Introduction
Ovarian cancer is the leading cause of death among
gynecological malignancies, and is also the fourth most common
malignancy in women in developed countries, following breast, lung,
and colorectal cancer (1,2). Apart from surgery and radiotherapy, a
substantial number of ovarian cancer patients commonly undergo
chemotherapy because of its high efficacy. However, some of these
patients frequently develop varying degrees of chemotherapeutic
resistance, which is closely associated with the histological
subtypes. Each of the ovarian cancers, represented by serous
carcinoma (SEC), endometrioid carcinoma (EMC), clear cell carcinoma
(CCC) and mucinous carcinoma (MUC), is known to have a specific
prognosis and chemotherapy sensitivity.
Histone deacetylases (HDACs) are chromatin-modifying
enzymes that are involved in regulation of many aspects of cell
biology, including tissue differentiation, autophagy, apoptosis,
migration, mitosis and angiogenesis via deacetylation of histone or
non-histone protein (3). The HDAC
family contains 18 enzymes and is divided into four classes based
on their sequences similar to the yeast. In terms of enzymatic
activity, HDAC1, 2, 3 and 8 for class I, HDAC4, 5, 6, 7, 9 and 10
for class II, and HDAC11 for class IV are zinc-dependent, and
SIRT1-SIRT7 for class III are NAD+ dependent. Class I HDACs are
considered as nuclear proteins and class II HDACs shuttle between
the nucleus and cytoplasm (4). In
several cancers including ovarian cancer, class I HDACs are
upregulated and high HDAC1 expression is associated with a poor
prognosis (5–10). In vitro, pan-HDAC inhibitor has
been demonstrated to have a cytotoxic effect for ovarian cancer
cell lines (11). However, the
clinical trials resulted in limited therapeutic effect of pan-HDAC
inhibitor because of the side toxicity (12). Therefore, more selective and effective
HDACs inhibitors are needed in the therapy for ovarian cancers.
This study was conducted to analyze the association
between immunohistochemical HDACs expression and
clinicopathological findings, especially focusing on histological
subtypes, prognosis and chemotherapy, with the aim at exploration
of the new possible therapeutic strategies for ovarian cancer.
Materials and methods
Patient data and clinicopathological
features (Table I)
Patient electronic medical charts from the Saitama
Medical University International Medical Center from 2008 to 2012
were reviewed under approval of the Institutional Review Board
(IRB) following the ethical standards of the responsible committee
on human experimentation and with the Helsinki Declaration of 1975,
as revised in 1983. A total of 201 epithelial ovarian cancer
patients (SEC, 100 cases; CCC, 56 cases; EMC, 36 cases; MUC, 9
cases) without preoperative chemotherapy, whose tumors were
surgically resected and pathologically confirmed, were recruited in
this study. We also obtained the specimens of 38 tumors (34 for
SEC; 1 for CCC; 3 for EMC) after chemotherapy in addition to before
chemotherapy in the same case. Clinicopathological characteristics
with these cases, such as age, menopause, parity, recurrence,
death, progression free survival (PFS), overall survival (OS), and
the International Federation of Obstetrics and Gynecology (FIGO)
stage, and treatment methods were reviewed.
Immunohistochemistry staining
Immunohistochemical expression of HDACs was analyzed
using tissue microarray (TMA: KIN-2; Azumaya, Tokyo, Japan) under
approval of the IRB. TMA was generated from 2 cylindrical cores 3.0
mm in diameter in each block, which were punched out of
paraffin-embedded tissue blocks corresponding to the representative
histological findings and were inserted into a recipient block. The
tissue blocks consisted of 201 cases with primary tumors which did
not undergo neo-adjuvant chemotherapy. In addition, 38 tissue
blocks in which the tumors had undergone chemotherapy were used.
The total of 239 tissue blocks were cut into 4-µm serial sections,
which were run through an automated system by Dako Autostainer Link
48 (Agilent Technologies, Inc., Santa Clara, CA, USA) as per
manufacturer's protocol. The primary antibodies used were as
follows: Polyclonal rabbit anti-HDAC1 (dilution:15,000; ab19845;
Abcam, Cambridge, UK); monoclonal rabbit anti-HDAC2 (dilution,
1:1,000; ab32117; Abcam); monoclonal rabbit anti-HDAC3 (dilution,
1:250; ab32369; Abcam); polyclonal rabbit anti-HDAC4 (dilution,
1:500; ab12172; Abcam); polyclonal rabbit anti-HDAC5 (dilution,
1:200; ab55403; Abcam); polyclonal rabbit anti-HDAC6 (dilution,
1:500; ab1440; Abcam); and polyclonal rabbit anti-HDAC7 (dilution,
1:100; NB100-61587; Novus Biological, Colorado, USA). For all
antibodies but HDAC7, Target Retrieval Solution (pH 9.0) was
applied for the antigen retrieval at 98°C for 20 min. Sections were
incubated with the primary antibodies at room temperature (RT) for
60 min, followed by incubation with a secondary antibody (EnVision
FLEX/HRP; Agilent Technologies, Inc.) at RT for 30 min. The
chromogen reaction was performed with diaminobenzidine plus the
H2O2 substrate at RT for 10 min. It was
confirmed that there are no significant differences in all of HDACs
expressions between TMA and the whole section, using 20 randomized
cases (data not shown).
Interpretation of immunohistochemical
results
Diagnoses were performed by one experienced
pathologist who was blind to clinical data and patient
characteristics, and one physician with a subspecialty in
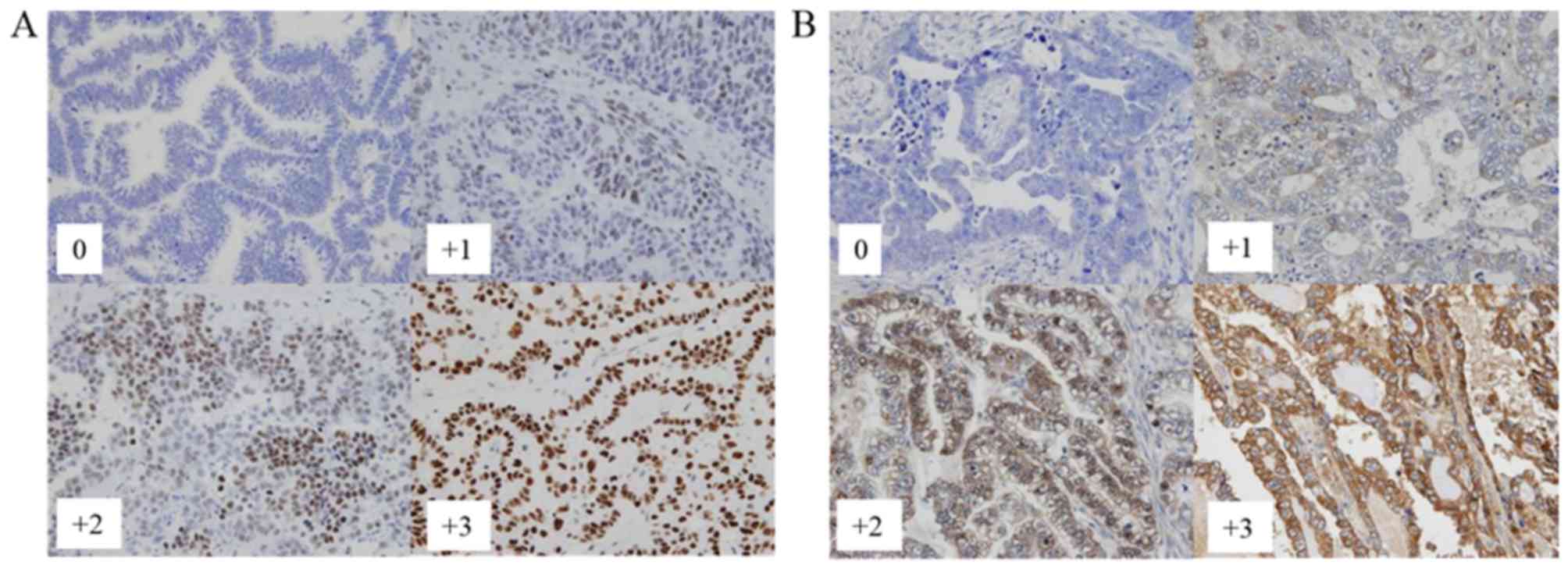
gynecological oncology. A four-tiered scoring scheme was used for
both nuclear expression (Fig. 1A) and
cytoplasmic expression (Fig. 1B),
respectively: 0 for negative; +1 for weak; +2 for moderate; and +3
for marked. To optimize for PFS and OS differences, the raw data
were binarized for statistical analysis as follows: The moderate
(+2) and marked (+3) cases were grouped as high-level expressers,
whereas the completely negative (0) and weak (+1) cases were
considered as low-level expressers.
Statistical analysis
Univariable survival analysis was performed by the
generation of Kaplan-Meier curves, and differences between the
groups were assessed using the log rank statistic. Univariable and
multivariable survival analysis was performed using the Cox
proportional hazards model. Kruskal-Wallis tests were used to
assess the change in the distribution of HDAC expression across
primary histological subtypes. Wilcoxon signed-rank test was used
to assess the change between before and after chemotherapy. All
analyses were performed using SPSS v24.0 (SPSS, Inc, Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Correlation of HDACs expression with
histological subtype
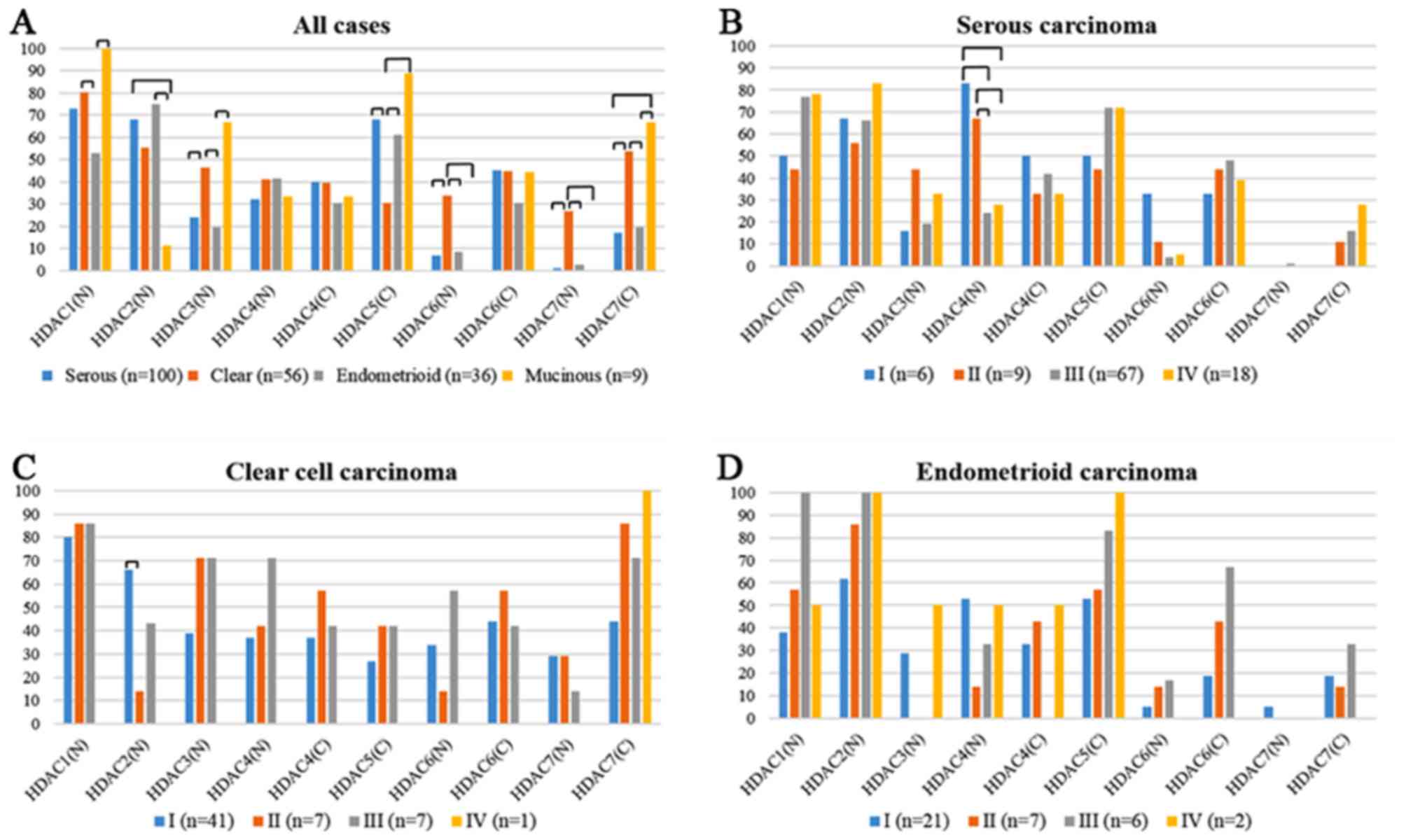
HDACs overexpressions of each histological subtype
are shown in Fig. 2A. Expressions of
HDAC1, 2, and 3 were observed only in the nucleus. Overexpression
of HDAC1 was detected in all cases with MUC, followed by CCC (80%),
SEC (73%), and EMC (53%). HDAC2 expression was observed in EMC
(75%) and SEC (68%). HDAC3 expression was done in MUC (67%) and CCC
(46%). CCC showed the highest frequency of HDAC7 (27%) and HDAC6
(34%) expression in the nucleus among all the subtypes. On the
other hand, CCC showed the lowest frequency of HDAC5 cytoplasmic
expression (30%). There were no significant differences in HDAC4
expression in both nucleus and cytoplasm and HDAC6 cytoplasmic
expression among the histological subtypes. We analyzed the HDACs
expressions of each FIGO stage in SEC (Fig. 2B), CCC (Fig.
2C), and EMC (Fig. 2D). In SEC,
FIGO stage I/II (83/67%) showed higher frequency of HDAC4 nuclear
expression than stage III/IV (24/28%). In CCC, FIGO stage I (66%)
showed higher frequency of HDAC2 nuclear expression than stage II
(14%). There were no significant differences in other HDACs
expression among each FIGO stage.
Correlation of HDACs expression with
chemotherapy (Table II)
The chemotherapy responses were evaluated as
follows: 38 ovarian cancers clinically are 3 for complete response
(CR), 31 for partial response (PR), 2 for stable disease (SD), and
2 for progressive disease (PD). In the comparison between before
and after chemotherapy, HDAC1 nuclear expression increased from 76
to 92% (P=0.03); HDAC7 expression in nucleus from 0 to 16% (P=0.01)
in cytoplasm from 16 to 66% (P=<0.01); HDAC6 cytoplasmic
expression increased from 39 to 74% (P=<0.01). No significant
changes were noted in other types of HDAC. We analyzed the CR+PR
group (n=35) and SD+PD group (n=3), and found that in the CR+PR
group, HDAC1, 6, and 7 nuclear expressions and HDAC7 cytoplasmic
expression increased in the comparison between before and after
chemotherapy. In PD+PR group, no significant changes were noted in
all types of HDAC.
Correlation of HDACs expression with
prognosis
In SEC, overexpression of HDAC1 in the nucleus was
significantly associated with the decrease in PFS (P=<0.01,
Fig. 3A) and OS (P=0.02, Fig. 3B), but overexpression of HDAC7 in the
cytoplasm had no significant adverse effect for PFS (P=0.35,
Fig. 3C) and OS (P=0.76, Fig. 3D). Also in EMC, overexpression of
HDAC1 in the nucleus was significantly associated with the decrease
in PFS (P=0.03, Fig. 3E) and OS
(P=0.03, Fig. 3F), but overexpression
of HDAC7 in the cytoplasm had no significant adverse effect for PFS
(P=0.42, Fig. 3G) and OS (P=0.18,
Fig. 3H). In CCC, however, nuclear
expression of HDAC1 showed no significant adverse effect for PFS
(P=0.17, Fig. 3I) and OS (P=0.68,
Fig. 3J); on the other hand,
cytoplasmic expression of HDAC7 was correlated with poor prognostic
factor (PFS, P=0.03; OS, P=0.06, Fig. 3K,
L). In the analysis focusing on the subgroup of FIGO stage I/II
and stage III/IV in SEC and CCC (Fig.
3M-T), SEC patients with HDAC1 nuclear overexpression tended to
have a poor prognosis in FIGO stage III/IV (PFS, P=0.06; OS,
P=0.15), but had no significant effect on a prognosis in stage I/II
(PFS, P=0.40; OS, P=0.21). CCC patients with HDAC7 cytoplasmic
overexpression showed a poor prognosis in FIGO stage I/II (PFS,
P=0.02; OS, P=0.047), but had no significant effect on a prognosis
in stage III/IV (PFS, P=0.76; OS, P=0.76). HDAC5 cytoplasmic
expression in EMC was associated with poor prognosis (PFS, P=0.01;
OS, P=0.05). HDAC4 nuclear expression in SEC was associated with
longer PFS (P=0.03), but no significant change of OS (P=0.13).
HDAC2, 3, and 6 in each of the histological types had no
significant effect on the prognosis.
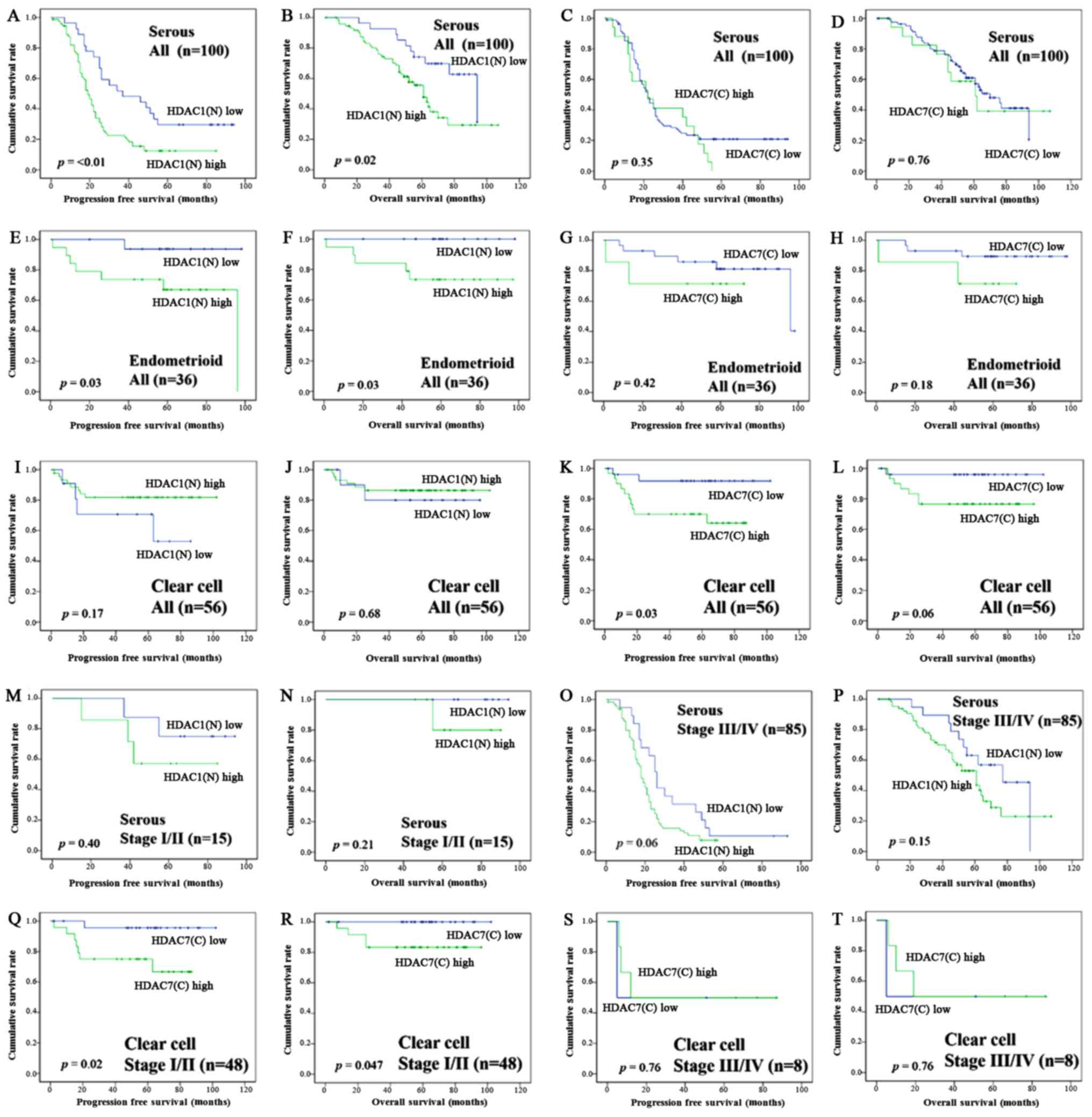 | Figure 3.Kaplan-Meier survival analysis:
Serous carcinoma patients according to the HDAC1 nuclear expression
(A, PFS; B, OS) and HDAC7 cytoplasmic expression (C, PFS; D, OS).
Endometrioid carcinoma patients according to the HDAC1 nuclear
expression (E, PFS; F, OS) and HDAC7 cytoplasmic expression (G,
PFS; H, OS). Clear cell carcinoma patients according to the HDAC1
nuclear expression (I, PFS; J, OS) and HDAC7 cytoplasmic expression
(K, PFS; L, OS). Serous carcinoma patients according to the HDAC1
nuclear expression in FIGO stage I/II (M, PFS; N, OS) and stage
III/IV (O, PFS; P, OS). Clear cell carcinoma patients according to
the HDAC1 nuclear expression in FIGO stage I/II (Q, PFS; R, OS) and
stage III/IV (S, PFS; T, OS). P-values, log-rank test. HDAC,
histone deacetylase; PFS, progression free survival; OS, overall
survival; FIGO, International Federation of Obstetrics and
Gynecology. |
Univariate and multivariate analyses
(Table III)
In univariate analysis, OS was associated with
histological subtype, FIGO stage, surgical residual tumor, HDAC1
nuclear expression (hazard ratio (HR)=2.06; 95% confidence interval
(CI)=1.10 to 3.87; P=0.02) and HDAC5 cytoplasmic expression
(HR=2.54; 95% CI, 1.46 to 4.42; P=<0.01). In multivariate
survival analysis performed under inclusion of age, histological
subtype, FIGO stage, surgical residual tumor, and HDACs expression,
FIGO stage (HR=10.8; 95% CI, 3.67 to 32.0; P=<0.01), surgical
residual tumor (HR=11.1; 95% CI, 3.32 to 37.4; P=<0.01),
histological subtype, and HDAC6 nuclear expression (HR=3.51; 95%
CI, 1.49 to 8.27, P=<0.01) were found to become the independent
prognostic factors. In the analysis with the subgroup of HDAC6
nuclear expression (Fig. 4),
overexpression of HDAC6 in the nucleus had no significant adverse
effect for OS in all cases (P=0.85, Fig.
4A), SEC (P=0.69, Fig. 4B), EMC
(P=0.35, Fig. 4D), FIGO stage I/II
(P=0.44, Fig. 4E), and optimal
surgery (P=0.34, Fig. 4G), but was
associated with the decrease OS in CCC (P=0.07, Fig. 4C), FIGO stage III/IV (P=0.07, Fig. 4F), and suboptimal surgery (P=<0.01,
Fig. 4H).
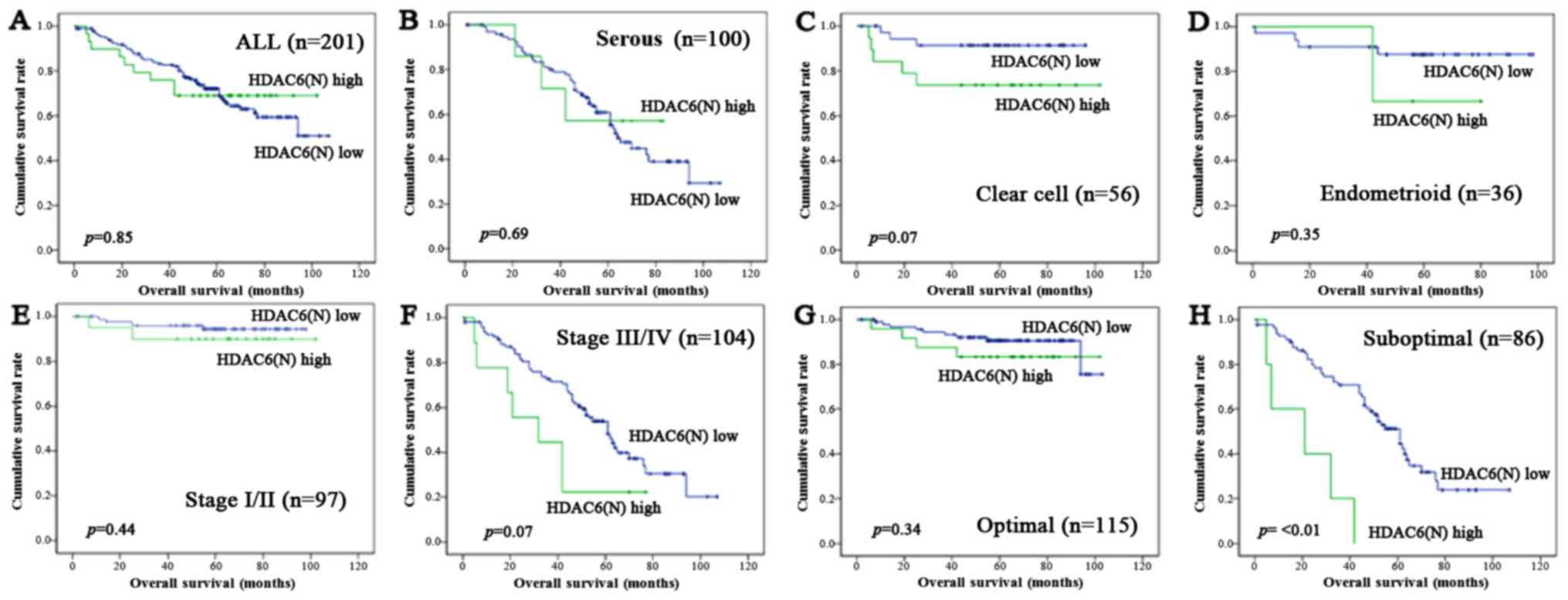 | Figure 4.Kaplan-Meier survival analysis: OS
according to the HDAC6 nuclear expression in all cases (A), SEC
(B), CCC (C), EMC (D), FIGO stage I/II (E), stage III/IV (F),
optimal surgery (G), and suboptimal surgery (H). P-values, log-rank
test. OS, overall survival; HDAC, histone deacetylase; FIGO,
International Federation of Obstetrics and Gynecology; SEC, serous
carcinoma; CCC, clear cell carcinoma; EMC, endometrioid
carcinoma. |
Discussion
It has been reported that class I HDACs are
upregulated and high HDAC1 expression is associated with poor
prognosis in several cancers including ovarian cancer (5–10). Class I
HDACs are involved in regulation of many aspects of cancer biology
including cell proliferation via p21, p27, and p57 (13,14),
apoptosis via p53, bcl2, caspase-3, −8, and −9 (15), metastasis via e-cadherin (10), angiogenesis via hypoxia inducible
factors-1α (HIF-1α) and vascular endothelial growth factor (VEGF)
(16,17), and anti-tumor immune responses via
programmed death-1 ligand (PD-L1) (18). Weichert et al reported that
overexpression of HDAC1 was an independent prognostic factor in
ovarian EMC (9); however, the
multivariable analysis does not contain the key prognostic factor
in ovarian cancer, such as surgical debulking status (optimal or
suboptimal). Hayashi et al (10) reported that overexpression of HDAC1
might be correlated with a poor prognosis in ovarian cancer, but
did not analyze each of the histological subtypes in detail.
Additionally, those two previous studies had not conducted an
evaluation about class II HDACs. The present study was designed to
supplement the deficiency in the previous studies and showed HDAC1
overexpression is a poor prognostic factor not only in EMC but also
in SEC. In CCC, HDAC6 and HDAC7 expressions were upregulated in
comparison with other histological subtypes, and that HDAC7
cytoplasmic expression is expected to become a poor prognostic
factor. Although HDAC6 nuclear expression had no significant effect
on a prognosis in univariate analysis, it was found to have the
significant as a poor prognostic factor in multivariate analysis
employing FIGO stage, histological subtype, and surgical debulking
status. By subgroup analysis, we found that HDAC6 nuclear
overexpression is associated with a poor prognosis especially in
surgical suboptimal cases. In SEC, the most prominent molecular
changes include the alternation in TP53, which was
exclusively mutations. HDAC1 provides the major enzymes for p53
deacetylation and form a Snail1/HDAC1/p53 tri-molecular complex,
and inactivates p53 (14,19,20). On
the other hand, in CCC, ARID1A mutation is the most common
event (57%) (21) and frequently
coexists with PIK3CA mutation (22). HDAC6 activity is essential in
ARID1A-mutated ovarian cancers and HDAC6 inhibition
selectively promoted apoptosis of ARID1A-mutated cells
(23). CCC is associated with Lynch
syndrome, which is characterized by germline mutations in MSH2
(24,25). HDAC6 deacetylates and ubiquitinates
MSH2, causing a cellular tolerance to DNA damage and decreased
cellular DNA mismatch repair activities (26). CCC is at a higher level of HIF-1α than
other histological subtypes (27),
and HDAC7 increases transcriptional activity of HIF-1α (28). In is suggested that the different HDAC
isoforms may become a prognostic factor in SEC, EMC, and CCC. HDAC6
and 7 have a potential of being a chemotherapeutic target
specifically for CCC.
HDAC1 and HDAC7 increased after chemotherapy.
Residual tumor cells after neo-adjuvant chemotherapy might have low
sensitivity or acquired resistance for chemotherapy. HDAC1 and
HDAC7 augment cancer stem cell (CSC) phenotype via MiR-34a and the
CSC markers such as CD44 and CD166, and the CSC phenotype is
associated with chemotherapy resistance, metastasis, and relapse
(29,30). HDAC1 directly deacetylates HIF-1α and
blocks degradation of the protein (16). HDAC7 increases transcriptional
activity of HIF-1α through the formation of a complex with HIF-1α,
HDAC7, and p300 in the nucleus (28).
Overexpression of HIF-1α reduced cisplatin-induced apoptosis in
cisplatin-sensitive cells (31). HDAC
inhibitor has been reported to have synergistic cytotoxicity with
cisplatin in ovarian carcinoma cells and can restore cisplatin
sensitivity in the acquired cisplatin-resistant cells (15,32). HDAC1
and 7 have a potential of being a chemotherapeutic target for
ovarian cancer with chemoresistance. It would be useful to clarify
the correlation between HDACs and chemoresistance-related
substances, such as HIF-1α, CD44, CD166, e-cadherin, MSH2, and
PD-L1 etc. A potential weakness of the present study is the small
population of several important subgroups, such as EMC (n=36), MUC
(n=9), chemotherapy SD+PD group (n=3). Further studies are needed
to clarify the precise associations with those factors
In conclusion, this immunohistochemical study of
HDACs expression revealed the correlation between the HDAC isoforms
and the prognosis and histological subtypes. Further studies,
especially focusing on HDAC1, 6, and 7, are needed in order to
explore the strategy for histological subtypes of ovarian cancer
with chemoresistance or low chemo-sensitivity.
Acknowledgements
This study was supported by the Hidaka Research
Projects in Saitama Medical University (grant no. 28-D-1-14) and
Grants-in-Aid from the Ministry of Education, Science, Sports and
Culture of Japan (Research Project no. 16K11157). We thank Koichi
Kamada and Tomomi Kato, Department of Pathology, Saitama Medical
University International Medical Center, for their technical
support.
Glossary
Abbreviations
Abbreviations:
|
HDAC
|
histone deacetylase
|
|
SEC
|
serous carcinoma
|
|
CCC
|
clear cell carcinoma
|
|
EMC
|
endometrioid carcinoma
|
|
MUC
|
mucinous carcinoma
|
|
PFS
|
progression free survival
|
|
OS
|
overall survival
|
|
FIGO
|
International Federation of Obstetrics
and Gynecology
|
|
TMA
|
tissue microarray
|
|
RT
|
room temperature
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
CSC
|
cancer stem cell
|
|
HIF-1α
|
hypoxia inducible factor-1α
|
|
VEGF
|
vascular endothelial growth factor
|
|
PD-L1
|
programmed death-1 ligand
|
References
|
1
|
Permuth-Wey J and Sellers TA: Epidemiology
of ovarian cancer. Methods Mol Biol. 472:413–437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gayther SA and Pharoah PD: The inherited
genetics of ovarian and endometrial cancer. Curr Opin Genet Dev.
20:231–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seto E and Yoshida M: Erasers of histone
acetylation: The histone deacetylase enzymes. Cold Spring Harb
Perspect Biol. 6:a0187132014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verdin E, Dequiedt F and Kasler HG: Class
II histone deacetylases: Versatile regulators. Trends Genet.
19:286–293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higashijima J, Kurita N, Miyatani T,
Yoshikawa K, Morimoto S, Nishioka M, Iwata T and Shimada M:
Expression of histone deacetylase 1 and metastasis-associated
protein 1 as prognostic factors in colon cancer. Oncol Rep.
26:343–348. 2011.PubMed/NCBI
|
|
6
|
Miyake K, Yoshizumi T, Imura S, Sugimoto
K, Batmunkh E, Kanemura H, Morine Y and Shimada M: Expression of
hypoxia-inducible factor-1alpha, histone deacetylase 1, and
metastasis-associated protein 1 in pancreatic carcinoma:
Correlation with poor prognosis with possible regulation. Pancreas.
36:e1–e9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minamiya Y, Ono T, Saito H, Takahashi N,
Ito M, Mitsui M, Motoyama S and Ogawa J: Expression of histone
deacetylase 1 correlates with a poor prognosis in patients with
adenocarcinoma of the lung. Lung Cancer. 74:300–304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khabele D, Son DS, Parl AK, Goldberg GL,
Augenlicht LH, Mariadason JM and Rice VM: Drug-induced inactivation
or gene silencing of class I histone deacetylases suppresses
ovarian cancer cell growth: Implications for therapy. Cancer Biol
Ther. 6:795–801. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weichert W, Denkert C, Noske A,
Darb-Esfahani S, Dietel M, Kalloger SE, Huntsman DG and Köbel M:
Expression of class I histone deacetylases indicates poor prognosis
in endometrioid subtypes of ovarian and endometrial carcinomas.
Neoplasia. 10:1021–1027. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayashi A, Horiuchi A, Kikuchi N, Hayashi
T, Fuseya C, Suzuki A, Konishi I and Shiozawa T: Type-specific
roles of histone deacetylase (HDAC) overexpression in ovarian
carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates
cell migration with downregulation of E-cadherin. Int J Cancer.
127:1332–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sonnemann J, Gänge J, Pilz S, Stötzer C,
Ohlinger R, Belau A, Lorenz G and Beck JF: Comparative evaluation
of the treatment efficacy of suberoylanilide hydroxamic acid (SAHA)
and paclitaxel in ovarian cancer cell lines and primary ovarian
cancer cells from patients. BMC Cancer. 6:1832006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Modesitt SC, Sill M, Hoffman JS and Bender
DP; Gynecologic Oncology Group, : A phase II study of vorinostat in
the treatment of persistent or recurrent epithelial ovarian or
primary peritoneal carcinoma: A Gynecologic Oncology Group study.
Gynecol Oncol. 109:182–186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamaguchi T, Cubizolles F, Zhang Y,
Reichert N, Kohler H, Seiser C and Matthias P: Histone deacetylases
1 and 2 act in concert to promote the G1-to-S progression. Genes
Dev. 24:455–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zupkovitz G, Grausenburger R, Brunmeir R,
Senese S, Tischler J, Jurkin J, Rembold M, Meunier D, Egger G,
Lagger S, et al: The cyclin-dependent kinase inhibitor p21 is a
crucial target for histone deacetylase 1 as a regulator of cellular
proliferation. Mol Cell Biol. 30:1171–1181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang JJ, Kim YS, Kim MJ, Jang S, Lee JH,
Choi J, Ro S, Hyun YL, Lee JS and Kim CS: A novel histone
deacetylase inhibitor, CG0006, induces cell death through both
extrinsic and intrinsic apoptotic pathways. Anticancer Drugs.
20:815–821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoo YG, Kong G and Lee MO:
Metastasis-associated protein 1 enhances stability of
hypoxia-inducible factor-1alpha protein by recruiting histone
deacetylase 1. EMBO J. 25:1231–1241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ray A, Alalem M and Ray BK: Loss of
epigenetic Kruppel-like factor 4 histone deacetylase
(KLF-4-HDAC)-mediated transcriptional suppression is crucial in
increasing vascular endothelial growth factor (VEGF) expression in
breast cancer. J Biol Chem. 288:27232–27242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Woods DM, Sodre AL, Villagra A, Sarnaik A,
Sotomayor EM and Weber J: HDAC Inhibition upregulates PD-1 ligands
in melanoma and augments immunotherapy with PD-1 blockade. Cancer
Immunol Res. 3:1375–1385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang H, Yan B, Liao D, Huang S and Qiu Y:
Acetylation of HDAC1 and degradation of SIRT1 form a positive
feedback loop to regulate p53 acetylation during heat-shock stress.
Cell Death Dis. 6:e17472015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ni T, Li XY, Lu N, An T, Liu ZP, Fu R, Lv
WC, Zhang YW, Xu XJ, Grant Rowe R, et al: Snail1-dependent p53
repression regulates expansion and activity of tumour-initiating
cells in breast cancer. Nat Cell Biol. 18:1221–1232. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones S, Wang TL, Shih IeM, Mao TL,
Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, et
al: Frequent mutations of chromatin remodeling gene ARID1A in
ovarian clear cell carcinoma. Science. 330:228–231. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamamoto S, Tsuda H, Takano M, Tamai S and
Matsubara O: Loss of ARID1A protein expression occurs as an early
event in ovarian clear-cell carcinoma development and frequently
coexists with PIK3CA mutations. Mod Pathol. 25:615–624. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bitler BG, Wu S, Park PH, Hai Y, Aird KM,
Wang Y, Zhai Y, Kossenkov AV, Vara-Ailor A, Rauscher FJ III, et al:
ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat Cell
Biol. 19:962–973. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ketabi Z, Bartuma K, Bernstein I, Malander
S, Grönberg H, Björck E, Holck S and Nilbert M: Ovarian cancer
linked to Lynch syndrome typically presents as early-onset,
non-serous epithelial tumors. Gynecol Oncol. 121:462–465. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jensen KC, Mariappan MR, Putcha GV, Husain
A, Chun N, Ford JM, Schrijver I and Longacre TA: Microsatellite
instability and mismatch repair protein defects in ovarian
epithelial neoplasms in patients 50 years of age and younger. Am J
Surg Pathol. 32:1029–1037. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang M, Xiang S, Joo HY, Wang L, Williams
KA, Liu W, Hu C, Tong D, Haakenson J, Wang C, et al: HDAC6
deacetylates and ubiquitinates MSH2 to maintain proper levels of
MutSα. Mol Cell. 55:31–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyazawa M, Yasuda M, Fujita M, Hirasawa
T, Kajiwara H, Hirabayashi K, Ogane N, Shimizu M, Asanuma H,
Murakami M, et al: Association of hypoxia-inducible factor-1
expression with histology in epithelial ovarian tumors: A
quantitative analysis of HIF-1. Arch Gynecol Obstet. 279:789–796.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kato H, Tamamizu-Kato S and Shibasaki F:
Histone deacetylase 7 associates with hypoxia-inducible factor
1alpha and increases transcriptional activity. J Biol Chem.
279:41966–41974. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Witt AE, Lee CW, Lee TI, Azzam DJ, Wang B,
Caslini C, Petrocca F, Grosso J, Jones M, Cohick EB, et al:
Identification of a cancer stem cell-specific function for the
histone deacetylases, HDAC1 and HDAC7, in breast and ovarian
cancer. Oncogene. 36:1707–1720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu MY, Fu J, Xiao X, Wu J and Wu RC:
MiR-34a regulates therapy resistance by targeting HDAC1 and HDAC7
in breast cancer. Cancer Lett. 354:311–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ai Z, Lu Y, Qiu S and Fan Z: Overcoming
cisplatin resistance of ovarian cancer cells by targeting
HIF-1-regulated cancer metabolism. Cancer Lett. 373:36–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin CT, Lai HC, Lee HY, Lin WH, Chang CC,
Chu TY, Lin YW, Lee KD and Yu MH: Valproic acid resensitizes
cisplatin-resistant ovarian cancer cells. Cancer Sci. 99:1218–1226.
2008. View Article : Google Scholar : PubMed/NCBI
|