Introduction
Cervical cancer is one of the common malignant
tumors in females, which seriously threatens their health and life.
Its incidence rate is second only to breast cancer among malignant
tumors in females. There are about 500,000 new cases each year
around the world, in which 267,000 new cases occur in Asia annually
(1). In recent years, cervical cancer
has frequently occured in young people. Cervical cancer is prone to
invasion and metastasis in patients, thus leading to poor prognosis
of patients with cervical cancer, and the 5-year survival rate is
only about from 30 to 60% (2).
Micro-ribonucleic acid (miRNA) is a non-coding small
RNA molecule with a length of about 22 nucleotides and can be
specifically paired with the three prime untranslated region
(3′-UTR) of the target gene, thus leading to degradation of the
target gene messenger RNA (mRNA) or inhibiting the translation
process so as to affect the expression of target genes (3). In 1993, Lee et al found miRNAs in
Caenorhabditis elegans for the first time, and discovered
that miRNAs play key roles in cell development, differentiation,
proliferation and apoptosis (4). At
the same time, it was found that miRNAs are abnormally expressed in
many kinds of tumors and are closely related to the occurrence and
development of tumors (5).
Previously, a study showed that miR-34a is an
important target for tumor suppressor protein p53 (6). miR-34a can specifically bind to mRNAs of
cyclin E2, cyclin-dependent kinase 4, c-Myc, and B-cell lymphoma 2
at 3′-UTR, which leads to mRNA degradation of these proteins, thus
blocking cell cycle, inhibiting cell proliferation and promoting
tumor cell apoptosis (7,8). The miR-218 gene is located on chromosome
5q16 and is downregulated in tissues and cells of tumors such as
gastric cancer, head and neck squamous cell carcinoma and breast
cancer. A study revealed that the miR-218 gene is associated with
tumor growth, invasion and metastasis (9). In addition, it has been found that
miR-218 expression is downregulated in cervical cancer cell lines
and cervical cancer, and it is speculated that the expression of
miR-218 is closely related to the occurrence and development of
cervical cancer (10).
A study showed that miRNAs are differently expressed
in different tumor tissues at different development stages of the
tumors (11). In addition, miRNAs
circulating in human serum or plasma are of great importance for
early diagnosis, staging and prognosis of tumors (12). Therefore, we are committed to the
study on the expression of miRNAs in the serum of patients with
cervical cancer, and confirm its possible effectiveness in the
early diagnosis and prognosis evaluation of cervical cancer. The
relationship between the expression of miR-34a and miR-218 in
peripheral blood of patients with cervical cancer and those in
tumor tissues and the correlation of the expression of miR-34a and
miR-218 with pathological parameters and the prognosis of cervical
cancer are rarely reported in the literature. Therefore, this study
preliminarily investigated the values of miR-34a and miR-218 in the
diagnosis of cervical cancer and the correlation of their
expression with clinicopathological parameters and the prognosis of
patients by detecting the expression levels of miR-34a and miR-218
in the serum and tumor tissues of patients with cervical cancer in
combination with the clinical data of patients.
Materials and methods
Materials
A total of 50 patients with cervical cancer admitted
to Dezhou People's Hospital from January, 2010 to December, 2016
were collected. They were aged 52.3±12.7 years, and none received
preoperative chemotherapy, radiotherapy or endocrine therapy.
Tissue specimens were pathologically diagnosed with cervical
cancer. A total of 30 patients with uterine fibroids receiving
uterine total resection who were admitted at the same period were
selected as the control group. They were aged 49.6±13.2 years, and
their tissue specimens were histopathologically confirmed with
normal cervical tissues. Materials were drawn from tissue specimens
in the operation within 30 min and then immediately placed in
liquid nitrogen for preservation. Peripheral venous blood (5 ml)
was collected before operation as peripheral blood samples. After
standing at room temperature for 30 min, the samples were
centrifuged at 10 min at 2,860 × g, and the supernatant was
separated and placed in at −80°C. The present study was approved by
the Clinical Ethics Committee, and all the patients or family
members signed the informed consent; TRIzol kit (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA); primer synthesis,
reverse transcription kits and quantitative real-time polymerase
chain reaction (qRT-PCR) kits (all from Takara Biotechnology Co.,
Ltd., Dalian, China).
Detection of the expression of miR-34a
and miR-218 in tissue specimens and serum of patients by
qRT-PCR
A total of 100 mg frozen tissues and 50 µl serum of
patients were selected, respectively. The total RNA was extracted
according to the instructions of TRIzol kit. The ratio of A260/A280
was measured using a spectrophotometer (Hitachi, Ltd., Tokyo,
Japan). The ratio of A260/A280 (1.8–2.0) could be used for
subsequent tests. Total RNA sample (1 µg) was extracted from
patients in the two groups, respectively, and complementary
deoxyribonucleic acid (cDNA) was obtained through reverse
transcription according to the instructions of reverse
transcription kits. Reaction conditions: At 16°C for 30 min, at
42°C for 30 min and at 75°C for 15 min. After that, with cDNA as a
template and U6 RNA as the control gene, the expression levels of
miR-218 and miR-34a were detected according to the instructions of
qRT-PCR kits. RT-PCR amplification conditions: At 95°C for 5 min,
at 95°C for 15 sec, at 60°C for 30 sec, and at 68°C for 30 sec; 30
cycles. In all reactions, three wells for repeated use were set up.
The primer sequences of miR-34a, miR-218 and U6 are shown in
Table I. Cq values were
from the instrument software, and the relative expression of the
target gene was expressed as 2−ΔCq.
 | Table I.Primer sequences of qRT-PCR. |
Table I.
Primer sequences of qRT-PCR.
| Gene | Primer sequence |
|---|
| miR-34a | F:
5′-CGGTATCATTTGGCAGTG-3′ |
|
| R:
5′-GTGCAGGGTCCGAGGT-3′ |
| miR-34a8 | F:
5′-GGAGTGGCGAATGGTAGTGGAGT-3′ |
|
| R:
5′-ACCAGGCTGGACAGTAGAGCG-3′ |
| U6 | F:
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
|
| R:
5′-C-CGCTTCACGAATTTGCGTGTCAT-3′ |
The correlation of the expression of
miR-34a and miR-218 in the serum of patients
According to the expression levels of miR-34a and
miR-218 in the serum of patients with cervical cancer, the patients
with cervical cancer were divided into: i) miR-34a high expression
group (≥2.54); ii) miR-34a low expression group (<2.54); iii)
miR-218 high expression group (≥2.63); iv) and miR-218 low
expression group (<2.63) with the average expression level as
the standard. The correlation of the expression of miR-34a and
miR-218 with pathological parameters and the prognosis of patients
was analyzed according to the clinicopathological data.
Statistical analysis
Data were analyzed using SPSS 18.0 software (SPSS,
Inc., Chicago, IL, USA). Measurement data were detected using the
t-test. Intergroup comparisons of count data were conducted using
the χ2 test. The correlation analysis was performed by
Pearson's correlation coefficient, and the receiver operator
characteristic (ROC) curve was plotted. P<0.05 was considered to
indicate a statistically significant difference.
Results
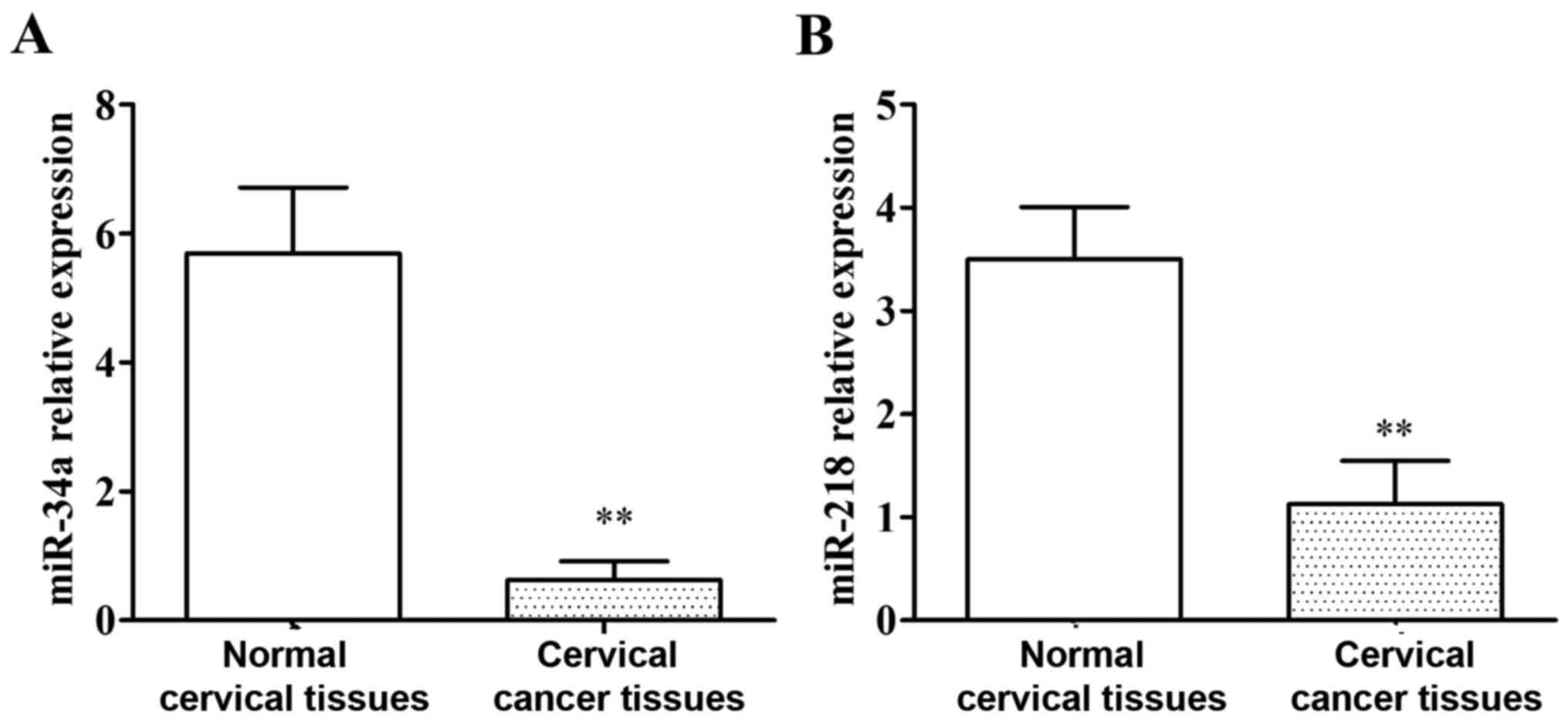
The expression of miR-34a and miR-218
in tissue specimens of patients
qRT-PCR results are shown in Fig. 1. The expression level of miR-34a in
cervical cancer tissues was significantly lower than that in normal
cervical tissues, and the difference was statistically significant
(P<0.01). The expression level of miR-218 in cervical cancer
tissues was significantly lower than that in normal cervical
tissues, and the difference was statistically significant
(P<0.01).
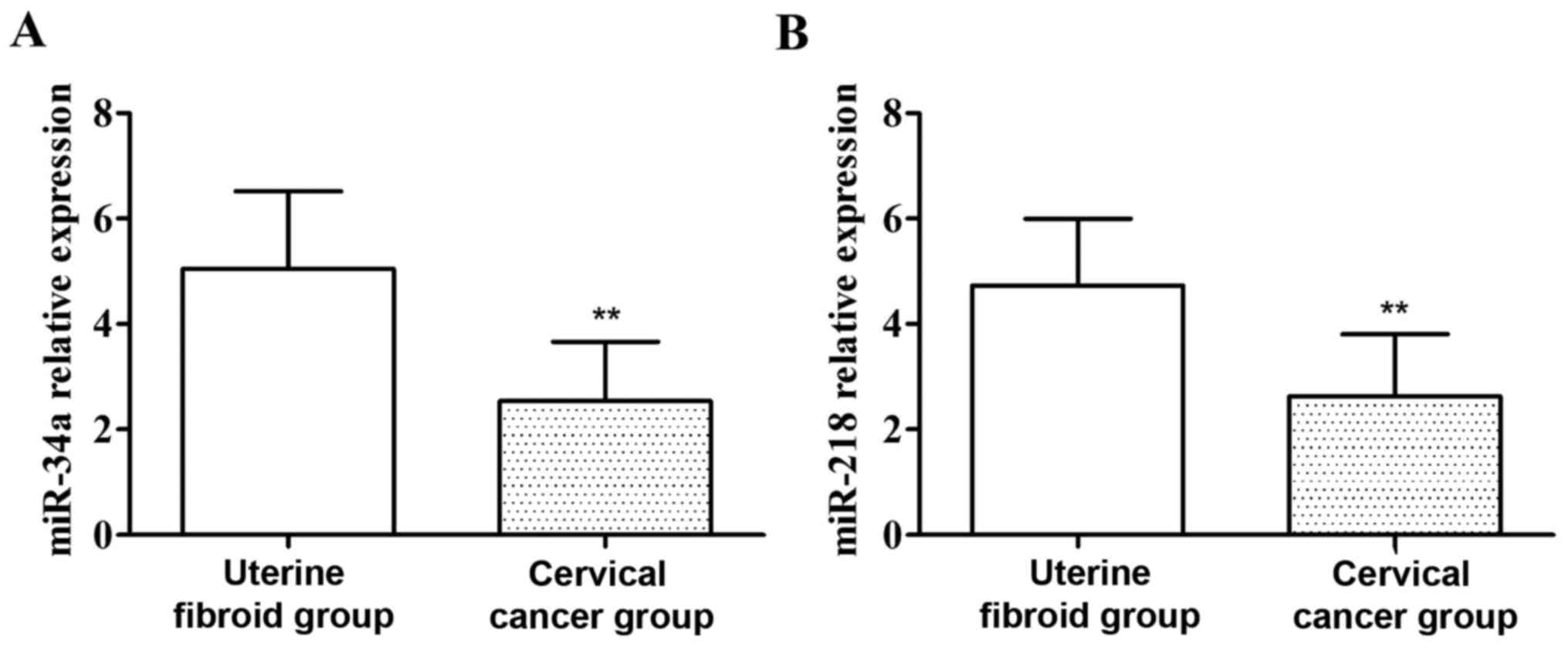
Detection of the expression of miR-34a
and miR-218 in the serum of patients by qRT-PCR
As shown in Fig. 2,
compared with those of patients in the uterine fibroid group, the
expression levels of miR-34a and miR-218 in the serum of patients
with cervical cancer were significantly decreased, and the
differences were statistically significant (P<0.01).
The correlation between the expression
of miR-34a and miR-218 in the serum and those in the tumor tissues
of patients with cervical cancer
Pearson's correlation coefficient was used to
analyze the correlation between the expression of miR-34a and
miR-218 in the serum and those in tumor tissues of patients. As
shown in Table II, the expression
levels of miR-34a and miR-218 in the serum were positively
correlated with those in tumor tissues of patients with cervical
cancer (P<0.01).
 | Table II.The correlation between the expression
of miR-34a and miR-218 in the serum and those in the tumor tissues
of patients with cervical cancer. |
Table II.
The correlation between the expression
of miR-34a and miR-218 in the serum and those in the tumor tissues
of patients with cervical cancer.
| Serum | Tumor tissues | r | P-values |
|---|
| miR-34a | miR-34a | 0.697 | <0.01 |
| miR-218 | miR-218 | 0.758 | <0.01 |
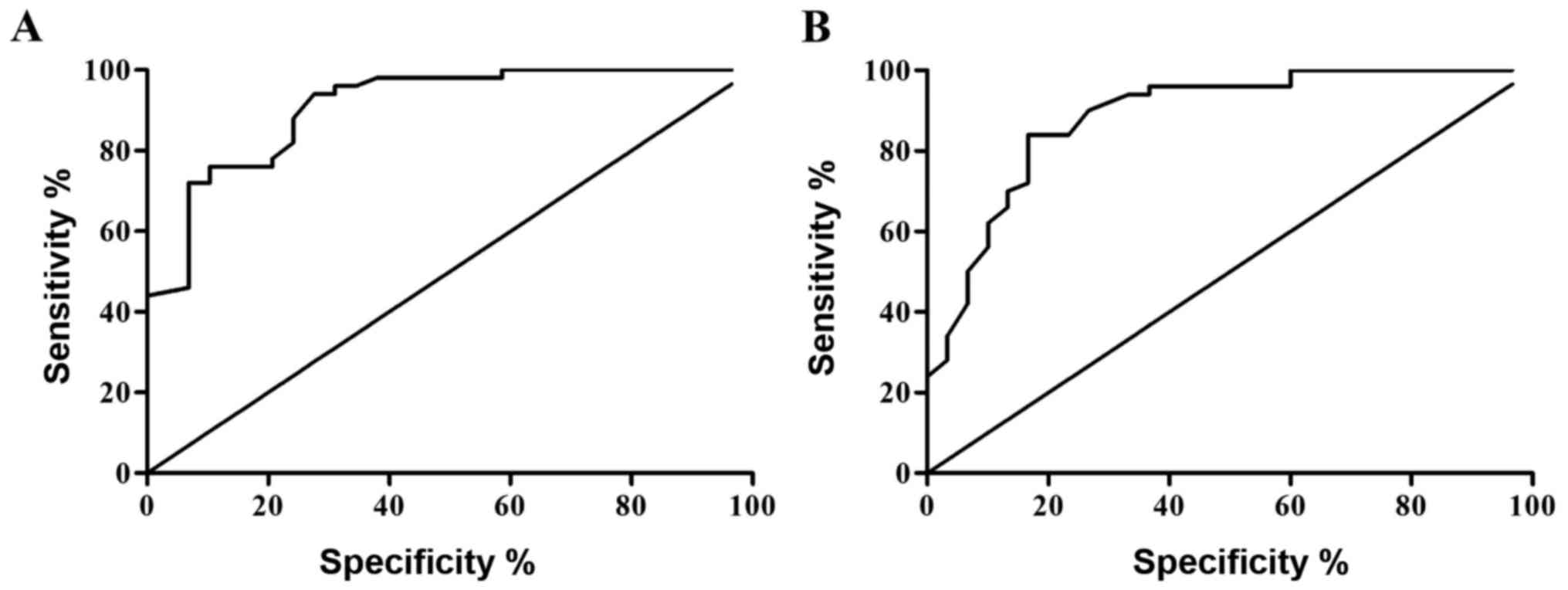
Values of the expression of miR-34a
and miR-218 in the serum of patients with cervical cancer in the
diagnosis of cervical cancer
The ROC curve was plotted according to the relative
expression levels of miR-34a and miR-218, and the area under curve
(AUC) 0.893±0.035 and 95% confidence interval (0.824–0.961) of
miR-34a for the diagnosis of cervical cancer were calculated, and
the diagnosis was relatively more accurate. The AUC 0.794±0.050 and
95% confidence interval (0.696–0.892) of miR-218 for the diagnosis
of cervical cancer were calculated, and the diagnosis was
relatively more accurate (Fig.
3).
The correlation of the expression of
miR-34a and miR-218 in the serum of patients with cervical cancer
with clinical pathology
Statistics showed that there were 36 with low
expression miR-34a (72.00%) and 31 with low expression miR-218
(62.00%) out of 50 patients with cervical cancer. The correlation
of the expression of miR-34a and miR-218 in the serum of patients
with cervical cancer is shown in Table
III. The χ2 test indicated that the low expression
of miR-34a in the serum was correlated with the degree of tumor
differentiation, invasion and metastasis and the International
Federation of Gynecology and Obstetrics (FIGO) staging, and the low
expression of miR-218 was associated with the degree of tumor
differentiation as well as invasion and metastasis.
 | Table III.The correlation of the expression of
miR-34a and miR-218 with the clinicopathological parameters of
cervical cancer. |
Table III.
The correlation of the expression of
miR-34a and miR-218 with the clinicopathological parameters of
cervical cancer.
|
|
| miR-34a | miR-218 |
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | Case (n) | Low expression | χ2
value | P-value | Low expression | χ2
value | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
| ≥40 | 32 | 24 | 0.40 | >0.05 | 21 | 0.50 | >0.05 |
|
<40 | 18 | 12 |
|
| 10 |
|
|
| Diameter of
tumor |
|
|
|
|
|
|
|
| ≥4
cm | 28 | 22 | 1.36 | >0.05 | 17 | 0.04 | >0.05 |
| <4
cm | 22 | 14 |
|
| 14 |
|
|
| Degree of tumor
differentiation |
|
|
|
|
|
|
|
| Low
differentiation | 21 | 9 | 15.25 | <0.01 | 8 | 8.78 | <0.01 |
|
Medium/high
differentiation | 29 | 27 |
|
| 23 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
| Yes | 35 | 30 | 5.14 | <0.05 | 26 | 7.74 | <0.01 |
| No | 15 | 6 |
|
| 5 |
|
|
| FIGO staging |
|
|
|
|
|
|
|
| I–II | 24 | 12 | 11.08 | <0.01 | 14 | 0.26 | >0.05 |
|
III–IV | 26 | 24 |
|
| 17 |
|
|
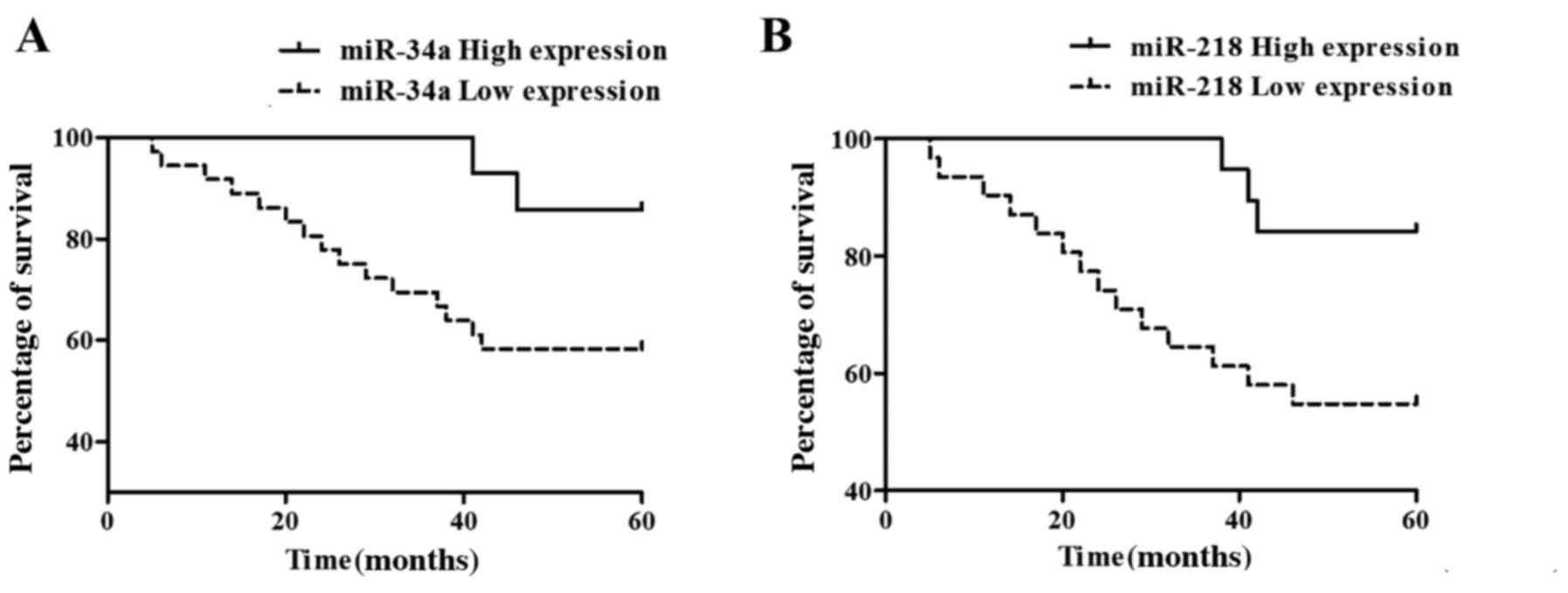
Analysis of the survival and prognosis
of patients
Statistical analysis of the follow-up results of
patients with cervical cancer showed that 33 patients survived and
17 died, and the 5-year overall survival rate was 66%, in which the
death of patients were caused by cervical cancer progress.
Kaplan-Meier survival curve analysis showed that miR-34a high
expression (Fig. 4A) and miR-218 low
expression (Fig. 4B) had worse
survival prognoses.
Discussion
In previous years, the incidence rate of cervical
cancer has significantly increased in China, and the pathogenesis
of cervical cancer is very complex, including the activation of
proto-oncogene, anti-cancer abnormal expression, human
papillomavirus infection and immune factors, as shown (13).
miRNAs play key roles in the occurrence and
development of various malignant tumors, and it has been found that
miRNAs are involved in the important processes, such as tumor cell
proliferation, apoptosis, metabolism and differentiation (14,15).
miR-34a, a member of the miR-34 family, is commonly found in
mammals and abnormally expressed in a variety of tumors, which can
induce cell apoptosis, prevent cell invasion and block cell cycle
progression (16–18). In recent years, the abnormal
expression of miR-218 in tumor tissue has become a hot topic in
tumor research. Studies have confirmed that the expression of
miR-218 was downregulated in cervical cancer, gastric cancer and
breast cancer, and miR-218 is closely related to the invasion and
metastasis, differentiation and staging of tumors (19,20).
In the serum of normal people, the expression of
miRNAs has a good consistency and stability. When lesions occur in
the body, the expression of miRNAs in the serum will also change
correspondingly, so detecting the abnormally expressed miRNAs in
the serum of cancer patients may become a simple, effective and
fast tumor diagnosis method (21).
Studies have confirmed that miR-34a and miR-218 have low expression
in cervical cancer and are closely related to clinicopathological
parameters and the prognosis of patients (22,23).
In order to further investigate the expression of
miR-34a and miR-218 in the serum and tumor tissues of patients with
cervical cancer and their influence on the pathological parameters
and prognosis of patients with cervical cancer, the expression
levels of miR-34a and miR-218 in the serum and tumor tissues of
patients with cervical cancer were detected using qRT-PCR in this
study. The results revealed that compared with those in normal
cervical tissues, the expression levels of miR-34a and miR-218 in
cervical cancer tissues were significantly decreased, and the
expression levels of miR-34a and miR-218 in the serum of patients
with cervical cancer were significantly lower than those of
patients with uterine fibroids. At the same time, Pearson's
correlation coefficient showed that the expression of miR-34a and
miR-218 in the serum were positively correlated with those in
cervical cancer tissues. The ROC curve analysis showed that the AUC
of miR-34a was 0.893 and the 95% confidence interval was
0.824–0.961; the AUC of miR-218 was 0.794, and the 95% confidence
interval was 0.696–0.892, indicating that the diagnosis of cervical
cancer using miR-34a and miR-218 is relatively more accurate. The
correlation analysis of clinicopathological parameters of patients
indicated that the low expression of miR-34a in the serum of
patients with cervical cancer was correlated with the degree of
tumor differentiation, lymph node metastasis and FIGO staging, and
the low expression of miR-218 in the serum was related to the
degree of differentiation as well as invasion and metastasis. The
5-year overall survival rate of patients was 66% (33/50), and the
low expressions of miR-34a and miR-218 presented a worse survival
prognosis.
In summary, the low expression of miR-34a and
miR-218 are closely related to the occurrence and development of
cervical cancer, especially to the degree of tumor differentiation
as well as invasion and metastasis. Tumor detection by miRNAs in
the serum of patients has many advantages. For example, it is
minimally invasive, repeatable and simple and feasible. The study
confirmed that miR-34a and miR-218 in the serum of patients with
cervical cancer could be used as important reference indicators in
the diagnosis and prognosis evaluation of cervical cancer.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: J Torre LA: Global cancer statistics. CA Cancer J
Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hisamatsu T, Mabuchi S, Yoshino K, Fujita
M, Enomoto T, Hamasaki T and Kimura T: Prediction of
progression-free survival and response to paclitaxel plus
carboplatin in patients with recurrent or advanced cervical cancer.
Int J Gynecol Cancer. 22:623–629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zamore PD and Haley B: Ribo-gnome: The big
world of small RNAs. Science. 309:1519–1524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He X, He L and Hannon GJ: The guardian's
little helper: microRNAs in the p53 tumor suppressor network.
Cancer Res. 67:11099–11101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hermeking H: p53 enters the microRNA
world. Cancer Cell. 12:414–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bommer GT, Gerin I, Feng Y, Kaczorowski
AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, et al:
p53-mediated activation of miRNA34 candidate tumor-suppressor
genes. Curr Biol. 17:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kinoshita T, Hanazawa T, Nohata N, Kikkawa
N, Enokida H, Yoshino H, Yamasaki T, Hidaka H, Nakagawa M, Okamoto
Y, et al: Tumor suppressive microRNA-218 inhibits cancer cell
migration and invasion through targeting laminin-332 in head and
neck squamous cell carcinoma. Oncotarget. 3:1386–1400. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinez I, Gardiner AS, Board KF, Monzon
FA, Edwards RP and Khan SA: Human papillomavirus type 16 reduces
the expression of microRNA-218 in cervical carcinoma cells.
Oncogene. 27:2575–2582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Võsa U, Vooder T, Kolde R, Vilo J,
Metspalu A and Annilo T: Meta-analysis of microRNA expression in
lung cancer. Int J Cancer. 132:2884–2893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schetter AJ, Okayama H and Harris CC: The
role of microRNAs in colorectal cancer. Cancer J. 18:244–252. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu J, Li X, Liang Y, Qiao L, Ran D, Lu Y,
Li X, Wei W and Zheng Q: Upregulation of URI/RMP gene expression in
cervical cancer by high-throughput tissue microarray analysis. Int
J Clin Exp Pathol. 6:669–677. 2013.PubMed/NCBI
|
|
14
|
Fassan M, Croce CM and Rugge M: miRNAs in
precancerous lesions of the gastrointestinal tract. World J
Gastroenterol. 17:5231–5239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao JJ, Lin J, Lwin T, Yang H, Guo J,
Kong W, Dessureault S, Moscinski LC, Rezania D, Dalton WS, et al:
MicroRNA expression profile and identification of miR-29 as a
prognostic marker and pathogenetic factor by targeting CDK6 in
mantle cell lymphoma. Blood. 115:2630–2639. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:13421–13426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tarasov V, Jung P, Verdoodt B, Lodygin D,
Epanchintsev A, Menssen A, Meister G and Hermeking H: Differential
regulation of microRNAs by p53 revealed by massively parallel
sequencing: miR-34a is a p53 target that induces apoptosis and
G1-arrest. Cell Cycle. 6:1586–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan D, Zhou X, Chen X, Hu DN, Dong XD,
Wang J, Lu F, Tu L and Qu J: MicroRNA-34a inhibits uveal melanoma
cell proliferation and migration through downregulation of c-Met.
Invest Ophthalmol Vis Sci. 50:1559–1565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao C, Zhang Z, Liu W, Xiao S, Gu W and Lu
H: Reduced microRNA-218 expression is associated with high nuclear
factor kappa B activation in gastric cancer. Cancer. 116:41–49.
2010.PubMed/NCBI
|
|
20
|
Iorio MV, Casalini P, Tagliabue E, Ménard
S and Croce CM: MicroRNA profiling as a tool to understand
prognosis, therapy response and resistance in breast cancer. Eur J
Cancer. 44:2753–2759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weng OL, Pourmand N and Patterson BK:
Patterns of known and novel small RNAS in human cervical cancer.
Cancer Res. 67:6031–6043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raver-Shapira N, Marciano E, Meiri E,
Spector Y, Rosenfeld N, Moskovits N, Bentwich Z and Oren M:
Transcriptional activation of miR-34a contributes to p53-mediated
apoptosis. Mol Cell. 26:731–743. 2007. View Article : Google Scholar : PubMed/NCBI
|