Introduction
Lung cancer is a global health problem with poor
clinical outcomes. According to the survey of Mayo Clinic in 2005,
non-small cell lung cancer (NSCLC) accounts for more than 80% of
5,628 patients with primary lung cancer out of all lung cancer
cases, and the majority of patients with NSCLC are diagnosed at the
advanced stage (1–3). Radiation therapy is regarded as the
primary treatment strategy for NSCLC. However, radio-resistance is
a key issue limiting its effectiveness (4). Therefore, screening for effective
molecular radiosensitizers is urgently required to provide
personalized therapy and improve patient survival.
MicroRNAs (miRs; miRNAs) are endogenous non-coding
RNAs that function as regulators of gene expression by targeting
mRNA degradation or inhibiting their translation (5). Over the previous decade, a number of
studies have demonstrated that the altered expression of miRs has
been associated with different types of cancer (6), including lung cancer (7). In addition, studies have also suggested
that there is an association between certain miRs and radiotherapy
(8,9).
The function of miR-9 in cancer cells has been considered to be
controversial since it serves as a tumor suppressor in melanoma
(10) and as a metastasis-promoter in
breast cancer (11). Reduced
expression of miR-9 is considered to be a marker of poor prognosis
in cervical cancer (12) and acute
lymphoblastic leukemia (13).
However, the function of miR-9 in NSCLC pathogenesis, and the
molecular mechanisms by which miR-9 exerts its functions and
modulates the malignant phenotypes of NSCLC cells have not been
fully determined.
Neuropilin 1 (NRP1) is a transmembrane glycoprotein
with large extracellular regions that is extensively expressed in
endothelial cells and a variety of tumor cells (14). NRP1 serves a crucial function in
tumorigenesis and radio-resistance. Brieger et al (15) identified that the addition of
recombinant vascular endothelial growth factor VEGF-121 and
VEGF-165 to squamous carcinoma cells increased resistance to
radiation-induced cell death by targeting NRP1 receptors. Glinka
et al (16) demonstrated that
the overexpression of NRP1 decreased the apoptosis rate of glioma
cells induced by radiation. In our previous study, NRP1 was highly
expressed in the radio-resistant NSCLC A549 cell line, and short
hairpin RNA-mediated NRP1 inhibition significantly enhanced
radio-sensitivity (17). However, the
mechanism by which NRP1 is regulated by certain miRs to exert its
functions remains unclear.
The present study demonstrated that miR-9 served a
tumor suppressor function in, and enhanced the radio-sensitivity
of, NSCLC cells by regulating NRP1. These data will provide novel
insights for understanding the mechanisms of NSCLC pathogenesis,
and present a potential biomarker in evaluating the effectiveness
of radio-therapy treatment.
Materials and methods
Cell lines and treatment
A549 cells were cultured in Dulbecco's modified
Eagle medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). All media were supplemented with 10% fetal bovine serum
(FBS; HyClone; GE Healthcare, Chicago, IL, USA), 100 U/ml
penicillin and 100 µg/ml streptomycin (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) in a humid incubator
with 5% CO2. Cells were harvested using 0.25% trypsin
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) and 0.03% EDTA solution at 37°C for 5–10 min. Media were
replaced 2–3 times per week.
A549 cells (Type Culture Collection of the Chinese
Academy of Sciences, Shanghai, China) were cultured in Dulbecco's
modified Eagle's medium (Gibco, Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% (vol/vol) fetal bovine
serum (HyClone, GE Healthcare) and 1% penicillin-streptomycin. A549
cells were transfected with 100 nM miR-9 mimic (Sense
5′-UCUUUGGUUAUCUAGCUGUAUGATT-3′, Anti-sense
5′-AUAAAGCUAGAUAACCGAAAGUTT-3) or negative control (NC; Sense
5′-UUCUCCGAACGUGUCACGUTT-3′, Anti-sense
5′-ACGUGACACGUUCGGAGAATT-3′) RNA (GenePharma, Shanghai, China)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) were sham-irradiated or exposed
to irradiation (IR). Subsequent experimentation was performed 24 h
after transfection.
Experimental animals
A total of 24 male 6-week-old Balb/c athymic nude
mice (Beijing HFK Bioscience Co., Ltd., Beijing, China), weighing
19–20 g were used in this study. Mice were maintained in a specific
pathogen free environment, with a 12 h light/dark cycle at 20–25°C
and a humidity of 40–70%, sterilized food and water were freely
available. Mice were subcutaneously injected in the right flank
with 1×106 cells in 0.1 ml PBS. Once the tumors had
formed, caliper measurements were performed daily and tumor volume
(V) was calculated using the formula: V=width2xlength/2.
When tumors volume reached ~100 mm3, the mice were
randomly divided into four groups (control, miR-9, IR and miR-9+IR
group; n=6), and the miR-9 and miR-9+IR groups received
intratumoral injection of miR-9 plasmids along with the DNA
transfection reagent (Entranster™-in vivo,
Engreen Biosystem Co., Ltd., Beijing, China) once weekly. For each
injection, 15 µg miRNA were mixed with 15 µl
Entranster™-in vivo. The IR and miR-9+IR group
were exposed to 20 Gy at the tumor site. The mice were euthanatized
15 days following IR, or if a humane endpoint was reached; defined
as a loss of more than 15% of body mass, a tumor volume greater
than 1.2 cm3, severe fever, vomiting or skin problems
(wounds or signs of inflammation) or inability to ambulate or rise
for food and water. Following euthanasia, the tumor mass and volume
were recorded, and tumor volumes did not exceed 219 mm3.
The dissected tumors were collected and prepared for subsequent
analyses. All animal experiments were performed in accordance with
the institutional guidelines of Jilin University (Changchun, China)
for the Care and Use of Laboratory Animals, and this board approved
the study protocol was.
Irradiation
A549 cells were sham-irradiated or exposed to IR at
a dose rate of 1.0 Gy/min (220 kV; 18 mA) by an X-ray generator
(Model X-RAD320, Precision X-ray, North Branford, CT, USA). The
male 6-week-old Balb/c athymic nude mice were exposed to a single
20 Gy dose of X rays at a dose rate of 1.55 Gy/min.
RNA isolation and quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from A549 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and reverse transcribed to generate cDNA
(PrimeScript RT-PCR kit; Takara Bio, Inc., Otsu, Japan) according
to the manufacturer's protocol. The primer sequences are as
follows: NPR1 forward, CCCCAAACCACTGATAACTCG, reverse,
AGACACCATACCCAACATTCC; GAPDH forward, ACATCGCTCAGACACCATG, reverse,
TGTAGTTGAGGTCAATGAAGGG (Sangon Biotech Co., Ltd., Shanghai, China).
The Thermocycling conditions were as follows: 95°C for 5 min, 40
cycles of 95°C for 10 sec follows by 60°C for 30 sec. U6 small
nuclear RNA and GAPDH were used as internal controls for miR-9 and
NRP1, respectively. All samples were normalized to the internal
controls and fold changes were calculated through relative
quantification (2−ΔΔCq) (18).
miRNA conserved target sites
prediction in 3′ UTR of NRP1
The TargetScan predictions (http://www.targetscan.org/vert_71/) were used to
identify the putative miRNA target sites in 3′ UTRs of NRP1
gene. The NRP1 gene symbol and human species were retrieved
from the database. The 3′ UTR of NRP1 transcript
ENST00000374875.1 was selected to analyze the potential binding
site of miRNAs.
Plasmid construction and luciferase
reporter assays
Gaussian luciferase and alkaline phosphatase
activities were measured by luminescence in conditioned medium
(DMEM; HyClone; GE Healthcare) without antibiotics and 10% FBS
(HyClone; GE Healthcare) 48 h after transfection, as previously
described (19). A 2,600 bp fragment
of DNA from the NRP1 3′ untranslated region (3′UTR) of A549 cells
was amplified by PCR using Q5® High-Fidelity DNA
Polymerase (M0491) from New England BioLabs, Inc. (Ipswich, MA,
USA). The primer sequences used were as follows: Forward,
ATGAACGGTACCAGGCAGACAGAGATGAAAAGACA, reverse,
GAACTTCTCGAGTCAGGTGTGGGATATTTTATGAAAATG. Thermocycling conditions
were 95°C for 3 min, followed by 35 cycles of 95°C for 15 sec, 58°C
for 30 sec, 72°C for 2 min 30 sec, 35 cycles, and one cycle of 72°C
for 10 min. DNA was then cloned into the pEZX-MT05 vector
(GeneCopoeia, Inc., Rockville, MD, USA). The vector was named
wild-type (wt) 3′UTR. Site-directed mutagenesis of the miR-9
binding site in the NRP1 3′UTR was performed using the GeneTailor
Site-Directed Mutagenesis System (Invitrogen; Thermo Fisher
Scientific, Inc.) and the construct was named mutant (mt) 3′UTR.
A549 cells were transfected with 100 ng wt/mt 3′UTR vectors and 100
nM miR-9 mimic/anti-miR-9/NC, using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Following
transfection for 48 h, the activity of Gaussia luciferase and
secreted alkaline phosphatase were examined using the Secrete-Pair
Dual Luminescence Assay kit (GeneCopoeia, Inc., Rockville, MD,
USA). Gaussian luciferase activity was normalized to alkaline
phosphatase activity. Normalized luciferase activity of treatment
group was compared with that of control group.
Western blot analysis
A total of 48 h after radiation exposure, media was
removed from the A549 cells, which were then washed twice with
ice-cold PBS. Lysis was performed using cell lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China), and cell lysates were
collected by scraping the plates prior to centrifugation at 10,000
× g at 4°C for 5 min. Proteins were quantified using a
bicinchoninic acid assay kit according to the manufacturers
protocol (Applygen Technologies Inc., Beijing, China). A total of
30 µg protein, denatured in gel loading buffer (Applygen
Technologies Inc., Beijing, China), was loaded per well and
separated using SDS-PAGE electrophoresis (5% stacking gel and 10%
separating gel) at 100 V voltage until bromophenol blue runs out of
separating gel, and then transferred onto polyvinylidene fluoride
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membranes were blocked in 5% skimmed milk at room temperature for 1
h, prior to incubation at 4°C overnight with the designated primary
antibodies (rabbit anti-Neuropilin 1 (Abcam, Cambridge, UK; cat.
no., ab81321), rabbit anti-phospho-p38 MAPK (CST Biological
Reagents Co., Ltd., Shanghai, China; cat. no., 9215), rabbit
anti-total-p38 MAPK (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany;
cat. no., M0800), rabbit anti-pERK1/2 (T202/Y204) (CST Biological
Reagents Co., cat. no., 4370), mouse anti-ERK1/2 (CST Biological
Reagents Co., cat. no., 9107), rabbit anti-pAKT (S473; CST
Biological Reagents Co., cat. no., 4060), mouse anti-AKT (CST
Biological Reagents Co. cat. no., 2966), rabbit PI3 Kinase p110α
(C73F8; CST Biological Reagents Co., cat. no., 4249) and mouse
anti-GAPDH (Santa Cruz Biotechnology, Inc., Dallas, TX, USA; cat.
no., sc-47724). All antibodies were used at a dilution of 1:1,000.
Membranes were then incubated with the corresponding horseradish
peroxidase-conjugated secondary antibodies (goat anti-rabbit IgG;
Beyotime Institute of Biotechnology) for 2 h at room temperature.
Densitometry was performed using ImageJ (version 1.50i; National
Institutes of Health, Bethesda, MD, USA).
Cell viability assay
A total of 5×103 A549 cells were seeded
into 96-well plates and transfected with miR-9 mimics or control
RNA. Following IR, the cells were cultured in DMEM (Hyclone; GE
Healthcare, Chicago, IL, USA) with 10% FBS (HyClone; GE Healthcare)
at 37°C for 0, 24 and 48 h. The effect of miR-9 on cell growth and
viability was determined by MTT assay (Cell Growth Determination
kit; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to
the manufacturer's instructions, DMSO was used to dissolve the
crystals, and the optical density was measured at 570 nm.
Cell-cycle analysis and Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection assay
For cell-cycle analysis, 1×106 cells
transfected with miRNAs or control RNA were cultured in complete
DMEM (Hyclone; GE Healthcare) at 37°C in triplicate in 6-well
plates. At 48 h post-transfection, cells were exposed to 10 Gy IR.
The cell-cycle distribution was analyzed by 0.05 mg/ml propidium
iodide staining (37°C for 30 min) and flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA). The cells were stained for
20 min at room temperature using the Annexin V-FITC Apoptosis
Detection kit I (BD Biosciences) according to the manufacturer's
protocol. The data obtained from this assay was analyzed using
Modfit software (version 3.3; Verity House Software, Topsham, ME,
USA).
Colony formation assays
Transfected and control A549 cells were plated into
6-well plates at specific cell densities (200 cells for 0 Gy, 500
cells for 2 Gy, 1,000 cells for 4 Gy, 3,000 cells for 6 Gy and
5,000 cells for 8 Gy) and received 0, 2, 4, 6 or 8 Gy X-ray 24 h
subsequent to seeding, and were then cultured in DMEM (Hyclone; GE
Healthcare) at 37°C for 7–14 days to allow colonies to grow. The
cells were then rinsed 3 times with PBS, fixed in 100% methanol (at
room temperature for 20 min), and stained with Giemsa stain (0.04%)
for 30 min at room temperature. The number of the colonies
containing at least 50 cells was counted using a light microscope
at magnification, ×40. The surviving fraction was calculated as the
number of colonies counted/(the number of cells seeded × plating
efficiency). Experiments were performed in triplicate and repeated
three times.
In vitro migration and invasion
assays
For the transwell migration assays,
2.5×104 A549 cells were plated in the top chamber on
non-coated membrane (24-well insert; Corning, NY, USA). For the
invasion assays, 5×104 A549 cells were plated in the top
chamber on Matrigel®-coated membranes (24-well insert;
BD Biosciences). The cells were plated in DMEM (Hyclone; GE
Healthcare) without serum, or supplemented 10% FBS (HyClone; GE
Healthcare) was used as the chemoattractant in the lower chamber.
The cells were then incubated for 12 h at 37°C. Cells remaining on
the upper side of the filter membrane were removed with PBS-rinsed
cotton swabs. The filters were then fixed in 100% ethanol for 20
min at room temperature, and stained with 0.1% crystal violet for
20 min at room temperature. The stained cells were counted under a
light microscope (magnification, ×40). The average count was
calculated in 6 random fields of view. Experiments were performed
in triplicate and repeated three times.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
(SPSS, Inc., Chicago, IL, USA). The results from three independent
experiments are presented as mean ± standard deviation. Differences
between the control and experimental groups were analyzed using
one-way analysis of variance, followed by between group comparisons
performed using the Student-Newman-Keuls test. P<0.05 was
considered to indicate a statistically significant difference.
Results
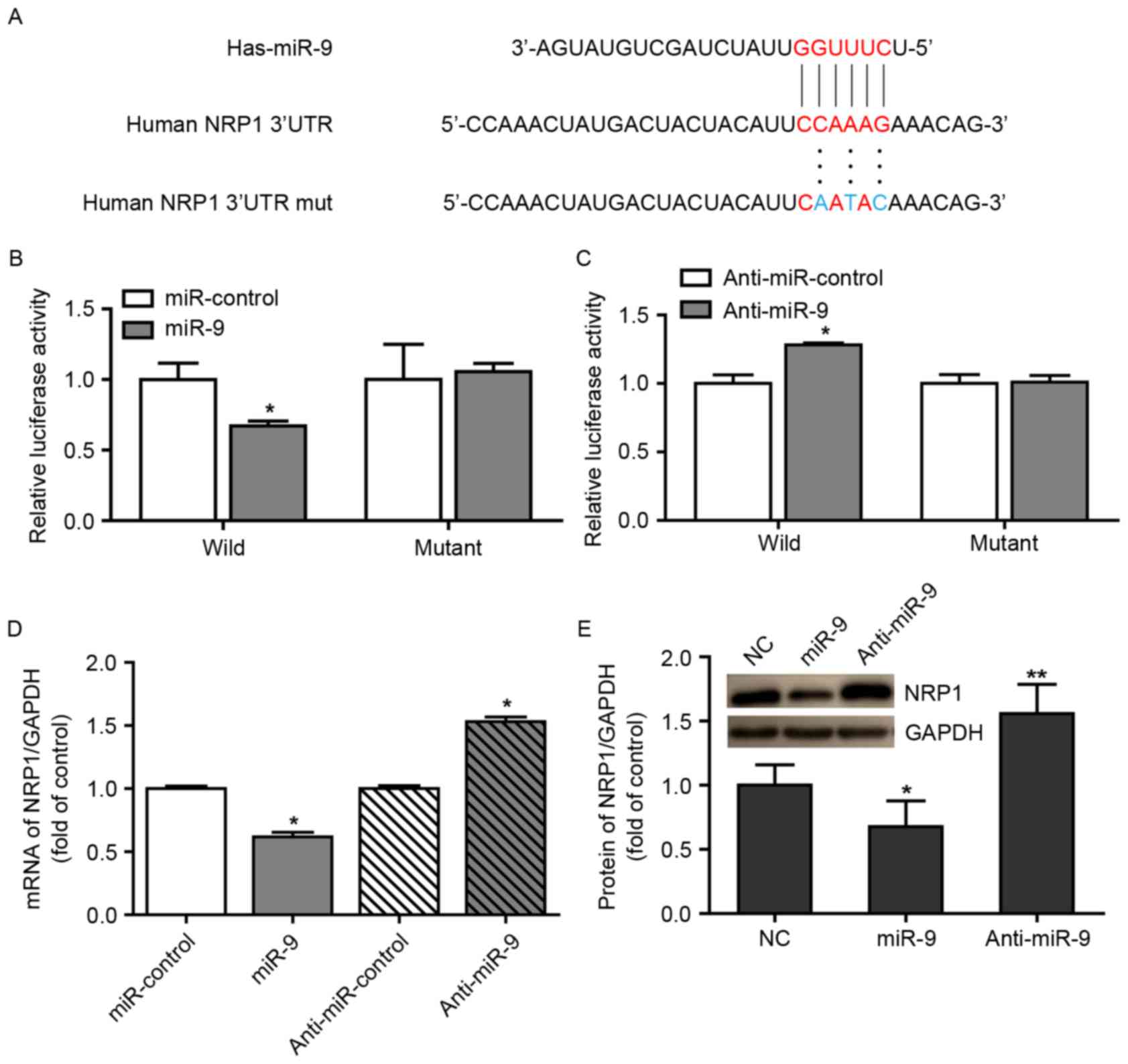
NRP1 is a direct target of miR-9 in
A549 cells
In our previous study, it had been demonstrated that
a high expression of NRP1 was correlated with the growth, survival
and radio-resistance of NSCLC cells via the VEGF-PI3K-NF-κB pathway
(17). We hypothesized that certain
miRNAs may be involved in these biological processes through
regulating NRP1 expression. In view of this consideration, the
present study used the TargetScan algorithm to determine the
potential miRNAs that may bind to the 3′UTR of NRP1 and it was
identified that NRP1 possessed a conserved miR-9 binding site
within its 3′UTR in the majority of species examined (Fig. 1A). A luciferase reporter assay was
additionally performed to identify the ability of miR-9 to bind to
the 3′UTR of NRP1 mRNA. Notably, co-transfecting the miR-9 mimics
with the luciferase-linked NRP1 wild-type 3′UTR construct
downregulated luciferase activity, whereas co-transfection with the
mutated 3′UTR construct did not (Fig.
1B). In addition, inhibition of endogenous miR-9 expression in
cells transduced with anti-miR-9 resulted in increased luciferase
activity from the wild-type construct, but had no effect on the
mutated NRP1 3′UTR (Fig. 1C). These
data suggest that miR-9 regulated the expression of NRP1 mRNA
(Fig. 1D) and protein (Fig. 1E) in A549 cells.
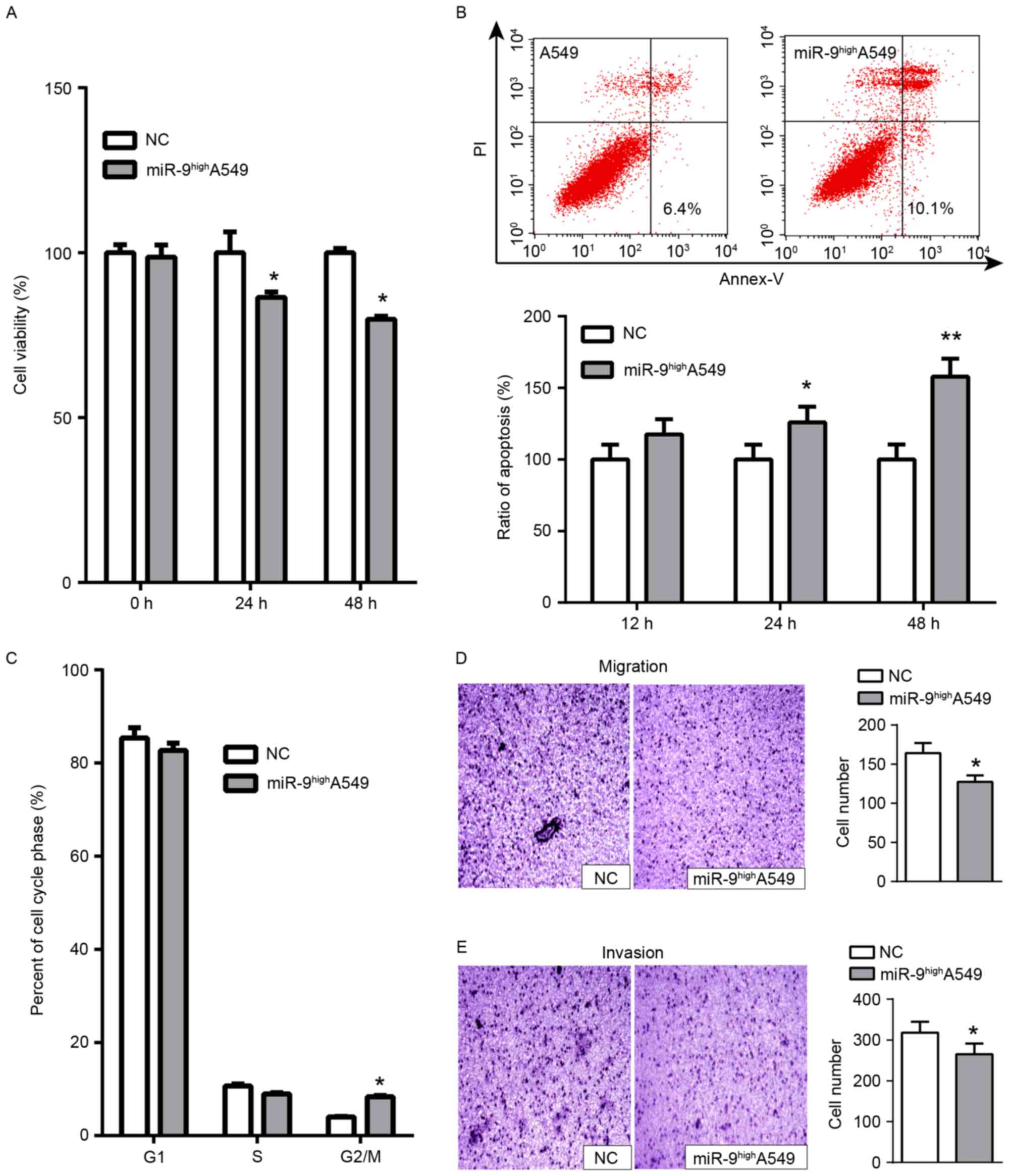
miR-9 serves a tumor suppressor role
in A549 cells in vitro and in vivo
The expression of NRP1 was detected in 5 NSCLC cell
lines including H358, H460, H1299, A549 and SK-MES-1 in a previous
study (17), and it was identified
that the A549 cell line demonstrated the highest expression of NRP1
and the strongest radio-resistance. In addition, the tumors formed
from NRP1-inhibited A549 cells grew at a decreased rate compared
with those from normal A549 cells (17). Therefore, A549 cells were selected as
the primary cell line. Whether miR-9 overexpression functioned
similarly to NRP1 inhibition was examined. miR-9 mimics were
transiently transfected into A549 cells for functional analysis. A
significant decrease in cell viability at 24 h and a more marked
decrease at 48 h was observed compared with the control (Fig. 2A). Concurrently, a significant
increase in apoptosis was detected with miR-9 treatment vs. the
control (Fig. 2B). Cell cycle assays
indicated that transfection with miR-9 mimics arrested cell cycle
progression in the G2/M phase at 48 h (Fig. 2C). In addition, we measured the
migratory and invasive abilities of A549 cells via transwell
migration and Matrigel® invasion assays. The results
indicated that the number of migratory (Fig. 2D) and invasive cells (Fig. 2E) decreased following transfection
with miR-9 mimics compared with untreated A549 cells. Furthermore,
the tumor formation assay in nude mice demonstrated that the growth
of tumors formed from miR-9-treated A549 cells was delayed compared
with the normal control group (Fig.
3C). Overall, these results indicated that miR-9 functioned as
a tumor suppressor in A549 cells by inhibiting cell proliferation,
promoting apoptosis and attenuating migratory and invasive
capacities.
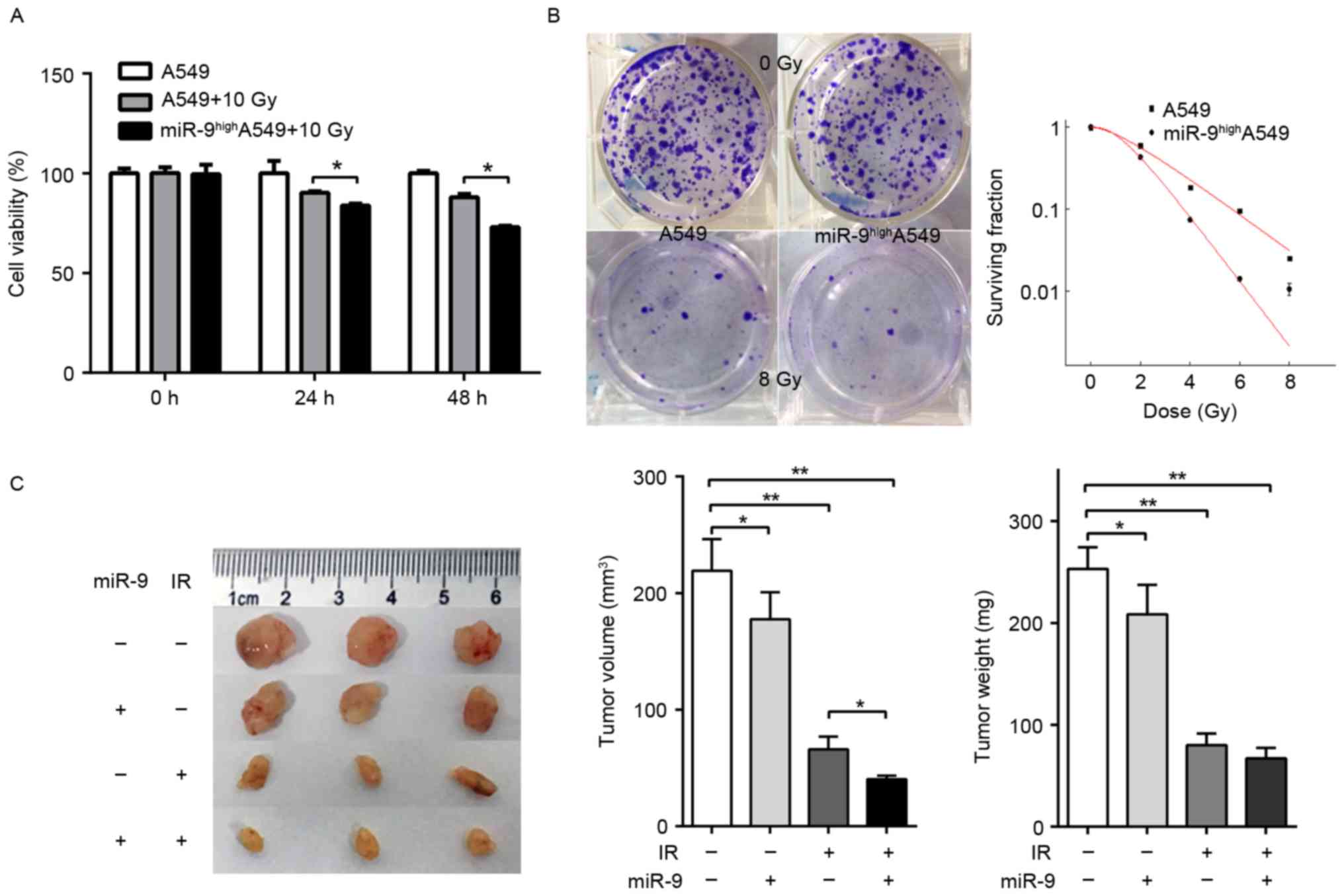
miR-9 overexpression enhances the
radio-sensitivity of A549 cells in vitro and in vivo
It had been demonstrated previously that knockdown
of endogenous NRP1 enhanced the radio-sensitivity of A549 cells
(17). Combined with the results of
the present study, we hypothesized that miR-9 was also able to
effect the radio-sensitivity of A549 cells. A549 cells were
transfected with or without miR-9 mimics and the effect upon IR was
observed. The viability of cells transfected with miR-9 mimics was
significantly decreased at 24 h, and even greater at 48 h compared
with the normal control (Fig. 3A).
Colony assays indicated that the survival fraction of A549 cells in
the miR-9-transfected group was decreased compared with that in the
miR-NC group upon IR with 0, 2, 4, 6 or 8 Gy (Fig. 3B). These data suggested that the
overexpression of miR-9 may significantly enhance the in
vitro radio-sensitivity of NSCLC cells. Next, the nude mouse
subcutaneous tumor model was constructed. The mice were randomly
divided into four groups (control, miR-9, IR and miR-9+IR group)
and the corresponding experimental treatment was administered. The
results indicated that the growth of the tumors treated with miR-9
and IR were significantly delayed compared with other groups
(Fig. 3C), which suggested that miR-9
overexpression combined with radiotherapy may induce a stronger
in vivo anti-tumor effect compared with radiotherapy
alone.
Exploration on the mechanism of
radio-sensitivity regulated by miR-9
The expression of NRP1 and miR-9 in IR- and
non-IR-treated tumors from the nude mice were additionally
examined. The NRP1 expression was significantly upregulated, while
the miR-9 was markedly downregulated, which indicates a marked
correlation with radiosensitivity (Fig.
4A). As a non-tyrosine kinase receptor, NRP1 may moderately
increase PI3K and phosphorylation of ERK P38 MAPK, ERK1/2, protein
kinase B (Akt) and NF-κB to promote tumor cell proliferation and
metastasis, and inhibit apoptosis and enhance radio-resistance
(17,20). Therefore, as NRP1 is a downstream
target of miR-9, miR-9 may also downregulate PI3K and the
phosphorylation of P38 MAPK, ERK1/2, Akt and NF-κB. Consequently,
it was identified that the overexpression of miR-9 inhibited PI3K
and phosphorylation of NF-κB, P38 MAPK, ERK1/2 and Akt, but the
relative expression of total protein was not significantly altered
(Fig. 4B). Therefore, one of the
mechanisms of miR-9-mediated radio-sensitivity may function by
regulating PI3K and the phosphorylation of NF-κB, ERK1/2, P38 MAPK
and Akt via NRP1.
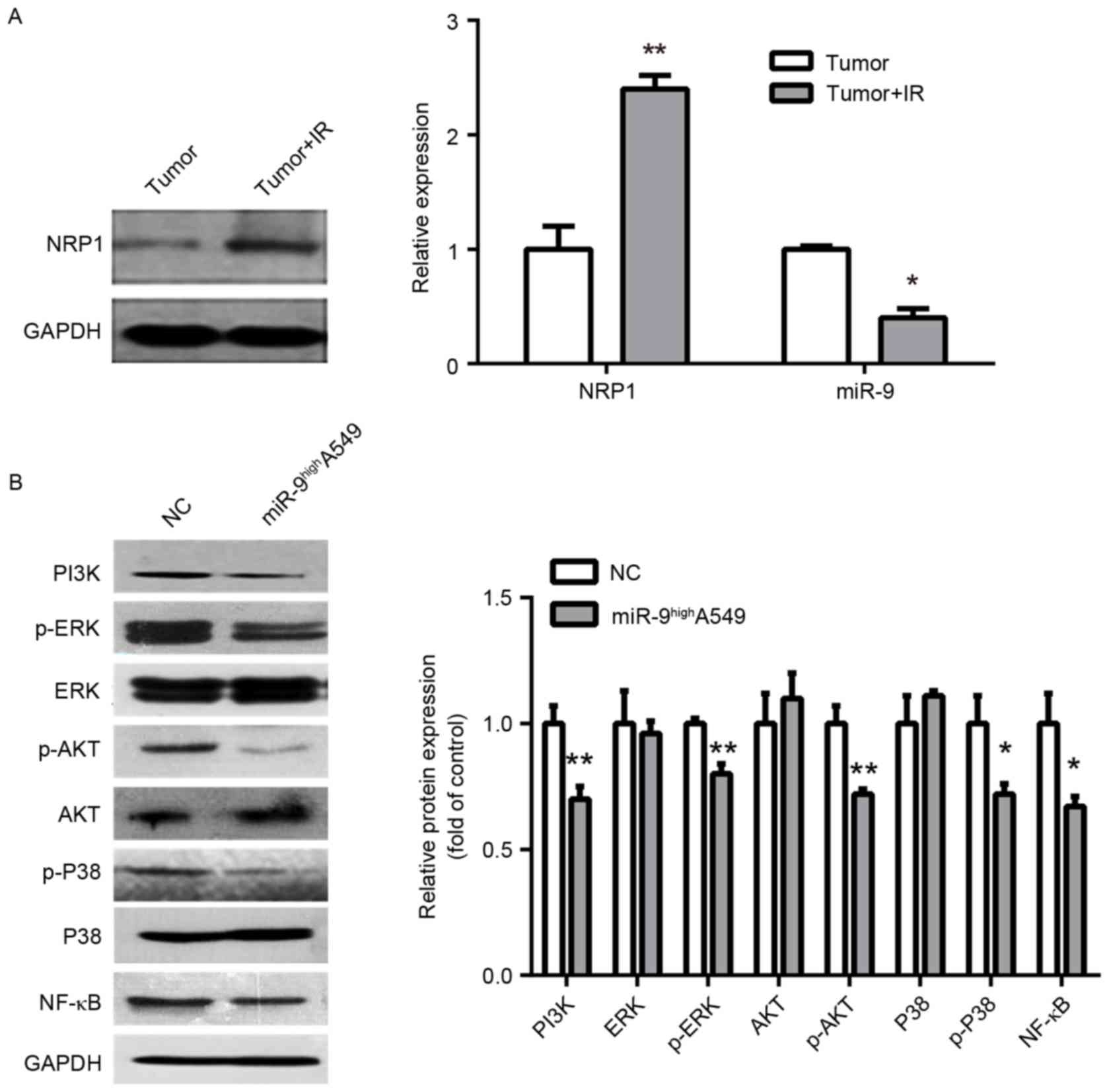 | Figure 4.Exploration of the mechanism of
radio-sensitivity. (A) Western blot analysis of NRP1 with IR and
non-IR treated tumors from nude mice (left panel). Histograms
demonstrate densitometric analysis of NRP1/GAPDH protein expression
by western blotting and miR-9 expression by reverse transcription
quantitative polymerase chain reaction from three separate
experiments (right panel). IR upregulated NRP1 protein expression
and downregulated miR-9 expression. (B) Representative immunoblots
(left panel) of A549 cells infected with miR-NC or miR-9 indicated
downregulation of PI3K/AKT, MAPK/ERK, NF-κB signaling protein by
miR-9. Right panel is densitometric analysis of NRP1/GAPDH from
three separate experiments. *P<0.05 and **P<0.01 vs. control.
NRP, Neuropilin; miR, microRNA; p, phosphorylated; PI3k,
phosphoinositide 3-kinase; ERK, extracellular signal-related
kinase; AKT, protein kinase B; P38, P38 mitogen-activated protein
kinase; NF-κB, nuclear factor κB; NC, negative control. |
Discussion
miRs serve a crucial function in controlling
proliferation, differentiation and tumorigenesis. A previous study
demonstrated the downregulation of miR-9 in gastric, colon and
ovarian cancer, and miR-9 upregulation in human breast cancer,
brain tumors and biliary tract cancer associated with the
controversial functions (21). miR-9
exhibits tumor-suppressive activity in human gastric cancer
(22) and colorectal cancer (23), whilst it serves an oncogenic role in
bladder cancer (24), osteosarcoma
(25) and mixed lineage
leukemia-rearranged leukemia (26).
These conflicting results are also reflected in studies on the same
types of tumors, such as ovarian cancer (27,28) and
NSCLC (21,29), in which miR-9 has exhibited
tumorigenesis-associated and anti-tumor activities in different
studies. The reasons for these contradicting conclusions may be due
to the heterogeneity of the tumors and the different materials,
such as cell lines, used. In the present study, it was identified
that miR-9 may inhibit the proliferation, invasion and migration in
A549 cells, which possessed marked radio-resistance compared with
other NSCLC cell lines. In addition, it was associated with the
high expression of NRP1 (17). In the
present study, it was demonstrated that miR-9 directly
downregulated expression of NRP1 by binding to its 3′UTR and
enhanced the radio-sensitivity of A549 cells.
To understand the clinicopathological significance
of miR-9, the effect of miR-9 overexpression in A549 lung cancer
cells was examined. The results indicated that miR-9 overexpression
significantly decreased the viability of A549 cells and arrested
cell cycle progression at the G2/M phase. As it has been
established that cells in the G2/M phase are sensitive to IR, with
sensitivity decreasing as cells proceed from G1 to S phase
(30), the results from the flow
cytometry analysis suggested that upregulation of miR-9 may enhance
A549 cell radio-sensitivity by disrupting the G2/M phase of the
cell cycle. In addition, the overexpression of miR-9 increased the
cell apoptosis rate and inhibited cell migration and invasion
compared with the transfected group. These results were consistent
with those observed in NRP1 knockdown cells in our previous study
(17), indicating that miR-9 serves a
role of tumor suppressor by targeting NRP1 in A549 cells. In fact,
NRP1 is an oncogenic promoter that is expressed in a wide variety
of human tumor cell lines, and it has been demonstrated that NRP1
may enhance the binding of VEGF to vascular endothelial growth
factor receptor 2 (VEGFR2), which resulted in promoting
VEGF165-mediated tumor angiogenesis, cell migration and
tumorigenicity (31).
It was also suggested that NRP1 may increase the
resistance of tumor cells to radiotherapy (17,32).
Therefore, we hypothesized that miR-9 may be also involved in the
regulation of radiosensitivity. It was identified that miR-9
overexpression significantly inhibited the viability and
colony-forming ability of A549 cells following IR, which
demonstrated that miR-9 enhanced the radio-sensitivity of this cell
line in vitro. Furthermore, a nude mouse subcutaneous tumor
model was constructed to analyze the effect of miR-9 to
radiotherapy. The results indicated that miR-9 overexpression,
combined with IR, exerted a more marked anti-tumor effect compared
with IR alone. All these data suggest that miR-9 enhanced the
radio-sensitivity in vitro and in vivo.
The intrinsic tumor radio-resistance relies on cell
survival and apoptosis, cell cycle and DNA repair pathways
(33). A total of 4 classical signal
transduction pathways, including PI3K/AKT, MAPK/ERK, NF-κB and
transforming growth factor-β, were involved in the regulation of
tumor radiation response, which may be activated either upon
radiation exposure or through the phosphorylation of receptor
tyrosine kinase such as EGFR and Insulin-like growth factor 1
receptor (34). The results of the
present study revealed that treatment with IR inhibited miR-9
expression, and correspondingly increased NRP1 expression in the
tumors formed from A549 cells. The upregulated NRP1 expression
increased the rate interaction between VEGFR2 and NRP1 and enhanced
radio-resistance by the activated VEGFR2-PI3K-NF-κB pathway
(17,20). It implied that increasing
radio-sensitivity by upregulating miR9, which targets NRP1, may be
a potential treatment strategy. Compared with the control group in
the present study, the expression levels of PI3K, NF-κB,
phosphorylated (p)ERK, pAkt and pP38 were all altered following the
overexpression miR-9, indicating that miR-9 may inhibit multiple
signaling pathways to enhance radio-sensitivity. These results may
offer insights for the improved understanding of the whole network
regulating radio-resistance, and may assist additional improvements
in the radiotherapy treatment strategy for NSCLC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81573085, 81672091,
81541142, 81371890 and 81201737) and Beijing Natural Science
Foundation (grant no. 7172232). An abstract for this study was
published following its acceptance for a poster presentation at the
Annual Meeting-American Society for Radiation Oncology 24–27
September 2017, San Diego, CA, USA (International Journal of
Radiation Oncology 99: E628, 2017).
References
|
1
|
Spiro SG and Silvestri GA: One hundred
years of lung cancer. Am J Respir Crit Care Med. 172:523–529. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics. 2002 CA Cancer J Clin. 55:1–108.
2005.
|
|
3
|
Yang P, Allen MS, Aubry MC, Wampfler JA,
Marks RS, Edell ES, Thibodeau S, Adjei AA, Jett J and Deschamps C:
Clinical features of 5,628 primary lung cancer patients: Experience
at Mayo Clinic from 1997 to 2003. Chest. 128:452–462. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ortholan C, Puissegur MP, Ilie M, Barbry
P, Mari B and Hofman P: MicroRNAs and lung cancer: New oncogenes
and tumor suppressors, new prognostic factors and potential
therapeutic targets. Curr Med Chem. 16:1047–1061. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salim H, Akbar NS, Zong D, Vaculova AH,
Lewensohn R, Moshfegh A, Viktorsson K and Zhivotovsky B: miRNA-214
modulates radiotherapy response of non-small cell lung cancer cells
through regulation of p38MAPK, apoptosis and senescence. Br J
Cancer. 107:1361–1373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh JS, Kim JJ, Byun JY and Kim IA:
Lin28-let7 modulates radiosensitivity of human cancer cells with
activation of K-Ras. Int J Radiat Oncol Biol Phys. 76:5–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu S, Kumar SM, Lu H, Liu A, Yang R,
Pushparajan A, Guo W and Xu X: MicroRNA-9 up-regulates E-cadherin
through inhibition of NF-κB1-Snail1 pathway in melanoma. J Pathol.
226:61–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma L, Young J, Prabhala H, Pan E, Mestdagh
P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S,
et al: miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin
and cancer metastasis. Nat Cell Biol. 12:247–256. 2010.PubMed/NCBI
|
|
12
|
Hu X, Schwarz JK, Lewis JS Jr, Huettner
PC, Rader JS, Deasy JO, Grigsby PW and Wang X: A microRNA
expression signature for cervical cancer prognosis. Cancer Res.
70:1441–1448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rodriguez-Otero P, Román-Gómez J,
Vilas-Zornoza A, José-Eneriz ES, Martín-Palanco V, Rifón J, Torres
A, Calasanz MJ, Agirre X and Prosper F: Deregulation of FGFR1 and
CDK6 oncogenic pathways in acute lymphoblastic leukaemia harbouring
epigenetic modifications of the MIR9 family. Br J Haematol.
155:73–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wild JR, Staton CA, Chapple K and Corfe
BM: Neuropilins: Expression and roles in the epithelium. Int J Exp
Pathol. 93:81–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brieger J, Schroeder P, Gosepath J and
Mann WJ: VEGF-subtype specific protection of SCC and HUVECs from
radiation induced cell death. Int J Mol Med. 15:145–151.
2005.PubMed/NCBI
|
|
16
|
Glinka Y, Mohammed N, Subramaniam V, Jothy
S and Prud'homme GJ: Neuropilin-1 is expressed by breast cancer
stem-like cells and is linked to NF-κB activation and tumor sphere
formation. Biochem Biophys Res Commun. 425:775–780. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong JC, Gao H, Zuo SY, Zhang HQ, Zhao G,
Sun SL, Han HL, Jin LL, Shao LH, Wei W and Jin SZ: Neuropilin 1
expression correlates with the Radio-resistance of human
non-small-cell lung cancer cells. J Cell Mol Med. 19:2286–2295.
2015.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hershkovitz-Rokah O, Modai S,
Pasmanik-Chor M, Toren A, Shomron N, Raanani P, Shpilberg O and
Granot G: MiR-30e induces apoptosis and sensitizes K562 cells to
imatinib treatment via regulation of the BCR-ABL protein. Cancer
Lett. 356:597–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong TM, Chen YL, Wu YY, Yuan A, Chao YC,
Chung YC, Wu MH, Yang SC, Pan SH, Shih JY, et al: Targeting
neuropilin 1 as an antitumor strategy in lung cancer. Clin Cancer
Res. 13:4759–4768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu T, Liu X, Han L, Shen H, Liu L and Shu
Y: Up-regulation of miR-9 expression as a poor prognostic biomarker
in patients with non-small cell lung cancer. Clin Transl Oncol.
16:469–475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wan HY, Guo LM, Liu T, Liu M, Li X and
Tang H: Regulation of the transcription factor NF-kappaB1 by
microRNA-9 in human gastric adenocarcinoma. Mol Cancer. 9:162010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu M, Xu Y, Ge M, Gui Z and Yan F:
Regulation of UHRF1 by microRNA-9 modulates colorectal cancer cell
proliferation and apoptosis. Cancer Sci. 106:833–839. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Zhang W, Zuo Y, Ding M, Ke C, Yan
R, Zhan H, Liu J and Wang J: miR-9 promotes cell proliferation and
inhibits apoptosis by targeting LASS2 in bladder cancer. Tumour
Biol. 36:9631–9640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu SW, Li JP, Ma XL, Ma JX, Yang Y, Chen
Y and Liu W: miR-9 modulates osteosarcoma cell growth by targeting
the GCIP tumor suppressor. Asian Pac J Cancer Prev. 16:4509–4513.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen P, Price C, Li Z, Li Y, Cao D, Wiley
A, He C, Gurbuxani S, Kunjamma RB, Huang H, et al: miR-9 is an
essential oncogenic microRNA specifically overexpressed in mixed
lineage leukemia-rearranged leukemia. Proc Natl Acad Sci USA.
110:pp. 11511–11516. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao HM, Wei W, Sun YH, Gao JH, Wang Q and
Zheng JH: MicroRNA-9 promotes tumorigenesis and mediates
sensitivity to cisplatin in primary epithelial ovarian cancer
cells. Tumour Biol. 36:6867–6873. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo LM, Pu Y, Han Z, Liu T, Li YX, Liu M,
Li X and Tang H: MicroRNA-9 inhibits ovarian cancer cell growth
through regulation of NF-kappaB1. FEBS J. 276:5537–5546. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Yang B, Han L, Li X, Tao H, Zhang
S and Hu Y: Demethylation of miR-9-3 and miR-193a genes suppresses
proliferation and promotes apoptosis in non-small cell lung cancer
cell lines. Cell Physiol Biochem. 32:1707–1719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murga M, Fernandez-Capetillo O and Tosato
G: Neuropilin-1 regulates attachment in human endothelial cells
independently of vascular endothelial growth factor receptor-2.
Blood. 105:1992–1999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawakami T, Tokunaga T, Hatanaka H, Kijima
H, Yamazaki H, Abe Y, Osamura Y, Inoue H, Ueyama Y and Nakamura M:
Neuropilin 1 and neuropilin 2 co-expression is significantly
correlated with increased vascularity and poor prognosis in
nonsmall cell lung carcinoma. Cancer. 95:2196–2201. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yahyanejad S, Theys J and Vooijs M:
Targeting Notch to overcome radiation resistance. Oncotarget.
7:7610–7628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao L, Lu X and Cao Y: MicroRNA and
signal transduction pathways in tumor radiation response. Cell
Signal. 25:1625–1634. 2013. View Article : Google Scholar : PubMed/NCBI
|