGAG | 146 |
|
|
| Reverse primer
GACCTAGTAGAGGAAGTCGATG |
|
| NM_001101.3 | β-actin | Forward primer
TGACGTGGACATCCGCAAAG | 186 |
|
|
| Reverse primer
CTGGAAGGTGGACAGCAGGG |
|
Statistical analysis
SPSS 19.0 Windows software was used for statistical
analysis. Quantitative data were presented as the mean ± SD. The
categorical variables were presented as frequency and percent
rates, and the positive rates from two groups were compared using
Chi-square (χ2) test or Fisher exact probabilities
(n<40 or T<1). Wilcoxon rank sum test was used to
perform the statistical analysis on ordinal data. Spearman rank
correlation coefficient was employed for the correlation analysis.
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Expressions of MAOA and EMT markers in
NSCLC and matched non-tumor adjacent lung tissues
Previous studies have demonstrated that prostate
cancer and HCC exhibit completely different MAOA expression levels
(24,31), and to date, MAOA expression has not
been reported in NSCLC. To investigate the expressions of MAOA and
EMT markers in NSCLC tissues, immunohistochemical staining was
performed in 45 pairs of NSCLC and patient-matched non-tumor
adjacent lung tissues. The protein expressions of MAOA, N-cadherin,
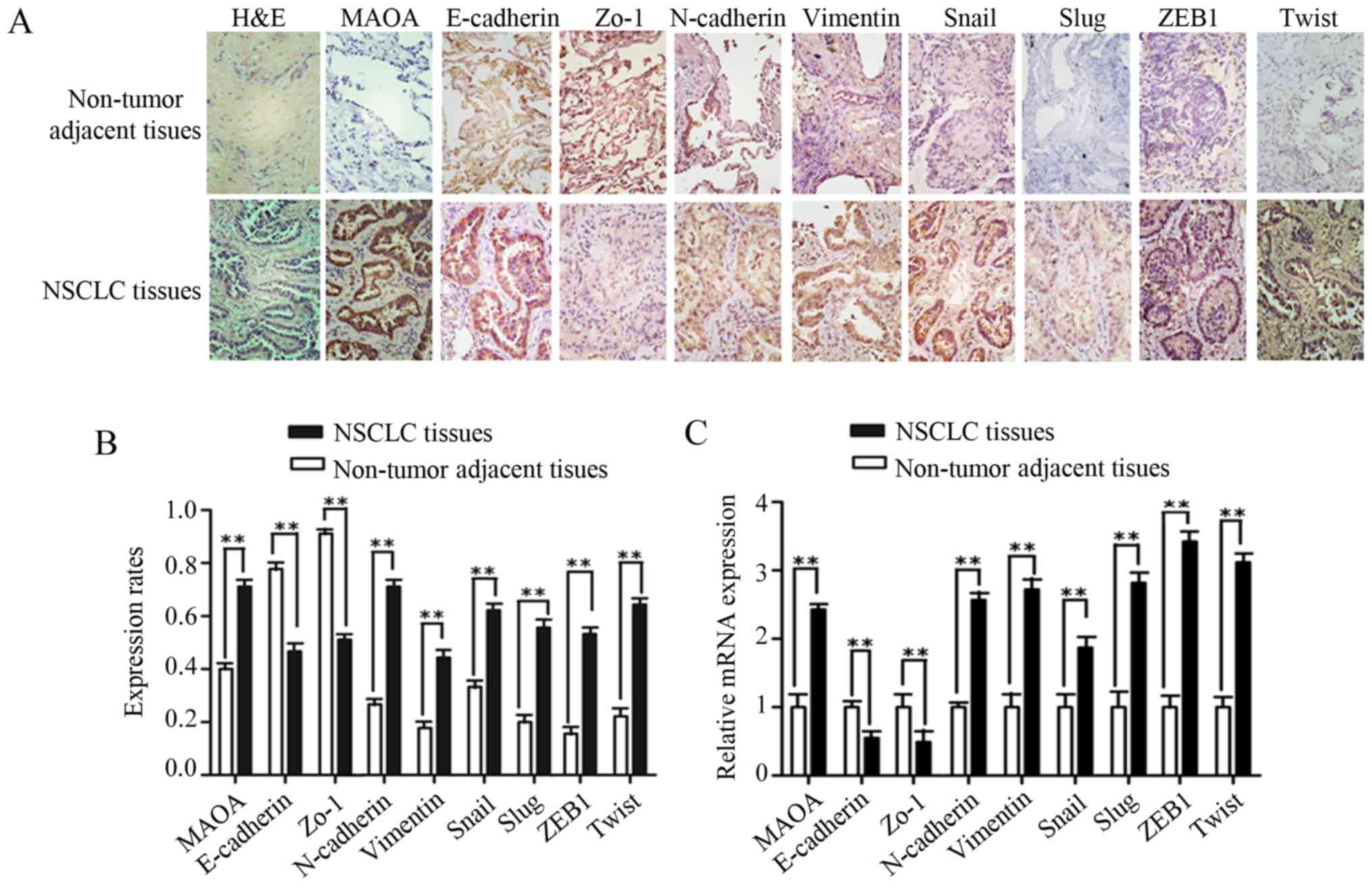
vimentin, Snail1, Slug, ZEB1, and Twist were obviously enhanced in
NSCLC tissues (Fig. 1A). The positive
expression rates of MAOA, N-cadherin, vimentin, Snail1, Slug, ZEB1,
and Twist in NSCLC tissues were higher than those observed in the
matched non-tumor adjacent lung tissues (P<0.01, Fig. 1B; Table
II), while the positive expression rates of E-cadherin, Zo-1,
and EMT epithelial makers determined in NSCLC tissues were lower
than those observed in adjacent normal lung tissues (Fig. 1A and B; Table II). To further investigate the mRNA
expressions of MAOA and EMT markers, NSCLC and matched adjacent
normal lung tissues from 30 cases were selected for RT-qPCR. Our
results showed that the mRNA levels of MAOA, N-cadherin, vimentin,
Snail1, Slug, ZEB1, and Twist in NSCLC tissues were significantly
higher than those detected in non-tumor adjacent tissues. As
expected, a significant decrease in E-cadherin and Zo-1 mRNA
expressions was observed in NSCLC tissues (P<0.01; Fig. 1C).
 | Table II.The positive rates of protein
expression of MAOA and EMT markers in NSCLC and non-tumor adjacent
lung tissues. |
Table II.
The positive rates of protein
expression of MAOA and EMT markers in NSCLC and non-tumor adjacent
lung tissues.
| Proteins | NSCLC tissues
(%) | Non-tumor adjacent
lung tissues (%) | χ2 | P-value |
|---|
| MAOA | 71.1 (32/45) | 40.0 (18/45) |
8.802 | 0.003 |
| E-cadherin | 46.7 (21/45) | 77.8 (35/45) |
9.265 | 0.002 |
| Zo-1 | 51.1 (23/45) | 91.1 (41/45) | 17.524 |
0.0001 |
| N-cadherin | 68.9 (31/45) | 26.7 (9/45) | 21.780 |
0.0001 |
| Vimentin | 44.4 (20/45) | 17.8 (8/45) |
7.465 | 0.006 |
| Snail1 | 62.2 (28/45) | 33.3 (15/45) |
7.526 | 0.006 |
| Slug | 55.6 (25/45) | 20.0 (9/45) | 12.101 | 0.001 |
| ZEB1 | 53.3 (24/45) | 15.6 (7/45) | 14.221 |
0.0001 |
| Twist | 64.4 (29/45) | 22.2 (10/45) | 16.335 |
0.0001 |
Moreover, the statistical distribution results
further demonstrated that MAOA, vimentin, and Snail1 protein
expressions were significantly stronger in NSCLC tissues than in
adjacent normal lung tissues, whereas E-cadherin protein expression
displayed the opposite trend (P<0.05; Fig. 2).
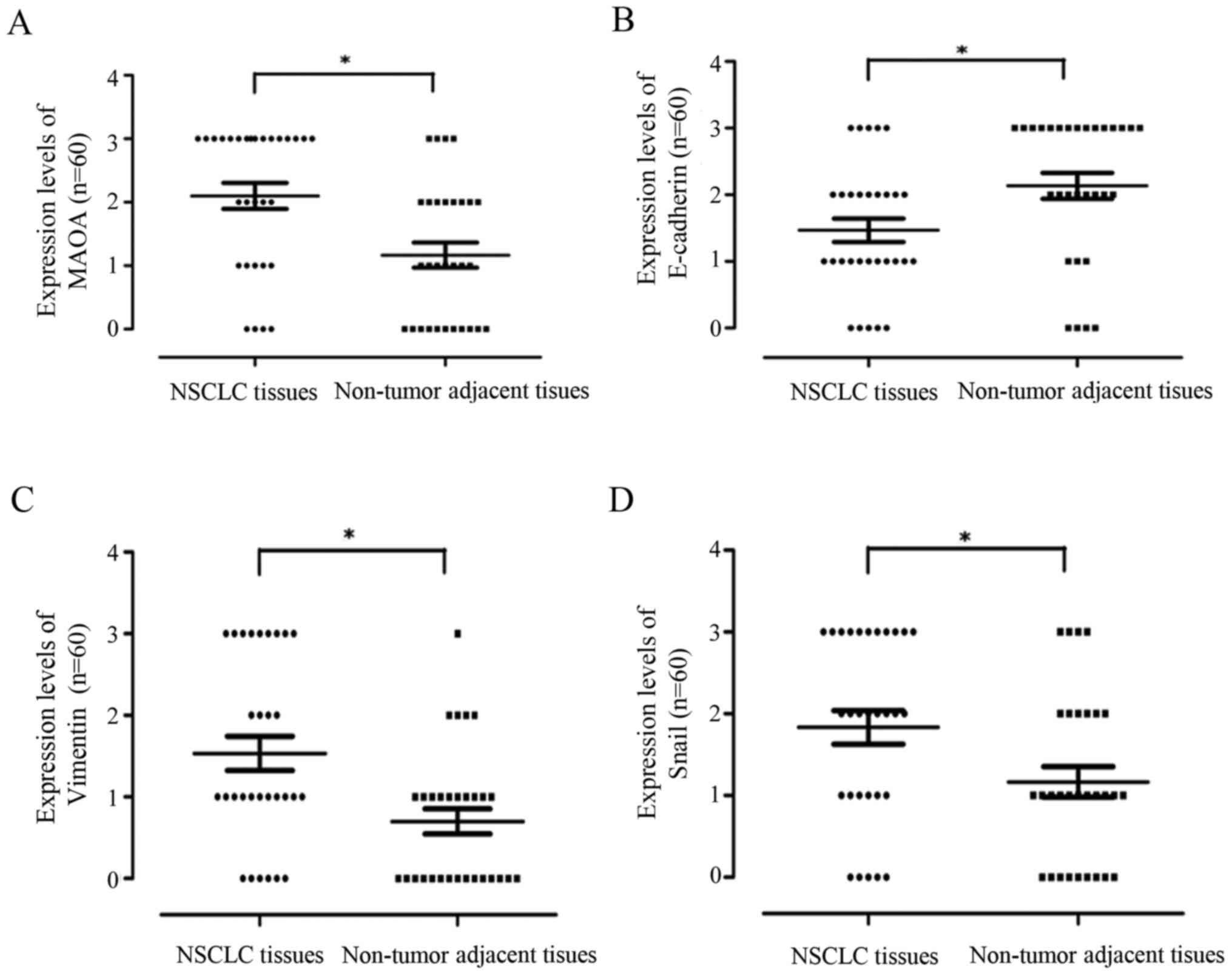 | Figure 2.The statistical distribution of MAOA,
E-cadherin, vimentin, and Snail1 expression levels in NSCLC and the
matched non-tumor adjacent lung tissues. Wilcoxon rank sum test was
employed for analysis of statistical distribution of protein
expression levels of MAOA, E-cadherin, vimentin, and Snail1. (A)
MAOA, (B) E-cadherin, (C) vimentin, and (D) Snail1. *P<0.05.
MAOA, monoamine oxidase A; NSCLC, non-small cell lung cancer. |
Correlation between the expressions of
MAOA and EMT markers in NSCLC
To study the role of MAOA expression in the EMT of
NSCLC, a Spearman rank correlation coefficient test was performed
to analyze the correlation between the expressions of MAOA and EMT
markers in NSCLC. As described in Table
III, although there was no relationship between the expression
of MAOA and the expressions of vimentin, Snail1, Zo-1, or ZEB1
(P>0.05; Table III), the
expression of MAOA was positively correlated with the expressions
of the EMT mesenchymal marker N-cadherin (r=0.525, P=0.002)
and the EMT transcription factors Slug (r=0.515, P=0.001)
and Twist (r=0.448, P=0.008). Accordingly, MAOA expression
was negatively correlated with the expression of the epithelial
maker E-cadherin (r=−0.387, P=0.01; Table III).
 | Table III.The correlation between MAOA
expression and EMT in NSCLC. |
Table III.
The correlation between MAOA
expression and EMT in NSCLC.
|
| MAOA |
|
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Proteins | + | − | Total | χ2 | P-value | r |
|---|
| E-cadherin |
|
|
| 6.724 | 0.010 | −0.387 |
| + | 11 | 10 | 21 |
|
|
|
| − | 21 | 3 | 24 |
|
|
|
| Zo-1 |
|
|
| 0.795 | 0.372 | −0.133 |
| + | 15 | 8 | 23 |
|
|
|
| − | 17 | 5 | 22 |
|
|
|
| N-cadherin |
|
|
| 10.020 | 0.002 | 0.525 |
| + | 27 | 4 | 31 |
|
|
|
| − | 5 | 9 | 14 |
|
|
|
| Vimentin |
|
|
| 3.380 | 0.066 | 0.274 |
| + | 17 | 3 | 20 |
|
|
|
| − | 15 | 10 | 25 |
|
|
|
| Snail1 |
|
|
| 0.160 | 0.690 | 0.11 |
| + | 21 | 7 | 28 |
|
|
|
| − | 11 | 6 | 17 |
|
|
|
| Slug |
|
|
| 11.948 | 0.001 | 0.515 |
| + | 23 | 2 | 25 |
|
|
|
| − | 9 | 11 | 20 |
|
|
|
| ZEB1 |
|
|
| 1.625 | 0.202 | 0.19 |
| + | 19 | 5 | 24 |
|
|
|
| − | 13 | 8 | 21 |
|
|
|
| Twist |
|
|
| 7.099 | 0.008 | 0.448 |
| + | 25 | 4 | 29 |
|
|
|
| − | 7 | 9 | 16 |
|
|
|
| Total |
| 32 | 13 | 45 |
|
|
Correlation between MAOA expression
and the development of clinicopathological features in NSCLC
Thirty of the patients who were definitively
diagnosed with NSCLC, and offered complete clinicopathological and
histopathological data, were enrolled to analyze the correlation
between MAOA expression and the clinicopathological features
observed in NSCLC. A Wilcoxon rank sum test was employed to carry
out the analysis of statistical distribution on the data obtained
regarding the protein expression levels. Our results showed that
the positive rate of MAOA expression in stage III was higher than
that measured in stages I and II (Z=−2.596, P=0.029; Table IV). Additionally, the lymph node
metastasis group exhibited a stronger MAOA expression level than
the controls with no metastasis (Z=−2.378, P=0.020; Table IV). These results indicated that MAOA
expression was significantly correlated with clinical stages and
lymph node metastases. However, MAOA expression was not influenced
by sex, age, degree of differentiation, or histological types
(P>0.05; Table IV).
 | Table IV.Correlationship between the
expression of MAOA and clinicopathologic characteristics. |
Table IV.
Correlationship between the
expression of MAOA and clinicopathologic characteristics.
|
|
| MAOA |
|
|---|
|
|
|
|
|
|---|
| Variables | n | − | + | P-value |
|---|
| Age (yeras) |
|
|
| 0.691 |
|
<60 | 13 | 3 | 10 |
|
|
≥60 | 17 | 6 | 11 |
|
| Sex |
|
|
| 1.000 |
|
Male | 21 | 6 | 15 |
|
|
Female | 9 | 3 | 6 |
|
| TNM stage |
|
|
| 0.029 |
|
I+II | 21 | 9 | 12 |
|
|
III | 9 | 0 | 9 |
|
|
Differentiation |
|
|
| 0.687 |
|
Poorly | 11 | 4 | 7 |
|
|
Moderatly | 19 | 5 | 14 |
|
| Pathologic
type |
|
|
| 0.704 |
|
Adenocarcinoma | 12 | 3 | 9 |
|
|
Squamous | 18 | 6 | 12 |
|
| Lymph node
metastasis |
|
|
| 0.020 |
|
Negative | 13 | 7 | 6 |
|
|
Positive | 17 | 2 | 15 |
|
|
Total | 30 | 9 | 21 |
|
Discussion
MAOA, a monoamine neurotransmitter degrading enzyme,
is well-known to be closely associated with impulsive aggressively,
anxiety, depression, among other emotions, and is considered as an
indicator of psychological status (22,23,35).
Recently, several studies have been focusing on the relationship
between MAOA expression and cancers (24–32).
However, conflicting results were reported across different types
of cancer, including prostate cancer (24–30), HCC
(31), and cholangiocarcinoma
(32). MAOA was demonstrated as being
highly expressed in high-grade aggressive prostate cancer, and
capable of mediating prostate tumorigenesis and metastasis
(24–27). Recently, MAOA was reported as a novel
decision maker in apoptosis and autophagy processes occurring
within hormone refractory neuroendocrine prostate cancer cells
(28). Moreover, clorgyline, a MAOA
inhibitor, was found to exhibit anti-oncogenic and
pro-differentiation effects on high-grade prostate cancer cells
(29), and the MAOA
inhibitor-near-infrared dye conjugate was reported to reduce
prostate tumor growth (30). These
findings suggest that MAOA might play a key role in mediating
prostate cancer progression. However, Li et al demonstrated
that MAOA expression was remarkably downregulated in clinical HCC
tissue samples (31), and that MAOA
suppressed HCC metastasis by inhibiting the adrenergic system and
its transactivation of EGFR signaling (31). Huang et al also found that MAOA
expression was inhibited by coordinated epigenetic and IL-6-driven
events in human cholangiocarcinoma (32), and that overexpression of MAOA
suppressed cholangiocarcinoma growth and invasion (32). In the present study, we demonstrated
for the first time to our knowledge that MAOA protein and mRNA
expression levels, positive rates, and statistical distribution in
NSCLC tissues were dramatically higher than those recorded in the
matched non-tumor adjacent lung tissues (Figs. 1 and 2;
Table II). Moreover, we further
found that MAOA expression was correlated with clinical stages and
lymph node metastases, while no relation could be established with
sex, age, degree of differentiation, and histological types
(Table IV). Taken together, our
results suggest that MAOA may play a role in promoting the
progression of NSCLC.
EMT, a key step in invasion and metastasis, plays a
crucial role in the progression of cancers, including NSCLC
(16–19). Wu et al (25) demonstrated that MAOA expression in
prostate cancer suppressed epithelial phenotype and promoted
mesenchymal transition by decreasing the expression of epithelial
marker E-cadherin, while increasing the expressions of mesenchymal
marker vimentin and transcription factor Twist, indicating its
association with EMT in prostate cancer. In the present study, we
showed that the increased MAOA expression in NSCLC tissues was
negatively correlated with E-cadherin expression, but positively
correlated with the expressions of N-cadherin, Slug, and Twist
(Table III), suggesting that MAOA
may mediate EMT, leading to the progression of NSCLC.
In conclusion, we demonstrated for the first time,
to the best of our knowledge, that MAOA expression was
significantly increased in NSCLC tissues, which was positively
associated with EMT, late stages and lymph node metastases of the
cancer, thus supporting the notion that MAOA may play a role in
NSCLC progression by regulating the EMT process.
Acknowledgements
This study was supported by the grants from National
Natural Science Foundation of China, 81372511) (to X.T.), Special
Fund for Scientific and Technological Development (Basic and
Applied Basic Research) of Guangdong Province (Natural Science
Foundation of Guangdong Province), 2017A030313539 (to X.T.), “Sail
plan” in Guangdong Province to cultivate high-level talents,
201635011 (to X.T.), Guangdong Provincial Department of Science and
Technology (Research and Development of Industrial Technology in
Guangdong Province), 2013B031100002 (to X.T.), and Zhanjiang
Municipal Governmental Specific Financial Fund Allocated for
Competitive Scientific and Technological Projects, 2012C0303-56 (to
X.T.). We would like to thank Professor Han-Guo Jiang (Department
of Pathology, Guangdong Medical University, China) and Dr. Ketao
Jin (Shaoxin Hospital, Zhejiang, China) for their diagnoses
performed on NSCLC tissues and their guidance regarding
immunohistochemical staining methods.
References
|
1
|
Islami F, Torre LA and Jemal A: Global
trends of lung cancer mortality and smoking prevalence. Transl Lung
Cancer Res. 4:327–338. 2015.PubMed/NCBI
|
|
2
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: The new IASLC/ATS/ERS international
multidisciplinary lung adenocarcinoma classification. J Thoracic
Oncol. 6:244–285. 2011. View Article : Google Scholar
|
|
3
|
Tang ER, Schreiner AM and Pua BB: Advances
in lung adenocarcinoma classification: A summary of the new
international multidisciplinary classification system
(IASLC/ATS/ERS). J Thorac Dis. 6 Suppl 5:S489–S501. 2014.PubMed/NCBI
|
|
4
|
Pikin OV, Ryabov AB, Trakhtenberg AK,
Glushko VA, Kolbanov KI, Amiraliev AM, Barmin VV and Tukvadze ZG:
Analysis of postoperative complications after pneumo-n-ectomy using
thoracic morbidity and mortality (tmm) system in nsclc patients for
a 5-year period. Khirurgiia (Mosk). 23–27. 2016.(In Russian).
PubMed/NCBI
|
|
5
|
Akthar AS, Ferguson MK, Koshy M,
Vigneswaran WT and Malik R: Limitations of PET/CT in the detection
of occult N1 metastasis in clinical stage I(T1-2aN0) non-small cell
lung cancer for staging prior to stereotactic body radiotherapy.
Technol Cancer Res Treat. 16:15–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Renaud S, Falcoz PE, Olland A, Reeb J,
Santelmo N and Massard G: Mediastinal downstaging after induction
treatment is not a significant prognostic factor to select patients
who would benefit from surgery: The clinical value of the lymph
node ratio. Interact Cardiovasc Thorac Surg. 20:222–227. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu S, Yan C, Yang X, He S, Liu J, Qin C,
Huang C, Lu Y, Tian Z and Jia L: Pharmacoproteomic analysis reveals
that metapristone (RU486 metabolite) intervenes E-cadherin and
vimentin to realize cancer metastasis chemoprevention. Sci Rep.
6:223882016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Liang D, Fan J, Lian X, Zhao Y,
Wang X, Chi ZH and Zhang P: Zinc attenuates tubulointerstitial
fibrosis in diabetic nephropathy via inhibition of HIF through
PI-3K signaling. Biol Trace Elem Res. 173:372–383. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu S, Yang H, Chen Y, He B and Chen Q:
Krüppel-like factor 4 enhances sensitivity of cisplatin to lung
cancer cells and inhibits regulating epithelial-to-mesenchymal
transition. Oncol Res. 24:81–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang SB, He XJ, Xia YJ, Hu WJ, Luo JG,
Zhang J and Tao HQ: MicroRNA-145-5p inhibits gastric cancer
invasiveness through targeting N-cadherin and ZEB2 to suppress
epithelial-mesenchymal transition. Onco Targets Ther. 9:2305–2315.
2016.PubMed/NCBI
|
|
11
|
Zhou JP, Gao ZL, Zhou ML, He MY, Xu XH,
Tao DT, Yang CC and Liu LK: Snail interacts with Id2 in the
regulation of TNF-α-induced cancer cell invasion and migration in
OSCC. Am J Cancer Res. 5:1680–1691. 2015.PubMed/NCBI
|
|
12
|
Li SP, Xu HX, Yu Y, He JD, Wang Z, Xu YJ,
Wang CY, Zhang HM, Zhang RX, Zhang JJ, et al: LncRNA HULC enhances
epithelial-mesenchymal transition to promote tumorigenesis and
metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1
signaling pathway. Oncotarget. 7:42431–42446. 2016.PubMed/NCBI
|
|
13
|
Ha JH, Ward JD, Radhakrishnan R, Jayaraman
M, Song YS and Dhanasekaran DN: Lysophosphatidic acid stimulates
epithelial to mesenchymal transition marker Slug/Snail2 in ovarian
cancer cells via Gαi2, Src, and HIF1α signaling nexus. Oncotarget.
7:37664–37679. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zidar N, Boštjančič E, Jerala M, Kojc N,
Drobne D, Štabuc B and Glavač D: Down-regulation of microRNAs of
the miR-200 family and up-regulation of Snail and Slug in
inflammatory bowel diseases - hallmark of epithelial-mesenchymal
transition. J Cell Mol Med. 20:1813–1820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Liu J, Ying X, Lin PC and Zhou BP:
Twist-mediated epithelial-mesenchymal transition promotes breast
tumor cell invasion via inhibition of hippo pathway. Sci Rep.
6:246062016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roy BC, Kohno T, Iwakawa R, Moriguchi T,
Kiyono T, Morishita K, Sanchez-Cespedes M, Akiyama T and Yokota J:
Involvement of LKB1 in epithelial-mesenchymal transition (EMT) of
human lung cancer cells. Lung Cancer. 70:136–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Che J, Yang Y, Xiao J, Zhao P, Yan B, Dong
S and Cao B: Decreased expression of claudin-3 is associated with a
poor prognosis and EMT in completely resected squamous cell lung
carcinoma. Tumour Biol. 36:6559–6568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang D, Duan H, Huang H, Tong X, Han Y,
Ru G, Qu L, Shou C and Zhao Z: Cisplatin resistance in gastric
cancer cells is associated with HER2 upregulation-induced
epithelial-mesenchymal transition. Sci Rep. 6:205022016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sung WJ, Kim H and Park KK: The biological
role of epithelial-mesenchymal transition in lung cancer (Review).
Oncol Rep. 36:1199–1206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edgnülü TG, Özge A, Erdal N, Kuru O and
Erdal ME: Association analysis of the functional MAOA gene promoter
and MAOB gene intron 13 polymorphisms in tension type headache
patients. Adv Clin Exp Med. 23:901–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nikolac Perkovic M, Svob Strac D, Nedic
Erjavec G, Uzun S, Podobnik J, Kozumplik O, Vlatkovic S and Pivac
N: Monoamine oxidase and agitation in psychiatric patients. Prog
Neuropsychopharmacol Biol Psychiatry. 69:131–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Huang L, Luo XJ, Wu L and Li M:
MAOA Variants and genetic susceptibility to major psychiatric
disorders. Mol Neurobiol. 53:4319–4327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Voltas N, Aparicio E, Arija V and Canals
J: Association study of monoamine oxidase-A gene promoter
polymorphism (MAOA-uVNTR) with self-reported anxiety and other
psychopathological symptoms in a community sample of early
adolescents. J Anxiety Disord. 31:65–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peehl DM, Coram M, Khine H, Reese S,
Nolley R and Zhao H: The significance of monoamine oxidase-A
expression in high grade prostate cancer. J Urol. 180:2206–2211.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu JB, Shao C, Li X, Li Q, Hu P, Shi C, Li
Y, Chen YT, Yin F, Liao CP, et al: Monoamine oxidase A mediates
prostate tumorigenesis and cancer metastasis. J Clin Invest.
124:2891–2908. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu JB, Yin L, Shi C, Li Q, Duan P, Huang
JM, Liu C, Wang F, Lewis M, Wang Y, et al: MAOA-dependent
activation of Shh-IL6-RANKL signaling network promotes prostate
cancer metastasis by engaging tumor-stromal cell interactions.
Cancer Cell. 31:368–382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stone L: Prostate cancer: Feel it in your
bones: MAOA mediates metastasis. Nat Rev Urol. 14:326–327. 2017.
View Article : Google Scholar
|
|
28
|
Lin YC, Chang YT, Campbell M, Lin TP, Pan
CC, Lee HC, Shih JC and Chang PC: MAOA - a novel decision maker of
apoptosis and autophagy in hormone refractory neuroendocrine
prostate cancer cells. Sci Rep. 7:463382017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao H, Flamand V and Peehl DM:
Anti-oncogenic and pro-differentiation effects of clorgyline, a
monoamine oxidase A inhibitor, on high grade prostate cancer cells.
BMC Med Genomics. 2:552009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu JB, Lin TP, Gallagher JD, Kushal S,
Chung LW, Zhau HE, Olenyuk BZ and Shih JC: Monoamine oxidase A
inhibitor-near-infrared dye conjugate reduces prostate tumor
growth. J Am Chem Soc. 137:2366–2374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Yang XM, Wang YH, Feng MX, Liu XJ,
Zhang YL, Huang S, Wu Z, Xue F, Qin WX, et al: Monoamine oxidase A
suppresses hepatocellular carcinoma metastasis by inhibiting the
adrenergic system and its transactivation of EGFR signaling. J
Hepatol. 60:1225–1234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang L, Frampton G, Rao A, Zhang KS, Chen
W, Lai JM, Yin XY, Walker K, Culbreath B, Leyva-Illades D, et al:
Monoamine oxidase A expression is suppressed in human
cholangiocarcinoma via coordinated epigenetic and IL-6-driven
events. Lab Invest. 92:1451–1460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Witalison EE, Cui X, Causey CP, Thompson
PR and Hofseth LJ: Molecular targeting of protein arginine
deiminases to suppress colitis and prevent colon cancer.
Oncotarget. 6:36053–36062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Li J, Shen J, Wang C, Yang L and
Zhang X: MicroRNA-182 downregulates metastasis suppressor 1 and
contributes to metastasis of hepatocellular carcinoma. BMC Cancer.
12:2272012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Różycka A, Słopień R, Słopień A,
Dorszewska J, Seremak-Mrozikiewicz A, Lianeri M, Maciukiewicz M,
Warenik-Szymankiewicz A, Grzelak T, Kurzawińska G, et al: The MAOA
COMT, MTHFR and ESR1 gene polymorphisms are associated with the
risk of depression in menopausal women. Maturitas. 84:42–54. 2016.
View Article : Google Scholar : PubMed/NCBI
|