Introduction
Prostate cancer (PCa) is one of the leading threats
to men's health, accounting for 25% of incident cases of cancer
diagnosed in adult males in the United States annually, and ~9% of
incidences of cancer-associated mortality in the same population,
according to statistical data from 2016 (1). PCa cells are considered to depend on
androgens for survival and growth in its early stages (2), and androgen ablation therapy may be a
sensitive and effective way of reducing tumor growth; however, the
treatment options for the late-stage disease termed
castration-resistant prostate cancer (CRPC), remain relatively
inefficient (3), as CRPC responds
poorly to chemotherapy (4).
Epirubicin (EPI) has been repeatedly found to exhibit activity as a
cytotoxic agent for prostate cancer patients, either administered
alone or in combination; however, drug resistance often leads to
treatment failure in CRPC patients (5).
As a non-selective Ca2+-permeable cation
channel, transient receptor potential cation channel subfamily M
member 8 (TRPM8) serves a key role in Ca2+ homoeostasis,
which is one of the vital factors in cancer-associated cell
signaling pathways. Fluctuations in Ca2+ homeostasis may
lead to an increase in cell proliferation (6,7), migration
(8) and may even induce
differentiation and apoptosis (9–12).
Therefore, TRPM8 has emerged as a promising prognostic marker and
putative therapeutic target in PCa for its vital role in
Ca2+ transportation (13–21). TRPM8
is abundantly expressed in the prostate, an expression that
increases in PCa, which indicates that there exists the potential
to treat prostate cancer via the specific gene-silencing of TRPM8.
Zhang and Barritt (2) reported that
TRPM8-knockdown could lead to the suppression of LNCaP cell
proliferation. Studies by Valero et al (17,22)
indicated that the small interfering RNA (siRNA) inhibition of
TRPM8 expression or small molecule inhibition of function using the
specific TRPM8 blockers
N-(3-aminopropyl)-2-{[(3-methylphenyl) methyl]
oxy}-N-(2-thienylmethyl)benzamide (AMTB) and JNJ41876666
reduced the rate of proliferation and proliferative fraction in
prostate cancer cells, but not in normal prostate cells. A previous
study demonstrated that
N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carboxamide
BCTC, a potent and representative antagonist of TRPM8, exerted an
antitumor effect on androgen-independent prostate cancer DU145
cells by altering levels of phosphorylated RAC
serine/threonine-protein kinase (p-AKT), cyclin D1,
cyclin-dependent kinase 2 (CDK2), CDK6, p-glycogen synthase
kinase-3β (p-GSK-3β) and proteins involved in the mitogen-activated
protein kinase (MAPK) signal pathways (23). Further, whether the knockdown of TRPM8
influence on the chemosensitivity of prostate cancer was assessed
in the present study. The anthracycline EPI, either alone or in
combination with other agents, has been extensively used in the
treatment of CRPC; however, chemoresistance increases as the
duration of treatment extends (24).
The present study investigated the potential effect of
TRPM8-knockdown on the chemosensitivity to EPI of prostate cancer
cells. The present study reports evidence that the knockdown of
TRPM8 enhanced the chemosensitivity of prostate cancer cells to
EPI, which would indicate the potential of a targeted
TRPM8-silencing therapeutic strategy to cure of PCa. To determine
the potential efficacy of this treatment approach, cell and
molecular analyses were performed following the silencing of the
TRPM8 gene in prostate cancer LNCaP and PC3 cells using a siRNA.
The results of these analyses revealed that the silencing of TRPM8
effectively inhibited cellular proliferation, yet failed to induce
apoptosis in LNCaP and PC3 cells. Nevertheless, siRNA treatment of
TRPM8 increased EPI-induced apoptosis in LNCaP and PC3 cells.
Silencing of TRPM8 was accompanied by the upregulation of p38 MAPK
(hereafter p38) and c-Jun N-terminal kinase (JNK) phosphorylation,
and ultimately results in the increased sensitivity of PCa cells to
EPI. Taken together, these results indicate that TRPM8 may
represent an effective target for the treating CRPC.
Materials and methods
Cell culture
All the prostate cancer cell lines, LNCaP, DU145,
PC3, and the non-cancer cell line PNT1A were obtained from the
American Type Culture Collection (Manassas, VA, USA). All the cells
were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS,
Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin-G
sodium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 100
mg/ml streptomycin sulfate (Sigma-Aldrich; Merck KGaA) at 37°C in a
humidified atmosphere containing 5% CO2.
RNA interference-mediated gene
silencing of TRPM8
The specific siRNA sequence targeting TRPM8
(siTRPM8) was 5′-UCUCUGAGCGCACUAUUCA(dTdT)-3′ (1); the sequence of negative control
scrambled siRNA (siCON) was 5′-UUCUCCGAACGUGUCACGUTT-3′ (not
homologous to any gene). These siRNAs were synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China). The LNCaP or PC3 cells were
seeded into 6-well plates at 30–40% confluence and cultured in 2 ml
of RPMI-1640 medium containing 10% FBS until the cells reached 70%
confluence. The siRNA-Lipofectamine 2000 (Thermo Fisher Scientific,
Inc.) complex was pre-mixed (10 nM siTRPM8 or siCON and 8 µl
Lipofectamine reagent per well) according to the manufacturer's
instructions and added to the 6-well plates. Cells transfected with
siTRPM8 and siCON are termed siTRPM8-LNCaP and siCON-LNCaP or
siTRPM8-PC3 and siCON-PC3 cells, respectively; untransfected cells
are termed parental LNCaP or PC3 cells. The expression of LNCaP or
PC3 cells transfected with siTRPM8 and siCON was evaluated by
RT-PCR and western blot analysis. EPI was added once cells had been
transfected for 24 h.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the parental PNT1A,
DU145, LNCaP and PC3 cells and transfected cells using the TRIzol
Reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. For RT analysis, 2 µg of total RNA
was reverse transcribed, using the Moloney Murine Leukemia Virus
Reverse Transcriptase system (cat. no. 28025013; Thermo Fisher
Scientific, Inc.). PCR was performed using Taq DNA polymerase
system (cat. no. 10342020; Thermo Fisher Scientific, Inc.) by
adding 2 µl RT reaction mixture in a final volume of 50 ul. DNA
amplification conditions included an initial 5 min denaturation
step at 94°C and 35 cycles of 30 sec at 94°C, 30 sec at 60°C, and
60 sec at 72°C. The PCR primers used were as follows: TRPM8
forward, 5′-GATTTTCACCAATGACCGCCG-3′ and reverse,
5′-CCCCAGCAGCATTGATGTCG-3′; β-actin forward,
5′-AGAAGGATTCCTATGTGGGCG-3′ and reverse,
5′-CATGTCGTCCCAGTTGGTGAC-3′.
Cell Counting kit-8 (CCK-8)
assays
Cell growth and viability were measured using cell
proliferation and cytotoxicity reagent CCK-8 (MedChemExpress, Cat.
HY-K0301), according to the manufacturers' instructions (Roche
Applied Science, Mannheim, Germany). The protocol was as follows:
LNCaP or PC3 transfected and untransfected cells (5×103
per well) were cultured in a 96-well plate, ten wells per group.
Following incubation in complete medium for 72 h,
siRNA-transfection for 24 h or EPI-incubation for 48 h, fresh
culture medium (which served as solvent for formazan generated from
WST-8 by 1-Methoxy PMS) with 10 µl CCK-8 working solution was added
and the mixture was incubated for another 4 h at 37°C. For the
examination of chemosensitivity, cells were incubated for 12 h to
allow cells to attach to the plate surface, and then cells were
treated with different concentrations (0, 200, 400, 600, 800 and
1,000 ng/ml respectively) of EPI for 72 h. To verify the role of
MAPK signal pathway, p38 inhibitor (SB203580, 20 µM) (cat. no.
S8307; Sigma-Aldrich; Merck KGaA,) and JNK inhibitor (SP600125; 10
µM; cat. no. S5567; Sigma-Aldrich; Merck KGaA) were added in the
culture medium 2 h prior to the addition of EPI. The optical
density at 490 nm was read using the enzyme-linked immunoassay
reader. The cell viability index was calculated using the optical
density (OD), according to the formula: Experimental OD
value/control OD value ×100. The experiments were repeated three
times.
Western blot assay
The parental PNT1A, DU145, LNCaP and PC3 cells and
transfected cells were washed twice with ice-cold PBS and
solubilized in 1% Triton X-100 lysis buffer (cat. no. 9803; Cell
Signaling Technology Inc., Danvers, MA, USA) on ice, then
quantified using a BCA kit (cat. no. 23209; Thermo Fisher
Scientific, Inc.). TRPM8 and GAPDH protein expression was assayed
by western blotting using 10% SDS-PAGE and transferred to a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA), with anti-TRPM8 antibodies (polyclonal, 1:500 in TBST), and
anti-GAPDH specific antibodies (1:3,000 in TBST). Membranes were
blocked by 5% skim milk solution at room temperature for 30 min and
then incubated with primary antibodies against TRPM8 (1:500; cat.
no. ab3243; Abcam, Cambridge, UK), p-JNK (1:1,000; cat. no. 4668P),
total JNK (1:1,000; cat. no. 9258P), p-p38 (1:1,000; cat. no.
4511P) and total p38 (1:1,000; cat. no. 9212P; all Cell Signaling
Technology, Inc.) and GAPDH (1:1,000; cat. no. sc-166574; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C,
followed by incubation with a secondary antibody (cat. no. sc-2005;
goat anti-mouse IgG-HRP, 1:2,000; Santa Cruz Biotechnology, Inc.)
for 1 h at room temperature. The membrane was washed three times in
TBST for 5 min each time. Immunoreactive proteins were detected
using the SuperSignal West Pico Chemiluminescent Substrate Western
Blot Detection system (cat. no. 34080; Thermo Fisher Scientific,
Inc.) according to the protocol of the manufacturer. All
experiments were repeated at least three times.
Flow cytometry analysis of
apoptosis
The parental LNCaP and PC3 cells and transfected
cells were incubated in a binding buffer (BD Pharmingen; BD
Biosciences, San Diego, CA, USA) containing fluorescein
isothiocyanate (FITC)-conjugated Annexin V (BD Pharmingen; BD
Biosciences) and propidium iodide (BD Pharmingen; BD Biosciences,
San Diego, CA, USA) at room temperature for 5 min in the dark. A
flow cytometer (FACSCanto II, BD Biosciences, Franklin Lakes, NJ,
USA) was used to detect the proportion of apoptotic cells and
analyzed using FACSDiva version 6.1.3 (BD Biosciences).
Statistical analysis
All data is presented as the mean ± standard
deviation. Statistical analysis was performed using SPSS for
Windows, version 16.0 (SPSS, Inc., Chicago, IL, USA); P<0.05 was
considered to indicate a statistically significant difference.
Statistical analysis was conducted using the one-way analysis of
variance followed by the SNK method.
Results
Expression of TRPM8 in different
prostate cancer cell lines
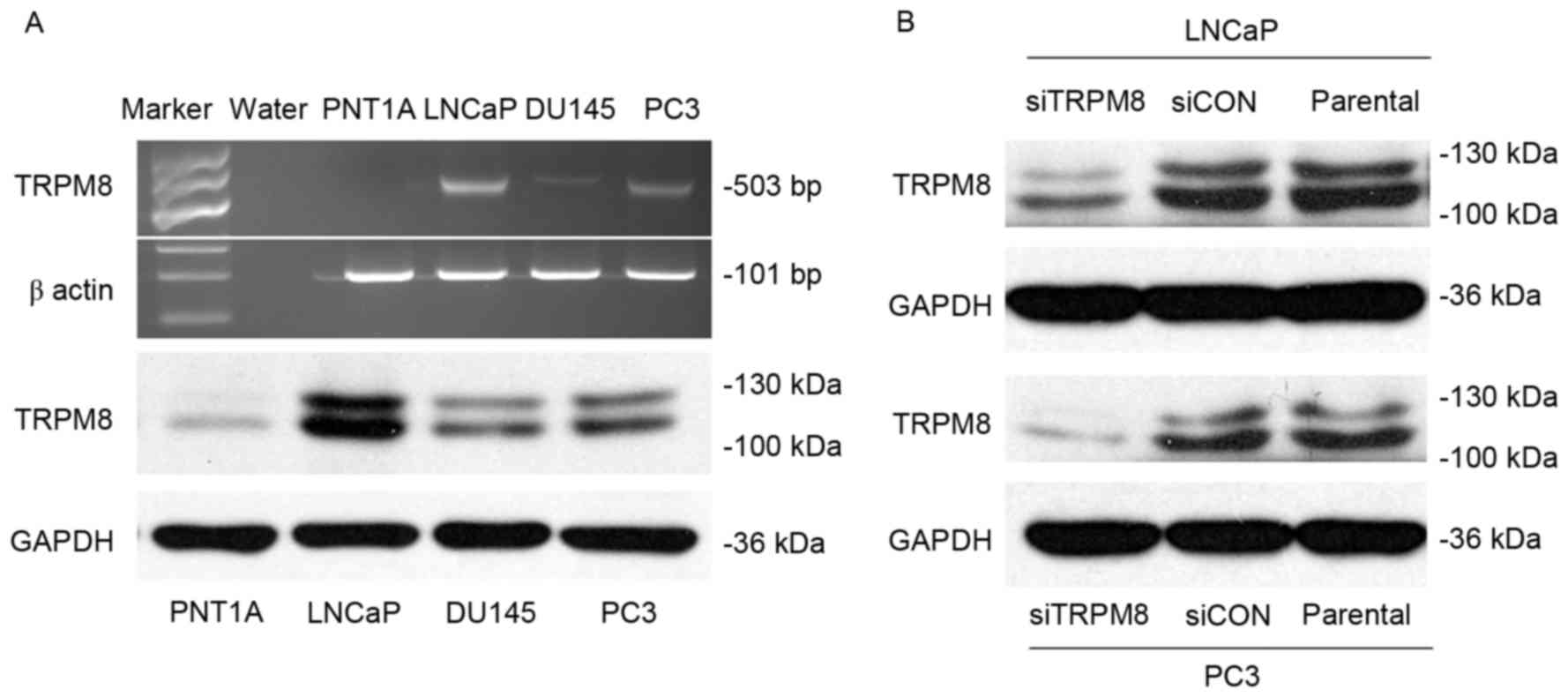
Using RT-PCR, and western blot analysis, the
expression of TRPM8 among different prostate cell lines, including
human immortalized prostate epithelia cell line PNT1A, and three
prostate cancer cell lines LNCaP, DU145, and PC3. The expression of
TRPM8 mRNA and protein was higher in LNCaP and PC3 cells than in
DU145; however, expression was almost undetectable in PNT1A cells
(Fig. 1A). The efficiency of
siRNA-mediated siTRPM8-knockdown was also investigated by western
blot analysis. Compared with parental and siCON cells, the
expression of TRPM8 was markedly downregulated in the cells
transfected with siTRPM8 in LNCaP and PC3 cells (Fig. 1B).
siTRPM8 inhibited cellular
proliferation and enhanced EPI chemosensitivity in LNCaP and PC3
cells
The effect of TRPM8 knockdown on the proliferation
of LNCaP and PC3 cells was assessed using CCK-8 assays. According
to the results of CCK-8 assay, there was no statistical difference
between parental and siCON cells, however the siTRPM8 cells grew
more slowly than the parental and siCON cells and the difference
was more pronounced after day 2 (Fig. 2A
and B).
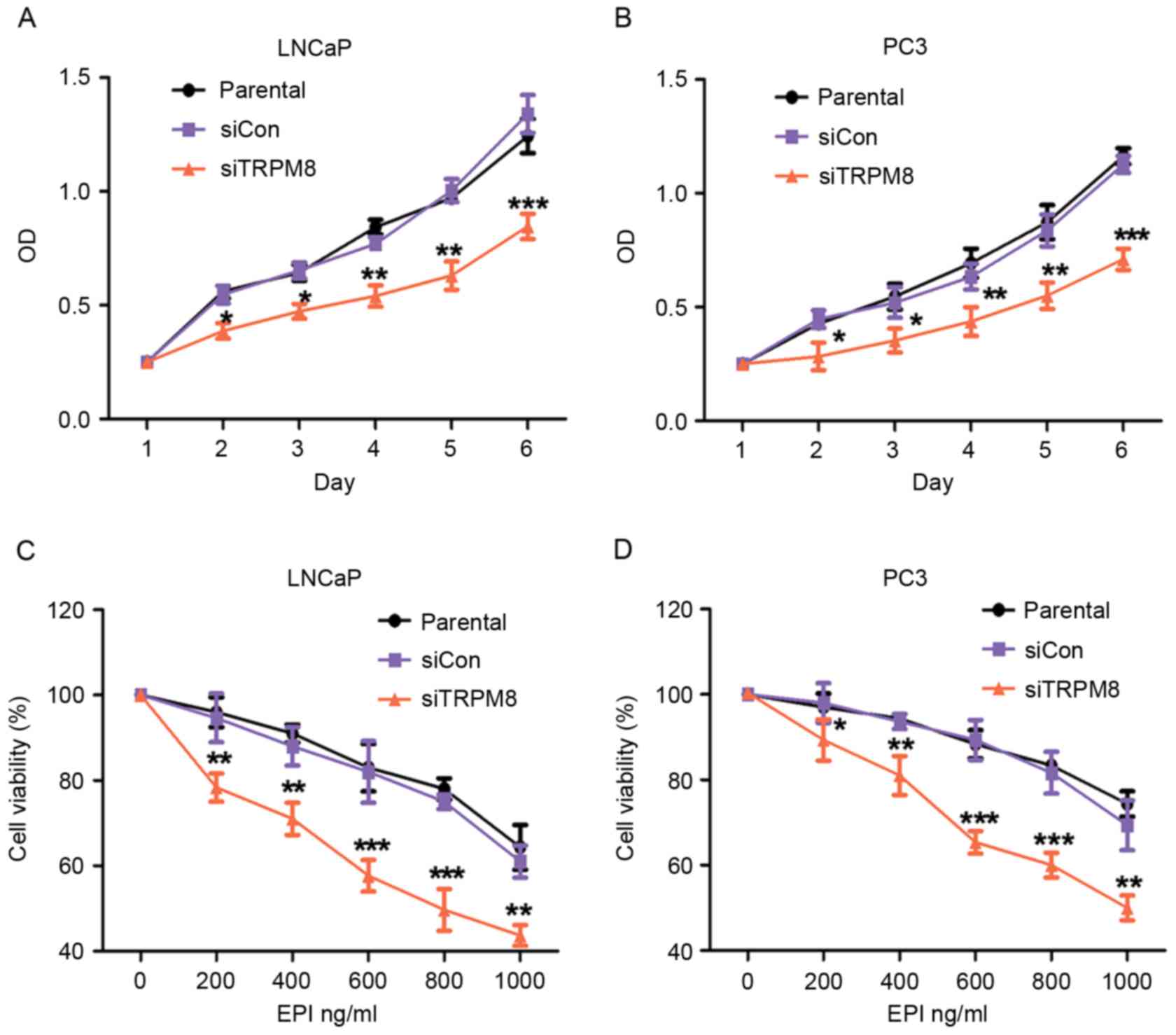 | Figure 2.Knockdown of TRPM8 suppressed growth
and enhanced EPI-induced growth inhibition of LNCaP and PC3 cells.
(A and B) The effect of TRPM8-knockdown on (A) LNCaP and (B) PC3
cells proliferation was measured by CCK-8 assay. Growth of siTRPM8
cells was significantly suppressed at day 2 compared with parental
and siCON cells. (C and D) Knockdown of TRPM8 significantly
enhanced EPI-induced inhibition of viability of (C) LNCaP and (D)
PC3 cells. Cells were incubated with vehicle (0.01% DMSO) or
different concentrations (0, 200, 400, 600, 800, 1,000 ng/ml) of
EPI for 48 h by CCK-8 assay, and are expressed as percentages
relative to the control, which was taken as 100%, and treated with
medium-containing vehicle (0.01% DMSO). *P<0.05, **P<0.01,
***P<0.001 vs. parental cells on indicated times or EPI
concentrations. siTRPM8, small interfering RNA targeting transient
receptor potential cation channel subfamily M member 8; EPI,
epirubicin; CON, negative control; CCK-8, Cell Counting kit-8;
DMSO, dimethyl sulfoxide. |
Next, the effect of siTRPM8 on chemosensitivity to
EPI was assessed using a drug sensitivity test by CCK-8 assays.
Compared with the parental and siCON cells, the viability of
siTRPM8 cells was markedly weakened in a dose-dependent manner
following incubation with EPI at the indicated concentration for 48
h (Fig. 2C and D). The viability of
siTRPM8 cells was significantly lower than the parental and siCON
cells when treated with 600 µM EPI, for LNCaP (siTRPM8, 58.37±2.14%
vs. parental, 82.31±7.60%, P<0.001; siCON, 84.03±3.80% vs.
parental cells, P>0.05) and PC3 (siTRPM8, 64.18±4.93% vs.
parental cells, 85.34±2.17%, P<0.001; siCON, 85.42±2.40% vs.
parental cells, P>0.05) cells (Fig. 2C
and D).
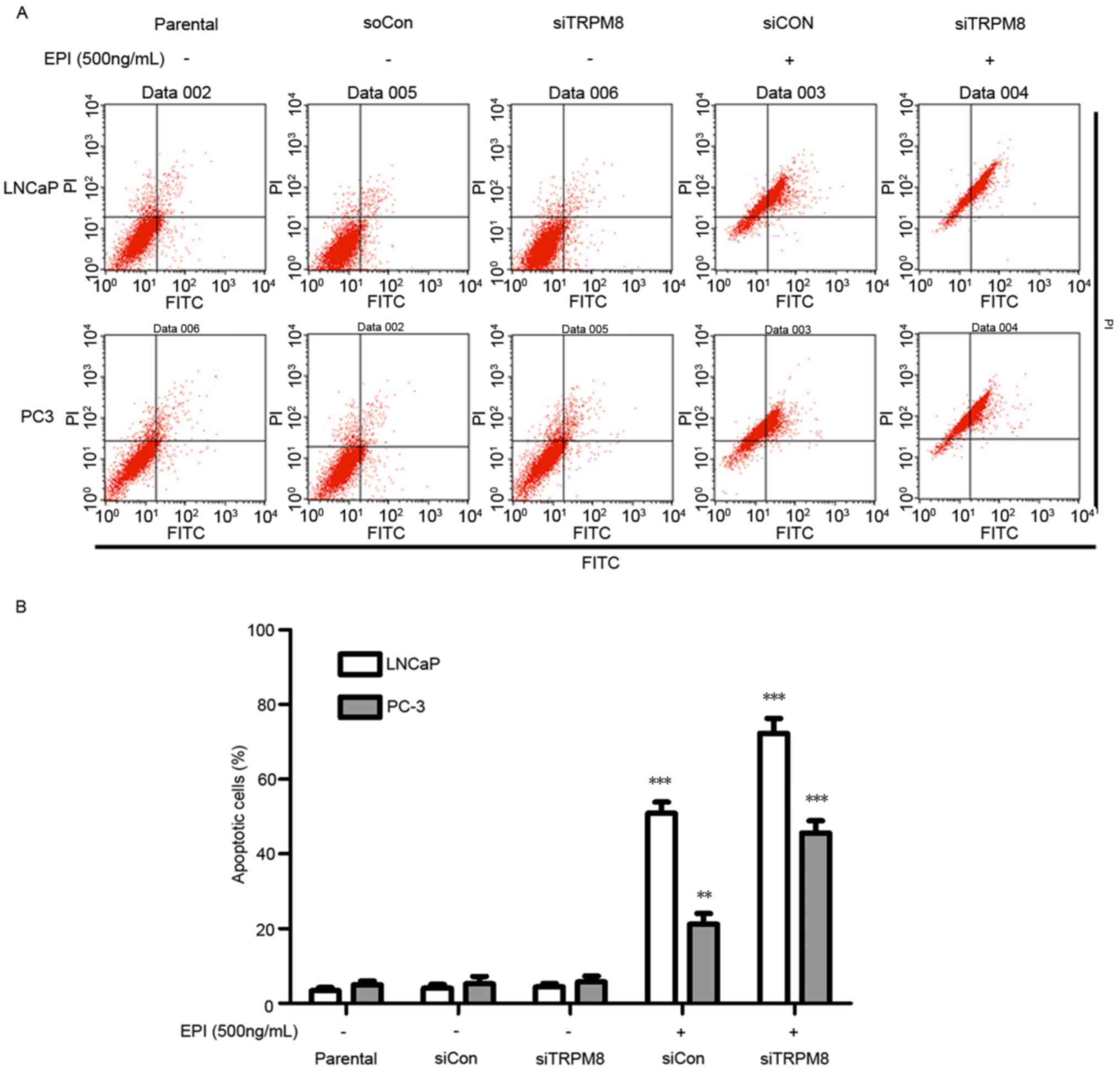
siTRPM8 promoted EPI-induced
apoptosis
When cells treated with siTRPM8 were compared with
the parental and siCON cells, no significant change in the
proportion of cells undergoing apoptosis was observed, as analyzed
by flow cytometry (Fig. 3).
Nevertheless, siTRPM8 facilitated cell apoptosis following
treatment with EPI, which was confirmed by the Annexin V-FITC flow
cytometry analysis. The concentration of EPI used in the following
experiments was set at 600 ng/ml, according to the results of the
CCK-8 assay (Fig. 3). This flow
cytometry analysis revealed that knockdown of TRPM8 facilitated
EPI-induced cell apoptosis when compared with the parental and
siCON cells in LNCaP (siTRPM8, 72.28±3.34%; siCON, 50.84±1.37%;
P<0.01) and PC3 (siTRPM8, 45.61±3.02%; siCON, 21.17±2.94%;
P<0.05) cells (Fig. 3A and B).
Silencing TRPM8 activated the MAPK
signal pathways
Whether MAPK pathways were involved in the action of
siTRPM8 was also investigated. Levels of p-p38 and p-JNK increased
in siTRPM8 cells, when compared with parental and siCON cells, with
levels of total protein remaining unchanged (Fig. 4). To confirm the involvement of the
MAPKs, the effects of the p38 inhibitor (SB203580, 20 µM) and JNK
inhibitor (SP600125, 10 µM) on the EPI-mediated proliferative
inhibition in siTRPM8 cells were analyzed by CCK-8 assays. These
specific inhibitors of p38 and JNK attenuated the enhancement of
EPI chemosensitivity of siTRPM8 (Fig.
5C). The efficiency of SB203580 and SP600125 were evaluated by
western blot; the phosphorylation of p38 and JNK was evidently
increased in siTRPM8 when compared with parental and siCON cells,
thus lowering the apoptotic thresholds. Taken together, these
results indicated that MAPK pathways at least partially
participated in the role of EPI sensitization via siTRPM8 treatment
in prostate cancer cells.
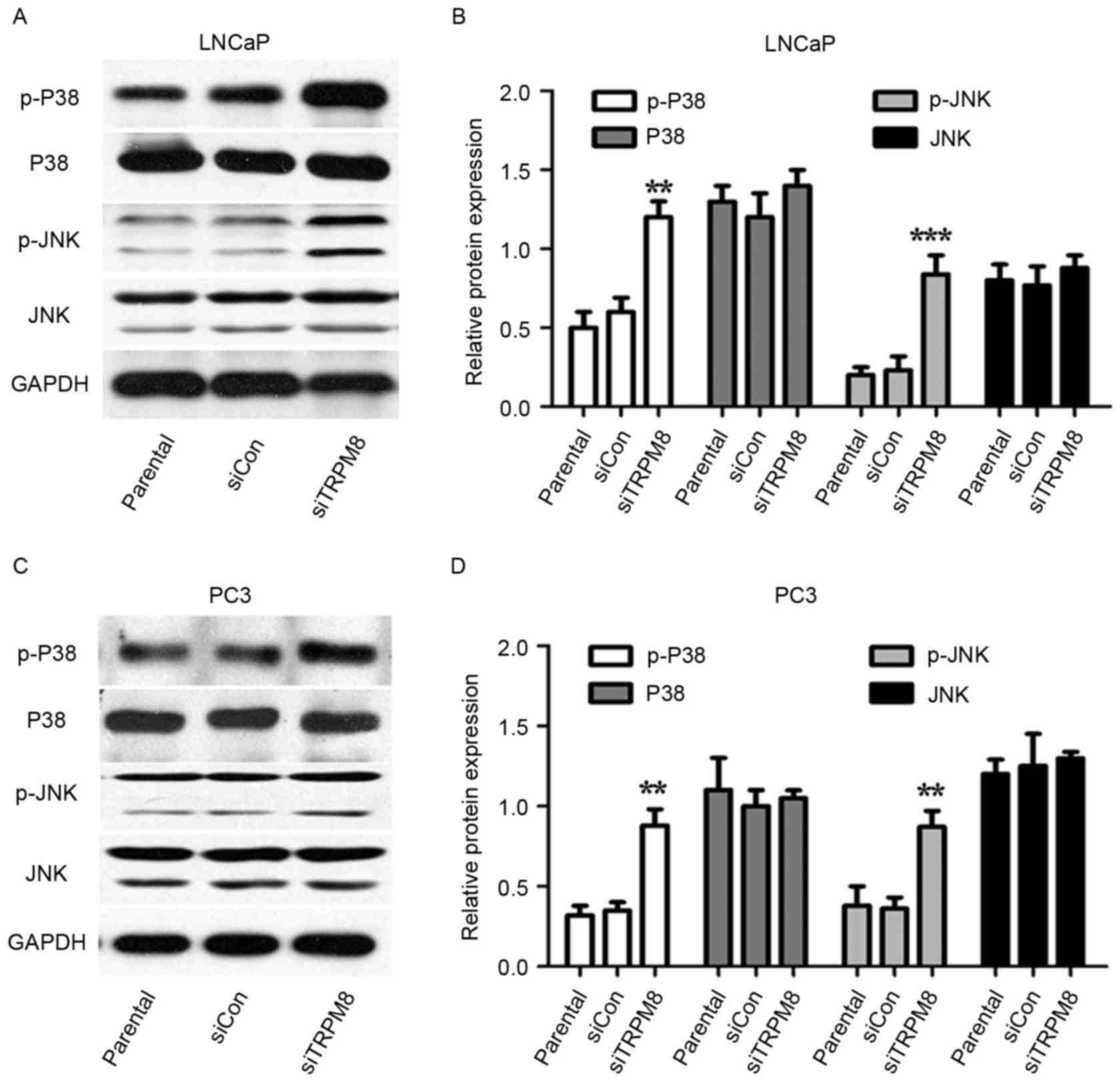 | Figure 4.Mitogen-activated protein kinase
signal pathways may partially involve in the sensitization activity
of siTRPM8 towards LNCaP and PC3 cells. (A-D) Western blot analysis
in (A) LNCaP cells, with (B) quantification and in (C) PC3 cells,
with (D) quantification. This analysis was performed performed to
investigate the expression of p-p38, p38, p-JNK, and JNK in
parental, siCON, siTRPM8 cells. **P<0.01, ***P<0.001,
compared with the parental group. p-p38, phosphorylated p38
mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase;
siTRPM8, small interfering RNA targeting transient receptor
potential cation channel subfamily M member 8; CON, negative
control. |
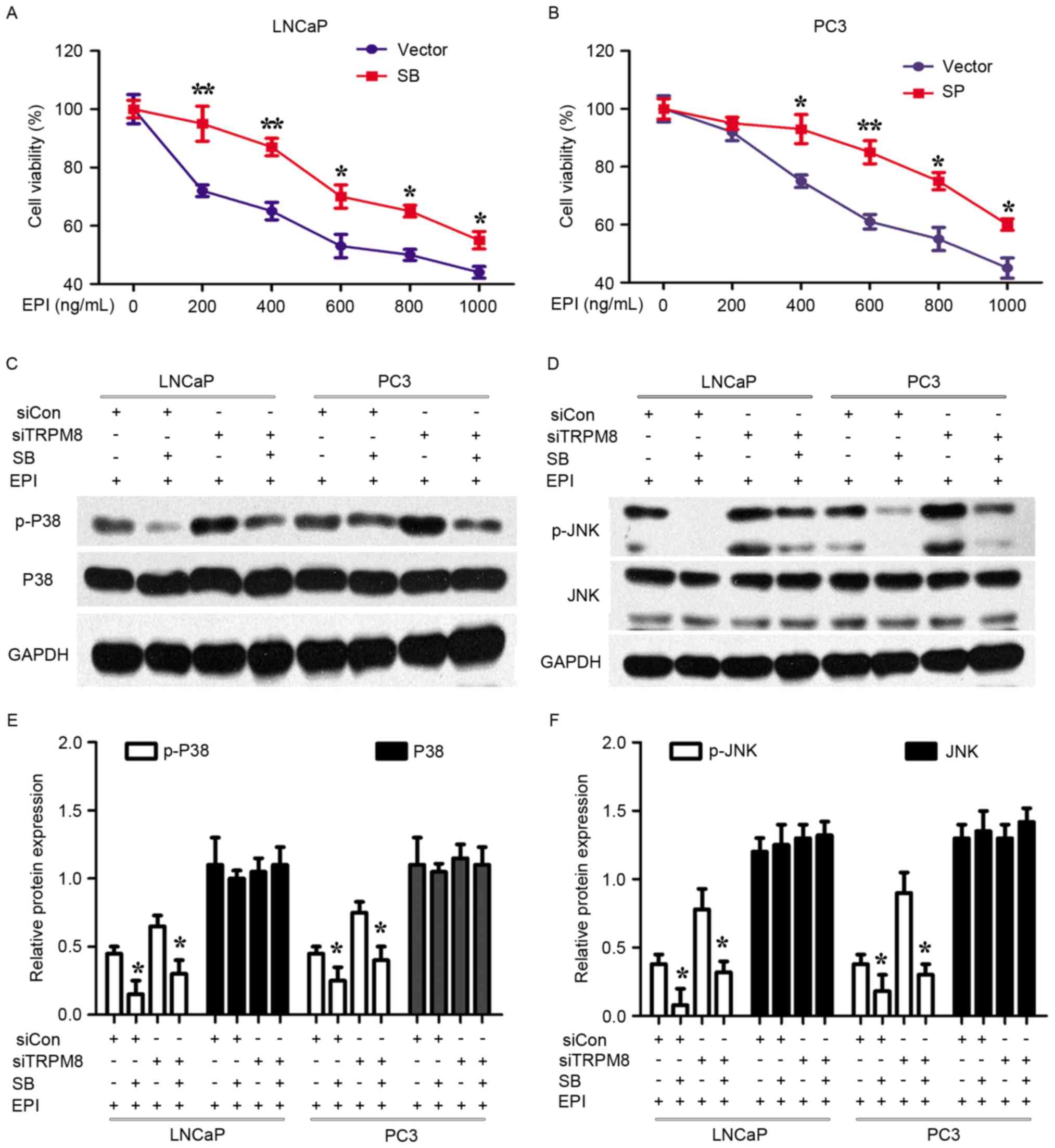 | Figure 5.Specific inhibitors of p38 and JNK
attenuated the enhancement of EPI chemosensitivity of siTRPM8. (A
and B) siTRPM8 Cells were treated with the p38 inhibitor SB203580
(20 µM) and JNK inhibitor SP600125 (10 µM) for 4 h, respectively,
and then treated with 500 ng/ml EPI in (A) LNCaP and (B) PC3 cells.
After 48 h treatment, cell viability was determined by Cell
Counting kit-8 assay. (C and D) Alteration of mitogen-activated
protein kinase signal pathways when (C) LNCaP and (D) PC3 cells
were treated with p38 and JNK inhibitors. siTRPM8 increased the
expression of p-P38 and p-JNK, which lowered the threshold of
EPI-induced apoptosis. (E and F) Quantification of the results of
(C and D), expressed in histograms. *P<0.05, **P<0.01,
compared with cells treated with the vehicle. p-p38, phosphorylated
p38 mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase;
siTRPM8, small interfering RNA targeting transient receptor
potential cation channel subfamily M member 8; CON, negative
control; EPI, epirubicin. |
Discussion
TRPM8 is considered to be a novel prostate-specific
marker; its expression is increase in PCa (2). Previous studies had indicated that TRPM8
expression increases only in early-stage PCa, decreasing markedly
as tumor progresses to the late, invasive, androgen-independent
stage (9,25). However, levels of TRPM8 expression in
PC3 cells have been disputed (26,27). Using
the nested RT-PCR method Kim et al (28) verified that the expression of TRPM8 in
LNCaP and PC3 cells was comparable. The present study investigated
the expression of TRPM8 in cancerous and non-cancerous prostate
epithelial cells by RT-PCR and western blotting (TRPM8 expression
levels: LNCaP>PC3>DU145>PNT1A).
A previous study revealed that BCTC, a potent and
representative antagonist of TRPM8, exerts an antitumor effect on
androgen-independent prostate cancer DU145 cells by p-AKT, cyclin
D1, CDK2 and CDK6, p-GSK-3β and also MAPK signal pathways (23). Zhang et al (2) and Valero et al (17) also demonstrated the antitumor effect
of knockdown or blockade of TRPM8 in PCa cells. Valero et al
(17) provided evidence that the
knockdown and antagonism (including by BCTC) of TRPM8 could inhibit
the proliferation, cell cycle progression and migration of PCa
cells. However, whether targeting TRPM8 can influence the
chemosensitivity of prostate cancer remains unknown. Therefore, the
present study uncovered the possible influence of TRPM8-knockdown
on the chemosensitivity of prostate cancer and the precise
mechanism involved.
The data produced by the present study revealed that
the RNA interference-mediated depletion of TRPM8 evidently
inhibited the proliferation of LNCaP and PC3 cells. Although
siTRPM8 treatment failed to induce apoptosis in prostate cancer
cells alone, it facilitated cell apoptosis when EPI was
administered, which indicated that the chemosensitivity of LNCaP
and PC3 cells was enhanced by TRPM8-knockdown. This result revealed
that EPI, which is routinely used as a therapeutic for treatment of
late-stage prostate cancer but to which resistance is readily
developed, could be used in combination with knockdown of
TRPM8.
MAPK family members are known to control cell cycle
progression at various stages in cell type- and context-specific
manners. The present study found that levels of p-p38 and p-JNK
increased in siTRPM8-LNCaP and siTRPM8-PC3 cells, compared with
parental and siCON cells. Furthermore, specific inhibitors of p38
and JNK attenuated the enhancement of EPI chemosensitivity induced
by siTRPM8, which indicated that MAPK pathways are partially
involved in the sensitization activity of siTRPM8 towards LNCaP and
PC3 cells.
In summary, the present study demonstrates that the
knockdown of TRPM8 using a specific siRNA reduced the proliferative
ability and enhanced the chemosensitivity of prostate cancer LNCaP
and PC3 cells. This finding is partially attributed to the
alteration of the MAPK signal pathways. These results reveal that
RNA interference-mediated depletion of TRPM8 is a therapeutic
strategy with substantial potential for treating prostate cancer,
including CRPC, which can provide novel insights into the
understanding of prostate cancer biology.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of China (nos. 81172434 and 81202027),
the Research Fund for the Doctoral Program of Higher Education of
China (no. 20130141110038) and the Project of Jingzhou Municipal
Science and Technology Bureau (no. 2013031 and 2014038).
Glossary
Abbreviations
Abbreviations:
|
TRPM8
|
transient receptor potential cation
channel subfamily M member 8
|
|
EPI
|
epirubicin
|
|
PCa
|
prostate cancer
|
|
siRNA
|
small interference RNA
|
|
siTRPM8
|
siRNA targeting TRPM8
|
|
siCON
|
negative control scrambled siRNA
|
|
CRPC
|
castration-resistant prostate
cancer
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang L and Barritt GJ: Evidence that
TRPM8 is an androgen-dependent Ca2+ channel required for
the survival of prostate cancer cells. Cancer Res. 64:8365–8373.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Egan A, Dong Y, Zhang H, Qi Y, Balk SP and
Sartor O: Castration-resistant prostate cancer: Adaptive responses
in the androgen axis. Cancer Treat Rev. 40:426–433. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katzenwadel A and Wolf P: Androgen
deprivation of prostate cancer: Leading to a therapeutic dead end.
Cancer Lett. 367:12–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petrioli R, Roviello G, Fiaschi AI, Laera
L, Miano ST, De Rubertis G, Barbanti G, Bianco V, Brozzetti S and
Francini E: Rechallenge of docetaxel combined with epirubicin given
on a weekly schedule in advanced castration-resistant prostate
cancer patients previously exposed to docetaxel and abiraterone
acetate: A single-institution experience. Med Oncol. 32:522015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thebault S, Flourakis M, Vanoverberghe K,
Vandermoere F, Roudbaraki M, Lehen'kyi V, Slomianny C, Beck B,
Mariot P, Bonnal JL, et al: Differential role of transient receptor
potential channels in Ca2+ entry and proliferation of
prostate cancer epithelial cells. Cancer Res. 66:2038–2047. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yee NS: Roles of TRPM8 ion channels in
cancer: Proliferation, survival and invasion. Cancers (Basel).
7:2134–2146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grolez GP and Gkika D: TRPM8 puts the
chill on prostate cancer. Pharmaceuticals (Basel). 9:E442016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Flourakis M and Prevarskaya N: Insights
into Ca2+ homeostasis of advanced prostate cancer cells. Biochim
Biophys Acta. 1793:1105–1109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vanoverberghe K, Vanden Abeele F, Mariot
P, Lepage G, Roudbaraki M, Bonnal JL, Mauroy B, Shuba Y, Skryma R
and Prevarskaya N: Ca2+ homeostasis and apoptotic
resistance of neuroendocrine-differentiated prostate cancer cells.
Cell Death Differ. 11:321–330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Skryma R, Mariot P, Bourhis XL, Coppenolle
FV, Shuba Y, Vanden Abeele F, Legrand G, Humez S, Boilly B and
Prevarskaya N: Store depletion and store-operated Ca2+
current in human prostate cancer LNCaP cells: Involvement in
apoptosis. J Physiol. 527:71–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vanden AF, Roudbaraki M, Shuba Y, Skryma R
and Prevarskaya N: Store-operated Ca2+ current in
prostate cancer epithelial cells. Role of endogenous
Ca2+ transporter type 1. J Biol Chem. 278:15381–15389.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu S, Xu Z, Zou C, Wu D, Wang Y, Yao X, Ng
CF and Chan FL: Ion channel TRPM8 promotes hypoxic growth of
prostate cancer cells via an O2-independent and RACK1-mediated
mechanism of HIF-1α stabilization. J Pathol. 234:514–525. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng M, Wang Z, Yang Z, Tao L, Liu Q, Yi
LU and Wang X: Overexpression of short TRPM8 variant alpha promotes
cell migration and invasion and decreases starvation-induced
apoptosis in prostate cancer LNCaP cells. Oncol Lett. 10:1378–1384.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bidaux G, Borowiec AS, Dubois C, Delcourt
P, Schulz C, Vanden Abeele F, Lepage G, Desruelles E, Bokhobza A,
Dewailly E, et al: Targeting of short TRPM8 isoforms induces
4TM-TRPM8-dependent apoptosis in prostate cancer cells. Oncotarget.
7:29063–29080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Asuthkar S, Velpula KK, Elustondo PA,
Demirkhanyan L and Zakharian E: TRPM8 channel as a novel molecular
target in androgen-regulated prostate cancer cells. Oncotarget.
6:17221–17236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valero ML, Mello de Queiroz F, Stühmer W,
Viana F and Pardo LA: TRPM8 ion channels differentially modulate
proliferation and cell cycle distribution of normal and cancer
prostate cells. PLoS One. 7:e518252012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grolez GP and Gkika D: TRPM8 puts the
chill on prostate cancer. Pharmaceuticals (Basel). 9:E442016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu G, Wang X, Yang Z, Cao H, Meng Z, Wang
Y and Chen D: Effects of TRPM8 on the proliferation and
angiogenesis of prostate cancer PC-3 cells in vivo. Oncol Lett.
2:1213–1217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang ZH, Wang XH, Wang HP and Hu LQ:
Effects of TRPM8 on the proliferation and motility of prostate
cancer PC-3 cells. Asian J Androl. 11:157–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng M, Wang Z, Yang Z, Tao L, Liu Q, Yi
LU and Wang X: Overexpression of short TRPM8 variant α promotes
cell migration and invasion and decreases starvation-induced
apoptosis in prostate cancer LNCaP cells. Oncol Lett. 10:1378–1384.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valero M, Morenilla-Palao C, Belmonte C
and Viana F: Pharmacological and functional properties of TRPM8
channels in prostate tumor cells. Pflugers Arch. 461:99–114. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu T, Fang Z, Wang G, Shi M, Wang X,
Jiang K, Yang Z, Cao R, Tao H, Wang X and Zhou J: Anti-tumor
activity of the TRPM8 inhibitor BCTC in prostate cancer DU145
cells. Oncol Lett. 11:182–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Petrioli R, Pascucci A, Conca R, Chiriacò
G, Francini E, Bargagli G, Fiaschi AI, Manganelli A, De Rubertis G
and Barbanti G: Docetaxel and epirubicin compared with docetaxel
and prednisone in advanced castrate-resistant prostate cancer: A
randomised phase II study. Br J Cancer. 104:613–619. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Henshall SM, Afar DE, Hiller J, Horvath
LG, Quinn DI, Rasiah KK, Gish K, Willhite D, Kench JG,
Gardiner-Garden M, et al: Survival analysis of genome-wide gene
expression profiles of prostate cancers identifies new prognostic
targets of disease relapse. Cancer Res. 63:4196–4203.
2003.PubMed/NCBI
|
|
26
|
Bidaux G, Flourakis M, Thebault S, Zholos
A, Beck B, Gkika D, Roudbaraki M, Bonnal JL, Mauroy B, Shuba Y, et
al: Prostate cell differentiation status determines transient
receptor potential melastatin member 8 channel subcellular
localization and function. J Clin Invest. 117:1647–1657. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thebault S, Lemonnier L, Bidaux G,
Flourakis M, Bavencoffe A, Gordienko D, Roudbaraki M, Delcourt P,
Panchin Y, Shuba Y, et al: Novel role of cold/menthol-sensitive
transient receptor potential melastatine family member 8 (TRPM8) in
the activation of store-operated channels in LNCaP human prostate
cancer epithelial cells. J Biol Chem. 280:39423–39435. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SH, Nam JH, Park EJ, Kim BJ, Kim SJ,
So I and Jeon JH: Menthol regulates TRPM8-independent processes in
PC-3 prostate cancer cells. Biochim Biophys Acta. 1792:33–38. 2009.
View Article : Google Scholar : PubMed/NCBI
|