Introduction
Laryngeal squamous cell carcinoma (LSCC) is a common
malignancy of the head and neck that is increasing in morbidity and
mortality worldwide (1). As
biomarkers for various cancers, tumor-node-metastasis stage and
grade may be insufficient to indicate a prognosis and treatment for
LSCC. The identification of prognostic and predictive biomarkers of
LSCC may help clinicians select more appropriate treatment for
individual patients. Invasion and metastasis are considered the
major clinical challenges in the treatment of cancer; the cellular
process of epithelial-mesenchymal transition (EMT), which is
characterized by the loss of epithelial markers (including adherens
junction proteins E-cadherin, α- and β-catenin) and increased
expression of mesenchymal markers (including N-cadherin and
vimentin), promotes the aggressive behavior of cancer (2–4).
Although certain molecular markers of the EMT
processes have been considered in numerous cancer cell models in
vitro (5–7), the association between EMT-associated
molecular alterations with LSCC clinicopathological characteristics
and prognosis requires further investigation. E-cadherin binding to
β-catenin on the cytomembrane represses tumor progression by
maintaining cellular adhesion to prevent EMT, cell motility and
tumor metastasis (8). Downregulation
or loss of E-cadherin and β-catenin from the cytomembrane and
nuclear β-catenin expression are frequently observed in multiple
cancer types, including head and neck cancer (9–11). EMT has
also been demonstrated to be induced by the expression of other
EMT-associated proteins, including N-cadherin and zinc finger E-box
binding homeobox 2 (ZEB2; also known as SIP1) in various different
cancer types, including head and neck squamous cell carcinoma
(HNSCC) (12). The decreased
expression of membranous E-cadherin accompanied by a simultaneous
increase in the expression of N-cadherin (termed the ‘cadherin
switch’) has been reported as a phenomenon that is associated with
lymph node metastasis in HNSCC, and disease recurrence in LSCC
(13–15). Furthermore, ZEB2, a major repressor of
E-cadherin, is associated with the initial stage of EMT, and
promotes tumor cell migration and invasion (11,12). The
overexpression of ZEB2 has been reported in different cancer types
and metastatic lymph nodes in HNSCC tissues, and has been suggested
as a candidate biomarker for poor prognosis (5,6,16).
Therefore, the primary objective of the present
study was to examine the expression patterns of EMT-associated
markers (E-cadherin, N-cadherin, β-catenin and ZEB2) in a cohort of
patients with LSCC treated with surgery, with and without lymph
node metastasis, using immunohistochemical analyses. The results of
the present study indicated significant differences in the
expression of the four EMT-associated markers between LSCC and the
adjacent non-neoplastic laryngeal tissue. The association of these
biomarkers with LSCC clinicopathological phenotype and prognosis
was analyzed. In particular, the clinicopathological significance
of the co-expression of E-cadherin/N-cadherin,
E-cadherin/β-catenin, and E-cadherin/ZEB2 in LSCC was assessed.
Materials and methods
Patient cohort
This retrospective study included 76 patients with
stage I–IVa LSCC treated from February 2007 to November 2013 at the
Ear, Nose, and Throat (ENT) Department of Drum Tower Hospital,
Nanjing University (Nanjing, China). All of the patients were male
and aged 34–87 years; none of the patients had been previously
treated. The data collected, including tumor characteristics and
the age at diagnosis, are reported in Table I, using the American Joint Committee
on Cancer Staging Manual (2002) (17). All of the patients in the study
provided written informed consent; the study protocol was performed
in accordance with institutional bioethics guidelines and was
approved by the Research and Ethics Committee of Drum Tower
Hospital.
 | Table I.Patient clinicopathological
characteristics. |
Table I.
Patient clinicopathological
characteristics.
| Variable | Value |
|---|
| Total, n | 76 |
| Age (years) |
|
|
Range | 34–87 |
|
Median | 64 |
| Lymph node
metastasis, n (%) |
|
|
Positive | 38 (50.00) |
|
Negative | 38 (50.00) |
| Tumor stage, n
(%) |
|
|
T1–3 | 45 (59.21) |
| T4 | 31 (40.79) |
| Tumor cell
differentiation, n (%) |
|
|
Good | 8
(10.53) |
|
Moderate | 52 (68.42) |
|
Poor | 16 (21.05) |
| Localization, n
(%) |
|
|
Supraglottic | 14 (18.42) |
|
Glottic | 46 (60.53) |
|
Subglottic | 7 (9.21) |
|
Hypopharynx invaded | 9
(11.84) |
| Surgery type, n
(%) |
|
| Partial
laryngectomy | 3 (3.95) |
| Total
laryngectomy | 73 (96.05) |
All of the patients underwent primary partial or
total laryngectomy, and unilateral or bilateral cervical lymph node
dissection at the ENT Department of Drum Tower Hospital. All of the
collected pathology materials were reviewed after excision by the
Department of Pathology of Drum Tower Hospital to confirm the
diagnosis of LSCC and assess the degree of differentiation.
Adjacent non-neoplastic laryngeal tissues were used as
controls.
Immunohistochemistry
Representative tissue sections of 2-µm thickness
were dewaxed in xylene and rehydrated in graded ethanol. Antigen
retrieval was performed by pressure heating the slides in 0.01 M pH
6.0 citrate buffer (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China). Endogenous peroxidase activity was quenched
by treatment with 3% H2O2 for 15 min at room
temperature, followed by incubation with non-specific protein
blocking solution 1% bovine serum albumin (cat. no., 11021037;
Thermo Fisher Scientific, Waltham, USA) in PBS for 45 min at room
temperature. Sections were subsequently incubated with primary
antibodies against E-cadherin (mouse monoclonal; cat. no., ab1416),
N-cadherin (rabbit polyclonal; cat. no., ab18203), β-catenin
(rabbit monoclonal; cat. no., ab32572), and ZEB2 (rabbit
polyclonal; cat. no., ab138222; all Abcam, Cambridge, UK) overnight
at 4°C. The secondary reactions for all antibodies were performed
using the Polink-1 HRP DAB Detection System kit (OriGene
Technologies, Inc., Beijing, China). The slides were rinsed,
counterstained with Harris hematoxylin for 15 sec at room
temperature, dehydrated and mounted. For negative controls,
blocking solution was added instead of the primary antibody.
Immunohistochemical evaluation
All slides were assessed by the following evaluation
method, based on other studies regarding LSCC, HNSCC and oral
squamous cell carcinoma (OSCC) (18–20). In
the assessment of E-cadherin and β-catenin staining, the focus was
on the cell membranes. For E-cadherin, the staining was considered
negative, and therefore, ‘low expression’, if <90% of the cells
were positive for membranous staining. For β-catenin, the staining
of the cell membranes was evaluated as negative, with
weak-to-extensive staining in the cytoplasm and nucleus considered
positive. When considering N-cadherin, the cells were negative, and
therefore exhibited ‘low expression,’ if <20% of the cells were
stained. Finally, for ZEB2, the intensity of nuclear staining was
evaluated as follows: 0= no staining, 1= weakly positive, 2=
moderately positive and 3= strongly positive, and the extent of
staining was based on the percentage of positive cells, where
1=1–25%, 2=26–50%, 3=51–75%, and 4=7 6–100%. The total ZEB2
immunoreactivity score was calculated as the product of the scores
for the intensity of nuclear staining and the extent of staining.
The scores were then divided into negative (<3) and positive
(≥3).
Statistical analysis
Statistical analysis was performed with SPSS 22.0
(IBM Corp., Armonk, NY, USA). The statistical associations of
protein expression levels with clinicopathological parameters were
analyzed with Pearson's χ2, or Fisher's exact test for
nominal data. Survival probability differences were compared with
the log-rank test, and the association of survival rates with the
four EMT biomarkers was illustrated using Kaplan-Meier survival
curves. A multivariate analysis was performed using the Cox
proportional hazards model. P<0.05 was considered to indicate a
statistically significant result.
Results
Clinicopathological characteristics
and outcomes of patients with LSCC
The main clinicopathological characteristics of the
patients with LSCC in the present study are included in Table I. The follow-up survival data were
available for 70/76 patients (92.11%), with a median survival time
of 38.5 months, and a maximum follow-up period of 98 months.
EMT-related markers are differentially
expressed between LSCC and non-neoplastic tissues
E-cadherin, N-cadherin, β-catenin and ZEB2 were
differentially expressed between LSCC and non-neoplastic tissues
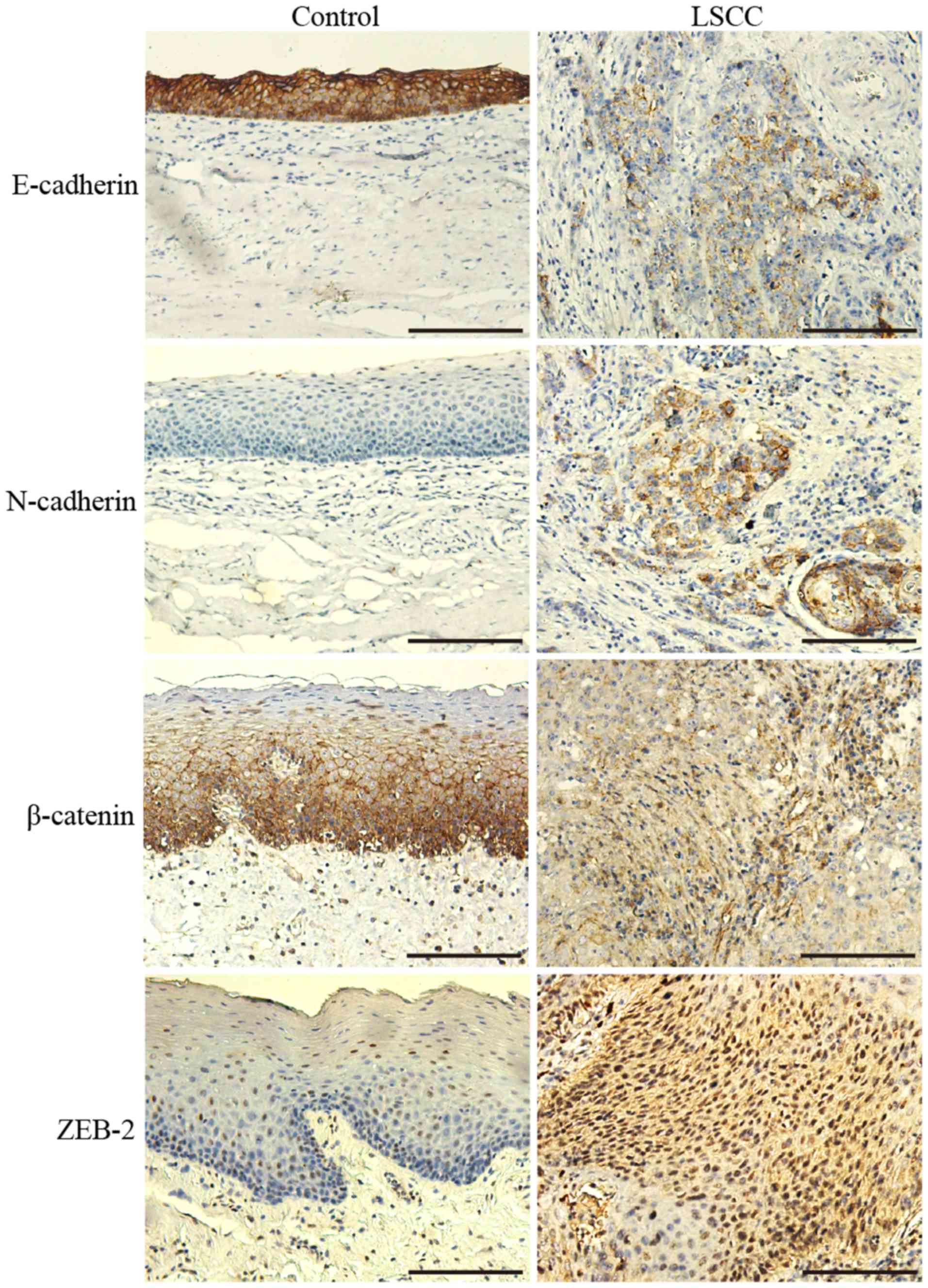
(Fig. 1). E-cadherin and β-catenin
were highly expressed in a membranous pattern in non-neoplastic
laryngeal tissues. In the majority of the cells of the tumor tissue
samples, their expression on the membrane was predominantly
reduced, and cytoplasmic expression patterns were diffuse (Fig. 1).
According to the immunohistochemical evaluation,
positive E-cadherin expression was observed in significantly fewer
LSCC tissue samples (42.11%; 32/76) than non-neoplastic tissue
samples (100%; 76/76; P<0.001).
The β-catenin staining pattern was also
significantly different between LSCC and non-neoplastic tissues
(Fig. 1); 40.79% (31/76) of the LSCC
tissues were considered to exhibit positive staining localized in
the cytoplasm and nucleus, whereas only 1.32% (1/76) of the
non-neoplastic tissues demonstrated similar staining
(P<0.001).
N-cadherin expression was observed in the cytoplasm
of the cells in a number of LSCC tissue samples (11/76), with a
positive rate of 14.47%, whereas its expression was not identified
in any of the non-neoplastic tissues (P<0.001).
The rate of the positive expression of ZEB2 in LSCC
tissue samples was 48.68% (37/76); there was frequently strong
staining in the nucleus and cytoplasm. However, the positive rate
in the control group was only 9.21% (7/76), which was significantly
different than the rate in the LSCC group (P<0.001); there was
weak nuclear staining in a limited number of cells in the
non-neoplastic tissues.
Correlation of EMT marker expression
with clinicopathological parameters
As included in Table
II, E-cadherin and β-catenin expression, a hallmark of EMT, was
significantly associated with lymph node metastases, T stage and
differentiation status (P=0.020, P=0.002; P=0.004, P=0.003;
P=0.028, P<0.001, respectively). It was identified that
relatively reduced E-cadherin staining in the membrane and
increased β-catenin staining in the cytoplasm and nucleus were
observed in the majority of LSCC cases with lymph node metastases,
stage T4 or poor differentiation. N-cadherin expression was
significantly associated with T stage and differentiation (P=0.003;
P=0.010).
 | Table II.Differential expression of
epithelial-mesenchymal transition-associated biomarkers in
laryngeal squamous cell carcinoma and non-neoplastic tissues. |
Table II.
Differential expression of
epithelial-mesenchymal transition-associated biomarkers in
laryngeal squamous cell carcinoma and non-neoplastic tissues.
| A, Association with
E-cadherin |
|---|
|
|---|
| Clinicopathological
parameter | Positive, n | Negative, n | χ2 | P-value |
|---|
| Lymph node
metastasis |
|
| 5.398 | 0.020 |
|
Positive | 11 | 27 |
|
|
|
Negative | 21 | 17 |
|
|
| T stage |
|
| 8.188 | 0.004 |
|
T1–3 | 25 | 20 |
|
|
| T4 | 7 | 24 |
|
|
| Tumor
differentiation |
|
| 7.164 | 0.028 |
|
Good | 2 | 14 |
|
|
|
Moderate | 26 | 26 |
|
|
|
Poor | 3 | 5 |
|
|
| Localization |
|
| 0.758 | 0.860 |
|
Supraglottic | 5 | 9 |
|
|
|
Glottic | 21 | 25 |
|
|
|
Subglottic | 3 | 4 |
|
|
|
Hypopharynx invasion | 3 | 6 |
|
|
|
| B, Association
with N-cadherin |
|
|
Clinicopathological parameter | Positive,
n | Negative,
n |
χ2 | P-value |
|
| Lymph node
metastasis |
|
|
| 0.516a |
|
Positive | 7 | 31 |
|
|
|
Negative | 4 | 34 |
|
|
| T stage |
|
|
| 0.006a |
|
T1–3 | 2 | 43 |
|
|
| T4 | 9 | 22 |
|
|
| Tumor
differentiation |
|
| 9.199 | 0.010 |
|
Good | 6 | 10 |
|
|
|
Moderate | 5 | 47 |
|
|
|
Poor | 0 | 8 |
|
|
| Localization |
|
| 3.069 | 0.381 |
|
Supraglottic | 2 | 12 |
|
|
|
Glottic | 5 | 41 |
|
|
|
Subglottic | 1 | 6 |
|
|
|
Hypopharynx invasion | 3 | 6 |
|
|
|
| C, Association
with β-catenin |
|
|
Clinicopathological parameter | Positive,
n | Negative,
n |
χ2 | P-value |
|
| Lymph node
metastasis |
|
| 9.207 | 0.002 |
|
Positive | 22 | 16 |
|
|
|
Negative | 9 | 29 |
|
|
| T stage |
|
| 9.111 | 0.003 |
|
T1–3 | 12 | 33 |
|
|
| T4 | 19 | 12 |
|
|
| Tumor
differentiation |
|
| 23.985 | <0.001 |
|
Good | 15 | 1 |
|
|
|
Moderate | 13 | 39 |
|
|
|
Poor | 3 | 5 |
|
|
| Localization |
|
| 4.678 | 0.197 |
|
Supraglottic | 8 | 6 |
|
|
|
Glottic | 17 | 29 |
|
|
|
Subglottic | 1 | 6 |
|
|
|
Hypopharynx invasion | 5 | 4 |
|
|
|
| D, Association
with zinc finger E-box binding homeobox 2 |
|
|
Clinicopathological parameter | Positive,
n | Negative,
n |
χ2 | P-value |
|
| Lymph node
metastasis |
|
| 23.227 | <0.001 |
|
Positive | 29 | 9 |
|
|
|
Negative | 8 | 30 |
|
|
| T stage |
|
| 13.637 | <0.001 |
|
T1–3 | 14 | 31 |
|
|
| T4 | 23 | 8 |
|
|
| Tumor
differentiation |
|
| 8.626 | 0.013 |
|
Good | 13 | 3 |
|
|
|
Moderate | 21 | 31 |
|
|
|
Poor | 3 | 5 |
|
|
| Localization |
|
| 2.128 | 0.546 |
|
Supraglottic | 9 | 5 |
|
|
|
Glottic | 20 | 26 |
|
|
|
Subglottic | 3 | 4 |
|
|
|
Hypopharynx invasion | 5 | 4 |
|
|
As ZEB2 may repress E-cadherin expression (5,16), it is
reasonable to expect its increased expression to be associated with
the tumor characteristics associated with E-cadherin loss. Indeed,
the expression of ZEB2 was associated with the lymph node
metastasis status, T stage and the differentiation status of tumor
cells in the present study (P<0.001; P<0.001; P=0.013).
In addition, the association of the co-expression of
E-cadherin and the other three EMT-associated biomarkers with
clinicopathological parameters was assessed in the present study,
as included in Table III.
E-cadherin/β-catenin co-expression was significantly associated
with the lymph node metastasis status (P=0.004), T stage (P=0.005)
and differentiation (P<0.001), whereas E-cadherin/N-cadherin or
E-cadherin/ZEB2 co-expression was significantly associated with
only two of the clinicopathological parameters (T stage, P=0.001;
differentiation, P=0.012). However, the co-expression of E-cadherin
with the other three EMT-associated biomarkers individually was not
associated with the localization of LSCC. Additionally, there was
no significant association between the individual expression of the
four EMT-associated biomarkers and tumor localization.
 | Table III.Co-expression of E-cadherin and the
other three epithelial-mesenchymal transition-associated biomarkers
in laryngeal squamous cell carcinoma. |
Table III.
Co-expression of E-cadherin and the
other three epithelial-mesenchymal transition-associated biomarkers
in laryngeal squamous cell carcinoma.
| A, Co-expression of
E-cadherin and N-cadherin |
|---|
|
|---|
|
|
E-cadherin/N-cadherin status, n |
|
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameter | +/− | +/+ | −/− | −/+ | χ2 | P-value |
|---|
| Lymph node
metastasis |
|
|
|
| 5.733 | 0.125 |
|
Positive | 10 | 1 | 21 | 6 |
|
|
|
Negative | 20 | 1 | 14 | 3 |
|
|
| T stage |
|
|
|
| 16.101 | 0.001 |
|
T1–3 | 25 | 0 | 18 | 2 |
|
|
| T4 | 5 | 2 | 17 | 7 |
|
|
| Tumor
differentiation |
|
|
|
| 16.265 | 0.012 |
|
Good | 2 | 0 | 8 | 6 |
|
|
|
Moderate | 24 | 2 | 23 | 3 |
|
|
|
Poor | 4 | 0 | 4 | 0 |
|
|
| Localization |
|
|
|
| 5.363 | 0.802 |
|
Supraglottic | 5 | 0 | 7 | 2 |
|
|
|
Glottic | 20 | 1 | 21 | 4 |
|
|
|
Subglottic | 3 | 0 | 3 | 1 |
|
|
|
Hypopharynx invasion | 2 | 1 | 4 | 2 |
|
|
|
| B, Co-expression
of E-cadherin and β-catenin |
|
|
|
E-cadherin/β-catenin status, n |
|
|
|
|
|
|
|
|
Clinicopathological parameter | +/− | +/+ | −/− | −/+ |
χ2 | P-value |
|
| Lymph node
metastasis |
|
|
|
| 13.444 | 0.004 |
|
Positive | 9 | 2 | 7 | 20 |
|
|
|
Negative | 17 | 4 | 12 | 5 |
|
|
| T stage |
|
|
|
| 12.749 | 0.005 |
|
T1–3 | 22 | 3 | 11 | 9 |
|
|
| T4 | 4 | 3 | 8 | 16 |
|
|
| Tumor
differentiation |
|
|
|
| 25.386 | <0.001 |
|
Good | 0 | 2 | 1 | 13 |
|
|
|
Moderate | 23 | 3 | 16 | 10 |
|
|
|
Poor | 3 | 1 | 2 | 2 |
|
|
| Localization |
|
|
|
| 7.495 | 0.586 |
|
Supraglottic | 3 | 2 | 3 | 6 |
|
|
|
Glottic | 17 | 4 | 12 | 13 |
|
|
|
Subglottic | 3 | 0 | 3 | 1 |
|
|
|
Hypopharynx invasion | 3 | 0 | 1 | 5 |
|
|
|
| C, Co-expression
of E-cadherin and ZEB2 |
|
|
| E-cadherin/ZEB2
status, n |
|
|
|
|
|
|
|
|
Clinicopathological parameter | +/− | +/+ | −/− | −/+ |
χ2 | P-value |
|
| Lymph node
metastasis |
|
|
|
| 27.44 | <0.001 |
|
Positive | 7 | 4 | 2 | 25 |
|
|
|
Negative | 17 | 4 | 13 | 4 |
|
|
| T stage |
|
|
|
| 16.590 | <0.001 |
|
T1–3 | 20 | 5 | 11 | 9 |
|
|
| T4 | 4 | 3 | 4 | 20 |
|
|
| Tumor
differentiation |
|
|
|
| 12.303 | 0.056 |
|
Good | 1 | 1 | 2 | 12 |
|
|
|
Moderate | 20 | 6 | 11 | 15 |
|
|
|
Poor | 3 | 1 | 2 | 2 |
|
|
| Localization |
|
|
|
| 4.558 | 0.871 |
|
Supraglottic | 3 | 2 | 2 | 7 |
|
|
|
Glottic | 16 | 5 | 10 | 15 |
|
|
|
Subglottic | 2 | 1 | 2 | 2 |
|
|
|
Hypopharynx invasion | 3 | 0 | 1 | 5 |
|
|
Association of EMT-associated marker
expression and clinicopathological parameters with clinical
outcome
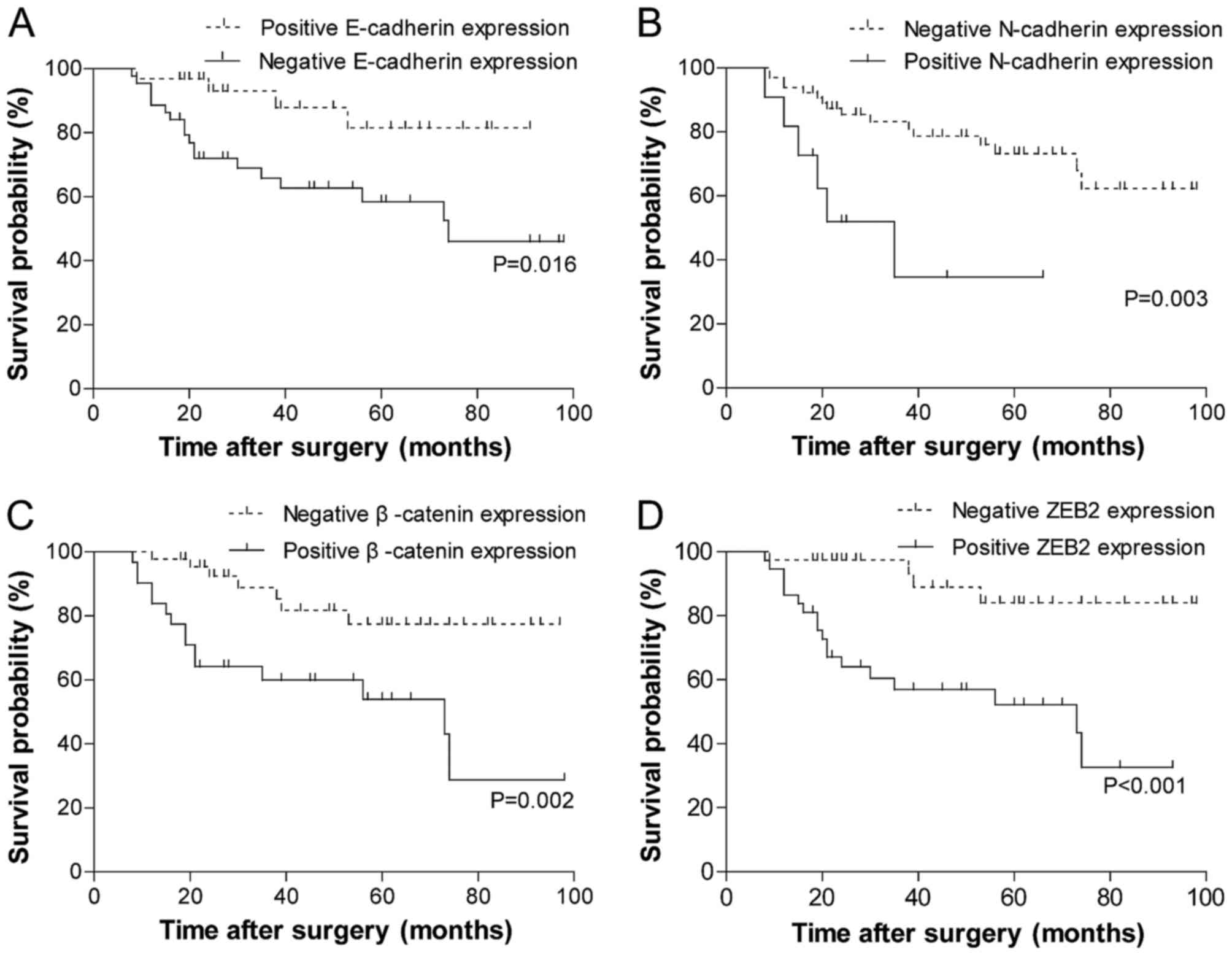
The results of the univariate analysis of the
biomarkers for overall survival (OS) are summarized in Fig. 2. Patients whose tumors exhibited the
negative membrane expression of E-cadherin (Fig. 2A; P=0.016) or positive expression of
the other three biomarkers experienced significantly reduced OS
time (Fig. 2B-D; N-cadherin, P=0.003;
β-catenin, P=0.002; ZEB2, P<0.001). The association between the
clinicopathological parameters and the clinical outcome was also
examined by log-rank analysis (data not shown). OS time was
significantly improved among patients with no lymph node
metastases, an early T stage (T1-3) and strong differentiation of
tumor cells (χ2=4.873, P=0.027; χ2=9.567,
P=0.023; χ2=6.126, P=0.047, respectively). Tumor
localization was not significantly associated with OS
(χ2=1.420, P=0.701).
Multivariate analysis
In the multivariate analysis, all of the analyzed
factors were those identified as significant in the univariate
analyses, with the exception of tumor localization, using the Cox
proportional hazards model (data not shown). The result revealed
that T stage and the positive expression of β-catenin or ZEB2 were
independent risk factors for OS in LCSS (HR, 3.004; 95% CI,
1.24–7.25; P=0.014; HR, 2.877; 95% CI, 1.15–7.23; P=0.025; HR,
5.278; 95% CI, 1.77–15.70; P=0.003; respectively).
Discussion
EMT, a cellular program in which epithelial cells
develop the motile and invasive properties typical of mesenchymal
cells, is an important process in the progression, invasion and
metastasis of cancer (1–3). At the molecular level, EMT, which is
indicated by changes in the expression of specific proteins,
involves the downregulation of epithelial-type markers, including
adherens junction proteins, and the expression of mesenchymal
proteins, including EMT-associated transcription factors (21–23). EMT
was previously reported to be associated with aggressive behavior
and a poor prognosis for several types of tumor, including HNSCC,
and other studies have investigated the involvement of EMT
specifically in LSCC (3,8,24,25). The present study was conducted to
provide preliminary clinicopathological data on this topic. At
present, the occurrence of EMT has been investigated in LSCC in
studies that largely focused on the loss of membranous E-cadherin
and the overexpression of cytoplasmic β-catenin (7,15,19,26,27). In
the present study, the expression of an extended panel of
EMT-associated markers, including E-cadherin, N-cadherin, β-catenin
and ZEB2, was examined in a larger cohort of patients with LSCC.
Additionally, analysis of the association between the expression of
these markers and clinicopathological and follow-up data was
performed to determine important prognostic information. The
findings of the present study indicated that four of these
EMT-associated proteins were differentially expressed between LSCC
and non-neoplastic mucosal epithelium. E-cadherin expression was
significantly reduced in the membrane, and there was a diffuse
cytoplasmic staining pattern in tumor tissue. Previous studies
reported a decrease in expression of the epithelial marker
E-cadherin in LSCC cell lines and resected samples, and some
provided evidence of cytoplasmic E-cadherin expression only in LSCC
(26,28–30). The
results of the present study indicated that the E-cadherin
expression pattern was altered in LSCC, and are therefore, was in
accord with the majority of previous studies.
E-cadherin is a cell surface glycoprotein which
mediates intercellular adhesion through the interactions of its
extracellular and cytoplasmic domains with β-catenin (31). The destabilization of cadherin/catenin
complex formation that results from the downregulation or loss of
E-cadherin expression may serve a role in tumor invasion and
metastasis (32,33). A previous study identified the loss of
membranous E-cadherin and β-catenin expression, in addition to
increases in cytoplasmic expression, irrespective of the lymph node
or distant metastasis status, in HNSCC (34). β-catenin expression was reported in
the membrane and cytoplasm in LSCC cells by Goulioumis et al
and Galera-Ruiz et al (14,33), who
observed a significant association between β-catenin expression and
localization (glottis and supraglottis LSCC). β-catenin exhibited
significantly different expression between LSCC and non-carcinoma
tissue in the present study, with positive staining identified in
the cytoplasm and nucleus of tumor tissue. Significant associations
were identified between β-catenin expression and lymph node
metastases, T stage and tumor cell differentiation, but not with
tumor localization.
It has been reported that cadherin switching (a
decrease in E-cadherin with an increase in N-cadherin) is a feature
of EMT in numerous types of malignant tumor and that an association
exists between cadherin switching and lymph node metastasis in a
number of tumor types, including HNSCC (4,11,13,14). In
the present study, it was only partially expressed in LSCC samples,
while N-cadherin expression was negative in the control group.
Although the N-cadherin positive rate of 14.47% detected in LSCC
tissue was low, the result of statistical analysis revealed that
there was a significant difference in expression between the LSCC
and non-neoplastic tissues, and N-cadherin expression was
significantly associated with T stage and differentiation.
Furthermore, there was no significant association between
N-cadherin expression and lymph node metastasis, whereas N-cadherin
expression was associated with T stage, tumor differentiation and
poor OS. Greco et al (27)
previously reported that N-cadherin expression was associated with
the tumor histological grade, but not OS. Taken together, these
results indicate that the cadherin switch between E-cadherin and
N-cadherin may be a classical phenomenon in tumor-associated EMT
rather than an individual criterion in LSCC, perhaps due to the low
rate of positive N-cadherin expression in this study.
ZEB2 is associated with EMT, and is therefore
proposed to be involved in this key step of the progression of
different types of tumor; as a repressor of E-cadherin, the
expression of ZEB2 is inversely associated with it (5,35).
Furthermore, it has been reported that the co-expression of ZEB2
and other EMT-related protein markers is associated with poor
prognosis in HNSCC and OSCC (6,20);
however, to the best of our knowledge, no clinicopathological
research has been conducted on the importance of ZEB2 in LSCC. The
present study confirmed that ZEB2 expression was significantly
increased in tumor tissue compared with non-carcinoma tissue, and
was directly associated with the status of lymph node metastases, T
stage and tumor cell differentiation in LSCC. It was also observed
that positive ZEB2 expression was associated with a poor prognosis
in patients with LSCC; therefore, it is reasonable to consider ZEB2
as an EMT biomarker in LSCC oncogenesis, development and metastasis
based on the conclusions of the present study.
EMT is a complex process that often involves several
types of EMT-associated proteins during malignant tumor progression
and metastasis in patients (22,36). The
four EMT biomarkers in the present study exhibited significantly
different expression between the LSCC and control tissues.
Considering E-cadherin to be a hallmark of EMT progression, the
co-expression of E-cadherin and the other three EMT-related
biomarkers was also taken into account. Among the three types of
co-expression, E-cadherin/β-catenin had the most significant
association with the clinicopathological characteristics of lymph
node metastases, T stage and tumor cell differentiation. To the
best of our knowledge, this is the first study to investigate EMT
in LSCC by assessing the co-expression of two biomarkers.
Each of the four EMT markers examined in the present
study had been previously demonstrated to have clinical
implications in other types of tumor (4–13), and
they were all further demonstrated to have prognostic implications
for OS by univariate analysis in LSCC in the present study. In
addition, patients with the loss of E-cadherin, expression of
N-cadherin and overexpression of β-catenin experienced a
significantly reduced OS time, in accord with previous results
derived from other tumors and LSCC (25,27,32).
Furthermore, the effect of ZEB2 on LSCC prognosis was elucidated
for the first time. By employing a Cox proportional hazards model,
it was identified that T-stage and positive β-catenin and ZEB2
expression were independent risk factors for adverse OS in the
multivariate analysis. Lopez-Gonzalez et al (36) reported that the overexpression of
cytoplasmic β-catenin was associated with poor tumor
differentiation. Greco et al (27) revealed that the reduced expression of
cytoplasmic β-catenin was associated with high histological grade;
however, they identified that cytoplasmic β-catenin overexpression
corresponded to significantly improved disease-specific survival in
certain patients with LSCC. Increasing tumor histological grade
should generally correspond to reduced survival time, which is in
accord with the results of the present study, which identified that
patients with the overexpression of β-catenin experienced worse OS.
Furthermore, in the study by Greco et al T stage was also an
independent prognostic predictor in the multivariate analysis,
which is consistent with the present study's results.
The present study, with a cohort of 76 patients,
was, to the best of our knowledge, the first systematic
investigation of LSCC that utilized immunohistochemistry analysis
to identify that positive ZEB2 expression is also an independent
risk factor for LSCC prognosis. ZEB2, a transcriptional repressor,
induces EMT by suppressing the expression of E-cadherin and
contributes to the invasiveness of malignant tumors; therefore, it
has been considered as a predictor of prognosis in numerous types
of cancer, including head and neck cancer; the high expression of
ZEB2 predicted a poor prognosis (6,37–39). The results of the present study also
indicated that ZEB2 expression could also be a critical factor in
predicting the prognosis of LSCC. Further junctional proteins were
identified as potential ZEB2 targets, and targeted treatment should
be investigated in a clinical setting.
In conclusion, EMT, which is mediated by several
biomarkers, serves a role in the prognosis of LSCC by increasing
the risk of tumor metastasis, therefore reducing OS time. The
reduction in membranous E-cadherin expression, and the increase in
cytoplasmic β-catenin expression, may be hallmarks of the EMT
process in LSCC. ZEB2 expression, as an independent prognostic
predictor, combined with its association with clinicopathological
parameters and OS, should be considered as an EMT biomarker in LSCC
based on the results of the present study. N-cadherin was also
indicated as an EMT biomarker on account of its association with
oncogenesis, development and metastasis in LSCC; however, there is
still controversy in the literature regarding how these biomarkers
affect survival. Therefore, more research is required to elucidate
the association between molecular biomarkers and the
clinicopathological characteristics of patients with LSCC.
Acknowledgements
The study was supported by the Nanjing Medical
Science Technology Development Programme (grant nos. YKK14062 and
QRX17012), the Project of Invigorating Health Care through Science,
Technology and Education (grant no. ZDXKB2016015). Additionally,
the authors would like to thank the Core Medical Laboratory of Drum
Tower Hospital, Nanjing University Medical School.
Glossary
Abbreviations
Abbreviations:
|
LSCC
|
laryngeal squamous cell carcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ZEB2
|
zinc finger E-box binding homeobox
2
|
|
ENT
|
ear, nose, and throat
|
|
OS
|
overall survival
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
T
|
tumor
|
|
OSCC
|
oral squamous cell carcinoma
|
References
|
1
|
de Vincentiis M, De Virgilio A, Bussu F,
Gallus R, Gallo A, Bastanza G, Parrilla C, Greco A, Galli J,
Turchetta R, et al: Oncologic results of the surgical salvage of
recurrent laryngeal squamous cell carcinoma in a multicentric
retrospective series: Emerging role of supracricoid partial
laryngectomy. Head Neck. 37:84–91. 2015. View Article : Google Scholar
|
|
2
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang Y and Massague J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vandewalle C, Comijn J, De Craene B,
Vermassen P, Bruyneel E, Andersen H, Tulchinsky E, Van Roy F and
Berx G: SIP1/ZEB2 induces EMT by repressing genes of different
epithelial cell-cell junctions. Nucleic Acids Res. 33:6566–6578.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chu PY, Hu FW, Yu CC, Tsai LL, Yu CH, Wu
BC, Chen YW, Huang PI and Lo WL: Epithelial-mesenchymal transition
transcription factor ZEB1/ZEB2 co-expression predicts poor
prognosis and maintains tumor-initiating properties in head and
neck cancer. Oral Oncol. 49:34–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li JJ, Zhang GH, Yang XM, Li SS, Liu X,
Yang QT, Li Y and Ye J: Reduced E-cadherin expression is associated
with lymph node metastases in laryngeal squamous cell carcinoma.
Auris Nasus Larynx. 39:186–192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Creighton CJ, Gibbons DL and Kurie JM: The
role of epithelial-mesenchymal transition programming in invasion
and metastasis: A clinical perspective. Cancer Manag Res.
5:187–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiery JP, Chua K, Sim WJ and Huang R:
Epithelial mesenchymal transition during development in fibrosis
and in the progression of carcinoma. Bull Cancer. 97:1285–1295.
2010.PubMed/NCBI
|
|
10
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nguyen PT, Kudo Y, Yoshida M, Kamata N,
Ogawa I and Takata T: N-cadherin expression is involved in
malignant behavior of head and neck cancer in relation to
epithelial-mesenchymal transition. Histol Histopathol. 26:147–156.
2011.PubMed/NCBI
|
|
14
|
Zidar N, Boštjančič E, Gale N, Kojc N,
Poljak M, Glavač D and Cardesa A: Down-regulation of microRNAs of
the miR-200 family and miR-205, and an altered expression of
classic and desmosomal cadherins in spindle cell carcinoma of the
head and neck-hallmark of epithelial-mesenchymal transition. Hum
Pathol. 42:482–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cappellesso R, Marioni G, Crescenzi M,
Giacomelli L, Guzzardo V, Mussato A, Staffieri A, Martini A,
Blandamura S and Fassina A: The prognostic role of the
epithelial-mesenchymal transition markers E-cadherin and Slug in
laryngeal squamous cell carcinoma. Histopathology. 67:491–500.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Comijn J, Berx G, Vermassen P, Verschueren
K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D and van Roy
F: The two-handed E box binding zinc finger protein SIP1
downregulates E-cadherin and induces invasion. Mol Cell.
7:1267–1278. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greene FL, Page DL, Fleming ID, Fritz A,
Balch CM, Haller DG and Morrow M: American Joint Committee on
Cancer: AJCC Cancer Staging Manual. 6th. Chapter 2. Springer; New
York, NY: pp. 47–57. 2002
|
|
18
|
Goulioumis AK, Varakis J, Goumas P and
Papadaki H: Differential beta-catenin expression between glottic
and supraglottic laryngeal carcinoma. Eur Arch Otorhinolaryngol.
267:1573–1578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahmed RA, Shawky Ael-A and Hamed RH:
Prognostic significance of cyclin D1 and E-cadherin expression in
laryngeal squamous cell carcinoma. Pathol Oncol Res. 20:625–633.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong YH, Syed Zanaruddin SN, Lau SH,
Ramanathan A, Kallarakkal TG, Vincent-Chong VK, Wan Mustafa WM,
Abraham MT, Abdul Rahman ZA, Zain RB and Cheong SC: Co-Expression
of TWIST1 and ZEB2 in oral squamous cell carcinoma is associated
with poor survival. PLoS One. 10:e01340452015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nieto MA: The ins and outs of the
epithelial to mesenchymal transition in health and disease. Annu
Rev Cell Dev Biol. 27:347–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: Mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
25
|
Kurtz KA, Hoffman HT, Zimmerman MB and
Robinson RA: Decreased E-cadherin but not beta-catenin expression
is associated with vascular invasion and decreased survival in head
and neck squamous carcinomas. Otolaryngol Head Neck Surg.
134:142–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Psyrri A, Kotoula V, Fountzilas E,
Alexopoulou Z, Bobos M, Televantou D, Karayannopoulou G, Krikelis
D, Markou K, Karasmanis I, et al: Prognostic significance of the
Wnt pathway in squamous cell laryngeal cancer. Oral Oncol.
50:298–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Greco A, De Virgilio A, Rizzo MI, Pandolfi
F, Rosati D and de Vincentiis M: The prognostic role of E-cadherin
and β-catenin overexpression in laryngeal squamous cell carcinoma.
Laryngoscope. 126:E148–E155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goulioumis AK, Fuxe J, Varakis J, Repanti
M, Goumas P and Papadaki H: Estrogen receptor-beta expression in
human laryngeal carcinoma: Correlation with the expression of
epithelial-mesenchymal transition specific biomarkers. Oncol Rep.
22:1063–1068. 2009.PubMed/NCBI
|
|
29
|
Yu L, Li HZ, Lu SM, Tian JJ, Ma JK, Wang
HB and Xu W: Down-regulation of TWIST decreases migration and
invasion of laryngeal carcinoma Hep-2 cells by regulating the
E-cadherin, N-cadherin expression. J Cancer Res Clin Oncol.
137:1487–1493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang N, Hui L, Wang Y, Yang H and Jiang X:
Overexpression of SOX2 promotes migration, invasion, and
epithelial-mesenchymal transition through the Wnt/β-catenin pathway
in laryngeal cancer Hep-2 cells. Tumour Biol. 35:7965–7973. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stappert J and Kemler R: A short core
region of E-cadherin is essential for catenin binding and is highly
phosphorylated. Cell Adhes Commun. 2:319–327. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Joo YE, Rew JS, Choi SK, Bom HS, Park CS
and Kim SJ: Expression of e-cadherin and catenins in early gastric
cancer. J Clin Gastroenterol. 35:35–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kallakury BV, Sheehan CE, Winn-Deen E,
Oliver J, Fisher HA, Kaufman RP Jr and Ross JS: Decreased
expression of catenins (alpha and beta), p120 CTN, and E-cadherin
cell adhesion proteins and E-cadherin gene promoter methylation in
prostatic adenocarcinomas. Cancer. 92:2786–2795. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Andrews NA, Jones AS, Helliwell TR and
Kinsella AR: Expression of the E-cadherin-catenin cell adhesion
complex in primary squamous cell carcinomas of the head and neck
and their nodal metastases. Br J Cancer. 75:1474–1480. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang CJ, Chao CH, Xia W, Yang JY, Xiong
Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, et al: p53 regulates
epithelial-mesenchymal transition and stem cell properties through
modulating miRNAs. Nat Cell Biol. 13:317–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ding W, You H, Dang H, LeBlanc F, Galicia
V, Lu SC, Stiles B and Rountree CB: Epithelial-to-mesenchymal
transition of murine liver tumor cells promotes invasion.
Hepatology. 52:945–953. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wiklund ED, Bramsen JB, Hulf T, Dyrskjøt
L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS,
Borre M, et al: Coordinated epigenetic repression of the miR-200
family and miR-205 in invasive bladder cancer. Int J Cancer.
128:1327–1334. 2011. View Article : Google Scholar : PubMed/NCBI
|