Introduction
Ankylosing spondylitis (AS), a prototype of
spondyloarthropathy, is a chronic autoimmune disease, which
manifests in its early stageas inflammatory back pain, restricts
the movements as it progresses and may eventually lead to
completedisability (1–3). Ankylosis, resulting from ectopic
ossification of tendons and ligaments, is generally accepted to be
the pathological hallmark of AS. However, the underlying mechanism
is still under investigation (4).
Matrix metalloproteinases (MMPs) are a family of
proteins that play an important role in the development of
inflammatory and immune diseases as well as in damaging cartilage
and bone (5,6). MMPs are structurally and functionally
related proteinases that share significant homology in their
cytoplasmic domains (7). MMPs are
responsible for the proteolytic degradation of the extracellular
matrix (8). MMP-2 was up-regulated in
numerous inflammatory processes and was involved in the degradation
and remodelling of extracellular matrix (9,10). We
speculated that elevated levels of MMP-2 protein expression were
likely to be associated with the development of AS, which is also
an inflammatory disease.
The principal aim of this study was to evaluate the
effect of silencing MMP-2 gene using siRNA technique, on the
MMP-2 expression levels in fibroblasts from patients with AS, and
also to investigate the effects of MMP-2 inhibition on the
activation of downstream signalling pathways. Endogenous siRNA is a
powerful tool that cells use to regulate developmental genes or
modify DNA and chromatin (5,11). In vitro inhibition of MMP-2
gene can be achieved by gene silencing.
Materials and methods
Primary fibroblast isolation and
culture
The research included 42 AS patients in Xinchang
People's Hospital between October 2014 and December 2015 at the
case group, which consisted of 31 males and 11 females. A total of
30 healthy volunteers who underwent routine physical examination in
the hospital were included as the control group, including 20 males
and 10 females. All the study subjects were aged from 20 to 43
years old, with the average of 31.18±6.28 (Table I). The diagnostic criteria for AS
patients complied with New York criteria (12). Basic clinical and pathological data of
these patients were collected with their written informed consents.
The present study was approved by the Ethics Committee of Xinchang
People's Hospital (Xinchang, China).
 | Table I.Clinical information of study
subjects. |
Table I.
Clinical information of study
subjects.
| Clinical items | Case group
(n=42) |
|---|
| Age of treatment
(median, years) | 26 |
| Age of onset (median,
years) | 21 |
| Course of disease
(median, years) | 5 |
| Male no. (%) | 31 (73.81) |
| Female no. (%) | 11 (26.19) |
| HLA-B27 positive rate
no. (%) | 36 (85.71) |
| Hip joint involvement
no. (%) | 12 (28.57) |
| Peripheral joints
involvement no. (%) | 20 (47.62) |
| Enthesitis no.
(%) | 8
(19.05) |
| Extra-articular
manifestations (iritis or urethritis) no. (%) | 2 (4.76) |
| Family history of
spondyloarthropathy no. (%) | 12 (28.57) |
| History of axial
joints and peripheral joints trauma no. (%) | 7
(16.67) |
| Bilateral
inflammation <II no. (%) | 5
(11.90) |
| Bilateral
inflammation in SIJ II no. (%) | 17 (40.48) |
| Bilateral or
unilateral inflammation in SIJ III no. (%) | 23 (54.76) |
| Bilateral l or
unilateral inflammation in SIJ IV no. (%) | 8
(19.05) |
Isolation and culture of both normal and AS
fibroblasts were carried out as previously reported (4,13).
Ligaments isolated from healthy volunteers and patients with AS,
were placed into sterile flasks containing DMEM/F-12 medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and cultured for 2 h at
4°C. The ligaments were washed with phosphate-buffered saline (PBS;
Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) to remove
any blood or attached tissue, and then cut into small blocks. After
centrifuged at 1,000 rpm for 5 min, the precipitated cells were
collected and cultured with Dulbecco's modified Eagle's
medium/Nutrient Mixture F-12 medium containing 1 mg/ml Type-I
collagenase (Beijing Solarbio Technology Co., Ltd., Beijing, China)
at 37°C for 4 h, incubated with 0.25% trypsin (Sinopharm Chemical
Reagent Co., Ltd.) for 15 min and shaken at 20 min intervals. The
ligament preparation was filtered with 200-mesh sieve (Shanghai
Yeasen Biotechnology Co., Ltd., Shanghai, China). The obtained
filtrate was centrifuged at 800 rpm for 5 min. The predicated cells
were re-suspended in DMEM/F-12 medium containing 20% fetal bovine
serum, 100 U/ml penicillin and 100 µg/ml streptomycin (Sinopharm
Chemical Reagent Co., Ltd.). The cells were seeded into plates at a
density of 1×103 cells per well. After approximately
12–15 days of culture at 37°C, a monolayer of fibroblasts was
obtained. The third-passage cells were used for the subsequent
experiments. Pictures of fibroblast morphology were captured using
a light microscope at a magnification of ×20 (Thermo Fisher
Scientific, Inc.).
Cell grouping and treatment
Fibroblasts isolated from patients with AS were
randomly divided into control, mock (empty vector transfected
controls) and siRNA-MMP-2 transfection group (vectors carrying
siRNA against MMP-2). Recombinant plasmids were obtained from
Nanjing Cobioer Biotechnology Co., Ltd. (Nanjing, China).
MTT assay
Fibroblasts in each group were seeded in 96-well
plates at a density of 2×103 cells per well and allowed
to attach overnight. Cells were cultured for 6 day(s), changing the
culture medium every 24 h. The celldensity was examined 6 times at
one-day interval using MTT assay (Shanghai Haling Biotechnology
Co., Ltd., Shanghai, China). In brief, the supernatant of each well
was removed at specific time points and replaced with 120 µl of MTT
solution (5 mg/ml in PBS) diluted 1:6 in medium, prior to use.
Cells were incubated in 5% CO2 incubator at 37°C for 3
h. Formazan, solubilized in 200 µl of DMSO (Sinopharm Chemical
Reagent Co., Ltd.), was added to the cultures and incubated at 37°C
for 5 min. Absorbance was read at 570 nm using a plate reader
(Thermo Fisher Scientific, Inc.). All experiments were performed in
triplicate. Cell growth rates were plotted.
RT-PCR
RNA was extracted by one-step TRIzol extraction as
per manufacture's instruction. The expression of MMP-2, Cbfa-1,
BMP-2, Smad1, Smad4 and p-Smad1/5/8 were determined by reverse
transcription. PCR primer pairs were designed based on the
sequences of different exons of the corresponding genes. All
primers in the study were designed by Shanghai Sangon Biotech Co.,
Ltd., Shanghai, China. The specific primer sequences for each gene
were listed as the follows: 5′TGT GTT GTC CAG AGG CAA TG3′ and
5′ATC ACT AGG CCA GCT GGT TG3′ for MMP-2 (product: 107 bp); 5′TCG
CCA GGC TTC ATA GCA AA3′ and 5′GGC CTT GGG TAA GGC AGA TT3′ for
Cbfa-1 (product: 170 bp); 5′CGC TGT CTT CTA GCG TTG CT3′ and 5′GGG
GTG GGT CTC TGT TTC AG3′ for BMP-2 (product: 191 bp); 5′ATT CGT GAG
TTC GCG GTT GA3′ and 5′CAC AGT TAC TCG GTT GCC CT3′ for Smad1
(product: 421 bp); 5′GCT GCA GAG CCC AGT TTA GA3′ and 5′CCC CAA AGC
AGA AGC TAC GA3′ for Smad4 (product: 147 bp); 5′GGC CGA GCT GCT AAT
AAA GTT G3′ and 5′AAA CAA GCT GGC CAT TGA CG3′ for p-Smad1/5/8
(product: 429 bp) and 5′GTC ATT CCA AAT ATG AGA TGC GT3′ and 5′GCT
ATC ACC TCC CCT GTG TG3′ for β-actin (product: 121 bp). The
reactions were incubated at 95°C for 10 min and amplified using the
following cycling parameters: 95°C for 10 sec, 58°C for 10 sec and
72°C for 30 sec. After totally 45 cycles, the primers were
elongated at 60°C for 1 min. The relative gene expression levels of
the target genes were analyzed through 2−ΔΔCq method.
β-actin was applied as the internal control to normalize the
expression level of each gene.
Western blotting
Total proteins were extracted with RIPA lysis buffer
containing 1 mM PMSF (Beijing Solarbio Technology Co., Ltd.). The
protein samples were boiled for 10 min in loading buffer and
subjected to SDS-PAGE/immunoblotting analysis. Separated protein
bands were transferred to polyvinylidene difluoride membranes
(Thermo Fisher Scientific, Inc.) at 25 V for 30 min. The membranes
were blocked with 5% skimmed milk powder in PBS. The membrane was
washed thrice with Tris buffered saline containing Tween-20 (TBST)
(Sinopharm Chemical Reagent Co., Ltd.), and incubated in secondary
antibodies diluted 1:1,000, at 37°C for 1 h. After incubation, it
was washed thrice, for 5 min each time, with TBST. Protein bands
were visualized using LumiPico® ECL Reagent.
Statistical analysis
All data were expressed as means ± standard
deviation (SD). Student's two-tailed t-test, and one-way ANOVA
followed by a Tukey's multiple comparison test were performed using
IBM SPSS version 20 for statistical comparisons. Statistical
significance was defined as P<0.05 and P<0.01.
Results
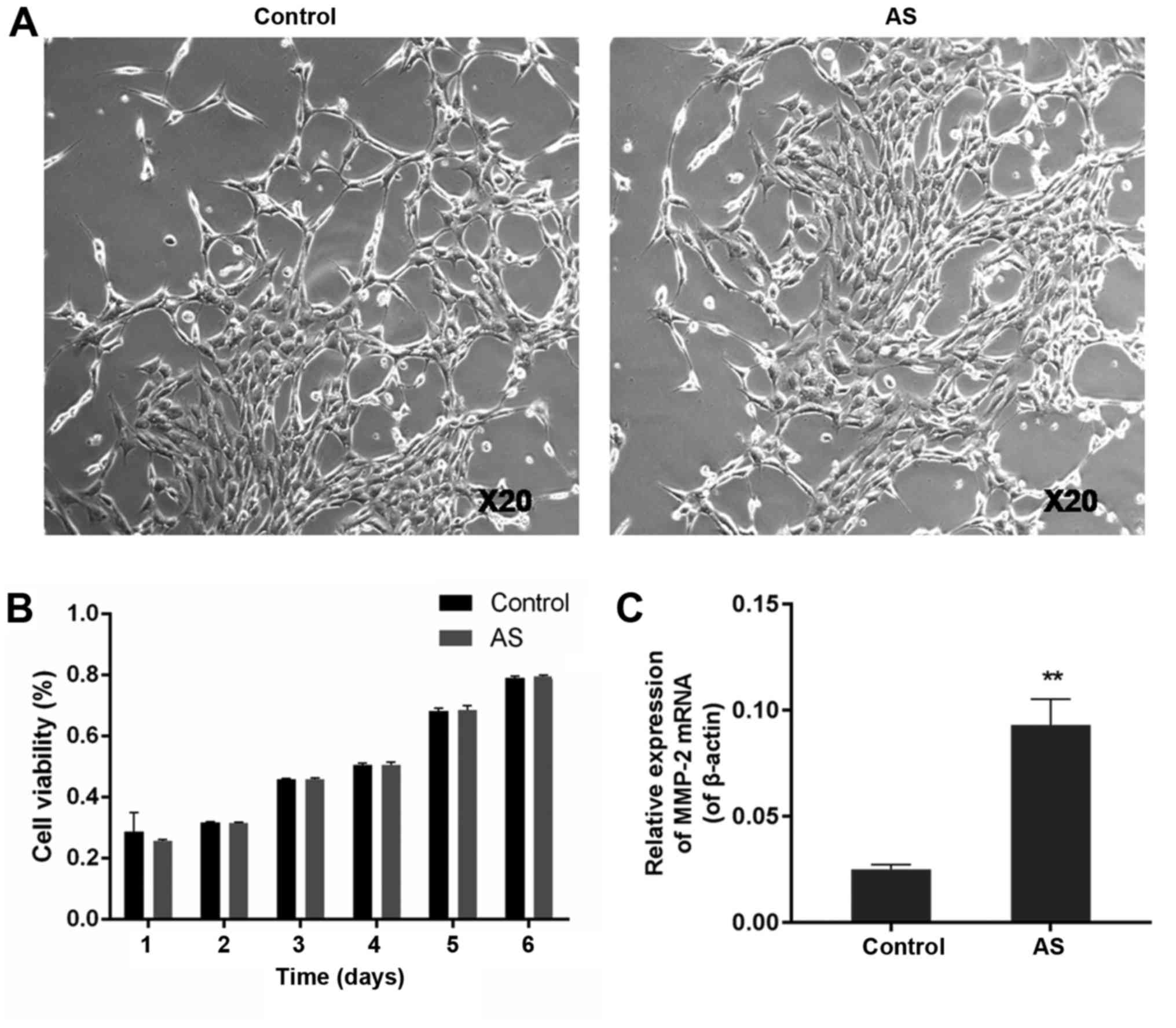
No obvious difference in the
morphology of normal and AS fibroblasts
Ligament fibroblasts in the control and AS groups
were isolated from tissues of healthy volunteers and patients with
AS, respectively. No significant difference in cell morphology was
observed between normal and AS fibroblasts. Gender difference was
not manifested in morphology of healthy or AS fibroblasts, but the
incidence rate of men was apparently higher than women (Table I). Cells in both control and AS groups
adhered to the culture flasks and were spindle-shaped, as observed
under a light microscope (Fig.
1A).
No significant difference in cell
viability between normal and AS fibroblasts
Cell proliferation profiles of normal and AS
fibroblasts were investigated using the MTT assay. The results
showed that both normal and AS fibroblasts had an initial lag
phase, followed by a steady increase in cell proliferation from day
2 to day 6 (Fig. 1B). There was no
significant difference in cell viability between the control and AS
groups at any of the time points tested (P>0.05).
MMP-2 mRNA expression level in AS
group was much higher than that in the control group
The expression levels of MMP-2 mRNA in both normal
and AS fibroblasts were measured using RT-PCR. The MMP-2 mRNA
expression level in AS fibroblasts at 0.093±0.012, was
approximately four times higher than that in normal cells
(0.025±0.0023) (P<0.01) (Fig.
1C).
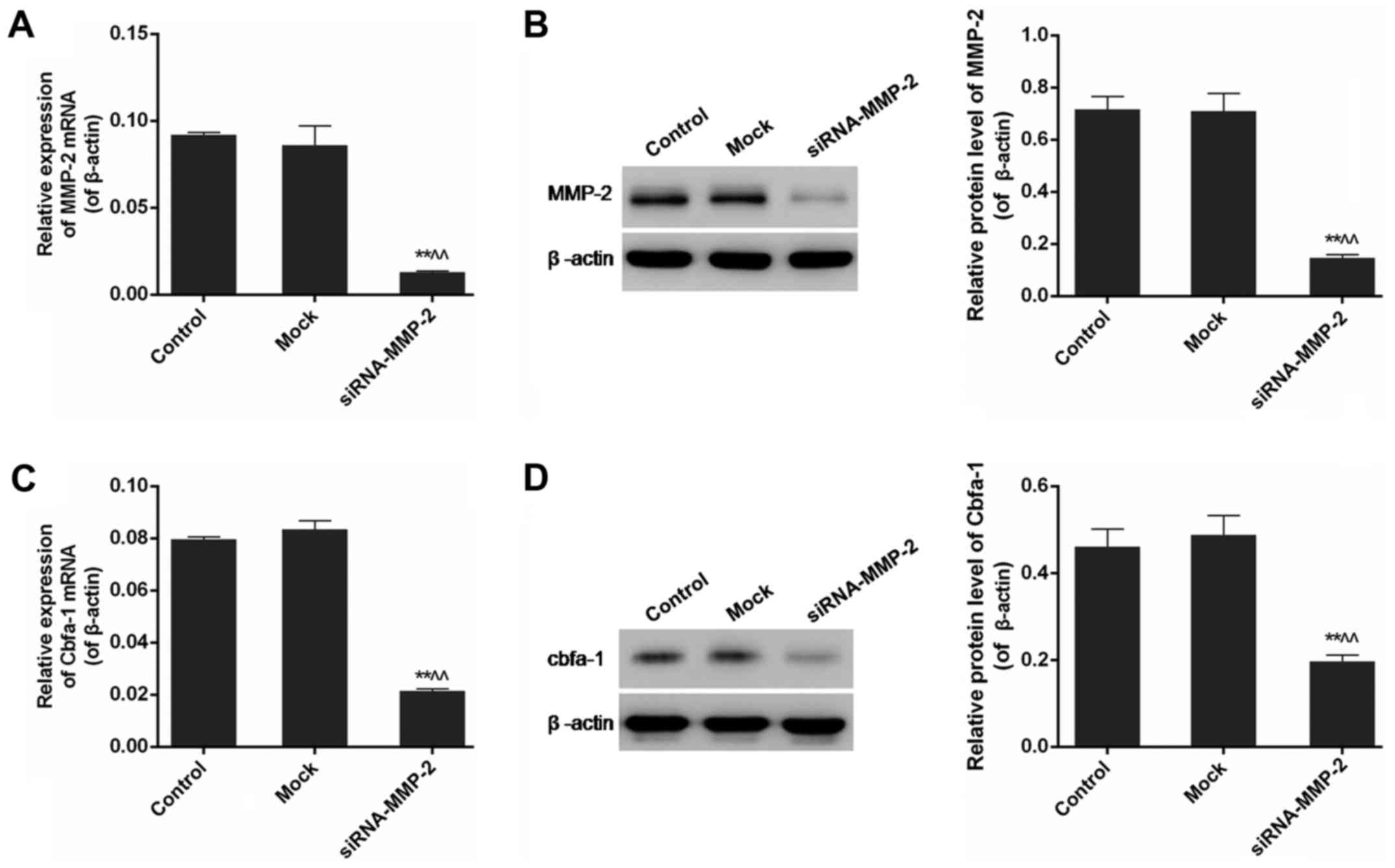
MMP-2 expression was substantially
inhibited in the siRNA-MMP-2 group
The expression levels of MMP-2 mRNA and protein were
examined in control, mock and siRNA-MMP-2 groups, using RT-PCR and
western blotting, respectively. There was no significant
quantitative differences in the protein levels of MMP-2 between the
control and mock group (P>0.05). However, MMP-2 gene
expression was dramatically inhibited in cells transfected with
siRNA-MMP-2, compared to that in normal cells, from 0.72±0.05 in
normal cells to 0.15±0.012 in siRNA-MMP-2 transfected cells
(P<0.01) (Fig. 2A and B).
MMP-2 gene silencing down-regulated
the expression of Cbfa-1in fibroblasts
The effect of MMP-2 gene silencing on the expression
of Cbfa-1 was analysed by RT-PCR and western blotting. Control and
mock groups did not differ significantly in the expression levels
of Cbfa-1 mRNA and protein (P>0.05). Results of both
RT-PCR and western blotting indicated that the expression of Cbfa-1
was markedly down-regulated by silencing the MMP-2 gene in
siRNA-MMP-2 group, which suggested the positive correlation between
the expressions of MMP-2 and Cbfa-1 (P<0.01) (Fig. 2C and D).
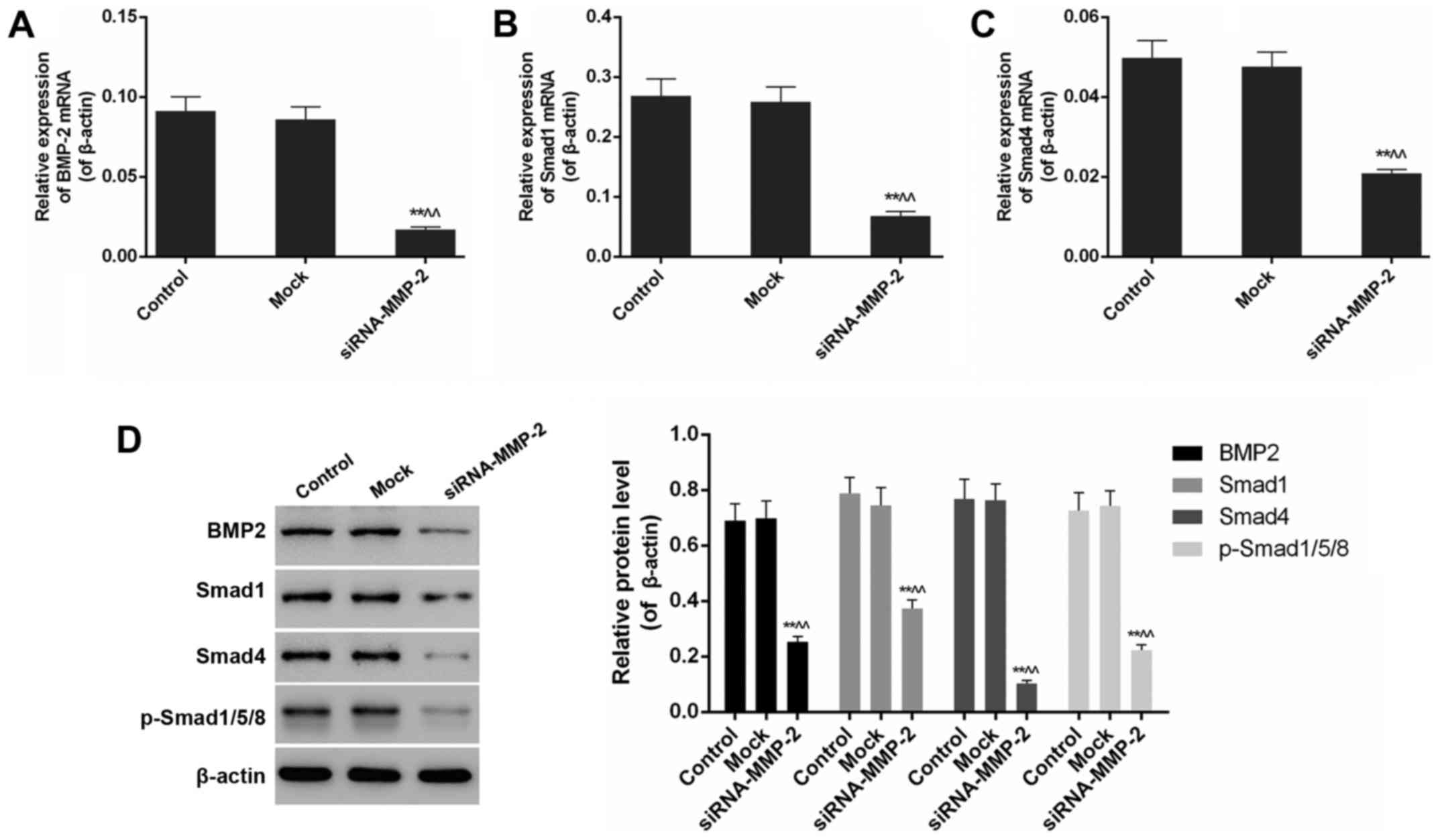
MMP-2 gene silencing had an inhibitory
effect on the BMP/Smad signalling pathway in AS fibroblasts
Quantitative analysis of the expression of BMP/Smad
pathway components was carried out using RT-PCR and western
blotting. The results of RT-PCR showed that the mRNA expression
levels of BMP-2, Smad1, Smad4, and Smad1/5/8 were all dramatically
reduced in the siRNA-MMP-2 group, compared to those in the control
as well as mock groups (P<0.01). Western blot analysis showed
the inhibitory effect of MMP-2 gene silencing on the expression
levels of BMP-2, Smad1, Smad4 and Smad1/5/8 proteins, as reflected
in the significant differences observed between siRNA-MMP-2 and
control groups (P<0.01) (Fig.
3).
Discussion
AS, a prototype of the spondyloarthritis group of
diseases, is a chronic systemic inflammatory and autoimmune
rheumatic disease with a high disability rate (14). The disease process is characterized by
ectopic ossification of spine and peripheral joints (15). In late stages of AS, which is regarded
as the beginning of irreversible disability, cartilage is
progressively replaced by bone, eventually leading to joint
ankyloses. Excessive MMP-2 production is associated with collagen
degradation in the joints, which is considered as one of the major
causes of AS (16). Fibroblasts from
patients with AS were isolated for the investigations described in
this report. First, we showed that the MMP-2 expression level
obviously differed between normal and AS fibroblasts, with the
levels in AS fibroblasts nearly four-fold higher compared to those
in normal cells. Silencing MMP-2 gene in AS fibroblasts
resulted in a remarkable reduction in the expressions of Cbfa-1 and
components of BMP/Smad signalling pathways.
Fibroblasts, one of the major cell types in
connective tissue, produce collagen-rich extracellular matrix and
play a key role in trauma repair as well as in pathologic ectopic
ossification (17,18). Fibroblasts can differentiate into
osteoblasts under specific conditions, although they are from the
same lineage. A variety of bone growth factors participate in the
regulation of cell proliferation, differentiation, and bone
metabolism (19). New bone formation
involves the recruitment of osteoprogenitor cells. The rate of
mature bone formation depends on the half-life of osteoblasts, cell
proliferation rate and their functional differentiation (20). Osteoblast differentiation is regulated
by signalling factors. According to recent reports, osteoblasts
express two osteogenic marker genes, Cbfa-1 and osteocalcin
(21,22). As a Runt-related osteoblast-specific
transcription factor, Cbfa-1 gene exerts its effects at earlier
stages of the disease, as opposed toosteocalcin, which is involved
in osteoblast differentiation (17,23,24). The
expression of Cbfa-1 is regulated by its upstream signalling
molecules (18).
BMPs are members of the transforming growth factor β
superfamily. Several lines of studies showed that BMPs could
activate the downstream signalling molecules in the Smad protein
family, stimulate mesenchymal cell differentiation, and
irreversibly induce bone and cartilage formation by regulating the
expression of Cbfa-1 (25–27). BMP-2 can modulate osteoblastic
differentiation through the canonical BMP/Smad pathway. This
signalling pathway is initiated by type II BMP receptors, which
after activation, propagate the BMP signals by phosphorylating
BMP-specific Smad1, Smad5 and Smad8. p-Smad1/5/8 then bindtoSmad4
to form a complex which gets translocated to the nucleus and
activates or represses the transcription of osteogenic genes. The
activation of BMP/Smads signalling pathway is an important
mechanism of the osteogenic differentiation of AS fibroblasts and
endochondral bone formation in ankylosing enthesitis. Untimely
activation of the signalling cascades may promote AS processes
(28–30).
We showed in this study that the expression of
MMP-2, which is responsible for the degradation of non-fibrillar
and denatured collagens, was obviously elevated at both
transcriptional and translational levels in fibroblasts from
patients with AS. Cbfa-1, one of the essential transcription
factors, which regulates osteoblast differentiation and bone
formation, was observed to be highly affected by the silencing of
MMP-2 gene, as demonstrated by its weak expression in the
siRNA-MMP-2 group (31). The
expression of Cbfa-1 was positively correlated with that of MMP-2.
It was also found that MMP-2 inhibition by siRNA technique resulted
in decreased expression of BMP-2, which in turn reduced the levels
of Smad signalling protein levels remarkably. BMP molecules induce
ligand-dependent type I and type II receptor heterodimerization,
which activates p-Smad1/5/8 that bind Smad4 (32). The inhibition of BMP/Smad signalling
by siRNA-MMP-2 was also associated with the inhibition of Cbfa-1
expression. Thus, inhibition of BMP/Smad signalling has the
potential to effectively prevent osteoblastic differentiation in
pathologic ectopic ossification (13). Our results showed that the level of
expression of MMP-2 in AS fibroblasts can be markedly inhibited by
siRNA-MMP-2. Therefore, MMP-2 silencing can be considered a
promising therapeutic strategy for AS treatment.
This study investigated in vitro, the
signalling mechanism of MMP-2 and the effect of its silencing in
fibroblasts isolated from patients with AS. Our findings
demonstrated that MMP-2 gene can be effectively down-regulated by
transfecting the AS fibroblasts with siRNA-MMP-2. MMP-2
down-regulation also led to decreased expression of Cbfa-1 and
BMP/Smad signalling proteins. The MMP-2 silencing, therefore, could
serve as a novel therapeutic approach for AS treatment.
References
|
1
|
Pompeu JE, Romano RS, Pompeu SM and Lima
SM: Static and dynamic balance in subjects with ankylosing
spondylitis: Literature review. Rev Bras Reumatol. 52:409–416.
2012.(In English, Portuguese). PubMed/NCBI
|
|
2
|
Akkoc N, van der Linden S and Khan MA:
Ankylosing spondylitis and symptom-modifying vs disease-modifying
therapy. Best Pract Res Clin Rheumatol. 20:539–557. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Machado P, Landewé R, Braun J, Hermann KG,
Baker D and van der Heijde D: Both structural damage and
inflammation of the spine contribute to impairment of spinal
mobility in patients with ankylosing spondylitis. Ann Rheum Dis.
69:1465–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang M, Yuan H, Miao M and Xu W: The
osteogenic potential of ligament fibroblasts is greater in
ankylosing spondylitis patients than in patients with
osteoarthritis. Z Rheumatol. 74:340–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Y, Liu M, Yang B, Li B and Lu J: Role
of siRNA silencing of MMP-2 gene on invasion and growth of
laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol.
265:1385–1391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noel A, Maillard C, Rocks N, Jost M,
Chabottaux V, Sounni NE, Maquoi E, Cataldo D and Foidart JM:
Membrane associated proteases and their inhibitors in tumour
angiogenesis. J Clin Pathol. 57:577–584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun R, Huang Y, Zhang H and Liu R: MMP-2,
TNF-α and NLRP1 polymorphisms in Chinese patients with ankylosing
spondylitis and rheumatoid arthritis. Mol Biol Rep. 40:6303–6308.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kargiotis O, Chetty C, Gondi CS, Tsung AJ,
Dinh DH, Gujrati M, Lakka SS, Kyritsis AP and Rao JS:
Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in
impaired invasion and tumor-induced angiogenesis, induces apoptosis
in vitro and inhibits tumor growth in vivo in glioblastoma.
Oncogene. 27:4830–4840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Badiga AV, Chetty C, Kesanakurti D, Are D,
Gujrati M, Klopfenstein JD, Dinh DH and Rao JS: MMP-2 siRNA
inhibits radiation-enhanced invasiveness in glioma cells. PLoS One.
6:e206142011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mello CC and Conte D Jr: Revealing the
world of RNA interference. Nature. 431:338–342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan MA: HLA-B27 and its subtypes in world
populations. Curr Opin Rheumatol. 7:263–269. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu HX, Jiang N, Liang HY, Zhou YY, Feng
XH, Feng XY, Zhang HQ, Wu ZK, Jiang Q, Fu J, et al: Bushen Qiangji
Granule () medicated serum inhibits osteogenic differentiation of
fibroblasts in ankylosing spondylitis by inhibiting the BMP/Smads
signal pathway in vitro. Chin J Integr Med. 22:817–822. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arnett FC: The seronegative
spondyloarthropathies. Curr Opin Rheumatol. 4:460–462.
1992.PubMed/NCBI
|
|
15
|
Ranganathan K, Loder S, Agarwal S, Wong
VW, Forsberg J, Davis TA, Wang S, James AW and Levi B: Heterotopic
ossification: Basic-science principles and clinical correlates. J
Bone Joint Surg Am. 97:1101–1111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Touaitahuata H, Cres G, de Rossi S, Vives
V and Blangy A: The mineral dissolution function of osteoclasts is
dispensable for hypertrophic cartilage degradation during long bone
development and growth. Dev Biol. 393:57–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schett G: Bone formation versus bone
resorption in ankylosing spondylitis. Adv Exp Med Biol.
649:114–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ogawa M and LaRue AC: Origin of fibroblast
colony-forming units. Exp Hematol. 35:1319–1320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanazawa I, Yamaguchi T, Yano S, Yamauchi
M, Yamamoto M and Sugimoto T: Adiponectin and AMP kinase activator
stimulate proliferation, differentiation, and mineralization of
osteoblastic MC3T3-E1 cells. BMC Cell Biol. 8:512007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jun JK and Kim SM: Association study of
fibroblast growth factor 2 and fibroblast growth factor receptors
gene polymorphism in korean ossification of the posterior
longitudinal ligament patients. J Korean Neurosurg Soc. 52:7–13.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ducy P, Schinke T and Karsenty G: The
osteoblast: A sophisticated fibroblast under central surveillance.
Science. 289:1501–1504. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang D, Haile A and Jones LC:
Dexamethasone-induced lipolysis increases the adverse effect of
adipocytes on osteoblasts using cells derived from human
mesenchymal stem cells. Bone. 53:520–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karsenty G: Role of Cbfa1 in osteoblast
differentiation and function. Semin Cell Dev Biol. 11:343–346.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ducy P, Starbuck M, Priemel M, Shen J,
Pinero G, Geoffroy V, Amling M and Karsenty G: A Cbfa1-dependent
genetic pathway controls bone formation beyond embryonic
development. Genes Dev. 13:1025–1036. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lories RJ, Derese I and Luyten FP:
Modulation of bone morphogenetic protein signaling inhibits the
onset and progression of ankylosing enthesitis. J Clin Invest.
115:1571–1579. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Massagué J: How cells read TGF-beta
signals. Nat Rev Mol Cell Biol. 1:169–178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Waite KA and Eng C: From developmental
disorder to heritable cancer: It's all in the BMP/TGF-beta family.
Nat Rev Genet. 4:763–773. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao X and Chen D: The BMP signaling and in
vivo bone formation. Gene. 357:1–8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pande V and Ramos MJ: NF-kappaB in human
disease: Current inhibitors and prospects for de novo structure
based design of inhibitors. Curr Med Chem. 12:357–374. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghosh S and Hayden MS: New regulators of
NF-kappaB in inflammation. Nat Rev Immunol. 8:837–848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harada H, Tagashira S, Fujiwara M, Ogawa
S, Katsumata T, Yamaguchi A, Komori T and Nakatsuka M: Cbfa1
isoforms exert functional differences in osteoblast
differentiation. J Biol Chem. 274:6972–6978. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang RL, Yuan Y, Tu J, Zou GM and Li Q:
Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways
converge on Runx2 to regulate BMP-2-induced osteoblastic
differentiation. Cell Death Dis. 5:e11872014. View Article : Google Scholar : PubMed/NCBI
|