Introduction
Breast cancer is one of the most common malignant
tumors in females, while metastases to the breast from non-breast
solid tumors are rare, accounting for only 0.3–2% of all malignant
mammary tumor types (1,2). To date, <500 cases of cancer with
secondary involvement of the breast from any non-breast solid
tumors have been reported (2,3). On the basis of the limited number of
studies available in the literature, excluding contralateral breast
cancer and lymphoma, the most frequent source of primary
malignancies with metastases to the breast in Western countries are
melanoma and lung cancer (3–5). Other reported primary sources include
the ovary, gastrointestinal tract, thyroid, kidney and sarcomas of
different origins (6–8). In general, symptoms of metastases to the
breast are similar to those of primary breast cancer, including the
presence of a palpable, freely-movable mass within the breast.
Pain, tenderness and inflammation are also observed occasionally.
Considering the rarity of extramammary lesions, it is challenging
to distinguish these lesions from primary breast cancer, even in
patients with a history of a primary non-breast solid tumor
(1–4).
However, the poor prognosis of patients with secondary breast
metastases and the contrast in appropriate treatments compared with
those for patients with primary breast cancer, in addition to the
fact that systemic treatment or palliative care is more appropriate
than extensive surgery in the majority of patients with secondary
breast metastases, emphasizes the importance of accurate diagnosis
(1–6).
In the present study, a single-institution retrospective review of
22 patients with pathologically confirmed extramammary metastases
to the breast, treated at the Sun Yat-sen University Cancer Center
(Guangzhou, China), was conducted. The clinical, radiological,
pathological and prognostic data of these patients were summarized
in order to identify their clinical characteristics, describe their
histological and immunohistochemical features and to assess their
clinical outcomes. The results may serve as a future reference for
the Southern Chinese population.
Patients and methods
Patients
Retrospective data were obtained from electronic
medical records and pathology databases at the Sun Yat-sen
University Cancer Center (Guangzhou, China) between January 2000
and December 2015 for patients with biopsy-diagnosed metastasis to
the breast from an on-breast primary solid tumor site. Tissues were
also obtained within the same time frame. Metastases from
contralateral primary breast cancer or hematological malignancies
were excluded. All histological slices were reviewed by two
pathologists.
Medical records from 22 patients (2 males, 20
females; mean age, 40.5 years; range, 10–62 years) were included in
the present study. Data collected from the records included the
following: Age, sex, initial symptoms, history of primary tumor,
interval from the diagnosis of the primary tumor to breast
metastases, other metastases status, tumor status at presentation
of breast metastasis, ultrasonographic and mammographic findings,
biopsy or post-surgery pathology results, clinical treatments and
follow-up. The breast imaging-reporting and data system (BI-RADS) I
to VI categorization (9), developed
by the American College of Radiology, was applied in present
study.
Statistical analysis
Descriptive statistics were applied to assess
frequency distributions. Overall survival interval probabilities
were calculated using the Kaplan-Meier method and differences in
survival were assessed by the log-rank test using SPSS 19.0 (IBM
Corp., Armonk, NY, USA). P<0.05 was determined to indicate
statistically significant difference.
Results
Demographic characteristics of
patients with metastases to the breast
Demographic characteristics of 22 patients with
metastases to the breast are summarized in Table I. Patients were predominately female
(2 males, 20 females). The mean and median onset of age for breast
metastasis was 40.5 and 43 years, respectively (range, 10–62
years). A total of 19 (86.4%) patients had a documented history of
a primary tumor with a mean interval of 6.5 months (range, 6–56
months) between diagnosis of the primary tumor to detection of
breast metastasis. In the other 3 patients (13.6%), the primary
tumor and breast metastasis were diagnosed simultaneously with
intact primary disease. A total of 7 patients (31.8%) presented
with other metastases and 11 patients (50.0%) exhibited no evidence
of a primary tumor or other metastasis when breast metastasis was
located. Pulmonary metastasis had been confirmed and treated in one
patient with melanoma when breast metastasis presented. Overall,
the breast was the only metastatic site in 6 (27.3%) patients.
 | Table I.Demographic characteristics of
patients with metastases to the breast (n=22). |
Table I.
Demographic characteristics of
patients with metastases to the breast (n=22).
|
Characteristics | Patients, n
(%) |
|---|
| Sex |
|
|
Male | 2 (9.1) |
|
Female | 20 (90.9) |
| Age, years |
|
|
Mean | 40.5 |
|
Median | 43 |
|
Range | 10–62 |
| History of primary
tumor |
|
|
Known | 19 (86.4) |
|
Unknown | 3
(13.6)a |
| Interval from
primary tumor to breast metastasis |
|
| Mean,
months | 16.5 |
| Range,
months | 6–56 |
| Other
metastases |
|
| Breast
only | 6 (27.3) |
| Other
metastases prior to breast | 9 (40.9) |
|
Simultaneous other
metastases | 2 (9.1) |
| Other
metastases following breast | 5 (22.7) |
| Tumor status at
presentation of breast metastasis |
|
|
Metastatic disease | 7 (31.8) |
| No
evidence of disease | 11 (50.0) |
| History
of other metastases, no NED at present | 1 (4.5) |
| Intact primary
disease | 3
(13.6)a |
Clinical characteristics of patients
with metastases to the breast
Breast metastasis were initially detected by
self-checking in 14 patients (63.6%; Table II). The mean tumor size was 2.9 cm
(range, 0.8–12.0 cm). A unilateral (45.5% left, 36.4% right), upper
outer quadrant (15/22, 68.2%) lesion of the breast was most
frequently diagnosed; bilateral lesions were present in 4 patients
(18.2%). Clinically, 14 patients (63.6%) presented with a palpable
painless solitary mass, 8 (36.4%) had multiple nodules, 1 (4.5%)
exhibited skin and nipple changes and 2 (9.1%) reported tenderness.
A total of 10 (45.5%) patients had palpable enlarged axillary lymph
nodes, of which 4 patients (18.2%) presented with enlarged
supraclavicular lymph nodes simultaneously.
 | Table II.Clinical characteristics of patients
with metastases to the breast. |
Table II.
Clinical characteristics of patients
with metastases to the breast.
|
Characteristics | Patients, n
(%) |
|---|
| Initial
detections |
|
|
Self.checking | 14 (63.6) |
|
Ultrasonography | 2 (9.1) |
|
Mammogram | 2 (9.1) |
| CT
(including PET.CT) | 3 (13.6) |
|
Unknown | 1 (4.5) |
| Clinical
symptoms |
|
| A
palpable painless solitary mass | 14 (63.6) |
|
Multiple nodules | 8 (36.4) |
| Skin
and nipple changes | 1 (4.5) |
|
Tendernessa | 2 (9.1) |
|
Enlarged axillary lymph
nodesb | 10 (45.5) |
| Tumor size, cm |
|
|
Mean | 2.9 |
|
Range | 0.8.12 |
| Breast
involvement |
|
|
Unilateral, left | 10 (45.5) |
|
Unilateral, right | 8 (36.4) |
|
Bilateral | 4 (18.2) |
|
Quadrant |
|
|
Innerc: Upper/mid/lower | 8 (30.8) |
|
Outerd: Upper/mid/lower | 18 (69.2) |
Ultrasonic and mammographic findings
of breast metastases
Among the 22 patients who underwent radiologic
imaging (Table III), 14 patients
underwent breast ultrasonography and 5 patients underwent a
mammography. In mammography, the most common finding was a single
mass with either circumscribed (3/5, 60%) or speculated (2/5, 40%)
margins. Only 1 (20%) patient who underwent a mammography presented
with a lesion classified as a BI-RADS category I which refers to a
negative examination by mammography, and the rest (4/5, 80%)
presented lesions categorized as BI-RADS category IVb or greater,
which refers to a high probability of malignancy. In
ultrasonography, breast lesions were primarily hypoechoic and
margins may be either circumscribed (8/14, 57.1%) or speculated
(6/14, 42.9%). The vascularity (8/14, 57.1%) of the lesions and
BI-RADS category V (7/14, 50%) were most commonly identified.
Calcifications were observed in only 2 cases in ultrasonography
(14.3%) and mammography (40%) each.
 | Table III.Ultrasonic and mammographic findings
of breast metastasesa. |
Table III.
Ultrasonic and mammographic findings
of breast metastasesa.
|
Characteristics | Patients, n |
|---|
| Ultrasonic findings
(n=14) |
|
|
Circumscribed/speculated
margins | 8/6 |
| Posterior echo |
|
| No
change/attenuation/enhancement | 8/3/3 |
| BI-RADS
category, II/III/IVa/IVb/IVc/V | 1/1/1/2/2/7 |
|
Calcifications | 2 |
|
Cooper's ligaments
involved | 1 |
|
Skin/subcutaneous nodule | 3 |
|
Vascularity | 8b |
| Mammographic
findings (n=5) |
|
| Margins,
circumscribed/speculated | 3/2 |
| BI-RADS
category, I/IVb/V | 1/3/1 |
|
Calcifications | 2 |
Histological features in different
types of carcinoma and non-carcinoma in patients with breast
metastases
Histological features of different tumor types and
primary tumor sites are presented in Tables IV and V. Carcinoma was the most common tumor type
for non-mammary metastases (16/22, 72.7%; Table IV), followed by melanoma (3/22,
13.6%; Table V) and sarcoma (2/22,
9.1%; Table V). The remaining patient
presented with a rare Wilms' tumor metastasis to the breast
(Table V). The most frequent primary
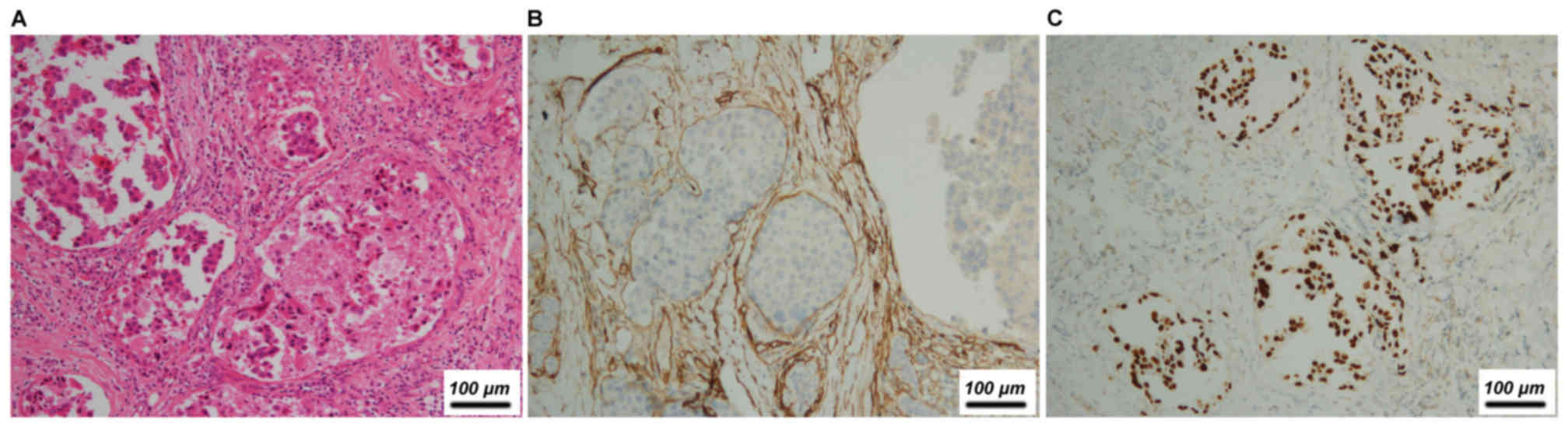
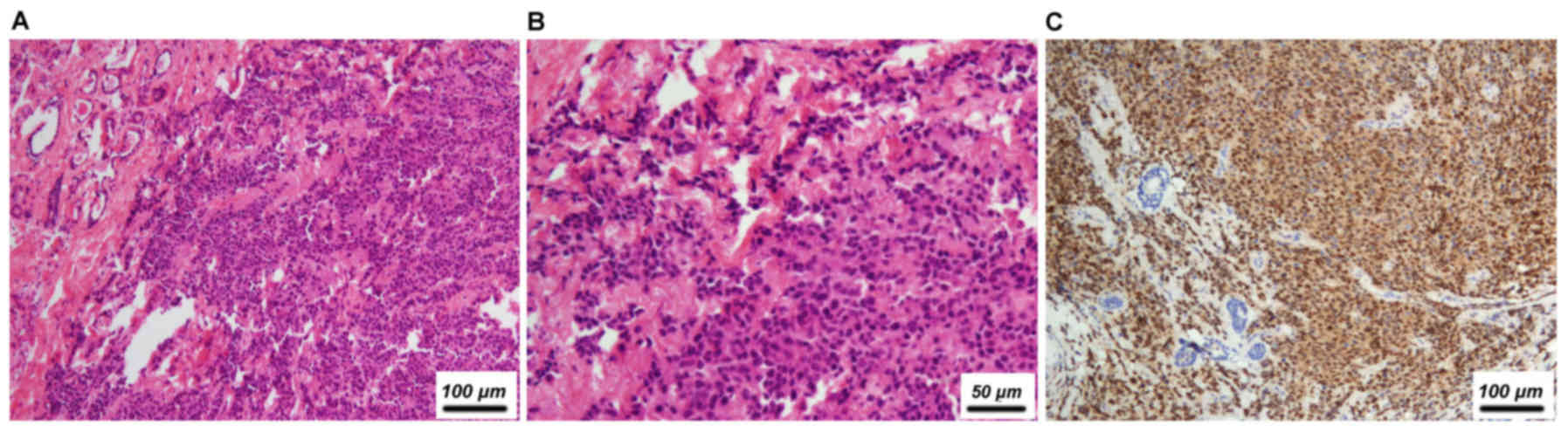
tumor site for carcinoma was the lungs (5/22, 22.7%, Fig. 1), followed by the nasopharynx (4/22,
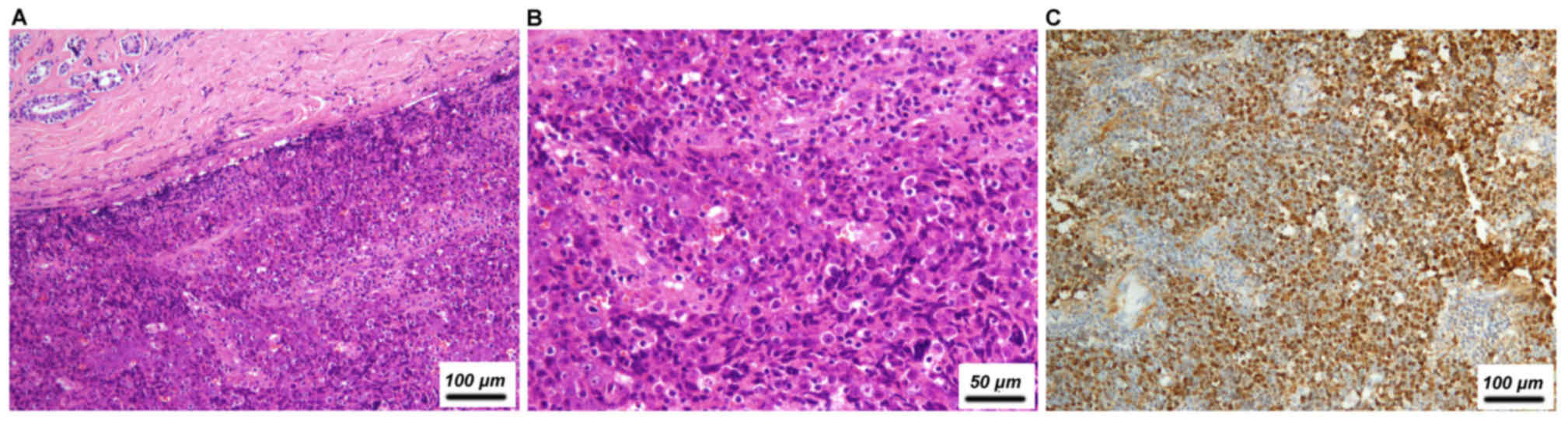
18.2%; Fig. 2) and ovary (3/22,
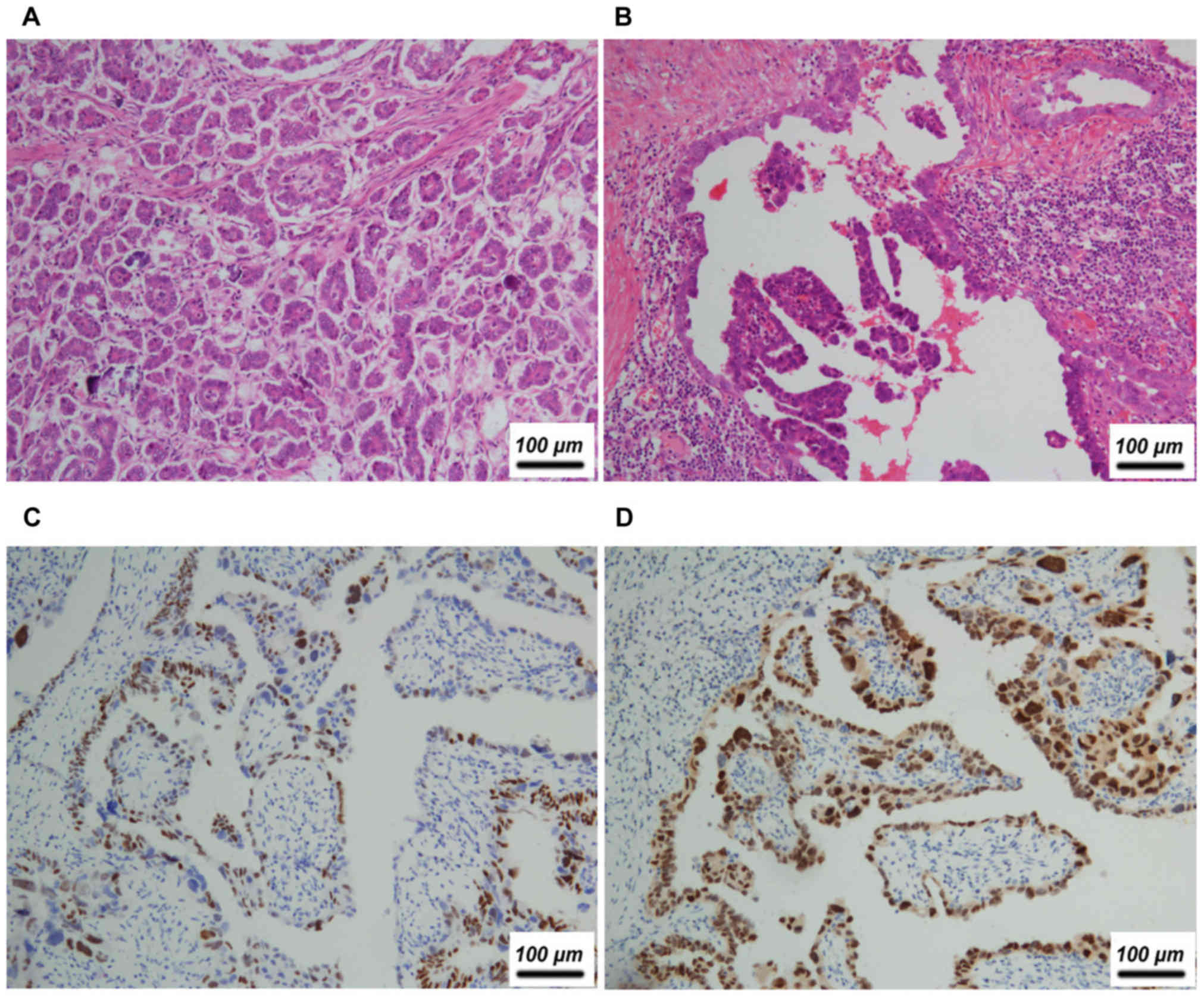
13.6%; Fig. 3). Other primary sites
included the gastrointestinal tract (2/22, 9.1%) and thyroid (1/22,
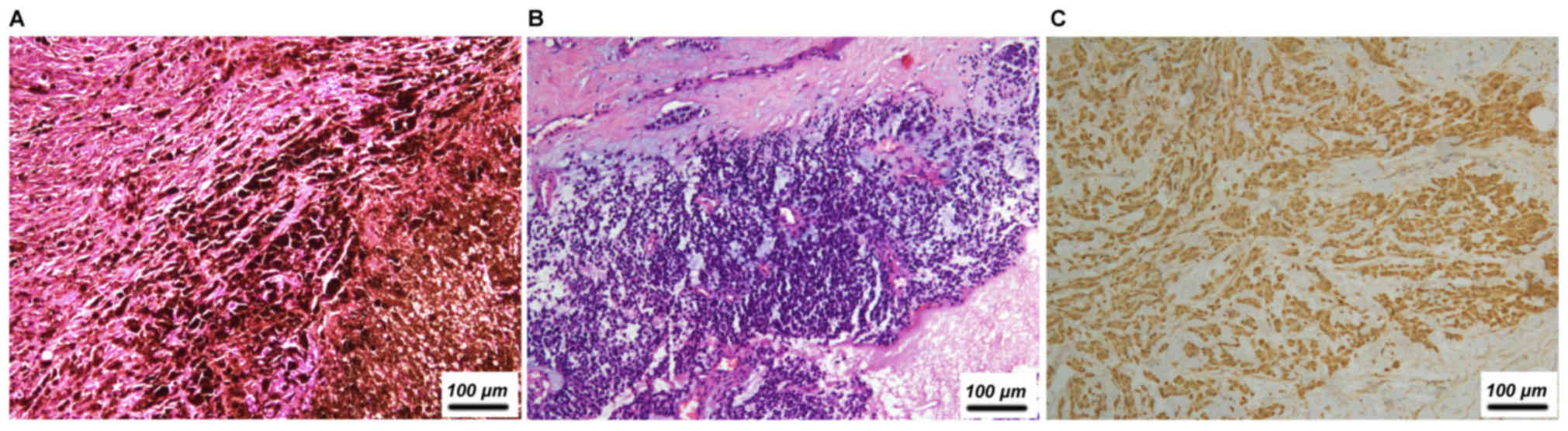
4.5%). Of the three melanoma cases, two initially occurred at
cutaneous sites and one was ocular (Fig.
4 and Table V). The two cases of
sarcoma were rhabdomyosarcoma (Fig.
5), with the primary sites being the palm and nasal cavity
(Table V).
 | Table IV.Histological features in different
types of carcinoma in patients with breast metastases. |
Table IV.
Histological features in different
types of carcinoma in patients with breast metastases.
| Primary site | Pathological
pattern | Sex | Age, year | Specific
histological features | Nipple/skin
invasiona | Lymphovascular
emboli | Axillary LN
metastasesa | Calcification | Necrosis | Other invasion | IHCb |
|---|
| Lung | Adenocarcinoma | F | 44 | No | + (nipple) | + | + | − | + | + (pectoralis
major; ribs) | + |
| Lung | Adenocarcinoma | F | 60 | No | + (nipple,
skin) | + | + | + | − | + (extra
nodal) | + |
| Lung |
Adenocarcinomac | F | 45 | No, spindle
cells | NA | − | NA | − | − | − | + |
| Lung | Adenocarcinoma | F | 62 | No | NA | − | NA | + | − | − | + |
| Lung | Adenocarcinoma | F | 44 | No | NA | − | NA | − | − | − | + |
| Nasopharynx | Undifferentiated
carcinoma | F | 41 | No | NA | − | NA | − | − | − | + |
| Nasopharynx | Undifferentiated
carcinoma | F | 24 | No | NA | − | NA | − | − | − | + |
| Nasopharynx | Undifferentiated
carcinoma | M | 46 | Yes, cells with
vesicular nuclei | NA | − | NA | − | − | − | − |
| Nasopharynx | Undifferentiated
carcinoma | M | 45 | Yes, cells with
vesicular nuclei | − | − | + | − | − | − | + |
| Ovary | Serous papillary
adenocarcinoma | F | 50 | Yes,
micropapillary | − | − | + | + | − | − | + |
| Ovary | Serous papillary
adenocarcinoma | F | 42 | Yes, papillary | NA | + | NA | − | + | + (pectoralis
major) | + |
| Ovary | Serous papillary
adenocarcinoma | F | 39 | Yes, papillary | NA | − | NA | − | − | − | − |
| Duodenum | Adenocarcinoma | F | 29 | Yes,
intracytoplasmic mucinous vacuoles | NA | − | NA | − | + | − | − |
| Stomach | Adenocarcinoma | F | 41 | No, rare
signet-ring cell | NA | − | NA | − | − | − | + |
| Thyroid | Papillary thyroid
carcinoma | F | 34 | Yes, papillary and
typical colloid | NA | + | NA | − | + (sternoclavicular
joint) | + | − |
| Cervix | Small cell
neuroendocrine carcinomad | F | 37 | Yes, small
cells | NA | − | NA | − | − | − | − |
 | Table V.Histological features in different
types of non-carcinoma in patients with breast metastases. |
Table V.
Histological features in different
types of non-carcinoma in patients with breast metastases.
| Primary site | Pathological
pattern | Sex | Age, years | Specific
histological features | Nipple (or skin)
invasiona | Lymphovascular
emboli | Axillary LN
metastasesa | Calcification | Necrosis | Other invasion | IHCb |
|---|
| Skin
(abdominal) | Melanoma | F | 49 | No | − | − | + | − | − | − | + |
| Skin (foot) | Melanoma | F | 46 | Yes, pigment | NA | − | NA | − | + | − | + |
| Eyes | Melanoma | F | 45 | Yes, pigment and
intranuclear inclusions | NA | − | NA | − | + | − | − |
| Palm | Rhabdomyosarcoma
(embryonic) | F | 20 | Yes, spindle cells,
eosinophilic cytoplasm | + (nipple,
skin) | − | + | − | − | − | − |
| Nasal cavity | Rhabdomyosarcoma
(alveolar) | F | 41 | No | − | − | − | − | − | − | + |
| Abdomen | Wilms' tumor | F | 10 | Yes,
epithelial-like cells and undifferentiated mesenchymal cells | NA | − | NA | − | + | − | + |
Immunohistochemistry (IHC) was performed in 15
patients (68.2%) in total. Of these, lung cancer was the most
frequent tumor identified (5/15, 33.3%), followed by tumors of
asopharynx (3/15, 20.0%), ovary (2/15, 13.3%), melanoma (2/15,
13.3%), rhabdomyosarcoma (1/15, 6.7%; data not shown), Wilms' tumor
(1/15, 6.7%) and thyroid (1/15, 6.7%; data not shown). The most
frequently used markers included the estrogen receptor (ER),
progesterone receptor (PR), human epidermal growth factor
receptor-2 (HER-2), mammaglobin, gross cystic disease fluid
protein-15 (GCDFP-15), cytokeratin, p63, transcription termination
factor-1 (TTF-1), S-100 by IHC and Epstein-Barr virus-encoded RNA
(EBER) by in situ hybridization, which together with the
primary tumor history, were conducive for accurate diagnosis.
Surgical resection of the breast was performed in 17
patients (77.3%): 7 patients (31.8%) had modified radical
mastectomy and 10 patients (45.5%) had breast-conserving surgery
(data not shown). Follow-up data were available in 20 cases. The
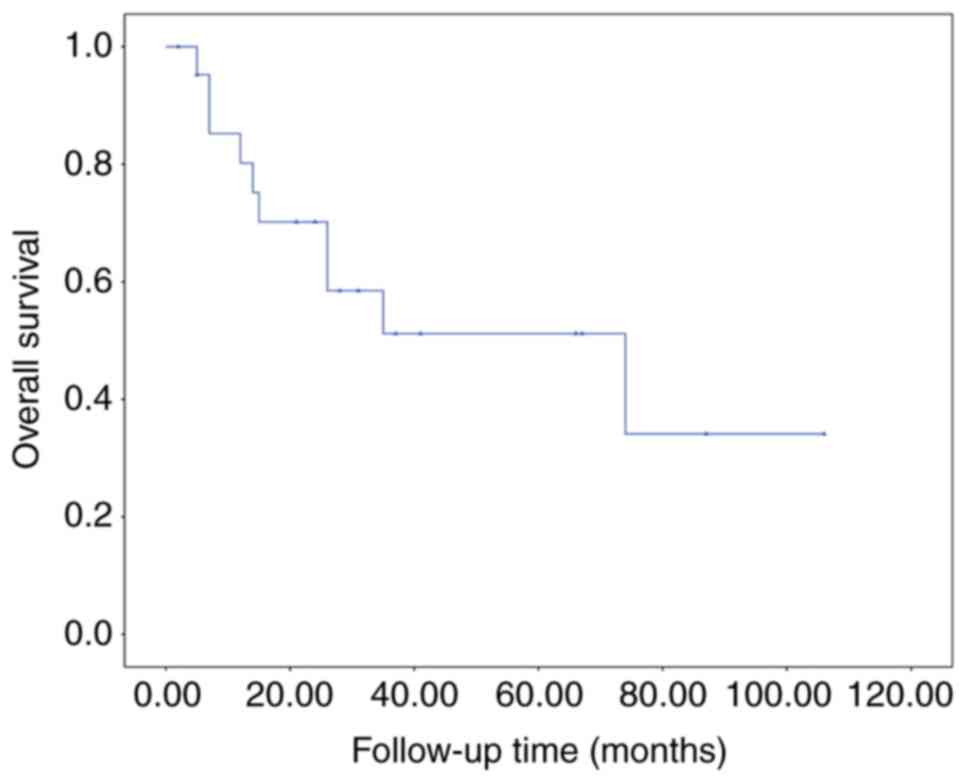
median survival duration from diagnosis of metastases to the breast
was 35 months (range, 2–106 months). A total of 10 patients (45.5%)
succumbed from the disease. Univariate analysis was applied to
evaluate different interventions on overall survival (Figs. 6–8).
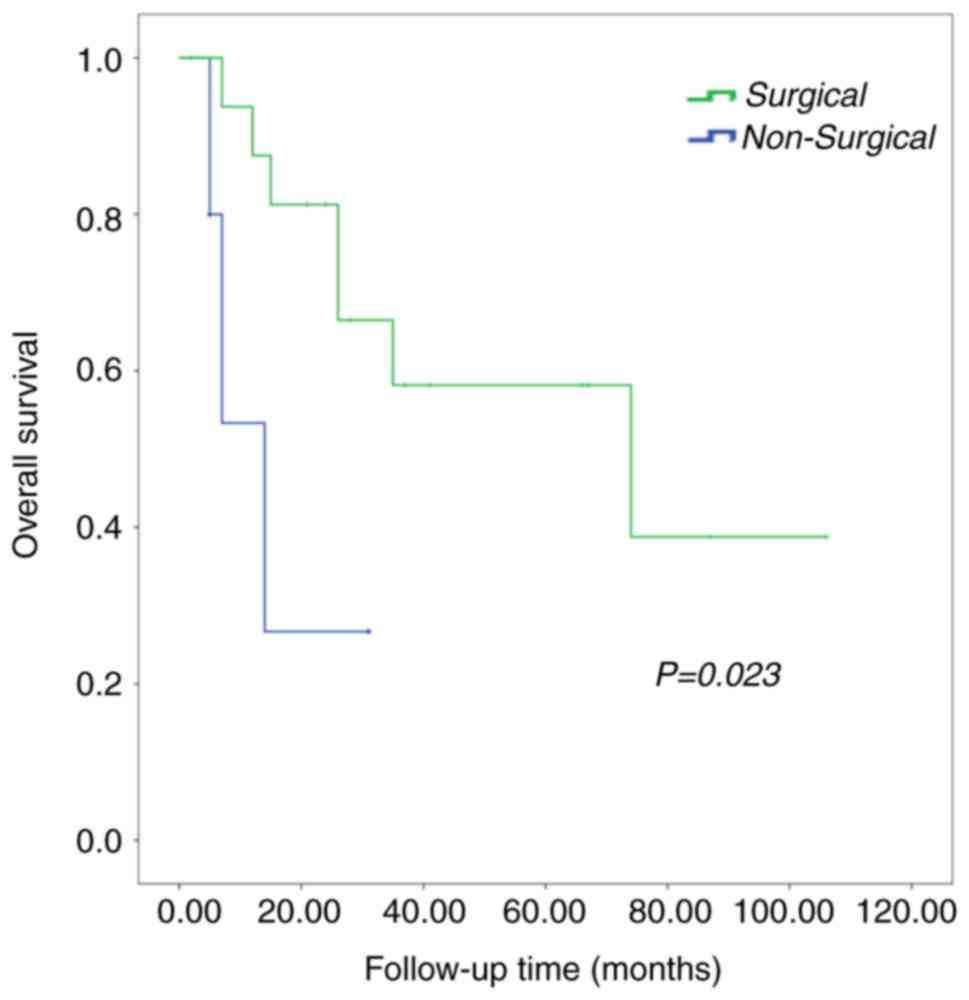
Patients who underwent surgery had a median survival duration of 74
months, while patients who did not undergo surgery survived for a
median duration of 12 months (P=0.023; Fig. 7). However, the difference in survival
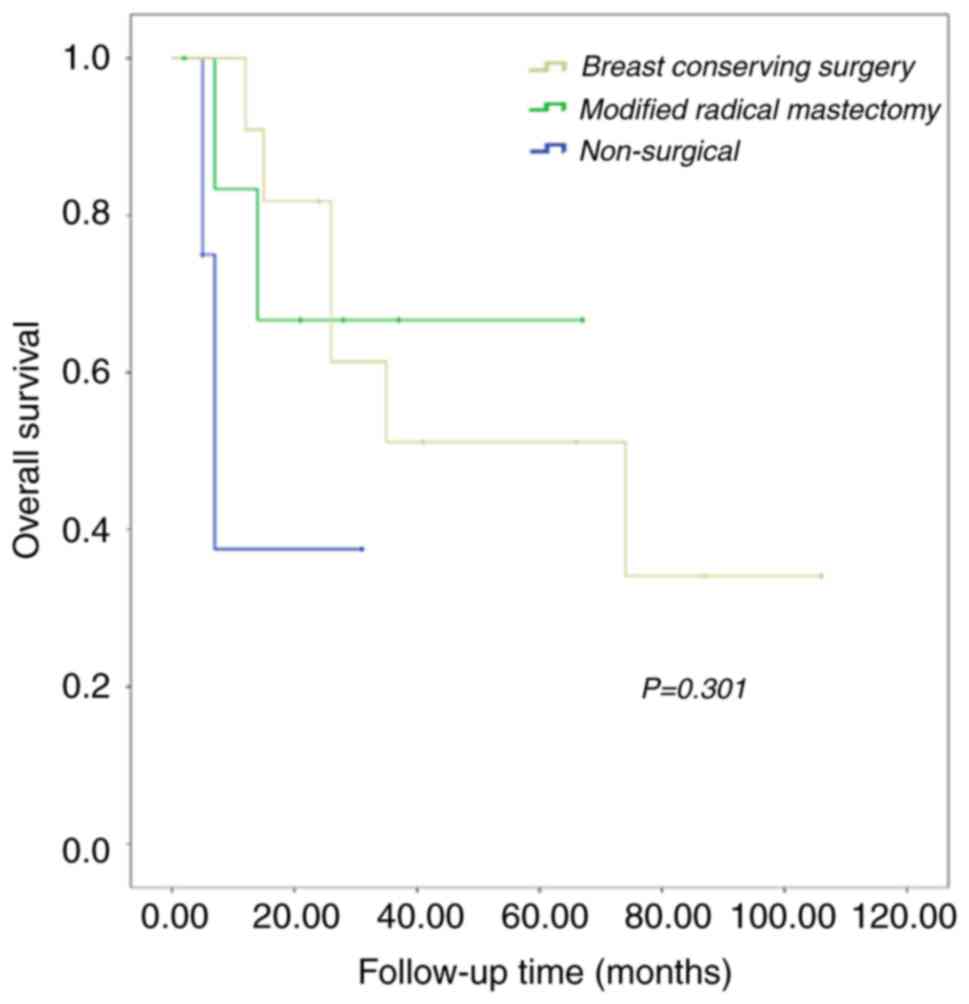
observed among patients who underwent modified radical mastectomy,
breast-conserving surgery and non-surgical intervention was not
significant (P=0.301; Fig. 8).
Discussion
Metastasis to the breast may occur in various types
of non-breast solid tumor; however, cases of this are extremely
rare (1–5,10).
Additionally, the therapeutic strategies used to treat these cases
are extremely different from those used to treat primary breast
cancer, making the history of the primary tumor particularly
important for the achievement of an accurate diagnosis (10). Williams et al (3) and Lee et al (4) demonstrated that a documented history of
a primary tumor was observed in 72–88% of patients with breast
metastases, and that these metastases more frequently occurred in
females, which is consistent with the results of the present study.
The results of the present study also demonstrated that the breast
lesion was the initial or only metastasis in half of the patients,
which is inconsistent with previous studies (4,10–13), which demonstrated that these
metastases are most likely to originate from carcinoma of genital
organs (3/4, 75.0%) and non-carcinoma (5/6, 83.3%). These
inconsistencies may be due to the variance in pathological cancer
types and the young onset age of 40.5 years in the patients used in
the current study, which is an age group that remains at high risk
of breast cancer (14). Other
previous studies have indicated that there is a higher incidence of
metastasis to the breast from non-breast solid tumors that occur in
adolescence (15), as well as during
lactation and pregnancy (16), owing
to the change in hormonal status during these periods. Longer
median time (2 years) intervals from the point of diagnosis of the
primary tumor to breast metastasis compared with those in the
current study have also been reported (17).
A single palpable painless mass located in the upper
outer quadrant of the breast was the most common initial clinical
feature reported in the current study. Akcay et al (2) demonstrated that the breast metastases
were frequently multiple and bilateral, which was observed in 36.4%
(8/22) and 18.2% (4/22) of patients in the current study,
respectively. Laterality was not present at either side of the
breast, which was inconsistent with earlier studies (3,4,17). Lee et al (4) suggested that a preponderant lymphatic
pathway to the breast from other organs may contribute to
laterality; however, further investigations are required.
Considering the rarity of breast metastases, a new primary breast
cancer may be considered ‘preferable’ for the breast lesion even in
patients with a history of definite primary extramammary malignancy
(3,4,17).
Radiological imaging also aids the establishment of a more accurate
diagnosis (18).
Previous ultrasonic and mammographic images studies
demonstrated that it is occasionally difficult to distinguish
breast metastases, which appeared as a hypoechoic or high-density
well-circumscribed and freely movable mass, from primary breast
cancer which displays a hypoechoic mass with speculated margin or a
diffuse lesions with or without calcifications (5,18,19). However, despite the absence of diffuse
lesions, 42.9% of the patients in the current study presented with
a mass with a speculated margin. Calcifications are rarely observed
in breast metastases (20,21), with the exception of metastatic
ovarian papillary carcinoma with psammoma bodies (22,23), which
is consistent with the results of the current study. Two cases of
breast metastasis from lung adenocarcinoma with calcifications was
also presented in the current study. Breast lesions were classified
as BI-RADS IVA or greater in the majority of cases in the present
study; the others were categorized as BI-RADS I–III, suggesting
that a metastatic mass from a solitary cyst should be distinguished
from a metastatic mass from a fibroadenoma, particularly in
postmenopausal women or women with a known history of cancer.
Posterior echo enhancement and vascularity demonstrated by Color
Doppler have previously been used to accurately identify a lesion
as either a metastatic melanoma or sarcoma (5,24) and the
results of the present study revealed that posterior echoic
enhancement and vascularity were observed in all three cases of
breast metastases from melanoma. Asian women have smaller breasts
with higher gland density and lower lipid content (25) than women from Western countries. The
lack of calcification may therefore lead to misdiagnosis of breast
lesions by mammography (5,22). Further research comparing the accuracy
of ultrasonic screening and mammography for diagnosis of breast
metastases in Asian females is required. Computerized tomography
and magnetic resonance imaging have also been used for diagnosis of
breast metastases (26,27); however, 95% of the patients in the
present study underwent a biopsy followed by radiological imaging,
confirming the diagnosis of breast metastasis.
Consistent with previous reports (3,15,17), lung adenocarcinoma, ovarian serous
papillary carcinoma and melanoma were the most common primary
carcinomas that metastasized to the breast. DeLair et al
(17) also reported that lung
adenocarcinoma and melanoma were more common in males. Previous
studies have reported the relatively high incidence of gastric
carcinoma (4) and lymphoma (28). To the best of our knowledge, the
present study is the first to report nasopharyngeal carcinoma (NPC)
as accounting for a high proportion (4/22) of primary tumors, with
half of these cases occurring in men. The inconsistency between the
results of the present and previous studies may be due to referral
(the Sun Yat-sen University Cancer Center is known for its
excellence in nasopharyngeal carcinomas care), geographical and/or
racial biases, as there is a higher disease prevalence of
nasopharyngeal carcinoma in southern Chinese Han males (29,30).
Typical morphological features of NPC, including cells with
vesicular nuclei and positive in situ hybridization staining
for EBERs, as well as immunostaining of cytokeratin 5/6 and p63,
aided the achievement of a diagnosis of NPC in the current study.
However, given the limited numbers of cases reported thus far,
further investigation is required.
Breast metastases and primary breast cancer have
common histological findings, including periductal and perilobular
distribution, absence of ductal carcinoma in situ, lack of
stromal reaction, including desmoplastic response and elastosis and
a large number of lymphovascular tumor emboli (4,15,31). The majority of metastatic lesions have
histological appearances consistent with their primary sites and
thus, pathologists often identify a metastasis to the breast by
comparing histological patterns to a previous sample from the
patient (4). This evaluation was
performed with metastatic melanoma, sarcoma, neuroendocrine
carcinoma and Wilms tumor in the present study. Immunohistochemical
staining for tumor-specific markers, including ER, PR, HER-2,
mammaglobin, GCDFP-15 and GATA binding protein 3 (GATA3) for breast
cancer; TTF-1 and Napsin A for lung adenocarcinoma; Wilms tumor
protein, paired box protein PAX-8 (PAX-8) and CA-125 for ovarian
carcinoma; CK20 and homeobox protein CDX-2 for gastrointestinal
tract adenocarcinoma; CgA, NSE and Synapsin for neuroendocrine
carcinoma; CK19 and thyroid peroxidase for thyroid carcinoma; S-100
and HMB-45 for melanoma; and myogenin for rhabdomyosarcoma aid
diagnosis. However, several overlaps exist, including ER, PR and
GCDFP-15 in histological appearance and immunophenotyping,
particularly between ovarian carcinoma and primary breast cancer,
often leading to difficulties in differential diagnosis (32). Prior studies have reported that
GCDFP-15 was rarely observed in ovarian carcinoma (33,34),
whereas ~30% of primary breast cancer also did not exhibit GCDFP-15
expression (34). Other studies have
reported that up to 95% of serous papillary carcinomas exhibit
nuclear expression of WT-1and membrane expression of CA125, which
is only present in <10% of different types of breast cancer
(35–37). A more recent study also suggested the
value of GATA-3 and PAX-8 as biomarkers for breast cancer (38,39).
Consistent with a previous study (3),
the results of the current study indicate that no single marker is
absolutely specific and its expression is always variable between
primary and metastatic lesions, particularly in tissue biopsies.
Thus, a panel of IHC markers with the same pathology as that of the
primary tumor, clinical history and imaging findings are required
in combination for accurate diagnosis.
The prognosis of metastases to the breast remains
poor (1–4,10,17). The median survival duration reported
by Williams et al (3) (169
cases), DeLair et al (17) (85
cases) and Lee et al (4) (30
cases) was 10, 15 and 13.9 months, respectively, which is
consistent with the results of the current study, in which the
shortest survival duration following diagnosis was >2 months.
Over 70% of patients had widely metastatic disease in combination
with the breast lesion, which is likely to be the main contributor
to the poor survival observed in the present study. Individualized
systemic therapeutic strategies for primary tumors should be
recommended as the primary therapy in a majority of cases (1–4,10,17);
however, the benefit of surgical resection of the breast remains
controversial. Consistent with the findings of Williams et
al (3), the results of the
current study indicated improved overall survival in patients who
underwent surgery compared with those who did not. However, there
are limitations to the current study: The sample size was extremely
small and there was selection bias, as surgery is not well
tolerated in patients with advanced disease or poor health
condition, as described previously (3). Previously, Rossfeld and Carson (40) proposed the benefit of metastasectomy
and suggested the re-evaluation of the approach of ‘sparing
patients unnecessary surgery’. Understanding the patients'
therapeutic goals should be the determinant factor in treating
metastatic lesions. Further multi-center clinical investigations
are therefore required to address the characteristics of breast
metastases that originate from non-breast solid tumor, as well as
the effect of surgery on patient prognosis.
In summary, breast metastases are rare and primarily
indicate a poor prognosis. Additionally, it may be easily
misdiagnosed as a primary breast cancer. Clinical manifestations,
radiologic findings and histopathological/immunohistochemical
features should all be considered in differentiating a secondary
mass from a primary breast cancer, even in patients without a
history of primary malignant tumors. Early and accurate diagnosis
is conducive to individualized treatment and improved prognosis
improvement. In addition, surgical resection of breast metastases
may result in a survival benefit, which remains yet to be further
studied.
References
|
1
|
Alva S and Shetty-Alva N: An update of
tumor metastasis to the breast data. Arch Surg. 134:4501999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akçay MN: Metastatic disease in the
breast. Breast. 11:526–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williams SA, Ehlers RA II, Hunt KK, Yi M,
Kuerer HM, Singletary SE, Ross MI, Feig BW, Symmans WF and
Meric-Bernstam F: Metastases to the breast from nonbreast solid
neoplasms: Presentation and determinants of survival. Cancer.
110:731–737. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee SK, Kim WW, Kim SH, Hur SM, Kim S,
Choi JH, Cho EY, Han SY, Hahn BK, Choe JH, et al: Characteristics
of metastasis in the breast from extramammary malignancies. J Surg
Oncol. 101:137–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sippo DA, Kulkarni K, Carlo PD, Lee B,
Eisner D, Cimino-Mathews A and Harvey SC: Metastatic disease to the
breast from extramammary malignancies: A multimodality pictorial
review. Curr Probl Diagn Radiol. 45:225–232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Manini C, Pietribiasi F, Sapino A and
Donadio S: Serous cystadenocarcinoma of the ovary with simultaneous
breast metastases. Description of a case. Pathologica. 90:152–155.
1998.(In Italian).
|
|
7
|
Hanna NN, O'Donnell K and Wolfe GR:
Alveolar soft part sarcoma metastatic to the breast. J Surg Oncol.
61:159–162. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oksüzoğlu B, Abali H, Güler N, Baltali E
and Ozişik Y: Metastasis to the breast from nonmammarian solid
neoplasms: A report of five cases. Med Oncol. 20:295–300. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liberman L and Menell JH: Breast imaging
reporting and data system (BI-RADS). Radiol Clin North Am.
40:409–430, v. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hajdu SI and Urban JA: Cancers metastatic
to the breast. Cancer. 29:1691–1696. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamby LS, McGrath PC, Cibull ML and
Schwartz RW: Gastric carcinoma metastatic to the breast. J Surg
Oncol. 48:117–121. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toombs BD and Kalisher L: Metastatic
disease to the breast: Clinical, pathologic, and radiographic
features. AJR Am J Roentgenol. 129:673–676. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mihai R, Christie-Brown J and Bristol J:
Breast metastases from colorectal carcinoma. Breast. 13:155–158.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brouckaert O, Rudolph A, Laenen A, Keeman
R, Bolla MK, Wang Q, Soubry A, Wildiers H, Andrulis IL, Arndt V, et
al: Reproductive profiles and risk of breast cancer subtypes: A
multi-center case-only study. Breast Cancer Res. 19:1192017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vergier B, Trojani M, de Mascarel I,
Coindre JM and Le Treut A: Metastases to the breast: Differential
diagnosis from primary breast carcinoma. J Surg Oncol. 48:112–116.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nayar M, Chandra M, Aggarwal R and Chander
S: Carcinoma cervix presenting as primary breast malignancy. Indian
J Pathol Microbiol. 30:283–286. 1987.PubMed/NCBI
|
|
17
|
DeLair DF, Corben AD, Catalano JP, Vallejo
CE, Brogi E and Tan LK: Non-mammary metastases to the breast and
axilla: A study of 85 cases. Mod Pathol. 26:343–349. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yeh CN, Lin CH and Chen MF: Clinical and
ultrasonographic characteristics of breast metastases from
extramammary malignancies. Am Surg. 70:287–290. 2004.PubMed/NCBI
|
|
19
|
Mun SH, Ko EY, Han BK, Shin JH, Kim SJ and
Cho EY: Breast metastases from extramammary malignancies: Typical
and atypical ultrasound features. Korean J Radiol. 15:20–28. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bohman LG, Bassett LW, Gold RH and Voet R:
Breast metastases from extramammary malignancies. Radiology.
144:309–312. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCrea ES, Johnston C and Haney PJ:
Metastases to the breast. AJR Am J Roentgenol. 141:685–690. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SH, Park JM, Kook SH, Han BK and Moon
WK: Metastatic tumors to the breast: Mammographic and
ultrasonographic findings. J Ultrasound Med. 19:257–262. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vizcaíno I, Torregrosa A, Higueras V,
Morote V, Cremades A, Torres V, Olmos S and Molins C: Metastasis to
the breast from extramammary malignancies: A report of four cases
and a review of literature. Eur Radiol. 11:1659–1665. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Surov A, Fiedler E, Holzhausen HJ, Ruschke
K, Schmoll HJ and Spielmann RP: Metastases to the breast from
non-mammary malignancies: Primary tumors, prevalence, clinical
signs, and radiological features. Acad Radiol. 18:565–574. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Torjesen I: Adding ultrasound to
mammography could increase breast cancer detection in Asian women.
BMJ. 351:h59262015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Phadke S, Thomas A, Yang L, Moore C, Xia C
and Schroeder MC: Frequency and clinical significance of
extramammary findings on breast magnetic resonance imaging. Clin
Breast Cancer. 16:424–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benveniste AP, Marom EM, Benveniste MF,
Mawlawi OR, Miranda RN and Yang W: Metastases to the breast from
extramammary malignancies-PET/CT findings. Eur J Radiol.
83:1106–1112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Buisman FE, van Gelder L, Menke-Pluijmers
MB, Bisschops BH, Plaisier PW and Westenend PJ: Non-primary breast
malignancies: A single institution's experience of a diagnostic
challenge with important therapeutic consequences-a retrospective
study. World J Surg Oncol. 14:1662016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leach BI, Sun B, Petrovic L and Liu SV:
Breast metastasis from nasopharyngeal carcinoma: A case report and
review of the literature. Oncol Lett. 5:1859–1861. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li S and Yang J: Nasopharyngeal carcinoma
metastasis to the mammary gland: A case report. Oncol Lett.
9:275–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Georgiannos SN, Chin J, Goode AW and
Sheaff M: Secondary neoplasms of the breast: A survey of the 20th
century. Cancer. 92:2259–2266. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tornos C, Soslow R, Chen S, Akram M,
Hummer AJ, Abu-Rustum N, Norton L and Tan LK: Expression of WT1, CA
125, and GCDFP-15 as useful markers in the differential diagnosis
of primary ovarian carcinomas versus metastatic breast cancer to
the ovary. Am J Surg Pathol. 29:1482–1489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dennis JL, Hvidsten TR, Wit EC, Komorowski
J, Bell AK, Downie I, Mooney J, Verbeke C, Bellamy C, Keith WN and
Oien KA: Markers of adenocarcinoma characteristic of the site of
origin: Development of a diagnostic algorithm. Clin Cancer Res.
11:3766–3772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moritani S, Ichihara S, Hasegawa M, Endo
T, Oiwa M, Yoshikawa K, Sato Y, Aoyama H, Hayashi T and Kushima R:
Serous papillary adenocarcinoma of the female genital organs and
invasive micropapillary carcinoma of the breast. Are WT1, CA125,
and GCDFP-15 useful in differential diagnosis? Hum Pathol.
39:666–671. 2008.
|
|
35
|
Lagendijk JH, Mullink H, van Diest PJ,
Meijer GA and Meijer CJ: Immunohistochemical differentiation
between primary adenocarcinomas of the ovary and ovarian metastases
of colonic and breast origin. Comparison between a statistical and
an intuitive approach. J Clin Pathol. 52:283–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Recine MA, Deavers MT, Middleton LP, Silva
EG and Malpica A: Serous carcinoma of the ovary and peritoneum with
metastases to the breast and axillary lymph nodes: A potential
pitfall. Am J Surg Pathol. 28:1646–1651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee BH, Hecht JL, Pinkus JL and Pinkus GS:
WT1, estrogen receptor, and progesterone receptor as markers for
breast or ovarian primary sites in metastatic adenocarcinoma to
body fluids. Am J Clin Pathol. 117:745–750. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Espinosa I, Gallardo A, D'Angelo E, Mozos
A, Lerma E and Prat J: Simultaneous carcinomas of the breast and
ovary: Utility of Pax-8, WT-1, and GATA3 for distinguishing
independent primary tumors from metastases. Int J Gynecol Pathol.
34:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tempfer CB, El Fizazi N, Ergonenc H and
Solass W: Metastasis of ovarian cancer to the breast: A report of
two cases and a review of the literature. Oncol Lett. 11:4008–4012.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rossfeld KK and Carson WE III: Surgical
management of ovarian carcinoma metastatic to the breast and
axilla: A role for metastasectomy? J Surg Oncol. 112:581–584. 2015.
View Article : Google Scholar : PubMed/NCBI
|