Introduction
Statins, which function as
3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) inhibitors,
are well-recognized for their efficacy in the prevention and
treatment of cardiovascular disease (1). However, a growing body of studies and
in vitro experiments suggested that statins may have an
unprecedented beneficial effect on cancer cell inhibition and thus
serve as an efficient treatment of various types of cancer,
including hepatocellular carcinoma (HCC), as well as prostate,
breast, lung and colorectal carcinomas (2–6). HCC is
the sixth most prevalent malignant tumor and the third most common
cause of cancer-associated mortality worldwide, with a poor 5-year
survival rate (7). However, there are
currently no effective chemotherapy treatments available for this
tumor (3). Therefore, it is necessary
to further investigate the pathogenesis of HCC and identify
efficient therapeutic protocols.
Receptor-interacting protein 140 (RIP140), also
known as nuclear receptor interacting protein 1, is a coregulator
of numerous transcription factors and a signal transduction
regulator (8,9). This molecule is mainly found in
metabolic tissues, including liver, muscle and adipose tissues.
RIP140 is able to negatively regulate the energy homeostasis by
affecting the storage of lipids and inhibiting the expression of
genes involved in fatty acid oxidation and glucose metabolism
(10). However, numerous studies had
identified that RIP140 served an important role in the development
of cancer through inhibiting the Wnt/β-catenin signaling pathway
(11,12). Wnt/β-catenin signaling inactivates
glycogen synthase kinase 3β (GSK3β) for the co-receptor
Frizzled/low-density lipoprotein receptor-related protein 1
stimulated by Wnt ligands. Inactivation of GSK3β results to
inability of β-catenin phosphorylation, which would decrease the
ubiquitination and proteolysis of β-catenin. Therefore, β-catenin
is accumulated by translocation from the cytoplasm into the
nucleus, where it forms a complex with T-cell factor 4 (TCF4), and
activates the transcription of the target genes, including c-myc
and cyclin D1. Consequently, this results in cell proliferation and
cancer development (11,12). Whereas, RIP140 can negative regulate
these genes expression by interact with the β-catenin, and inhibit
the activity of β-catenin (13).
As statins are able to induce cell apoptosis, RIP140
simultaneously inhibits cell proliferation through the
Wnt/β-catenin signaling pathway. However, whether simvastatin (SV)
affects the Wnt/β-catenin signaling and RIP140 expression in HCC
remains unclear and requires further investigation. Therefore, in
the present study, a RIP140 overexpression cell model was
established in the HCC SMCC-7721 cell line. These cells were then
treated with the SV, and the results revealed that SV was able to
inhibit cell proliferation by increasing the expression of RIP140
and inhibiting the Wnt/β-catenin signaling.
Materials and methods
Determination the IC50 of
SV concentration to SMCC-7721 cells by cell counting kit-8
SMCC-7721 cells were purchased from the Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China), and were cultured in Dulbecco's modified Eagle's
medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Tianjin Haoyang
Biological Products Technology Co., Ltd., Tianjin, China) and
penicillin and streptomycin (100 U/ml and 0.1 mg/ml, respectively;
P1400, Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China), and incubated at 37°C in a humidified atmosphere
containing 5% CO2. For cell growth and viability assays,
SMCC-7721 cells, at the same confluence (30–40%) for every well,
were plated onto 96-well flat-bottomed plates (Beaver
Nano-Technologies Co., Ltd., Suzhou, China). Next, different
concentrations of SV, including 0, 4, 8, 12, 16 and 20 µmol/l, were
added into each well and cultured to measure the cell growth and
viability. Following incubation for 48 h, 20 µl cell counting kit-8
(CCK-8; Beijing Zoman Biotechnology Co., Ltd., Beijing, China)
solution was added into each well and incubated at 37°C in the dark
for 2 h. The absorbance of each well was measured using a
microplate reader (Multiskan FC; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 450 nm. Furthermore, the half maximal
inhibitory concentration (IC50) of SV was calculated.
Each assay was repeated at least three times.
Transfection and grouping
In order to construct a RIP140 overexpression cell
model, RIP140-overexpressing plasmids [pcDNA3.1(+)], which were
constructed by Magus Technology (Shanghai, China), were transfected
into SMCC-7721 cells. Transfection was performed according to the
manufacturer's protocol. At 12 h before transfection, cells were
seeded onto 6-well plates containing antibiotic-free medium and
were allowed to reach 60–70% confluence prior to transfection. The
RIP140-overexpressing (4 µg) or negative (4 µg; control group)
plasmids and 5 µl Entranster™-D-4000 (Engreen Biosystem New Zealand
Ltd., Auckland, New Zealand) were diluted with 50 µl DMEM (FBS- and
antibiotic-free). After 5 min, the diluted plasmids (4 µg) and
Entranster™-D-4000 were mixed together, and were incubated at 37°C
for 20 min, prior to being added to the wells. The DMEM medium was
replaced after 6 h with DMEM supplemented with 10% FBS, and cells
were cultured at 37°C in a humidified atmosphere containing 5%
CO2 for 48 h. The Control and RIP140 groups were then
further divided into groups treated with or without 8 µmol/l SV,
and the following groups were obtained: Control, RIP140, Control +
SV (referred to as the SV group) and RIP140 + SV groups. After 48 h
of incubation, all cells were harvested and used in subsequent
experiments.
CCK-8 and flow cytometry analysis for
cell growth and viability
In order to identify whether RIP140 and SV are able
to alter the HCC cell proliferation, the growth and viability of
SMCC-7721 cells was determined by CCK-8, while apoptosis was
examined by flow cytometry (14). For
cell growth and viability determination, the CCK-8 assay was
conducted as described earlier, with the exception of the groups
investigated, which included the Control, RIP140, SV and RIP140 +
SV groups, and the SV concentration (8 µmol/l) used. For the
determination of cell apoptosis by flow cytometry analysis, cells
(1×106/ml) were seeded into 6-well plates and cultured
for 12 h, followed by treatment with SV at 8 µmol/l for 48 h.
Following trypsin digestion, cells were collected, washed twice by
PBS and analyzed using a BD FACSCalibur™ flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocol of the FITC Annexin V Apoptosis Detection
kit (cat. no. 556547; Promega Corporation, Madison, WI, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from the cells using the
RNAiso Plus reagent (Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's protocol. Next, total RNA (2 µg)
was reverse transcribed into cDNA in a total reaction volume of 20
µl using a RevertAid First Strand cDNA Synthesis kit (cat. no.
K1622; Thermo Fisher Scientific, Inc.) following qualification by
NanoDrop 2000 (Thermo Fisher Scientific, Inc.). Primers for
β-actin, RIP140, cyclin D1 and c-myc were synthesized by Shenggong
Biology Engineering Technology Service, Ltd. (Shanghai, China), and
their sequences are displayed in Table
I (15,16). In order to determine the expression of
these genes, SYBR® Premix Ex Taq™ was used as a
fluorescent dye (cat. no. RR42LR; Takara Biotechnology Co., Ltd.).
qPCR was conducted with an ABI 7500 sequence detection system
(Thermo Fisher Scientific, Inc.) as follows: 95°C for 3 min, and 40
cycles of 95°C for 10 sec and 60°C for 1 min. Fluorescent
information and melting curves were obtained. All samples were
analyzed in triplicate, and the quantification cycle (Cq) value was
defined as the number of cycles required for the fluorescent signal
to reach the threshold. Using the comparative Cq method, the
relative expression levels were calculated using the formula for
2−ΔΔCt (17). Experimental
data represent the mean ± standard deviation of three biological
replicates. In the current study, the gene expression was
normalized to the expression of β-actin.
 | Table I.Primers sequences used in polymerase
chain reaction. |
Table I.
Primers sequences used in polymerase
chain reaction.
| Gene | Primer sequence |
|---|
| β-actin |
5′-GATCATTGCTCCTCCTGAGC-3′ (forward) |
|
|
5′-ACTCCTGCTTGCTGATCCAC-3′ (reverse) |
| RIP140 |
5′-ATAGCCCTCAGTCATGATT-3′ (forward) |
|
|
5′-CAGCACATGACAACGGTTCA-3′ (reverse) |
| CyclinD1 |
5′-GGCGGAGGAGAACAAACAGA-3′ (forward) |
|
|
5′-TGTGAGGCGGTAGTAGGACA-3′ (reverse) |
| c-myc |
5′-CCCTCCACTCGGAAGGACTA-3′ (forward) |
|
|
5′-GCTGGTGCATTTTCGGTTGT-3′ (reverse) |
Western blot analysis
Total proteins were extracted from the cells using a
radioimmunoprecipitation assay buffer, and the protein
concentration was measured using an bicinchoninic acid assay (cat.
no. CW0014; Kangwei Century Biotechnology Co., Ltd., Beijing,
China). Equal amounts of clear lysates (~50 µg protein) were
separated by SDS-PAGE on 10% gel, and then transferred onto
polyvinylidene fluoride membranes (PVDF; Immobilon-P; EMD
Millipore, Billerica, MA, USA). Equal transfer was validated by
staining with Ponceau red staining (cat. no. CW0057S; Kangwei
Century Biotechnology Co., Ltd.). The membranes were blocked at
room temperature for 1 h with 10% skimmed milk in Tris-buffered
saline (TBS), prior to being incubated with primary antibodies in
TBS containing 0.05% Tween 20, 2% bovine serum albumin (cat. no.
A8010; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) and 0.05% sodium azide overnight at 4°C. The
following primary antibodies were used: RIP140 (cat. no. sc-8997;
dilution, 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA); apoptosis-associated proteins B-cell lymphoma 2 (Bcl-2; cat.
no. 12789-1-AP; dilution, 1:2,000; ProteinTech Group, Inc.,
Chicago, IL, USA) and Bcl-2-associated X protein (Bax; cat. no.
50599-2-Ig; dilution, 1:2,000; ProteinTech Group, Inc.); and the
Wnt/β-catenin signaling pathway-associated proteins, β-catenin
(cat. no. 51067-2-AP; dilution, 1:2,000; ProteinTech Group, Inc.),
c-myc (cat. no. 10828-1-AP; dilution, 1:2,000; ProteinTech Group,
Inc.) and cyclin D1 (cat. no. 60186-1-Ig; dilution, 1:5,000;
ProteinTech Group, Inc.). A β-actin antibody (cat. no. 60008-1-Ig;
dilution, 1:10,000; ProteinTech Group, Inc.) served as the internal
control. Subsequently, the PVDF membranes were incubated at room
temperature for 1 h with secondary horseradish peroxidase-coupled
rabbit antibodies (dilution, 1:10,000; ProteinTech Group, Inc.) in
TBS containing 0.05% Tween 20. Signals were revealed using an
enhanced chemiluminescence reagent (cat. no. CW0049M; Century
Biotech Co., Ltd.) and an autoradiography system (Chemiluminescence
Imaging System; Bio-Rad Laboratories, Inc., Hercules, CA, USA)
(18). The Image Lab software
(version 5.1; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was
used to analyze the protein bands and the results were normalized
by the b-actin. Each assay was repeated at least three times.
Immunofluorescence analysis of
β-catenin expression
SMCC-7721 cells cultured in a 24-well plate were
transfected with RIP140 plasmids and were then treated by SV,
followed by fixation in 4% (w/v) paraformaldehyde for 20 min at
room temperature. Next, a β-catenin antibody (cat. no. 51067-2-AP;
dilution, 1:100; ProteinTech Group, Inc.) was added to the cells
and incubated overnight at 4°C. Subsequent to washing with PBS,
cells were stained with FITC-conjugated secondary antibodies (cat.
no. BA1105; dilution, 1:50; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) for 20 min. DAPI staining (cat. no. AR1177;
Wuhan Boster Biological Technology, Ltd.) was then performed at a
concentration of 300 nM for 5 min (19). Finally, an inverted fluorescence
microscope (Olympus IX71; Olympus Corp., Tokyo, Japan) was used to
analyze the results.
Statistical analysis
Data are presented as the mean ± standard deviation.
All statistical analyses were performed using SPSS version 17.0
software (SPSS, Inc., Chicago, IL, USA). The Student's t-test was
used for single comparisons. For multiple comparisons, one-way
analysis of variance with Tukey's or Games-Howell post-hoc analysis
was used. Statistically significant differences were considered to
be indicated by P<0.05.
Results
Appropriate concentration of SV
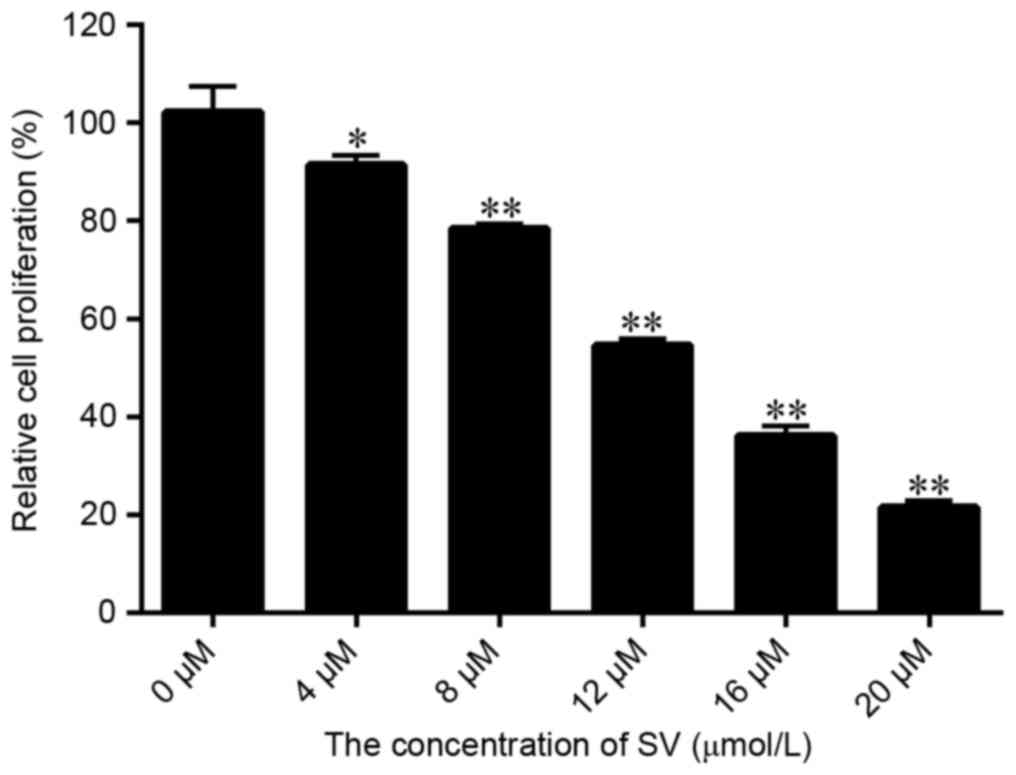
The results displayed in Fig. 1A indicated that SV treatment
significantly inhibited the SMCC-7721 cell growth, with the
proliferation rate decreasing from 91.58 to 21.56% upon increase of
the SV dose between 4 and 20 µmol/l, respectively. Therefore, the
inhibition efficiency of SV was dose dependent. Furthermore, the
IC50 of SV in SMCC-7721 cells was calculated to be 12.57
µmol/l. However, to significantly demonstrate whether RIP140 is
able to improve the sensibility of SV to SMCC-7721 cells, the
treatment concentration of SV selected for further experiments in
the present study was 8.0 µmol/l.
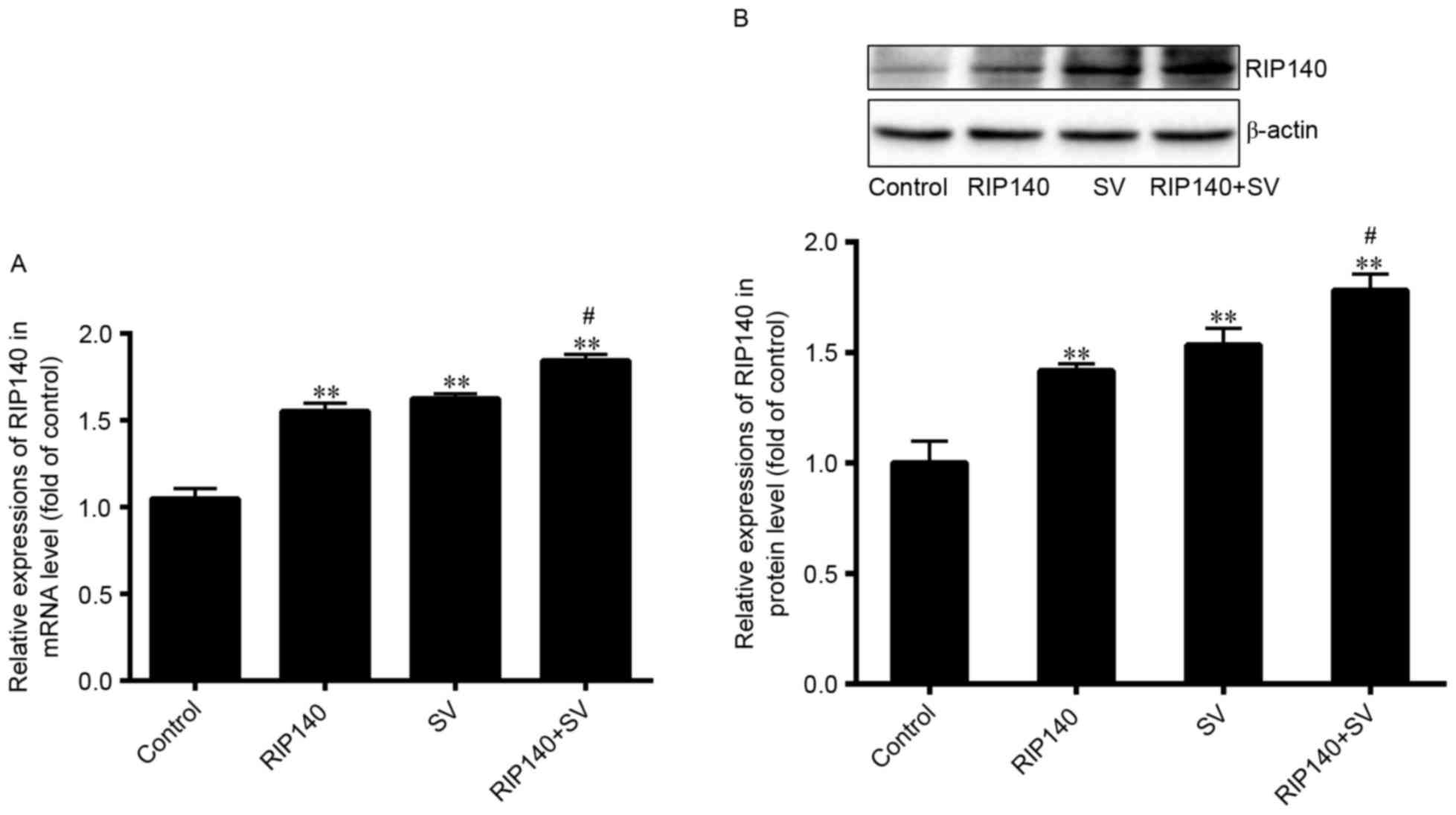
RIP140 expression in
plasmid-transfected and SV-treated cells
In the current study, RIP140 overexpression plasmids
were transfected into SMCC-7721 cells. Additionally, the present
study determined the expression of RIP140 following treatment with
SV, using RT-qPCR and western blot analysis. As demonstrated in
Fig. 2, the mRNA and protein
expression levels of RIP140 were significantly upregulated in the
RIP140, SV and RIP140 + SV groups as compared with those in the
control group (P<0.001; n=4). The mRNA level of RIP140 increased
by ~55.32, 62.48 and 84.36% in the RIP140, SV and RIP140 + SV
groups, respectively, whereas the protein levels increased by
~41.71, 53.39 and 78.24%, respectively. In addition, the RIP140
expression in the RIP140 + SV group was further increased in
comparison with that in the RIP140 or SV groups (P<0.05; n=4).
These results suggested that both the RIP140 plasmid transfection
and SV treatment were able to increase the expression of RIP140 in
SMCC-7721 cells (P<0.001; n=4).
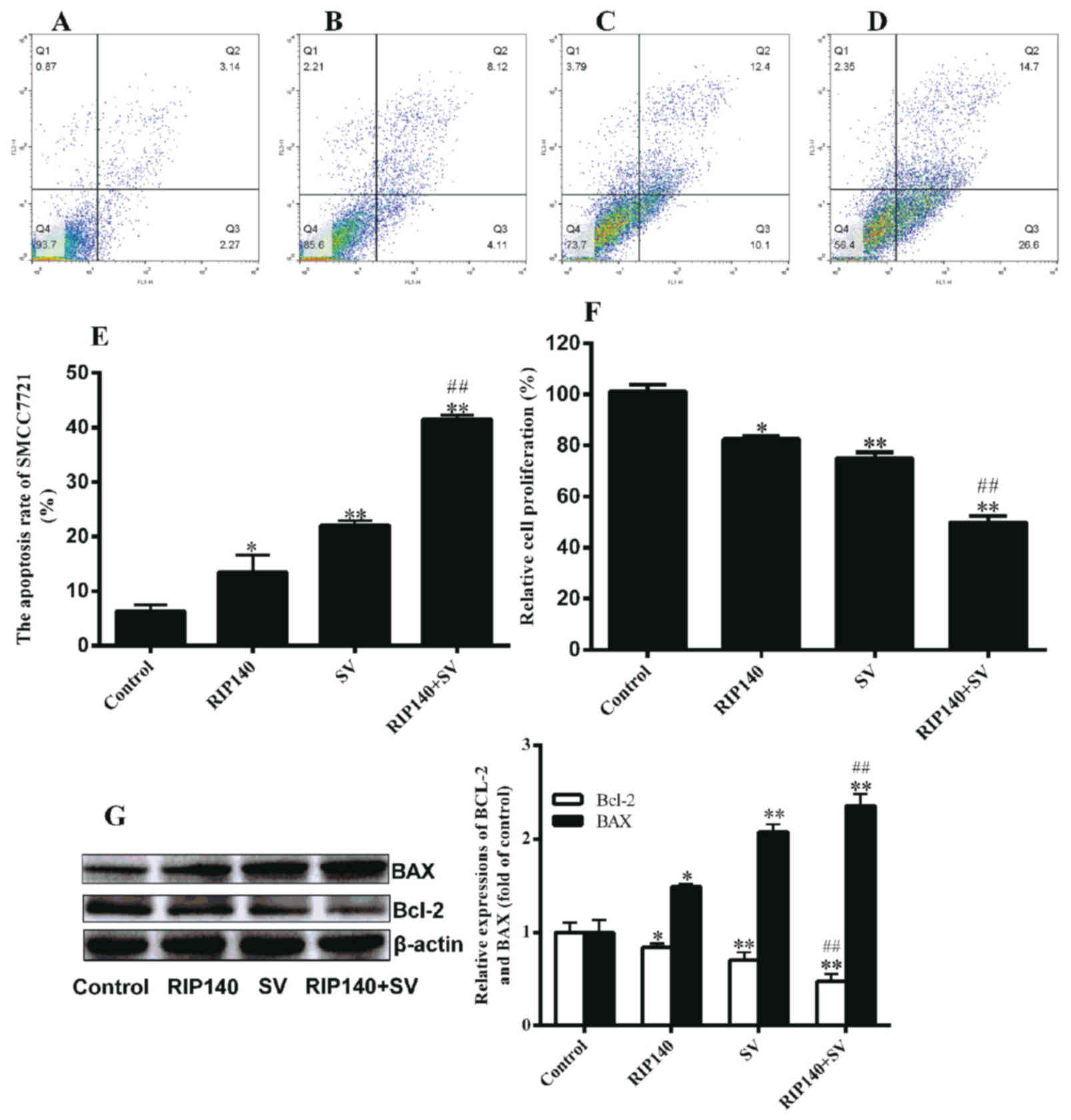
HCC cell proliferation and apoptosis
are affected by RIP140 overexpression or and SV treatment
SV induces cell apoptosis, while RIP140 is also able
to inhibit the cell proliferation; thus, the present study
investigated the combined effect of RIP140 and SV on the growth and
viability of the HCC SMCC-7721 cells by CCK-8 and flow cytometry
assays, respectively. To examine the apoptosis of SMCC-7721 cells,
flow cytometry assays were conducted in each group (Fig. 3A-D), and the apoptosis rate was
calculated (Fig. 3E). The apoptosis
rates in the RIP140, SV and RIP140 + SV groups were 13.44, 22.08
and 41.70%, respectively. The data shown in Fig. 3E indicated that RIP140 overexpression
or SV treatment alone led to evidently enhanced cell apoptosis
compared with the control group cells (P<0.05 and P<0.001,
respectively; n=3). It was also observed that the apoptosis rate in
the RIP140 + SV group was significantly higher when compared with
that in the RIP140 or SV group alone (P<0.001; n=3). Notably,
the rate increase in the RIP140 + SV group was higher than the
combined increase observed in the RIP140 and SV groups by 6.18%
(Fig. 3E). As observed in Fig. 3F, RIP140 overexpression or SV
treatment alone were able to decrease the proliferation of
SMCC-7721 cells (P<0.05 and P<0.001, respectively; n=6).
However, in the RIP140 + SV group, overexpression of RIP140
significantly promoted the proliferation inhibition induced by SV
(Fig. 3F; P<0.001; n=6). The
inhibition rates in the RIP140, SV and RIP140 + SV groups were
17.54, 25.99 and 50.01, respectively. Furthermore, the rate
increase in the RIP140 + SV group was higher by 6.48% than the
combined total increase of the RIP140 and SV groups (Fig. 3F). These findings confirmed that both
RIP140 overexpression and SV treatment were able to induce
apoptosis and decrease the proliferation of SMCC-7721 cells, while
an enhanced effect was observed in the RIP140 + SV group.
These results were further verified by examining the
expression levels of two apoptosis-associated proteins, Bcl-2 and
Bax. As displayed in Fig. 3G, RIP140
overexpression or SV treatment alone were able to increase the
expression of Bax protein, as well as decrease the expression of
Bcl-2 (P<0.05 and P<0.001). Similarly, the effect in the
RIP140 + SV group was markedly higher compared with that in the SV
or RIP140 group alone (P<0.001; n=4; Fig. 3G).
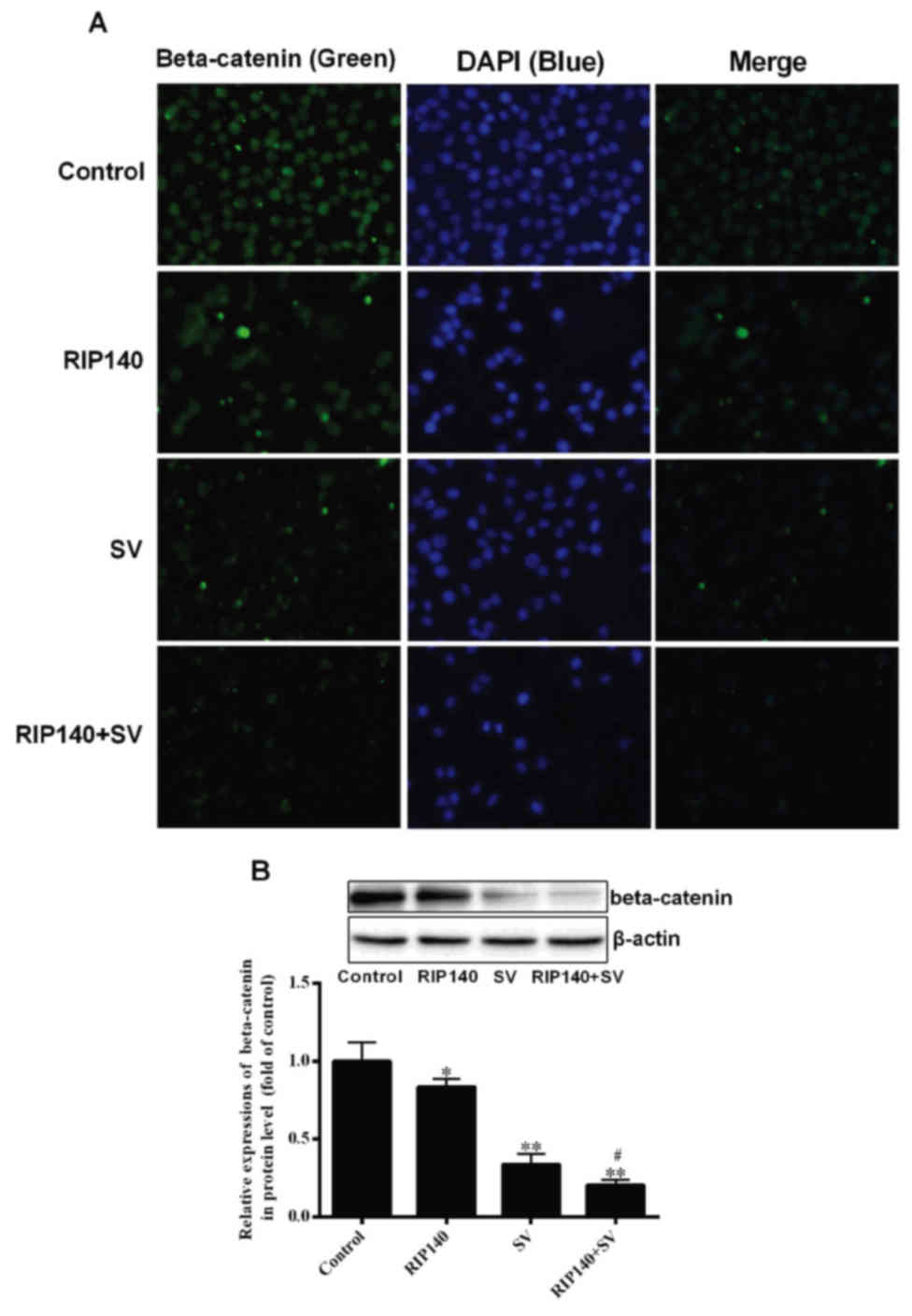
β-catenin content is decreased by
RIP140 and SV treatment
β-catenin is able to regulate the transcription of
several genes associated with cell proliferation (11,12);
therefore, the present study analyzed the expression of β-catenin
in SMCC-7721 cells. The relative content of β-catenin was initially
analyzed by immunofluorescence. As demonstrated in Fig. 4A, the relative content of β-catenin in
the RIP140, SV and RIP140 + SV group was reduced when compared with
the control group. It was also observed that the RIP140 + SV group
exhibited lower fluorescence in comparison with the groups treated
with RIP140 overexpression or SV alone. Furthermore, western blot
analysis revealed that the expression of β-catenin was
significantly decreased in the RIP140, SV and RIP140 + SV groups as
compared with the control group (P<0.05 and P<0.001; n=4),
while expression in the RIP140 + SV group was also markedly lower
in comparison with the RIP140 or SV group alone (P<0.05; n=4;
Fig. 4B). Thus, the western blot
analysis results were in agreement with the immunofluorescence
results.
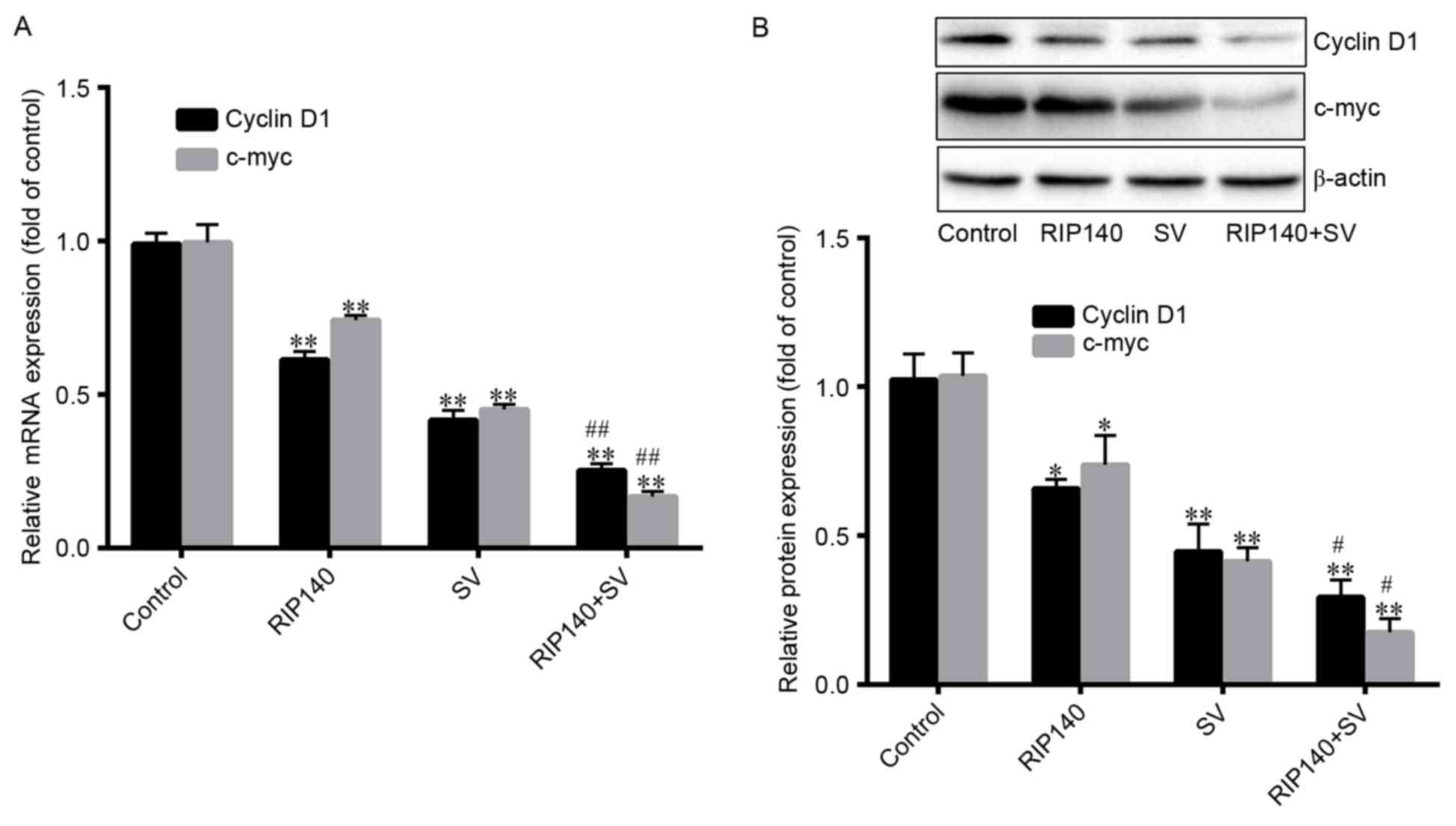
Expression of c-myc and cyclin D1
β-catenin is known to activate the transcription of
the target genes, including cyclin D1 and c-myc. Thus, the current
study investigated the expression levels of cyclin D1 and c-myc. As
demonstrated in Fig. 5A, cyclin D1
mRNA expression levels were significantly decreased by ~38.60,
58.29 and 74.72% in the RIP140, SV and RIP140 + SV groups,
respectively (P<0.001; n=4), compared with the control groups.
However, the protein levels of cyclin D were significantly
decreased by ~34.21, 55.37 and 71.73%, respectively (P<0.001;
n=4; Fig. 5B). Furthermore, Fig. 5A also demonstrates that the mRNA
levels of c-myc were significantly decreased by ~25.32, 54.46 and
82.83% (P<0.001; n=4), in the RIP140, SV and RIP140 + SV groups,
respectively, compared with the control groups. Additionally, c-myc
protein levels were significantly decreased by ~29.88, 62.37,
86.19%, respectively (P<0.001; n=4; Fig. 5B). Finally, the protein and mRNA
expression levels in the RIP140 + SV group were significantly lower
in comparison with those in the RIP140 or SV groups alone
(P<0.001; n=4; Fig. 5A and B).
Discussion
In the present study, a RIP140 overexpression cell
model was initially constructed and then cells were treated by the
SV. The results of growth and viability assays displayed that
RIP140 overexpression and SV treatment alone were able to inhibit
SMCC-7721 cell proliferation. However, RIP140 overexpression
enhanced the effect of SV treatment on these cells when these two
were applied in combination. In addition, RIP140 overexpression and
SV treatment applied together or separately on the cells resulted
in decreased β-catenin, c-myc and cyclin D1 levels as compared with
the control cells. These results suggested that β-catenin
participated in the growth and viability regulation of the SV on
the SMCC-7721 cells.
Statins are widely used to treat cardiovascular
diseases since they decrease the biosynthesis of cholesterol
through inhibition the enzyme HMGCR (1). In addition, statins have been
investigated for carcinoma prevention or as cancer treatments
(2–6).
However, certain studies have suggested that statin-induced
inhibition of cancer growth is due to the decrease of the
cholesterol content in the plasma or cells (20,21).
Cholesterol is vital for cell membrane integrity, cellular
metabolism and cell signaling in cellular proliferation (21). Therefore, inhibition of certain signal
transmissions is the primary mechanism underpinning the anticancer
activity of statins. For instance, Huang et al (22) indicated that SV significantly promoted
apoptosis in HCC cells through a mechanism that may involve the
upregulation of the Notch1 gene in the Akt-dependent signaling
pathway. Lee et al (23) also
revealed that NS398 and SV co-administration produced greater
anti-proliferative and pro-apoptotic effects against Hep3B and
Huh-7 cells via inhibition of the NF-κB and Akt pathways, and
activation of the caspase cascade. Besides these signaling pathway,
statins are also able to regulate other signaling pathways,
including the Hippo and PKC pathways (24,25).
In the present study, it was demonstrated that
RIP140 and SV reduced the content of β-catenin (Fig. 4), suggesting that this protein may be
involved in the apoptosis of SMCC-7721 cells. β-catenin is a
subunit of the cadherin protein complex and functions as an
intracellular signal transducer in the Wnt signaling pathway, which
is involved in the expression of certain genes associated with cell
proliferation (11,12). The findings of the present study also
indicated that SV treatment in the SMCC-7721 cells increased the
expression of RIP140 (Fig. 2). RIP140
is a transcriptional co-regulator that is involved in the negative
regulation of energy homeostasis by affecting the storage of lipids
and inhibiting the expression of genes involved in fatty acid
oxidation and glucose metabolism (9–11), thus
negatively regulating carcinomas, obesity, diabetes,
atherosclerosis and other metabolic diseases (12,13,26). The
possible mechanism underlying the negative regulation of carcinomas
is the suppressive role of RIP140 on the pathogenesis of carcinomas
by interacting with β-catenin and negatively regulating
Wnt/β-catenin/TCF signaling (15,26). In
the present study, the overexpression of RIP140 would strengthen
the suppression of β-catenin expression and, eventually, of the
β-catenin/TCF signaling. Furthermore, when SMCC-7721 cells were
treated with SV, the content of β-catenin was also significantly
decreased (Fig. 4). The results
further revealed that the apoptosis rate of the RIP140 + SV group
was higher when compared with RIP140 or SV group alone (Figs. 3 and 4).
Therefore, it is suggested that RIP140 and SV exerted a synergistic
effect on the apoptosis of SMCC-7721 cells, and that RIP140- and
SV-induced apoptosis was associated with the Wnt/β-catenin
signaling pathway due to the fact that the content of β-catenin was
decreased in cells following combined treatment, compared with
monotherapy (Figs. 2 and 4).
In conclusion, the present study results revealed
that SV induced SMCC-7721 cell apoptosis through increasing the
expression of RIP140 and decreasing the expression of β-catenin,
and this effect may also be associated with the Wnt/β-catenin
signaling pathway. Furthermore, RIP140 exerted a synergistic effect
along with SV on the inhibition of the HCC cell proliferation and
survival.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81560151)
and the Jiangxi Provincial Department of Science and Technology
(grant no. 20142BAB205014).
References
|
1
|
Istvan ES and Deisenhofer J: Structural
mechanism for statin inhibition of HMG-CoA reductase. Science.
292:1160–1164. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee Y, Lee KH, Lee GK, Lee SH, Lim KY, Joo
J, Go YJ, Lee JS and Han JY: Randomized phase II study of afatinib
plus simvastatin versus afatinib alone in previously treated
patients with advanced nonadenocarcinomatous non-small cell lung
cancer. Cancer Res Treat. 49:1001–1011. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou YY, Zhu GQ, Wang Y, Zheng JN, Ruan
LY, Cheng Z, Hu B, Fu SW and Zheng MH: Systematic review with
network meta-analysis: Statins and risk of hepatocellular
carcinoma. Oncotarget. 7:21753–21762. 2016.PubMed/NCBI
|
|
4
|
Demierre MF, Higgins PD, Gruber SB, Hawk E
and Lippman SM: Statins and cancer prevention. Nat Rev Cancer.
5:930–942. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blanc-Brude OP, Mesri M, Wall NR, Plescia
J, Dohi T and Altieri DC: Therapeutic targeting of the survivin
pathway in cancer: Initiation of mitochondrial apoptosis and
suppression of tumor-associated angiogenesis. Clin Cancer Res.
9:2683–2692. 2003.PubMed/NCBI
|
|
6
|
Li G, Zheng J, Xu B, Ling J, Qiu W and
Wang Y: Simvastatin inhibits tumor angiogenesis in
HER2-overexpressing human colorectal cancer. Biomed Pharmacother.
85:418–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Z, Zhang Y, Wang W, Hua Y, Liu L,
Shen S and Peng B: Thrombocytopenia and the outcomes of hepatectomy
for hepatocellular carcinoma: A meta-analysis. J Surg Res.
210:99–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferreira-Silva GÁ, Lages CC, Sartorelli P,
Hasegawa FR, Soares MG and Ionta M: Casearin D inhibits ERK
phosphorylation and induces downregulation of cyclin D1 in HepG2
cells. Toxicol In Vitro. 38:27–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu WH, Lee YM, Lam KK, Chen YF, Wang JJ,
Yen MH and Cheng PY: The role of receptor-interacting protein 140
in the accumulation of fat in ovariectomised rats. Obes Surg.
21:935–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mejhert N, Laurencikiene J, Pettersson AT,
Kaaman M, Stenson BM, Rydén M and Dahlman I: Role of
receptor-interacting protein 140 in human fat cells. BMC Endocr
Disord. 10:12010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Catalán V, Gómez-Ambrosi J, Lizanzu A,
Rodríguez A, Silva C, Rotellar F, Gil MJ, Cienfuegos JA, Salvador J
and Frühbeck G: RIP140 gene and protein expression levels are
downregulated in visceral adipose tissue in human morbid obesity.
Obes Surg. 19:771–776. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lapierre M, Docquier A, Castet-Nicolas A,
Gitenay D, Jalaguier S, Teyssier C and Cavaillès V: The emerging
role of the transcriptional coregulator RIP140 in solid tumors.
Biochim Biophys Acta. 1856:144–150. 2015.PubMed/NCBI
|
|
13
|
Aziz MH, Chen X, Zhang Q, DeFrain C,
Osland J, Luo Y, Shi X and Yuan R: Suppressing NRIP1 inhibits
growth of breast cancer cells in vitro and in vivo. Oncotarget.
6:39714–39724. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang CR, Jin ZX, Dong L, Tong XP, Yue S,
Kawanami T, Sawaki T, Sakai T, Miki M, Iwao H, et al: Cisplatin
augments FAS-mediated apoptosis through lipid rafts. Anticancer
Res. 30:2065–2071. 2010.PubMed/NCBI
|
|
15
|
Zhang D, Wang Y, Dai Y, Wang J, Suo T, Pan
H and Liu H, Shen S and Liu H: Downregulation of RIP140 in
hepatocellular carcinoma promoted the growth and migration of the
cancer cells. Tumour Biol. 36:2077–2085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jafari N, Zargar SJ, Yassa N and Delnavazi
MR: Induction of apoptosis and cell cycle arrest by dorema glabrum
root extracts in a gastric adenocarcinoma (AGS) cell line. Asian
Pac J Cancer Prev. 17:5189–5193. 2016.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu S, Ding Y, Gong J and Yan N:
Sphingomyelin synthase 2 affects CD14-associated induction of NF-κB
by lipopolysaccharides in acute lung injury in mice. Mol Med Rep.
14:3301–3306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berwick DC, Javaheri B, Wetzel A,
Hopkinson M, Nixon-Abell J, Grannò S, Pitsillides AA and Harvey K:
Pathogenic LRRK2 variants are gain-of-function mutations that
enhance LRRK2-mediated repression of β-catenin signaling. Mol
Neurodegener. 12:92017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sohda T, Iwata K, Hirano G, Sakurai K,
Yokoyama K, Morihara D, Takeyama Y, Irie M, Shakado S and Sakisaka
S: 3-Hydroxyl-3-methylglutaryl-coenzyme A reductase is up regulated
in hepatocellular carcinoma associated with paraneoplastic
hypercholesterolemia. Med Mol Morphol. 46:239–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Furuya Y, Sekine Y, Kato H, Miyazawa Y,
Koike H and Suzuki K: Low-density lipoprotein receptors play an
important role in the inhibition of prostate cancer cell
proliferation by statins. Prostate Int. 4:56–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang X, Ma J, Xu J, Su Q and Zhao J:
Simvastatin induces growth inhibition and apoptosis in HepG2 and
Huh7 hepatocellular carcinoma cells via upregulation of Notch1
expression. Mol Med Rep. 11:2334–2340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SJ, Hwang JW, Yim H, Yim HJ, Woo SU,
Suh SJ, Hyun JJ, Jung SW, Koo JS, Kim JH, et al: Synergistic effect
of simvastatin plus NS398 on inhibition of proliferation and
survival in hepatocellular carcinoma cell line. J Gastroenterol
Hepatol. 29:1299–1307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Higashi T, Hayashi H, Kitano Y, Yamamura
K, Kaida T, Arima K, Taki K, Nakagawa S, Okabe H, Nitta H, et al:
Statin attenuates cell proliferative ability via TAZ (WWTR1) in
hepatocellular carcinoma. Med Oncol. 33:1232016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim W, Yoon JH, Kim JR, Jang IJ, Bang YJ,
Kim YJ and Lee HS: Synergistic anti-tumor efficacy of lovastatin
and protein kinase C-beta inhibitor in hepatocellular carcinoma.
Cancer Chemother Pharmacol. 64:497–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lei JJ, Peng RJ, Kuang BH, Yuan ZY, Qin T,
Liu WS, Guo YM, Han HQ, Lian YF, Deng CC, et al: NOP14 suppresses
breast cancer progression by inhibiting NRIP1/Wnt/β-catenin
pathway. Oncotarget. 6:25701–25714. 2015. View Article : Google Scholar : PubMed/NCBI
|