Introduction
Glioma is one of the most common malignant tumor
types globally with high morbidity and mortality. To date, surgical
resection and radiotherapy remain the primary methods used for the
treatment of glioma (1,2). Radiotherapy requires at least 55 Gy of
external radiation to control the tumor, however, a radiation dose
of >60 Gy often results in normal brain tissue necrosis
(3–5).
Therefore, novel therapeutic strategies are required.
Sodium iodide symporter (NIS) is a type of cell
membrane glycoprotein that mediates the active uptake of iodine in
the thyroid and other types of tissues (6). Gene therapy is an important alternative
for the treatment of tumors; for example, radioiodine treatment of
extra-thyroidal cancer via the ectopic transfer of the NIS gene
into otherwise non-NIS-expressing cancer. A number of studies have
successfully obtained ectopic expression of NIS in different tumors
through gene transfer (7–10). The dual function of NIS as a
diagnostic and therapeutic gene thereby allows easy monitoring of
functional NIS expression using γ camera imaging techniques prior
to the application of a therapeutic radionuclide dose (11). Our previous studies (12–15)
demonstrated that baculovirus-mediated human NIS (hNIS) expression
may mediate multiple tumor uptake of 131I, providing a
promising target for gene therapy. In previous studies,
extra-thyroidal tissues have generally not been able to mediate
iodide organification following NIS gene transfer, therefore rapid
outflow of 131I resulted in a decreased radiation dose
able to affect the activity of tumor cells, thus decreasing the
curative effect (16). The use of
alternative NIS-transported radioisotopes with a higher energy than
131I may improve the efficacy of NIS-mediated
radionuclide targeted therapy (17,18).
188Re is a type of short physical half-life radionuclide
(0.71 days), which has been applied to treat various diseases,
including prostate carcinoma, hepatoma carcinoma, breast carcinoma,
bladder carcinoma, refractory arthritis and coronary
beta-brachytherapy (19–22). Compared with 131I (0.192
MeV), due to its high energy, 188Re (23) is an ideal therapeutic alternative
(0.778 MeV). Our previous study (24)
suggested that lentiviruses mediate hNIS expression that results in
188Re uptake in glioma and provides a promising gene
therapy strategy. However, the physical half-life of
188Re is shorter (0.71 days) compared with that of
131I (8.02 days). Therefore, the purpose of the present
study was to compare the therapeutic effects of 188Re-
and 131I-mediated gene therapy in vitro and in
vivo.
Materials and methods
Plasmid construction, lentivirus
preparation and U87 cell transfection with lentivirus
Human NIS gene was obtained in the form of a
pcDNA3.1-hNIS plasmid (provided by Dr Sissy Jhiang from Ohio
University, OH, USA). U87 glioma cell line [American Type Culture
Collection (ATCC), Manassas, VA, USA] was maintained in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.; 5%
CO2, 37°C). The plasmid containing hNIS was packaged
into lentivirus vector (Thermo Fisher Scientific, Inc.) and named
as Lenti-CMV-hNIS. To generate cell line expressing hNIS controlled
by cytomegalovirus-enhancer/promoter, Lenti-CMV-hNIS was added to
U87 cells at a multiplicity of infection of 2.0, then were selected
for further study after 3 weeks. The transfected cell line stably
expressing hNIS was named U87-hNIS. U87-hNIS was validated by
radionuclide uptake experiments. Cells were inoculated with DMEM
containing 10% fetal bovine serum, 100 U/ml penicillin and 100
µg/ml streptomycin in 24-well plates 24 h prior to the experiment
to achieve a density of 105 cells per well at the day of
experiment.
In vitro radioisotope uptake
experiments
188Re was eluted from a
188W/188Re generator (Jiangsu ReTai
Pharmaceutical Biotechnology Co., Ltd., Changzhou, China) using
0.9% saline. 131I was supplied by Shanghai Kexin Biotech
Co., Ltd. (Shanghai, China). In vitro
188Re/131I uptake experiments of U87-hNIS
cells expressing hNIS were performed as Weiss and Grollman
(25) described previously, with
minor modifications. All data were corrected for attenuation.
U87-hNIS cells were washed with 0.5 ml buffered Hanks' balanced
salt solution (bHBSS; containing 10 µM HBSS and buffered with
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.3),
incubated in 0.5 ml bHBSS (containing 3.7 kBq
188Re/131I) liquid for 1, 2, 5, 10, 20, 30,
60 and 120 min respectively, then washed two times with ice-cold
bHBSS, incubated with 1 ml of 100% dehydrated ethanol for 20 min,
followed by cell lysis (using trypsin and 0.25% EDTA; Gibco; Thermo
Fisher Scientific, Inc.). Finally, the radioactivity (count per
min) was detected using a well gamma-counter (Shanghai Institute of
Nuclear Research Rihuan Instrument Co., Shanghai, China) and
compared with background, in order to achieve a ratio. All the
following experiments were conducted in triplicate.
Sodium perchlorate inhibition
study
The specificity of 188Re/131I
uptake was investigated in the present study. U87-hNIS cells (at a
density of 105) were incubated with sodium perchlorate
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 3.7 kBq
188Re/131I in DMEM containing 10% fetal
bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin (5%
CO2, 37°C) for 30 min, in which the concentration of
sodium perchlorate was 0, 1, 2, 5, 10, 20, 50 and 100 µM,
respectively. Thereafter, the cells were washed with bHBSS twice,
lysed (using trypsin and 0.25% EDTA; Gibco; Thermo Fisher
Scientific, Inc.) at room temperature for three min, and their
radioactivity measured, according to the methods
aforementioned.
Radioisotope efflux study in
vitro
The 188Re/131I efflux kinetics
of U87-hNIS cells were investigated as previously described
(25). U87-hNIS cells (at a density
of 105) were incubated with
188Re/131I (3.7 kBq/well) at 37°C for ~30
min. The culture medium (DMEM containing 10% fetal bovine serum)
was then replaced with nonradioactive bHBSS. Cells were incubated
for 2, 4, 6, 8, 10, 12, 14, 16, 18 or 20 min and immediately lysed
using trypsin and 0.25% EDTA (Gibco; Thermo Fisher Scientific,
Inc.). Subsequently, cells were extracted with 1 ml dehydrated
alcohol and the radioactivity in the cells was measured as
aforementioned.
In vitro assessment of radioisotope
toxicity by clonogenic assay
Prior to the experiment, U87-hNIS cells were
cultured in 24-well plates, in order to achieve a density of
105 cells/well at the day of study. As presented in
Table I, cells were divided into five
groups. Group 1 comprised U87-hNIS cells washed with bHBSS with 740
kBq 188Re, then cultured (5% CO2, 37°C) for 7
h. Group 2 was processed much the same as group 1, with the
exception that 188Re was replaced with 131I.
Groups 3 and 4 were non-transfected U87 cells with 188Re
and 131I, respectively. Group 5 comprised
non-transfected U87 cells without the addition of any radionuclide.
Groups 3, 4 and 5 all served as controls. The 5 groups of cells
were then washed and lysed as described above, and seeded in 6-well
plates at a density of 200 cells/well. The cells were cultured for
7 days (5% CO2 at 37°C). Following removal of the
culture medium as aforementioned, cells were stained with crystal
violet solution (0.1%) at room temperature for 10 min and the
colonies >30 cells were counted using an optical microscope
(×100 magnification; CKX41; Olympus Corporation, Tokyo, Japan).
Results were expressed as the percentage of surviving cells
compared with untreated U87 cells (Group 5).
 | Table I.Subgroup of in vitro
188Re and 131I clonogenic assay. |
Table I.
Subgroup of in vitro
188Re and 131I clonogenic assay.
| Characteristic | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|
| U87-human |
|
|
|
|
|
| sodium iodide |
|
|
|
|
|
| symporter | + | + |
|
|
|
| U87 |
|
| + | + | + |
|
188Re | + |
| + |
|
|
|
131I |
| + |
| + |
|
Animal models
A total of 18 female 5-week-old athymic Balb/c nude
mice (18–20 g in weight) were used (Shanghai Laboratory Animal
Centre Chinese Academy of Sciences, Shanghai, China). A full
light-dark cycle with 10 h of light and 14 h of darkness each day
was applied, with conditions including a temperature of 26–28°C, a
humidity of 40–60%, and general feed and drinking water provided.
Mice were sacrificed 4 weeks after the first intravenous injections
of 188Re or 131I. Animal experiments were
approved by the Ethics Committee and Animal Care Committee of
Shanghai JiaoTong University, School of Medicine (Shanghai, China).
A xenograft model was generated by subcutaneous injection of
U87-hNIS cells (5×106 cells, suspended in 150 µl PBS)
into the right armpit of the mice. Subsequently, ~6 weeks following
inoculation, the tumor diameters reached 0.8–1.0 cm, which could be
used for imaging and therapies.
Tumor imaging and therapy study in a
xenograft model
Tumor imaging studies were performed in six U87-hNIS
nude mice. 188Re (55.5 MBq) or 131I (55.5
MBq) was injected through the caudal vein. Tumor images were
performed 15 min, 1, 2, 4 and 24 h after injection using a
high-resolution gamma camera equipped with a pinhole collimator (GE
Healthcare Life Sciences, Little Chalfont, UK) immediately
following 188Re or 131I injection. Each image
lasted for 3 min. The matrix size was 256×256. Region of interest
was placed on the area of tumor and the contralateral axillary, and
the ratio of radioactivity counts between tumor and non-tumor sites
(T/NT) at each time point was measured.
Tumor therapy studies were performed in U87-hNIS
nude mice at the same time. In the present study, no animal
presented with multiple tumors, and when the tumor diameter of nude
mice grew to 0.8–1.0 cm in diameter (the minimum and maximum
diameter exhibited by a single tumor in the present study were 0.82
and 0.96 cm, respectively), they were randomly divided into three
groups (n=6). Group 1 was given two intravenous injections of 55.5
MBq 188Re with an interval of 1 week. Group 2 was
treated intravenously with 2 injections of 55.5 MBq 131I
with a 1 week interval. Group 3 was left untreated. In order to
evaluate the therapeutic effect of hNIS mediated radionuclides,
tumor size was measured with calipers prior to and following the
administration of 188Re or 131I every week,
in three dimensions. Tumor volume was estimated by the following
formula: Length × width2x0.52.
Statistical methods
Origin 7.5 (OriginLab, Northampton, UK) and SPSS
16.0 (SPSS, Inc., Chicago, IL, USA) software were used in the
present study. All experiments were performed in triplicates unless
otherwise indicated. Numeric data are expressed as mean ± standard
deviation. One-way analysis of variance was performed to evaluate
the difference amongst groups followed by Tukeys post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Preparation of the lentiviruses
The lentiviral vector Lenti-cytomegalovirus
(CMV)-hNIS containing CMV promoter and hNIS gene was packed
successfully, and the Lenti-CMV-0 vector was packed as control.
Stable cell lines U87-hNIS and U87-0 were established 3 weeks after
infection with recombinant lentivirus. For the purpose of comparing
hNIS mediated uptake of radioisotope in the cell line,
188Re and 131I uptake experiments were
performed and compared in the following studies.
hNIS mediated in vitro radioisotope
uptake
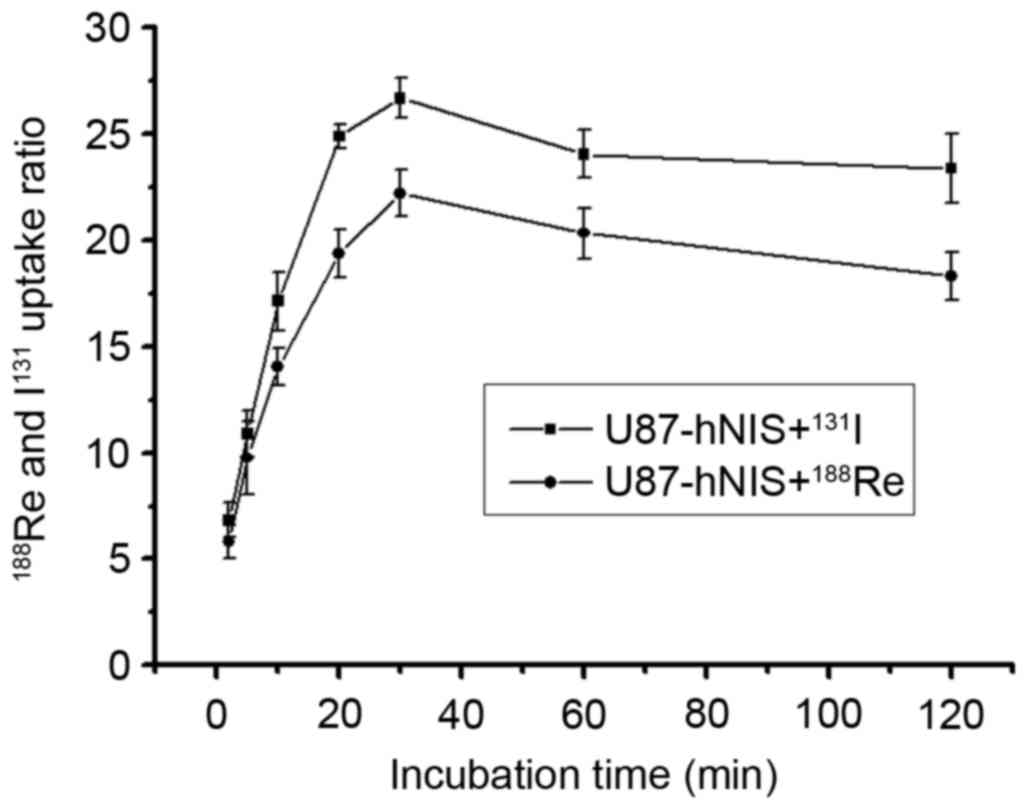
In U87-hNIS cells, uptake of
188Re/131I in relation to incubation time is
presented in Fig. 1. The results
reveal the initial 188Re/131I uptake
dependent on incubation time. The results demonstrated that
188Re/131I influx rapidly into U87-hNIS
cells, reaching a peak at 30 min. The
188Re/131I uptake of U87-hNIS cells was
measured to be 21.3 and 25.9-fold higher compared with that of
U87-0 cells. The radioactivity measured 2 h after
188Re/131I incubation was 84.7 and 88.5% of
the maximal uptake. 131I experienced an increased uptake
compared with 188Re in U87-hNIS cells. In a similar
study performed by Zuckier et al (26), 188Re and 131I
were reported to demonstrate a high uptake.
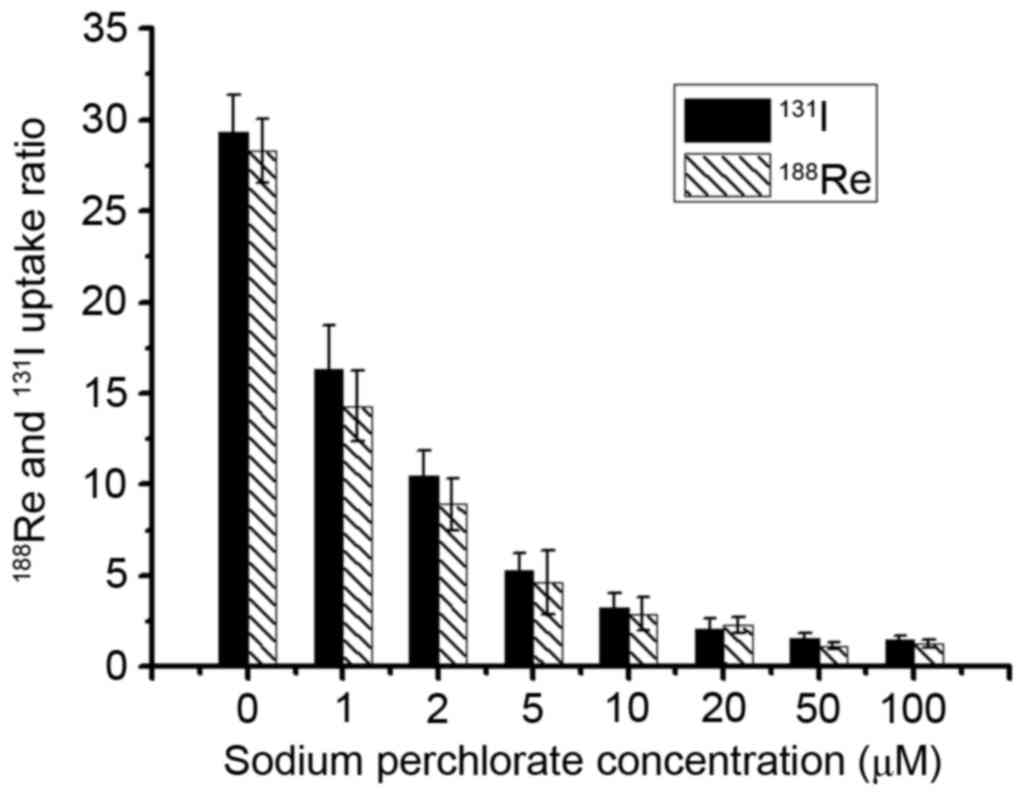
Sodium perchlorate inhibition
study
In order to verify the specificity of hNIS mediated
188Re/131I uptake,
188Re/131I inhibition test was performed in
U87-hNIS cells in the presence of varying concentrations of sodium
perchlorate, an already confirmed competitive inhibitor. Fig. 2 presents the effect of distinct
concentrations of sodium perchlorate on
188Re/131I uptake in U87-hNIS cells. When
cells were treated with sodium perchlorate (1–100 µM),
188Re/131I uptake in U87-hNIS cells was
inhibited in a dose-dependent manner. At 5 µM, sodium perchlorate
decreased 188Re/131I uptake to 15.3 and 18.1%
of original levels, respectively, compared with and an inhibition
rate of 96 and 95.3% at 50 µM. These results demonstrate that
sodium perchlorate may specifically block
188Re/131I uptake in U87-hNIS cells,
suggesting that 188Re/131I uptake in U87-hNIS
cells is mediated by functional hNIS expression.
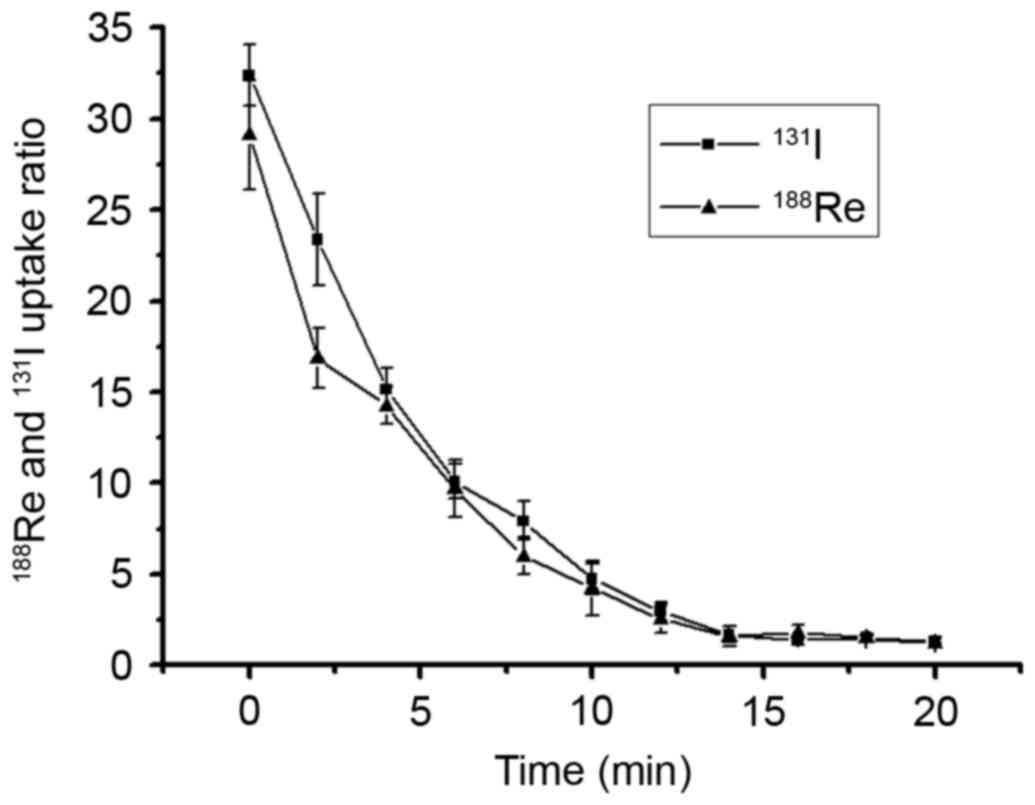
Radionuclide efflux assay
188Re/131I efflux assays were
performed 30 min after the addition of
188Re/131I into the cultured cells (the
aforementioned cell uptake experiment identified that the highest
intracellular uptake was obtained at 30 min). The culture medium
containing 188Re/131I was replaced by
nonradioactive DMEM, the remaining 188Re/131I
activity in U87-hNIS cell lysate was determined. As presented in
Fig. 3, the intracellular
radionuclides were released rapidly into the medium, with >50%
of radionuclides excreted out of the cells within the first 4 min,
whereas a limited amount of residual radionuclide was observed 20
min after 188Re/131I was replaced by
nonradioactive DMEM. These results suggested that radioactivity is
rarely retained in U87-hNIS cells without reuptaking.
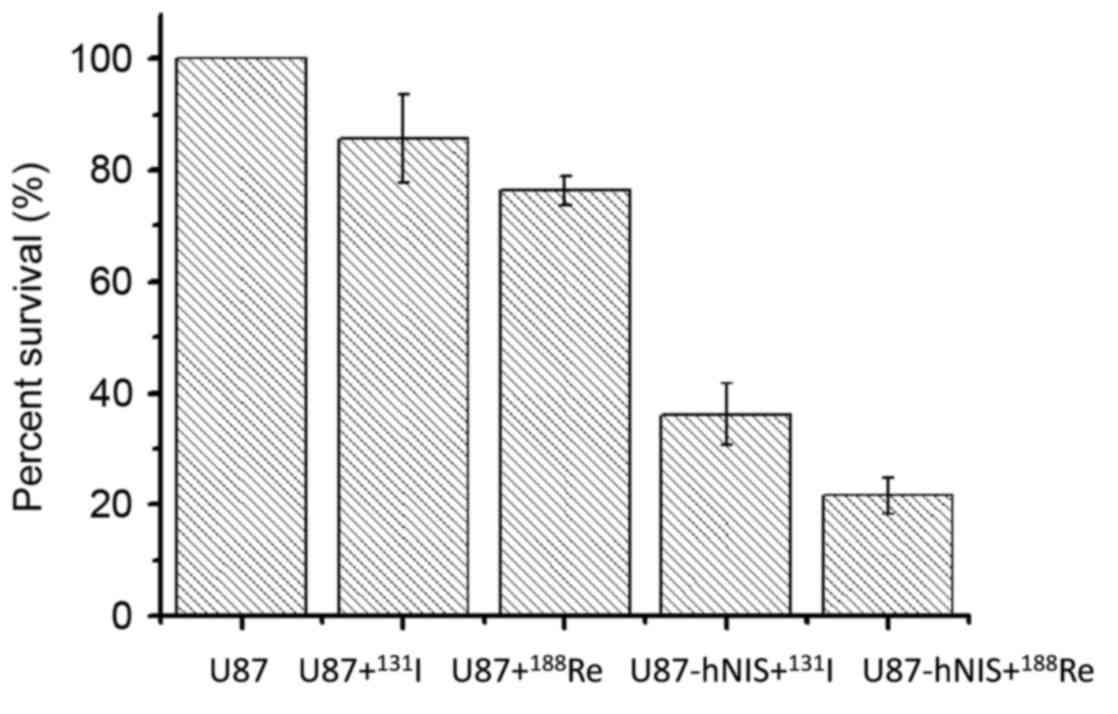
Radio toxicity assessed by
colony-forming assay
Following treatment with
188Re/131I, a colony-forming experiment was
performed to calculate the survival rate of the cells. The results
are presented in Fig. 4. The data are
expressed as the percentage of surviving cells. In Groups 3 and 4,
U87-0 cells were treated with 188Re/131I,
with the number of colonies representing 76.3 and 85.7% of the
blank control group (Group 5), indicating that the two
radionuclides exhibit nonspecific killing effects on U87-0 cells.
Following treatment with 188Re/131I, survival
of Groups 1 and 2 were markedly decreased compared with that of the
other 3 groups, with survival rates of only 21.6 and 36.2%,
respectively. These results suggest that
188Re/131I exhibits a selective killing
effect on U87-hNIS cells, with an increased killing capacity of
188Re compared with that of 131I.
Tumor imaging and therapy study
The results of in vivo uptake and efflux of
188Re/131I were different from those observed
in vitro. U87-hNIS tumor demonstrated an efficient
188Re/131I uptake in vivo, which
accumulated 188Re/131I rapidly and
significantly as indicated by the arrows in imaging results of the
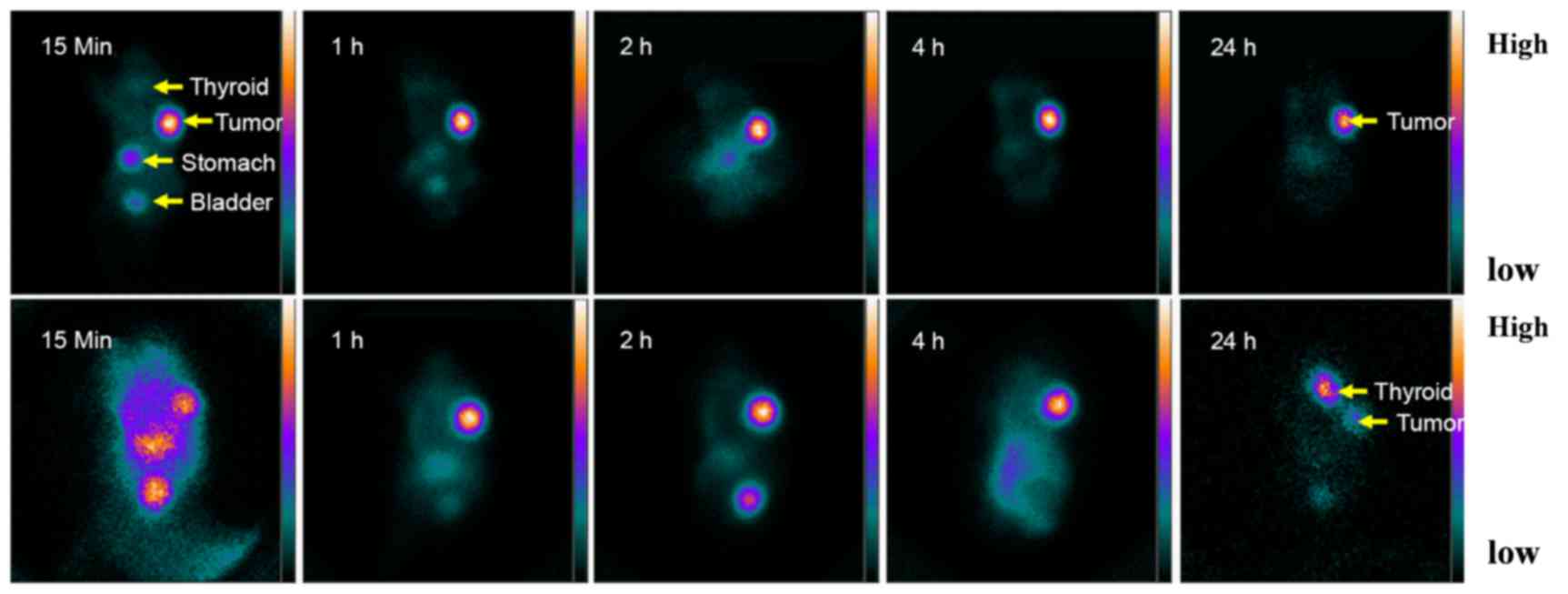
right armpit in Fig. 5. Organs
naturally expressing hNIS (such as the thyroid and stomach) and
organs participating in 188Re/131I excretion
(such as the kidneys and bladder) were able to be visualized.
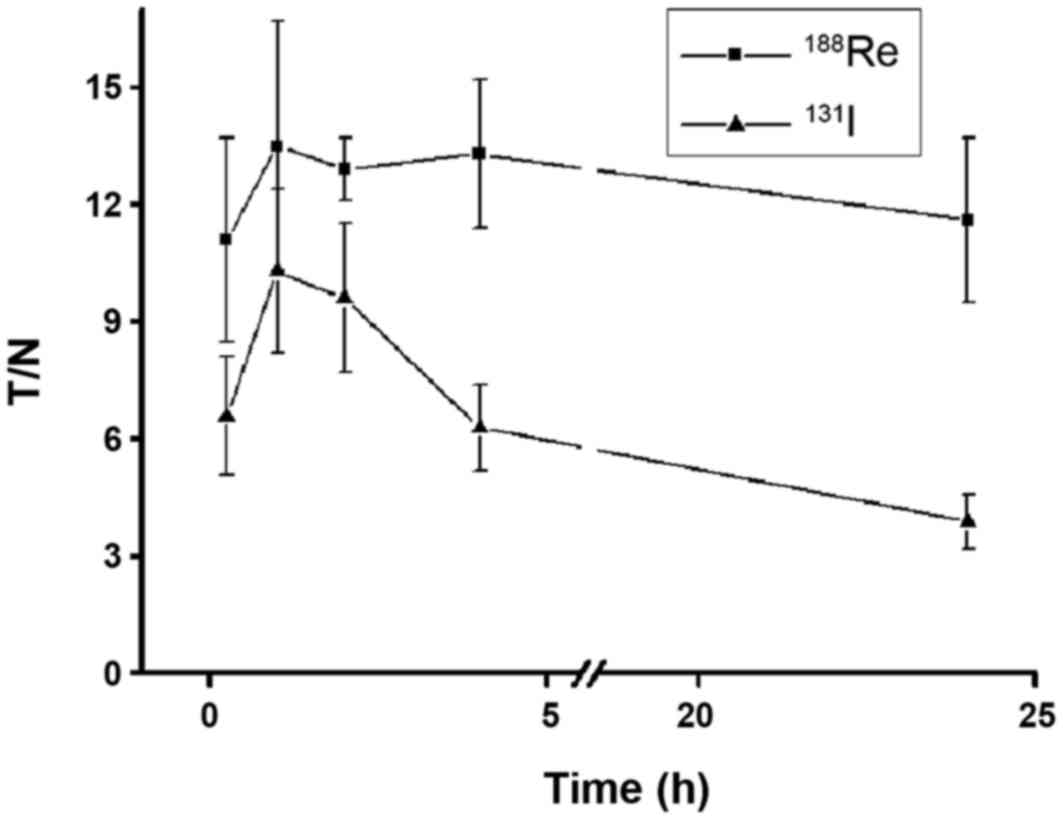
Quantified results are presented in Fig.
6. The accumulation of 188Re/131I reached
a peak 1 h after administration (T/NT, 13.5±3.2 and 10.3±2.1,
respectively). 188Re remained at a steady level until 24
h (T/NT, 11.6±2.1), whereas 131I experienced limited
retention at 24 h (T/NT, 3.9±0.7). Thyroid naturally expressing NIS
markedly accumulated 131I 24 h after injection, whereas
188Re demonstrated limited accumulation (Fig. 5). As a result of lacking
organification, 188Re cannot be retained in the thyroid
for a prolonged period of time, therefore its half-life is
relatively short in the thyroid and may protect the thyroid from
hypothyroidism. In contrast, 131I can be organified by
the thyroid, which alongside a long half-life may increase the risk
of hypothyroidism.
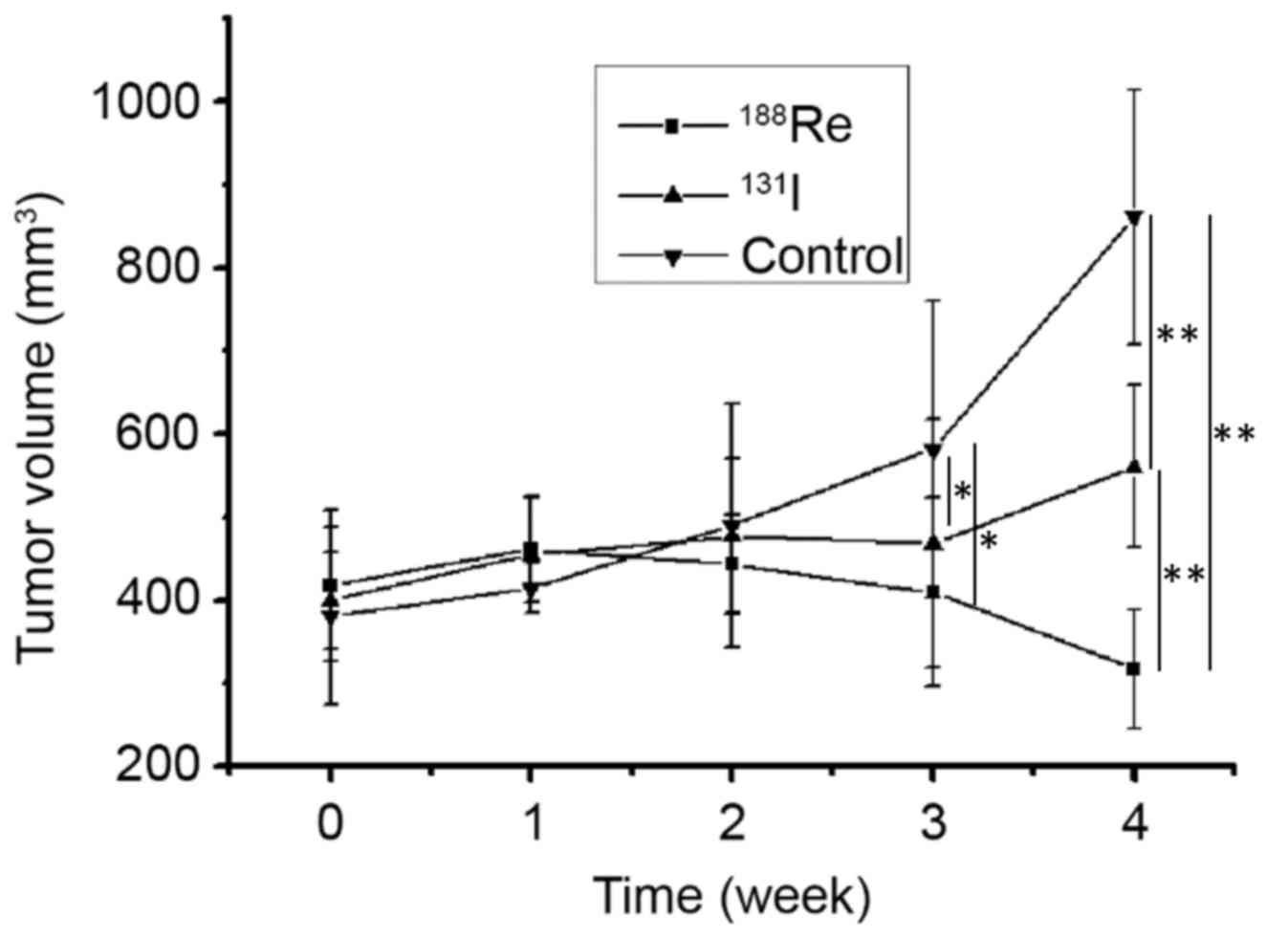
A total of 3 weeks following treatment, a difference
in tumor volume was demonstrated among mice treated with
188Re (409.6±113.4 mm3), 131I
(468.4±148.9 mm3) and non-treated animals (582.1±178.8
mm3) (P<0.05). No difference was observed between the
188Re and 131I treatment group at 3 weeks.
The association between treatment group and tumor volume was more
significant at 4 weeks post treatment. The tumor volume was
significantly different between mice treated with 188Re
(317.2±72.1 mm3), 131I (560.8±97.4
mm3) and non-treated (861.1±153.8 mm3)
(P<0.01; Fig. 7). Following
therapy, 188Re treated U87-hNIS tumors experienced a
volume reduction of 24.1%, whereas 131I treated U87-hNIS
tumors demonstrated a volume increase of 28.8%. 188Re
and 131I were demonstrated to be effective at decreasing
the tumor volume compared with the untreated control, with
188Re revealed to exhibit an increased effect compared
with 131I (P<0.01).
Discussion
NIS-mediated radionuclide therapy possesses several
advantages (27), making it an
attractive approach for glioma imaging and therapies. It does not
require complex radiolabeling procedures, it also is small-sized
allowing it to penetrate the blood brain barrier and diffuse into
the tumor (28). Radioisotopes
exhibit the potential of a bystander effect, which may destroy
tumor cells without hNIS expression by radionuclides emitted from
surrounding hNIS expression tumor cells (29). The hNIS gene is not expressed in
normal cells however is in glioma cells (30), this indicates that it may assist in
improving the specificity of tumor therapy and decreasing the
damage to normal brain tissue.
131I is an isotope commonly used in
NIS-associated radionuclide therapy studies (31). However, as the radiation energy is not
high enough, therapeutic efficacy of 131I is limited
(3). The use of a high-energy
radionuclide also transported by NIS including 188Re,
may be an effective strategy to improve therapeutic effect. A
previous study demonstrated that 188Re and
125I share similar biodistribution patterns in mice,
with the exception of the thyroid gland, as the thyroid gland has
an organic function of iodine, which can retain 125I
(26). With lower γ-photons energy,
188Re (155 keV) (32) is
suitable for imaging, unlike 131I (364 keV).
188Re can be easily generated using a
188W/188Re generator (33). These attractive physical properties
make 188Re a promising candidate for tumor imaging and
therapy (19). 188Re
possesses a higher β energy, but relatively shorter physical
half-life compared with 131I (34). Therefore, in the current study, the
effects of 188Re and 131I in the treatment of
glioma were compared in vivo and in vitro.
In the present study, it was demonstrated that a
lentivirus containing CMV-hNIS expression cassette is able to
transfect U87 cells, as revealed by efficient
188Re/131I uptake in U87-hNIS cells.
188Re/131I exhibit rapid uptake in U87-hNIS
cells, and the dynamic uptake pattern of
188Re/131I was similar to that of previous
studies (30,31). 188Re uptake of U87-hNIS was
up to 21.3-times that of the U87-0 control group, whereas
131I uptake was up to 25.9-fold of that of the control
group. The two isotopes experienced a plateau phase akin to
previous studies (35,36), when the uptake and efflux of the
isotopes reached a state of equilibrium (25). As with previous NIS studies,
radionuclide retention time was short, and rapid outflow remained a
problem in the in vitro studies, in the present study.
Neither 188Re nor 131I experienced a long
retention time in vitro.
In the in vitro experiments, isotope flow
was rapid, with the outflow kinetics of the two radionuclides being
similar. In addition, the colony formation assay demonstrated that
U87-hNIS cells could be killed effectively by 188Re and
131I. The absorbed dose of 188Re was
sufficiently high to selectively kill 78.4% of the cells, whereas
131I was able to kill up to 63.8% of the cells. These
data are sufficient to suggest that even with a lower uptake than
that of 131I, 188Re may also be able to
deliver a greater radiation dose to tumor cells, which is similar
to the results reported by Kang et al (37) who identified that 188Re
uptake increased 87-times, whereas 125I uptake increased
150-times in NIS transfected hepatocellular carcinoma cells,
compared with untransfected cells. Following treatment with
188Re or 131I, the survival rate of cells was
28.9 and 46.3% respectively. Therefore, a superior therapeutic
effect of 188Re was demonstrated.
In the in vitro study, cells in the culture
dishes were arranged in a single layer, which is not a
three-dimensional structure. Conclusions from the cells cannot be
applied to solid glioma with a three-dimensional structure, which
requires further confirmation in xenografts. In vivo,
U87-hNIS glioma cells are close to each other, which allows for
rapid reuptake of radionuclides in the cells and serves as a
mechanism for isotope trapping by glioma. The results of the in
vivo imaging investigations in the present study suggested that
188Re remained in U87-hNIS glioma cells even 24 h after
administration, whereas a limited amount of 131I was
retained in the cells 24 h after treatment. The results of the
present study suggest that 188Re cannot be retained in
the thyroid gland, as it cannot be organified. A previous study has
reported a similar biodistribution pattern for 131I and
188Re (38). In fact, the
thyroid will decrease the competitive uptake of 188Re,
thereby decreasing thyroid injury and increasing tumor reuptake of
188Re, which is an advantage of hNIS-mediated
radionuclide therapy in non-thyroid tumors. In addition, the
results of the present study demonstrated that the degree of
188Re/131I uptake and retention are
sufficient to achieve tumor therapeutic effects. Following
treatment for 4 weeks, there was a significant difference in tumor
size between 188Re treated U87-hNIS mice and
131I treated U87-hNIS mice.
The present study also possessed a number of
limitations. A stable hNIS transfected cell line was used to
express hNIS, which could not be used directly in clinical
practice. As a result of the variation of hNIS transfection
efficiency and the difference in specificity, actual clinical
efficacy of 188Re and 131I requires further
evaluation. It is reported that U87 MG ATCC cell line has been
contaminated/misidentified, however it is most probably also a
glioblastoma cell line (39). hNIS
mediates uptake of iodine in thyroid and numerous types of
non-thyroid cells (6), as a result, a
number of previous studies have also successfully obtained ectopic
expression of hNIS in different tumors by gene transfer (7–10). The
main purpose of the present study was to compare the therapeutic
effects of 188Re and 131I mediated by hNIS,
rather than a study into the underlying molecular mechanisms of
their effects, therefore the U87 cell line could be replaced with a
variety of cell lines that express hNIS, as such the aforementioned
misidentification/contamination issue is unlikely to have affected
the outcomes of the present study.
In conclusion, the results of the present study
demonstrated that 188Re appeared to be more efficient
than 131I in the treatment of gliomas. The strategy
appears to be a novel method for tumor imaging and therapy.
However, improvements to decrease efflux and achieve increased
radiation doses in the target tissue are still required.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81101071 and
81471687) and the Medical Engineering (Science) Cross Foundation of
Shanghai JiaoTong University (grant no. YG2013MS27).
References
|
1
|
D'Amico RS, Englander ZK, Canoll P and
Bruce JN: Extent of resection in glioma-a review of the cutting
edge. World Neurosurg. 103:538–549. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sulman EP, Ismaila N, Armstrong TS, Tsien
C, Batchelor TT, Cloughesy T, Galanis E, Gilbert M, Gondi V, Lovely
M, et al: Radiation therapy for glioblastoma: American society of
clinical oncology clinical practice guideline endorsement of the
American society for radiation oncology guideline. J Clin Oncol.
35:361–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prados MD and Levin V: Biology and
treatment of malignant glioma. Semin Oncol. 27(3 Suppl 6): S1–S10.
2000.
|
|
4
|
Brandes AA, Rigon A and Monfardini S:
Radiotherapy of the brain in elderly patients. Contra. Eur J
Cancer. 36:447–452. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grau JJ and Verger E: Radiotherapy of the
brain in elderly patients. Pro. Eur J Cancer. 36:443–447. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tazebay U, Wapnir IL, Levy O, Dohan O,
Zuckier LS, Zhao QH, Deng HF, Amenta PS, Fineberg S, Pestell RG and
Carrasco N: The mammary gland iodide transporter is expressed
duringlactation and in breast cancer. Nat Med. 6:871–878. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spitzweg C, Zhang S, Bergert ER, Castro
MR, McIver B, Heufelder AE, Tindall DJ, Young CY and Morris JC:
Prostate-specific antigen (PSA) promoter-driven androgen-inducible
expression of sodium iodide symporter in prostate cancer cell
lines. Cancer Res. 59:2136–2141. 1999.PubMed/NCBI
|
|
8
|
Mandell RB, Mandell LZ and Link CJ Jr:
Radioisotope concentrator gene therapy using the sodium/iodide
symporter gene. Cancer Res. 59:661–668. 1999.PubMed/NCBI
|
|
9
|
Cho J, Xing S, Liu X, Buckwalter TL, Hwa
L, Sferra TJ, Chiu IM and Jhiang SM: Expression and activity of
human Na+/I-symporter in human glioma cells by adenovirus-mediated
gene delivery. Gene Ther. 7:740–749. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dingli D, Diaz RM, Bergert ER, O'Connor
MK, Morris JC and Russell SJ: Genetically targeted radiotherapy for
multiple myeloma. Blood. 102:489–496. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmohl KA, Gupta A, Grünwald GK,
Trajkovic-Arsic M, Klutz K, Braren R, Schwaiger M, Nelson PJ, Ogris
M, Wagner E, et al: Imaging and targeted therapy of pancreatic
ductal adenocarcinoma using the theranostic sodium iodide symporter
(NIS) gene. Oncotarget. 16:33393–33404. 2017.
|
|
12
|
Zhang M, Guo R, Xu H, Zhang M and Li B:
Retinoic acid and tributyrin induce in-vitro radioiodine uptake and
inhibition of cell proliferation in a poorly differentiated
follicular thyroid carcinoma. Nucl Med Commun. 32:605–610. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo R, Zhang R, Pan Y, Xu H, Zhang M,
Liang S, Wang L, Zhang Y and Li B: Feasibility of a novel positive
feedback effect of 131I-promoted Bac-Egr1-hNIS expression in
malignant glioma through baculovirus: A comparative study with
Bac-CMV-hNIS. Nucl Med Commun. 32:402–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo R, Tian L, Han B, Xu H, Zhang M and Li
B: Feasibility of a novel positive feedback effect of 131I-promoted
Bac-Egr1-hNIS expression in malignant glioma via baculovirus. Nucl
Med Biol. 38:599–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo R, Zhang Y, Liang S, Xu H, Zhang M and
Li B: Sodium butyrate enhances the expression of
baculovirus-mediated sodium/iodide symporter gene in A549 lung
adenocarcinoma cells. Nucl Med Commun. 31:916–921. 2010.PubMed/NCBI
|
|
16
|
Mallick UK and Charalambous H: Current
issues in the management of differentiated thyroid cancer. Nucl Med
Commun. 25:873–881. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamoto Y, Saga T, Misaki T, Kobayashi H,
Sato N, Ishimori T, Kosugi S, Sakahara H and Konishi J:
Establishment and characterization of a breast cancer cell line
expressing Na+/I-symporters for radioiodide concentrator gene
therapy. J Nucl Med. 41:1898–1904. 2000.PubMed/NCBI
|
|
18
|
Dadachova E, Bouzahzah B, Zuckier LS and
Pestell RG: Rhenium-188 as an alternative to iodine-131 for
treatment of breast tumors expressing the sodium/iodide symporter
(NIS). Nucl Med Biol. 29:13–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lambert B and de Klerk JM: Clinical
applications of 188Re-labelled radiopharmaceuticals for
radionuclide therapy. Nucl Med Commun. 27:223–229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dadachova E, Nguyen A, Lin EY, Gnatovskiy
L, Lu P and Pollard JW: Treatment with rhenium-188-perrhenate and
iodine-131 of NIS-expressing mammary cancer in a mouse model
remarkably inhibited tumor growth. Nucl Med Biol. 32:695–700. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Höher M, Wöhrle J, Wohlfrom M, Kamenz J,
Nusser T, Grebe OC, Hanke H, Kochs M, Reske SN, Hombach V and
Kotzerke J: Intracoronary beta-irradiation with a
rhenium-188-filled balloon catheter: A randomized trial in patients
with de novo and restenotic lesions. Circulation. 107:3022–3027.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murray A, Simms MS, Scholfield DP, Vincent
RM, Denton G, Bishop MC, Price MR and Perkins AC: Production and
characterization of 188Re-C595 antibody for radioimmunotherapy of
transitional cell bladder cancer. J Nucl Med. 42:726–732.
2001.PubMed/NCBI
|
|
23
|
Dadachova E, Bouzahzah B, Zuckier LS and
Pestell RG: Rhenium-188 as an alternative to Iodine-131 for
treatment of breast tumors expressing the sodium iodide/symporter
(NIS). Nucl Med Biol. 29:13–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo R, Zhang M, Xi Y, Ma Y, Liang S, Shi
S, Miao Y and Li B: Theranostic studies of human sodium iodide
symporter imaging and therapy using 188Re: A human glioma study in
mice. PLoS One. 9:e1020112014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weiss SJ, Philp NJ and Grollman EF: Iodide
transport in a continuous line of cultured cells from rat thyroid.
Endocrinology. 114:1090–1098. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zuckier LS, Dohan O, Li Y, Chang CJ,
Carrasco N and Dadachova E: Kinetics of perrhenate uptake and
comparative biodistribution of perrhenate, pertechnetate, and
iodide by NaI symporter-expressing tissues in vivo. J Nucl Med.
45:500–507. 2004.PubMed/NCBI
|
|
27
|
Ahn BC: Requisites for successful
theranostics with radionuclide-based reporter gene imaging. J Drug
Target. 22:295–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dadachova E and Carrasco N: The Na/I
symporter (NIS): Imaging and therapeutic applications. Semin Nucl
Med. 34:23–31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carlin S, Cunningham SH, Boyd M, McCluskey
AG and Mairs RJ: Experimental targeted radioiodide therapy
following transfection of the sodium iodide symporter gene: Effect
on clonogenicity in both two-and three-dimensional models. Cancer
Gene Ther. 7:1529–1536. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Opyrchal M, Allen C, Iankov I, Aderca I,
Schroeder M, Sarkaria J and Galanis E: Effective radiovirotherapy
for malignant gliomas by using oncolytic measles virus strains
encoding the sodium iodide symporter (MV-NIS). Hum Gene Ther.
23:419–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chung JK and Cheon GJ: Radioiodine therapy
in differentiated thyroid cancer. The first targeted therapy in
oncology. Endocrinol Metab (Seoul). 29:233–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
O'Donoghue JA, Bardiès M and Wheldon TE:
Relationships between tumor size and curability for uniformly
targeted therapy with beta-emitting radionuclides. J Nucl Med.
36:1902–1909. 1995.PubMed/NCBI
|
|
33
|
Sundram FX, Jeong JM, Zanzonico P, Divgi
C, Bernal P, Chau T, Onkhuudai P, Knapp FF Jr, Buscombe J and Padhy
AK: Trans-arterial rhenium-188 lipiodol in the treatment of
inoperable hepatocellular carcinoma: An IAEA sponsored multi-centre
phase 1 study. World J Nucl Med. 1:5–11. 2002.
|
|
34
|
De Ruyck K, Lambert B, Bacher K, Gemmel F,
De Vos F, Vral A, de Ridder L, Dierckx RA and Thierens H: Biologic
dosimetry of 188Re-HDD/lipiodol versus 131I-lipiodol therapy in
patients with hepatocellular carcinoma. J Nucl Med. 45:612–618.
2004.PubMed/NCBI
|
|
35
|
Chen L, Altman A, Mier W, Lu H, Zhu R and
Haberkorn U: 99mTc-pertechnetate uptake in hepatoma cells due to
tissue-specific human sodium iodide symporter gene expression. Nucl
Med Biol. 33:575–580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boland A, Ricard M, Opolon P, Bidart JM,
Yeh P, Filetti S, Schlumberger M and Perricaudet M:
Adenovirus-mediated transfer of the thyroid sodium/iodide symporter
gene into tumors for a targeted radiotherapy. Cancer Res.
60:3484–3492. 2000.PubMed/NCBI
|
|
37
|
Kang JH, Chung JK, Lee YJ, Shin JH, Jeong
JM, Lee DS and Lee MC: Establishment of a human hepatocellular
carcinoma cell line highly expressing sodium iodide symporter for
radionuclide gene therapy. J Nucl Med. 45:1571–1576.
2004.PubMed/NCBI
|
|
38
|
Willhauck MJ, Sharif Samani BR, Gildehaus
FJ, Wolf I, Senekowitsch-Schmidtke R, Stark HJ, Göke B, Morris JC
and Spitzweg C: Application of 188rhenium as an alternative
radionuclide for treatment of prostate cancer after tumor-specific
sodium iodide symporter gene expression. J Clin Endocrinol Metab.
92:4451–4458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|