Introduction
Despite a recent decline in the occurrence of
gastric cancer worldwide, it remains one of the most common
malignant tumors in China, and the second and third cause of
morbidity and mortality in 2010, respectively (1). Great progress has been made in the
treatment of gastric cancer; however, the survival rate in patients
with gastric cancer remains low. Recently target-orientated
therapies have become one of the hot topics in cancer-related
research, but such studies on gastric cancer remain rare.
Distant organ metastasis is a sign of poor prognosis
in patients with gastric cancer. Liver is a common target organ of
gastric cancer metastasis (2).
Hepatic stellate cells (HSCs) are a type of liver-specific
mesenchymal cells. HSCs are postulated as a component of the
prometastatic liver microenvironment; HSCs can be activated by
tumor-derived factors to then promote the metastatic growth of
tumor cells (3). A previous study has
demonstrated that the expression of metastasis-associated in colon
cancer 1 (MACC1) is significantly higher in activated HSCs
(4). MACC1 is reported to be
associated with distant metastasis in gastric cancer (5). MACC1 enhances migration, invasion and
metastasis of cancer cells by activating the hepatocyte growth
factor (HGF)/MET proto-oncogene (c-Met) signaling pathway (6). Overexpression of MACC1 increases
invasion in cancer cells by enhancing epithelial-mesenchymal
transition (EMT) (7), and is
significantly correlated to decreased overall survival (OS) and
disease-free survival (DFS) (8).
These previous studies, therefore, suggest that MACC1 may have a
significant role in promoting tumor metastases.
A previous study from our group has demonstrated
that MACC1 is highly expressed in activated HSCs, and that MACC1
suppression decreased the expression of HGF/c-Met and the progress
of EMT, which suggested that MACC1 may be involved in HSCs
activation and promotion of metastatic growth. In the present
study, expression of MACC1, HGF and c-Met were detected in both
human gastric cancer tissues (GTs) and adjacent normal tissues
(ATs) by immunohistochemistry (IHC), and then the relationship of
protein expression for these three proteins and the
clinicopathological parameters and clinical outcomes of the tumors
were statistically analyzed. Furthermore, MACC1 overexpressing
lentiviral vectors were used to infect HSCs, and to detect the
effect of MACC1-overexpressing HSCs on the migration and invasion
of gastric carcinoma cells.
Materials and methods
Patient tissue samples
Pathology-confirmed, primary gastric cancer tissue
samples (n=129) and their adjacent non-tumor tissues (n=129) were
acquired from patients whose tumors were removed thoroughly at the
Affiliated Hospital of Xuzhou Medical University (Xuzhou, China)
from January 2009 through December 2010. None of the patients
received chemotherapy or radiotherapy prior to surgical resection
and collection of samples. All the patients were successfully
followed-up for 5 years. The Ethics Review Committee of Xuzhou
Medical University (Xuzhou, China) approved this study, and
informed consent was obtained from all patients.
IHC
Expression of MACC1, HGF and c-Met in GTs and ATs
was detected by IHC. Specimens were fixed by 4% formaldehyde
solution for a week at room temperature and embedded in paraffin
and 4 µm sections were prepared. Antigen recovery was performed by
boiling the sections in citrate buffer (pH=6.0) for 2 min and
subsequent cooling at room temperature (RT). To deactivate
endogenous peroxidases, 3% H2O2 was added.
Goat serum (OriGene Technologies, Inc., Beijing, China) was used
for blocking at RT for 15 min. Antibodies (all from Abcam,
Cambridge, MA, USA) targeting MACC1 (rabbit polyclonal; cat. no.
ab106579; 1:500), HGF (rabbit polyclonal; cat. no. ab83760; 1:100)
and c-Met (rabbit monoclonal; cat. no. ab51067; 1:250) were added
to the sections and incubated at 4°C overnight. Histostain-Plus kit
(OriGene Technologies, Inc.) was used for primary antibody
detection, according to the manufacturer's instructions. Positive
cells were visualized by 3,3′-diaminobenzidine staining. As a
control, some sections were incubated with non-immune goat serum in
place of the primary antibodies.
All the sections were observed by two experienced
pathologists separately using Olympus CX31 (Olympus Corporation,
Tokyo, Japan). For conflict diagnosis, a third opinion was pursued.
For each section, 10 non-contiguous optical fields (magnification,
×400) were randomly captured, 100 cells from each field were
observed for % calculation, and finally the mean of the 10 fields
per section was acquired. The quantification criteria were as
follows: i) Degree of staining: 0 points (negative staining), 1
point (yellow), 2 points (brown), 3 points (tan); ii) percentage of
stained cells: 0 points (no positive cells), 1 point (≤10%), 2
points (11–50%), 3 points (51–75%), 4 points (>75%). If the
product of these two scores was >3, it was considered as
positive.
Cell lines and cell culture
The normal HSC line LX2 was donated by Jiangsu Key
Laboratory of Immunity and Metabolism (Xuzhou, China). The human
gastric cells lines MKN45 (poorly differentiated adenocarcinoma)
and MKN74 (moderately differentiated adenocarcinoma) were donated
by the Tumor Laboratory of Nanjing Medical University (Nanjing,
China) and Shanghai Jiao Tong University (Shanghai, China),
respectively. All cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% mycoplasma-free fetal
bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.),
penicillin (100 U/ml) and streptomycin (100 µg/ml), and incubated
at 37°C in 5% CO2.
Lentiviral infection
LX2 cells were seeded into 6-well plates and
incubated at 37°C with 5% CO2 overnight. The lentiviral
vector overexpressing MACC1, LV5-MACC1, and its scrambled negative
control, LV5-NC, were synthesized by GenePharma Co., Ltd.
(Shanghai, China). LX2 cells were infected with 200 µl
(1×108 TU/ml) LV5-MACC1 or 100 µl (2×108
TU/ml) LV5-NC, according to the manufacturer's instructions.
Western blotting
For western blotting, cell lysates were prepared
using radioimmunoprecipitation assay buffer and PMSF (1:100) (both
from Beyotime Institute of Biotechnology, Shanghai, China). Protein
concentrations were measured using the bicinchoninic acid assay
(Beyotime Institute of Biotechnology), according to the
manufacturer's instructions. Proteins (30 µg/lane) were separated
with 10 or 7.5% SDS-PAGE and transferred to a nitrocellulose
membrane. The membrane was blocked with 5% skimmed milk for 2 h at
room temperature, then incubated with primary antibodies against
MACC1 (rabbit polyclonal; cat. no. ab106579; 1:2,000; Abcam),
α-smooth muscle actin (goat polyclonal; cat. no. ab21027; 1:1,000),
HGF (rabbit polyclonal; cat. no. ab83760; 1:1,000) (both from
Abcam), matrix metallopeptidase (MMP)-2 (rabbit polyclonal; cat.
no. 4022S; 1:1,000), MMP-9 (rabbit polyclonal; cat. no. 3852S;
1:1,000) (both from Cell Signaling Technology, Inc., Danvers, MA,
USA) and β-actin (mouse monoclonal; cat. no. TA-09; 1:4,000;
ZSGB-Bio, Beijing, China) at 4°C overnight, followed by secondary
antibody (goat anti-mouse, cat. no. 925-68020; goat anti-rabbit,
cat. no. 925-68021; donkey anti-goat, cat. no. 926-68024; 1:10,000;
LI-COR Biosciences, Lincoln, NE, USA) incubation for 2 h at room
temperature. Results were detected using an Odyssey scanner (LI-COR
Biosciences) and were analyzed with ImageJ (1.6.024; National
Institutes of Health, Bethesda, MD, USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using the Total RNA
Extraction kit (Tiangen Biotech Co., Ltd., Beijing, China),
according to the manufacturer's instructions. RNA concentration was
determined by UV spectrophotometry, genomic DNA was removed and RNA
was converted to cDNA using PrimeScript™ RT Reagent kit with gDNA
Eraser (cat. no. RR047A; Takara Bio, Inc., Otsu, Japan). qPCR was
performed using the SYBR® Premix Dimer Eraser (cat. no.
RR091A; Takara Bio, Inc.) on a 7900HT Fast Real-Time PCR system
(Thermo Fisher Scientific, Inc.). The PCR conditions were as
follows: 95°C for 30 sec; 40 cycles at 95°C for 5 sec and 55°C for
30 sec 72°C for 34 sec; melt curve: 95°C for 15 sec, 60°C for 1
min, 95°C for 15 sec. The sequence-specific primer pairs were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) and are
listed in Table I. β-actin was used
as an internal control. Relative quantification was calculated
using the comparative threshold cycle (ΔΔCq) method (9). To exclude any potential contamination,
negative controls were also performed with dH2O instead
of cDNA during each run. No amplification product was detected in
the negative controls. qPCR reactions were run at least three times
for each sample.
 | Table I.Sequences of primers used for
polymerase chain reaction analysis. |
Table I.
Sequences of primers used for
polymerase chain reaction analysis.
| Gene | GenBank ID | Primer | Sequence (5′-3′) | Product size
(bp) |
|---|
| MACC1 | NM_182762.3 | Forward |
TGGACATTTTAGACGACACAGC | 238 |
|
|
| Reverse |
CCTCCTTGATGGTTTACTTTGC |
|
| α-SMA | NM_001100.3 | Forward |
ATGTGCGACGAAGACGAGAC | 156 |
|
|
| Reverse |
TTTCTGACCCATACCGACCA |
|
| HGF | NM_000601.4 | Forward |
CGAGGGAAGGTGACTCTGAA | 154 |
|
|
| Reverse |
CACATCCACGACCAGGAAC |
|
| MMP-2 | NM_004530.4 | Forward |
TATGGCTTCTGCCCTGAGAC | 142 |
|
|
| Reverse |
CACACCACATCTTTCCGTCA |
|
| MMP-9 | NM_004994.2 | Forward |
AGTCCACCCTTGTGCTCTTC | 117 |
|
|
| Reverse |
ACTCTCCACGCATCTCTGC |
|
| β-actin | NM_001101.3 | Forward |
CTTAGTTGCGTTACACCCTTTC | 154 |
|
|
| Reverse |
GTCACCTTCACCGTTCCAGT |
|
Cell invasion and migration
assays
LX2 cells infected with LV5-MACC1 or LV5-NC were
harvested following puromycin (0.05 µg/ml) selection. Gastric
adenocarcinoma cell lines MKN45 and MKN74 were harvested for the
cell invasion assays. For the invasion assays, 24-well 8.0 µm pore
Transwells (Corning Inc., Corning, NY, USA) coated with fibronectin
(30 µl/well; BD Biosciences, Franklin Lakes, NJ, USA) were used,
according to the manufacturer's instructions. MKN45 or MKN74 cells
in serum-free DMEM (200 µl of a 1×105/ml cell
suspension) were seeded in the upper chambers of the Transwells.
HSCs infected with LV5-MACC1 or LV5-NC in 10% FBS/DMEM (600 µl of a
1×105/ml cell suspension) were seeded in the bottom
chambers coated with Matrigel (25 µl/well; BD Biosciences). After
24 h of incubation, cells remaining on the top side of the membrane
were removed with a cotton swab, while cells on the bottom side of
the membrane were fixed in methanol and stained with 0.1% crystal
violet (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Invading
cells were observed by Olympus CX31 and counted using ImageJ. For
each section, 10 non-contiguous optical fields (magnification,
×200) were randomly captured.
The migration assay was performed in the same manner
as the invasion assay, but with uncoated Transwells and for a 12 h
incubation period.
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for
all data analyses. The results were presented as the mean ±
standard deviation. Pearson, χ2 and Cox methods were
used for analyzing the relationship between protein expression and
clinicopathological parameters. One-way ANOVA with Student's t-test
method for homogeneity of variance and Welch with Dunnett-t3 method
for missing variance were performed for analysis among groups.
P<0.05 was considered to indicate a statistically significant
difference. All graphs were generated using GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
MACC1 and HGF expression are
correlated with survival in gastric cancer patients
To investigate MACC1, HGF and c-Met expression in
gastric cancer, IHC analysis was performed on matched tumor tissues
(GTs) and adjacent normal tissues (ATs) from 129 patients with
gastric cancer. Among these 129 cases, 89 were male and 40 were
female. MACC1 was positively expressed in 104 GTs (80.6%) and 41
ATs (31.8%); HGF was positively expressed in 103 GTs (79.8%) and 59
ATs (45.7%); c-Met was positively expressed in 107 GTs (82.9%) and
32 ATs (24.8%). Overall, the number of MACC1, HGF or c-Met-positive
patients was significantly higher in the GTs compared with the ATs
(P<0.05; Table II). In GTs, the
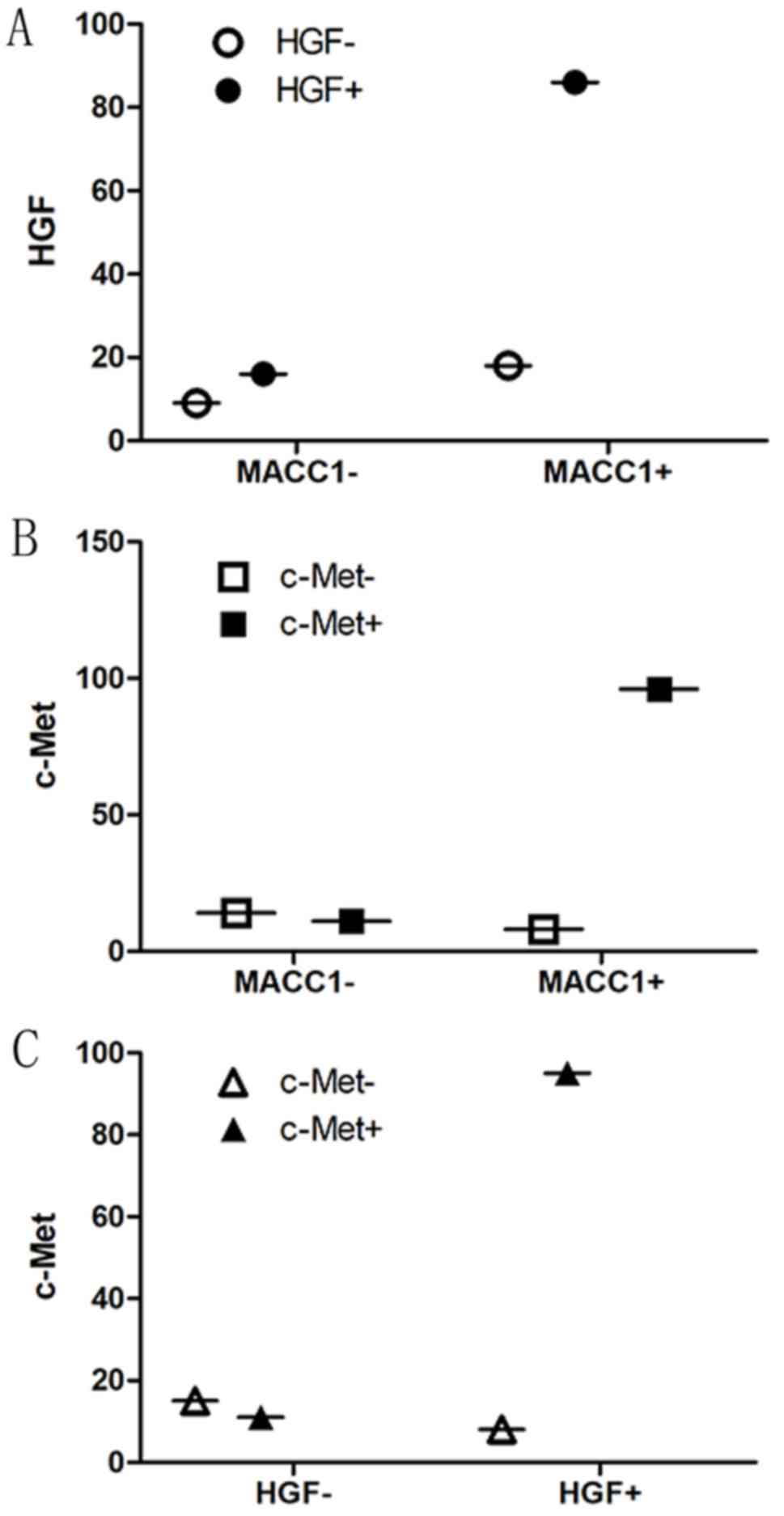
number of MACC1-positive patients was significantly correlated with
the number of HGF-positive patients (r=0.182, P=0.039) and the
number of c-Met-positive patients (r=0.508, P<0.001). Similarly,
the number of HGF-positive patients was significantly correlated
with the number of c-Met-positive patients (r=0.523, P<0.001;
Fig. 1). The present results
indicated that the expression levels of MACC1, HGF and c-Met are
higher in gastric cancer tissues compared with adjacent normal
tissues, and that they are significantly related to each other in
gastric cancer.
 | Table II.Correlation analysis between positive
expression of MACC1, HGF and c-Met and the clinicopathological
parameters of gastric tumors. |
Table II.
Correlation analysis between positive
expression of MACC1, HGF and c-Met and the clinicopathological
parameters of gastric tumors.
|
|
| MACC1 expression |
| HGF expression |
| c-Met expression |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Patients, n | − | + | P-value | − | + | P-value | − | + | P-value |
|---|
| Sex |
|
|
| 0.071 |
|
| 0.328 |
|
| 0.677 |
| Male | 89 | 21 | 68 |
| 20 | 69 |
| 16 | 73 |
|
|
Female | 40 | 4 | 36 |
| 6 | 34 |
| 6 | 34 |
|
| Age, years |
|
|
| 0.284 |
|
| 0.965 |
|
| 0.172 |
|
<60 | 65 | 15 | 50 |
| 13 | 52 |
| 14 | 51 |
|
|
≥60 | 64 | 10 | 54 |
| 13 | 51 |
| 8 | 56 |
|
| Tumor size, cm |
|
|
| 0.425 |
|
| 0.006 |
|
| 0.291 |
|
<5 | 63 | 14 | 49 |
| 19 | 44 |
| 13 | 50 |
|
| ≥5 | 66 | 11 | 55 |
| 7 | 59 |
| 9 | 57 |
|
| Invasion depth |
|
|
| 0.001 |
|
| <0.001 |
|
| <0.001 |
|
T1+T2 | 29 | 12 | 17 |
| 14 | 15 |
| 14 | 15 |
|
|
T3+T4 | 100 | 13 | 87 |
| 12 | 88 |
| 8 | 92 |
|
| Lymph
metastasis |
|
|
| 0.144 |
|
| <0.001 |
|
| <0.001 |
| − | 41 | 11 | 30 |
| 16 | 25 |
| 14 | 27 |
|
| + | 88 | 14 | 74 |
| 10 | 78 |
| 8 | 80 |
|
| Periconeal
metastasis |
|
|
| 0.279 |
|
| 0.070 |
|
| 0.383 |
| − | 113 | 24 | 89 |
| 26 | 87 |
| 21 | 92 |
|
| + | 16 | 1 | 15 |
| 0 | 16 |
| 1 | 15 |
|
| TNM stage |
|
|
| 0.009 |
|
| 0.001 |
|
| <0.001 |
|
I+II | 48 | 15 | 33 |
| 17 | 31 |
| 17 | 31 |
|
|
III+IV | 81 | 10 | 71 |
| 9 | 72 |
| 5 | 76 |
|
| Differentiation
level |
|
|
| 0.009 |
|
| 0.005 |
|
| 0.005 |
|
High | 53 | 16 | 37 |
| 17 | 36 |
| 15 | 38 |
|
|
Low | 76 | 9 | 67 |
| 9 | 67 |
| 7 | 69 |
|
| Location |
|
|
| 0.391 |
|
| 0.566 |
|
| 0.533 |
|
Cardia-fundus+gastric
body | 51 | 8 | 43 |
| 9 | 42 |
| 10 | 41 |
|
|
Antrum | 78 | 17 | 61 |
| 17 | 61 |
| 12 | 66 |
|
| OS, years |
|
|
| 0.381 |
|
| 0.001 |
|
| 0.003 |
|
<5 | 72 | 12 | 60 |
| 7 | 65 |
| 6 | 66 |
|
| ≥5 | 57 | 13 | 44 |
| 19 | 38 |
| 16 | 41 |
|
| DFS, years |
|
|
| 0.292 |
|
| 0.000 |
|
| 0.002 |
|
<5 | 74 | 12 | 62 |
| 7 | 67 |
| 6 | 68 |
|
| ≥5 | 55 | 13 | 42 |
| 19 | 36 |
| 16 | 39 |
|
| Expression |
|
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
| Tumor
tissues | 129 | 25 | 104 |
| 26 | 103 |
| 22 | 107 |
|
|
Adjacent normal tissues | 129 | 88 | 41 |
| 70 | 59 |
| 97 | 32 |
|
Next, the relationship of positive expression for
the MACC1, HGF and c-Met proteins and the various
clinicopathological parameters of the gastric cancer patients were
analyzed. The results of the statistical analysis are presented in
Table II. Positive expression of
MACC1 was significantly higher in T3+T4 GTs compared with T1+T2 GTs
(P=0.001). Positive expression of MACC1 in III+IV grade GTs was
significantly higher compared with grade I+II GTs (P=0.009).
Positive expression of MACC1 was significantly higher in low
differentiated GTs compared with well differentiated GTs (P=0.009).
In addition, positive expression of HGF and c-Met was significantly
increased in GTs with T3+T4 stage, positive lymph node metastasis,
III+IV grade, low differentiation status, and poor OS (<5 years)
and DFS (<5 years), compared with GTs of T1+T2 stage, no lymph
node metastasis, I+II grade, well-differentiated status, and good
OS (>5 years) and DSF (>5 years), respectively (Table II). The present data indicated that
high HGF and c-Met expression levels were associated with
metastasis, life span, invasion depth, TNM stage and
differentiation level; MACC1 expression levels were associated with
invasion depth, TNM stage and differentiation level, but not
significantly associated with metastasis and life span.
MACC1 overexpression activates
HSCs
The overexpression of MACC1 in lentivirally
transfected cells was confirmed by western blotting (data not
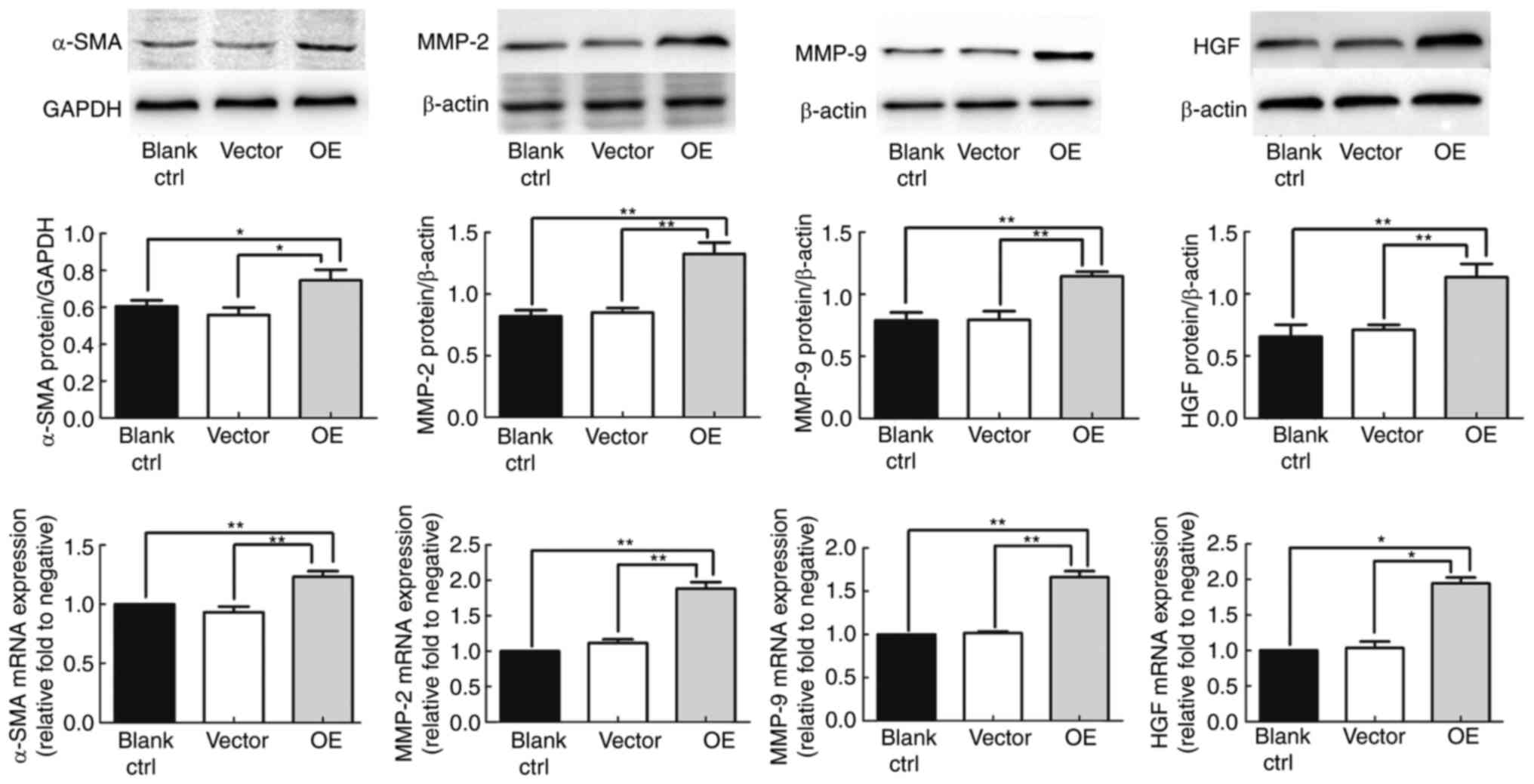
shown). To examine the effect of MACC1 on LX2 HSCs, the protein and
mRNA expression levels of α-SMA were detected. α-SMA is a
well-recognized marker of HSC activation (10). The results demonstrated that α-SMA
protein was expressed in LX2 HSCs (non-infected blank group) and in
LV5-NC-infected HSCs (vector negative control group), but was
significantly overexpressed in LV5-MACC1-infected HSCs (OE group);
the mRNA expression levels of α-SMA were also significantly higher
in the OE group compared with the blank and vector groups
(P<0.01; Fig. 2). No significant
difference was observed between the blank and the vector group
(P>0.05; Fig. 2). The present data
suggested that MACC1 stimulated the α-SMA expression and thus
activation of the LX2 HSCs.
MACC1 overexpression upregulates the
levels of MMP-2 and MMP-9 in HSCs
MMP-2 and MMP-9 are involved in extracellular matrix
(ECM) degradation and vascularization, processes that contribute to
tumor metastasis. The results demonstrated that the mRNA and
protein expression levels of MMP-2 and MMP-9 were significantly
increased in the OE group compared with the blank and vector groups
(P<0.01; Fig. 2). No significant
difference was observed between the blank and the vector group
(P>0.05; Fig. 2). These results
indicated that MACC1 overexpression resulted in upregulated levels
of MMP-2 and MMP-9 in LX2 HSCs.
MACC1 overexpression increases the
expression of HGF in HSCs
The expression of HGF was detected in LX2 HSCs
(blank), LV5-NC-infected HSCs (vector) and LV5-MACC1-infected HSCs
(OE). The results demonstrated that the HGF protein expression
levels were significantly increased in the OE group compared with
the blank and vector group (P<0.01; Fig. 2); similarly, the mRNA expression
levels were also increased in the OE group compared with the blank
and the vector group (P<0.05; Fig.
2). No significant difference was observed between the blank
and the vector group (P>0.05; Fig.
2). The present data suggested that MACC1 overexpression
results in increased HGF expression in LX2 HSCs.
MACC1 overexpression enhances
migration and invasion in gastric cancer cells
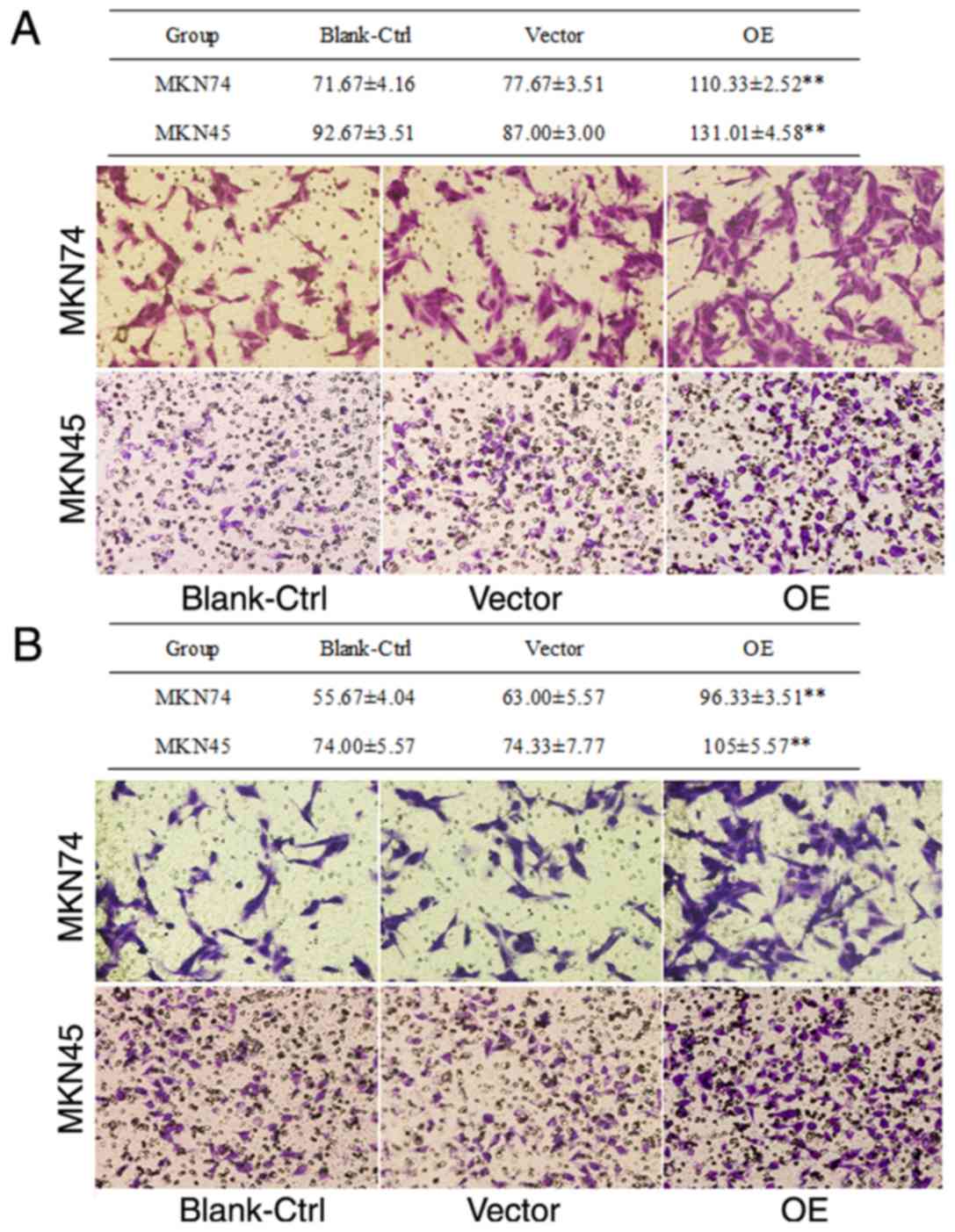
To determine the effect of the activated HSCs on the
invasion and migration potential of tumor cells, Transwell assays
were performed. Migration and invasion assay results demonstrated
that the number of MKN45 or MKN74 cells that crossed the membrane
were significantly higher in the assays where the cells migrated
towards the OE HSC group compared with the blank or vector groups
(P<0.01; Fig. 3). Of note, the
number of MKN45 cells that crossed the membrane was significantly
higher compared with that of MKN74 cells (P<0.01; Fig. 3). No significant difference was
observed between the blank group and the vector group (P>0.05;
Fig. 3). The present data suggested
that MACC1-overexpressing activated HSCs promoted migration and
invasion of gastric tumor cells, with the effect being more
prominent in poorly differentiated tumor cells.
Discussion
MACC1 was first reported by Stein et al
(11) as an oncogene regulating colon
cancer metastasis, but MACC1 has been since reported to be highly
expressed in several types of cancer cells (12,13). The
expression levels of MACC1 have been demonstrated to be
significantly associated with peritoneal metastasis and TNM stage
(14), as well as invasion depth and
α-fetoprotein levels (15).
Therefore, MACC1 has been suggested as a potential biomarker for
metastasis and invasion of colorectal cancer and primary
hepatocellular carcinoma (16). High
levels of MACC1 are also associated with lymph node metastasis and
TNM stage in esophageal cancer, as well as a lower OS and a higher
risk of death (17). The present
study demonstrated that positive expression of MACC1 was
significantly higher in GTs compared with ATs, and significantly
correlated with invasion depth, TNM stage and differentiation
status. These results implied that MACC1 might accelerate the
progression and metastasis of gastric cancer and may serve as a new
parameter for the prognostic prediction of gastric cancer.
Metastasis and recurrence are the main causes of
death in patients with gastric cancer (5). Metastases to distant areas, resulting
from primary gastric cancer, are localized mainly in the liver.
α-SMA-positive HSCs were described in human hepatocellular
carcinoma and in liver metastases from primary gastric cancer
(18). In the present study, first it
was confirmed that MACC1 overexpression could activate HSCs, by
upregulating α-SMA expression. Activated HSCs displayed increased
expression levels of MMP-2 and MMP-9, which are involved in ECM
degradation and vascularization, allowing cancer cells to migrate
out of the primary tumor and to form metastases (19). The present results are consistent with
Chen et al (20), that
reported that MACC1 downregulation inhibited α-SMA, MMP-2, and
MMP-9 mRNA or protein expression levels following transfection of
endometrial carcinoma cells with MACC1 small interfering RNA. In
addition, the present study used HSCs as a chemoattractant source
for gastric cancer cells in Transwell assays. The results
demonstrated that the number of MKN45 or MKN74 gastric cancer cells
migrating through the membrane towards MACC1-overexpressing HSCs
was significantly increased compared with non-activated HSCs.
Furthermore, the number of migratory and invasive MKN45 cells was
significantly higher than MKN74 cells. Consistent with the present
results, knockdown of MACC1 has been reported to significantly
suppress cell migration and invasion in melanoma cells (21). Thus, it is hypothesized that HSCs,
activated by MACC1 overexpression, may promote migration and
invasion of gastric cancer and this effect may be more pronounced
in poorly differentiated cancer cells.
Further investigation may reveal the effects of
MACC1 on HGF and c-Met expression. Both HGF and c-Met are
associated with progression, metastasis and survival in gastric
cancer (22). Previous studies have
demonstrated that MACC1, similar with c-Met, is upregulated in
hepatocellular carcinoma, and that the mRNA levels of MACC1 and
c-Met are significantly associated (23). In the present study, analysis of
gastric cancer clinical data demonstrated that positive expression
of MACC1, HGF and c-Met was significantly higher in GTs compared
with ATs. This result is consistent with a previous study (24). Expression of HGF was also detected in
LX2 HSCs, LV5-NC-infected HSCs and LV5-MACC1-infected HSCs. The
results demonstrated that both the protein and mRNA levels of HGF
were increased in the MACC1-overexpressing cells. Therefore, this
data suggested that MACC1 may increase the expression of HGF.
Preclinical models have demonstrated that activation of MET
signaling by HGF in gastric cancer cell lines promotes
tumorigenesis and metastasis (25).
Taken together, these results suggest that the effect of MACC1 on
migration and invasion may occur through the HGF/c-Met signaling
pathway.
In conclusion, the present investigation
demonstrated that MACC1 and HGF expression were associated with
survival in gastric cancer patients. MACC1 may promote the
progression, metastasis and poor outcome of gastric cancer, through
activation of the HGF/c-Met signaling pathway. Therefore, MACC1,
HGF and c-Met may serve as potential cancer biomarkers in gastric
cancer. Identification of inhibitors for the HGF/c-Met pathway
might contribute to the therapy of gastric cancer. The present
study might provide basic evidence for the diagnosis, therapy and
prognosis assessment of gastric cancer, and may be helpful for the
development of novel drug targets and biomarkers.
Acknowledgements
This study was supported by the Natural Science Fund
Project of Colleges in Jiangsu (grant no. 16KJB340002), the Jiangsu
Health Bureau Project (grant no. H201323), the Xuzhou Science and
Technology Plan Project (grant no. KC15SM047), and the Xuzhou
Medical University Scientific Research Fund for Talents (grant nos.
D2016004 and D2016005).
References
|
1
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H
and Zou X: Report of cancer incidence and mortality in China, 2010.
Ann Transl Med. 2:612014.PubMed/NCBI
|
|
2
|
Fock KM: Review article: The epidemiology
and prevention of gastric cancer. Aliment Pharmacol Ther.
40:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mikuriya Y, Tashiro H, Kuroda S, Nambu J,
Kobayashi T, Amano H, Tanaka Y and Ohdan H: Fatty liver creates a
pro-metastatic microenvironment for hepatocellular carcinoma
through activation of hepatic stellate cells. Int J Cancer.
136:E3–E13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Q, Shan H and Zhu Z: Regulation of
epithelial-mesenchymal transition by MACC1 via the HGF/c-Met
signaling pathway and its effects on ability of migration and
invasion of gastric carcinoma cells. J Shanghai Jiaotong Univ (Med
Sci). 34:1325–1331. 2014.
|
|
5
|
Xie QP, Xiang C, Wang G, Lei KF and Wang
Y: MACC1 upregulation promotes gastric cancer tumor cell metastasis
and predicts a poor prognosis. J Zhejiang Univ Sci B. 17:361–366.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stein U, Dahlmann M and Walther W:
MACC1-more than metastasis? Facts and predictions about a novel
gene. J Mol Med (Berl). 88:11–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Wu Y, Lin L, Liu P, Huang H, Liao
W, Zheng D, Zuo Q, Sun L, Huang N, et al: Metastasis-associated in
colon cancer-1 upregulation predicts a poor prognosis of gastric
cancer, and promotes tumor cell proliferation and invasion. Int J
Cancer. 133:1419–1430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang G, Fu Z and Li D: MACC1
overexpression and survival in solid tumors: A meta-analysis.
Tumour Biol. 36:1055–1065. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Navidshad B, Liang JB and Jahromi MF:
Correlation coefficients between different methods of expressing
bacterial quantification using real time PCR. Int J Mol Sci.
13:2119–2132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobrevals L, Enguita M, Quiroga J, Prieto
J and Fortes P: Insulin-like growth factor I (IGF-I) expressed from
an AAV1 vector leads to a complete reversion of liver cirrhosis in
rats. PLoS One. 11:e01629552016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stein U, Walther W, Arlt F, Schwabe H,
Smith J, Fichtner I, Birchmeier W and Schlag PM: MACC1, a newly
identified key regulator of HGF-MET signaling, predicts colon
cancer metastasis. Nat Med. 15:59–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ge Y, Meng X, Zhou Y, Zhang J and Ding Y:
Positive MACC1 expression correlates with invasive behaviors and
postoperative liver metastasis in colon cancer. Int J Clin Exp Med.
8:1094–1100. 2015.PubMed/NCBI
|
|
13
|
Li H, Zhang H, Zhao S, Shi Y, Yao J, Zhang
Y, Guo H and Liu X: Overexpression of MACC1 and the association
with hepatocyte growth factor/c-Met in epithelial ovarian cancer.
Oncol Lett. 9:1989–1996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shirahata A, Shinmura K, Kitamura Y,
Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K,
Kigawa G, et al: MACC1 as a marker for advanced colorectal
carcinoma. Anticancer Res. 30:2689–2692. 2010.PubMed/NCBI
|
|
15
|
Shirahata A, Fan W, Sakuraba K, Yokomizo
K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, et
al: MACC 1 as a marker for vascular invasive hepatocellular
carcinoma. Anticancer Res. 31:777–780. 2011.PubMed/NCBI
|
|
16
|
Tang J, Chen JX, Chen L, Tang JY, Cui Z,
Liu CH and Wang Z: Metastasis associated in colon cancer 1 (MACC1)
promotes growth and metastasis processes of colon cancer cells. Eur
Rev Med Pharmacol Sci. 20:2825–2834. 2016.PubMed/NCBI
|
|
17
|
Zhu M, Xu Y, Mao X, Gao Y, Shao L and Yan
F: Overexpression of metastasis-associated in colon cancer-1
associated with poor prognosis in patients with esophageal cancer.
Pathol Oncol Res. 19:749–753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yanagihara K, Takigahira M, Kubo T, Ochiya
T, Hamaguchi T and Matsumura Y: Marked antitumor effect of NK012, a
SN-38-incorporating micelle formulation, in a newly developed mouse
model of liver metastasis resulting from gastric cancer. Ther
Deliv. 5:129–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rink M, Chun FK, Robinson B, Sun M,
Karakiewicz PI, Bensalah K, Fisch M, Scherr DS, Lee RK, Margulis V
and Shariat SF: Tissue-based molecular markers for renal cell
carcinoma. Minerva Urol Nefrol. 63:293–308. 2011.PubMed/NCBI
|
|
20
|
Chen S, Zong ZH, Wu DD, Sun KX, Liu BL and
Zhao Y: The role of metastasis-associated in colon cancer 1 (MACC1)
in endometrial carcinoma tumorigenesis and progression. Mol
Carcinog. 56:1361–1371. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding Y, Li X, Hong D, Jiang L, He Y and
Fang H: Silence of MACC1 decreases cell migration and invasion in
human malignant melanoma through inhibiting the EMT. Biosci Trends.
10:258–264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo T, Yang J, Yao J, Zhang Y, Da M and
Duan Y: Expression of MACC1 and c-Met in human gastric cancer and
its clinical significance. Cancer Cell Int. 13:1212013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiu J, Huang P, Liu Q, Hong J, Li B, Lu C,
Wang L, Wang J and Yuan Y: Identification of MACC1 as a novel
prognostic marker in hepatocellular carcinoma. J Transl Med.
9:1662011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Lin L, Chen X, Sun L, Liao Y,
Huang N and Liao W: Metastasis-associated in colon cancer-1
promotes vasculogenic mimicry in gastric cancer by upregulating
TWIST1/2. Oncotarget. 6:11492–11506. 2015.PubMed/NCBI
|
|
25
|
Toiyama Y, Yasuda H, Saigusa S, Matushita
K, Fujikawa H, Tanaka K, Mohri Y, Inoue Y, Goel A and Kusunoki M:
Co-expression of hepatocyte growth factor and c-Met predicts
peritoneal dissemination established by autocrine hepatocyte growth
factor/c-Met signaling in gastric cancer. Int J Cancer.
130:2912–2921. 2012. View Article : Google Scholar : PubMed/NCBI
|